Submitted:
26 June 2023
Posted:
26 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Transgenic Mouse Models for HTLV-1 Research
3. Development of Severely Immunodeficient Mice
4. Immunodeficient Mouse Models for HTLV-1 Research
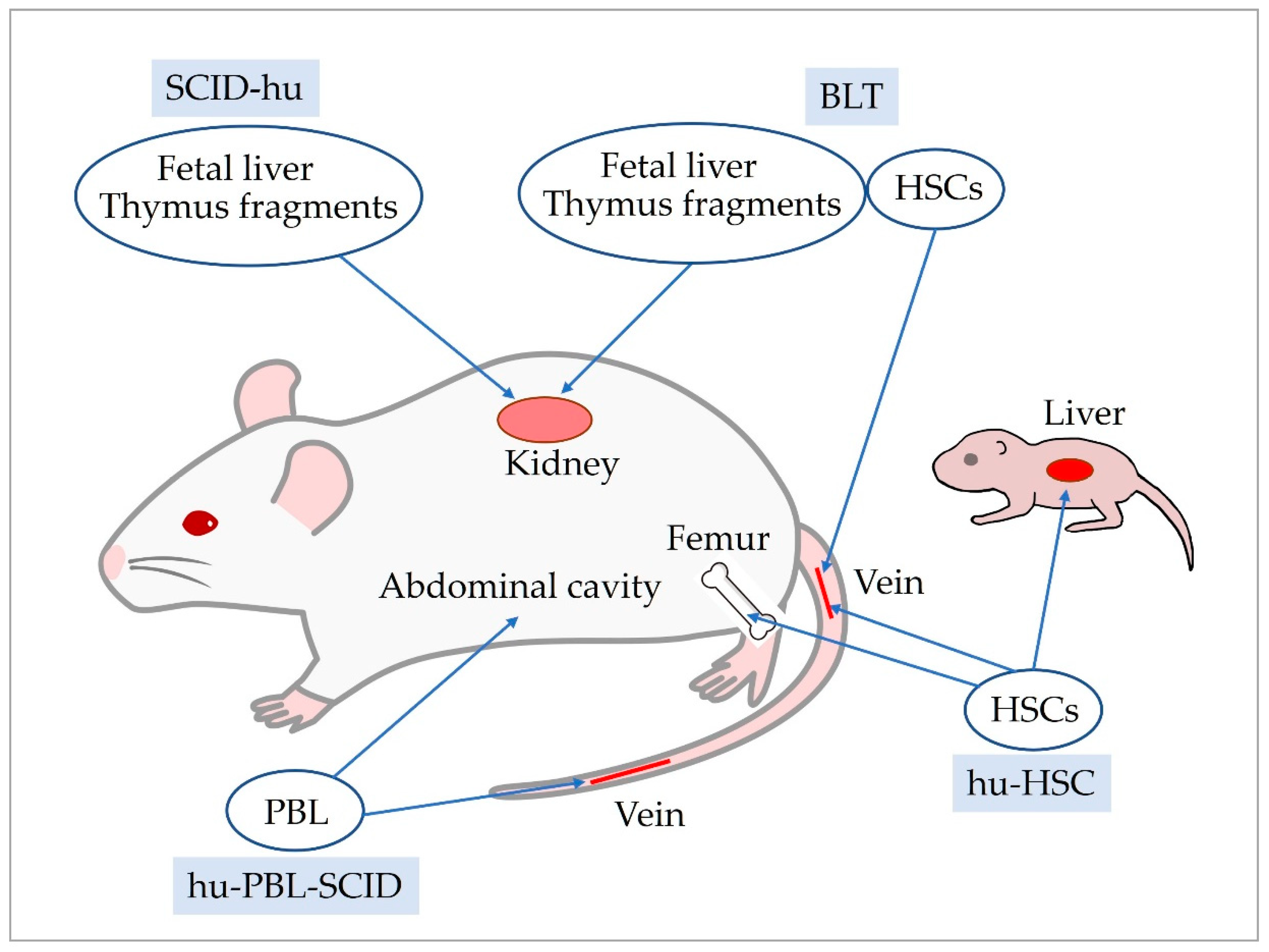
5. Development of Human Immune System Mice
6. Improvements in the hu-HSC Mice
7. Human Immune System Mouse Models for HTLV-1 Research
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and Isolation of Type C Retrovirus Particles from Fresh and Cultured Lymphocytes of a Patient with Cutaneous T-Cell Lymphoma. Proc. Natl. Acad. Sci. U. S. A. 1980, 77, 7415–7419. [Google Scholar] [CrossRef]
- Poiesz, B.J.; Ruscetti, F.W.; Reitz, M.S.; Kalyanaraman, V.S.; Gallo, R.C. Isolation of a New Type C Retrovirus (HTLV) in Primary Uncultured Cells of a Patient with Sézary T-Cell Leukaemia. Nature 1981, 294, 268–271. [Google Scholar] [CrossRef]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Takatsuki, K.; Uchiyama, T.; Sagawa, K.; Yodoi, J.; Seno, S.; Takaku, F.; Irino, S. Topics in Hematology. In Proceedings of the The 16th International Congress of Hematology; Excerpta Medica: Amsterdam, 1977; pp. 73–77.
- Uchiyama, T.; Yodoi, J.; Sagawa, K.; Takatsuki, K.; Uchino, H. Adult T-Cell Leukemia: Clinical and Hematologic Features of 16 Cases. Blood 1977, 50, 481–492. [Google Scholar] [CrossRef]
- Gessain, A.; Barin, F.; Vernant, J.C.; Gout, O.; Maurs, L.; Calender, A.; de Thé, G. Antibodies to Human T-Lymphotropic Virus Type-I in Patients with Tropical Spastic Paraparesis. Lancet (London, England) 1985, 2, 407–410. [Google Scholar] [CrossRef]
- Iwanaga, M.; Watanabe, T.; Yamaguchi, K. Adult T-Cell Leukemia: A Review of Epidemiological Evidence. Front. Microbiol. 2012, 3, 322. [Google Scholar] [CrossRef]
- Yamano, Y.; Sato, T. Clinical Pathophysiology of Human T-Lymphotropic Virus-Type 1-Associated Myelopathy/Tropical Spastic Paraparesis. Front. Microbiol. 2012, 3, 389. [Google Scholar] [CrossRef]
- Shimoyama, M. Diagnostic Criteria and Classification of Clinical Subtypes of Adult T-Cell Leukaemia-Lymphoma. A Report from the Lymphoma Study Group (1984-87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef]
- Katsuya, H.; Ishitsuka, K.; Utsunomiya, A.; Hanada, S.; Eto, T.; Moriuchi, Y.; Saburi, Y.; Miyahara, M.; Sueoka, E.; Uike, N.; et al. Treatment and Survival among 1594 Patients with ATL. Blood 2015, 126, 2570–2577. [Google Scholar] [CrossRef]
- Mahgoub, M.; Yasunaga, J.-I.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off Switching of HTLV-1 Tax Expression Is Crucial to Maintain the Whole Population of Virus-Induced Leukemic Cells. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E1269–E1278. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kubota, R.; Tara, M.; Izumo, S.; Osame, M. Existence of Escape Mutant in HTLV-I Tax during the Development of Adult T-Cell Leukemia. Blood 2001, 97, 987–993. [Google Scholar] [CrossRef]
- Koiwa, T.; Hamano-Usami, A.; Ishida, T.; Okayama, A.; Yamaguchi, K.; Kamihira, S.; Watanabe, T. 5’-Long Terminal Repeat-Selective CpG Methylation of Latent Human T-Cell Leukemia Virus Type 1 Provirus in Vitro and in Vivo. J. Virol. 2002, 76, 9389–9397. [Google Scholar] [CrossRef]
- Satou, Y.; Yasunaga, J.-I.; Zhao, T.; Yoshida, M.; Miyazato, P.; Takai, K.; Shimizu, K.; Ohshima, K.; Green, P.L.; Ohkura, N.; et al. HTLV-1 BZIP Factor Induces T-Cell Lymphoma and Systemic Inflammation in Vivo. PLoS Pathog. 2011, 7, e1001274. [Google Scholar] [CrossRef]
- Satou, Y.; Yasunaga, J.; Yoshida, M.; Matsuoka, M. HTLV-I Basic Leucine Zipper Factor Gene MRNA Supports Proliferation of Adult T Cell Leukemia Cells. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 720–725. [Google Scholar] [CrossRef]
- Tanaka-Nakanishi, A.; Yasunaga, J.; Takai, K.; Matsuoka, M. HTLV-1 BZIP Factor Suppresses Apoptosis by Attenuating the Function of FoxO3a and Altering Its Localization. Cancer Res. 2014, 74, 188–200. [Google Scholar] [CrossRef]
- Vernin, C.; Thenoz, M.; Pinatel, C.; Gessain, A.; Gout, O.; Delfau-Larue, M.-H.; Nazaret, N.; Legras-Lachuer, C.; Wattel, E.; Mortreux, F. HTLV-1 BZIP Factor HBZ Promotes Cell Proliferation and Genetic Instability by Activating OncomiRs. Cancer Res. 2014, 74, 6082–6093. [Google Scholar] [CrossRef]
- Gill, P.S.; Harrington, W.J.; Kaplan, M.H.; Ribeiro, R.C.; Bennett, J.M.; Liebman, H.A.; Bernstein-Singer, M.; Espina, B.M.; Cabral, L.; Allen, S. Treatment of Adult T-Cell Leukemia-Lymphoma with a Combination of Interferon Alfa and Zidovudine. N. Engl. J. Med. 1995, 332, 1744–1748. [Google Scholar] [CrossRef]
- Hermine, O.; Bouscary, D.; Gessain, A.; Turlure, P.; Leblond, V.; Franck, N.; Buzyn-Veil, A.; Rio, B.; Macintyre, E.; Dreyfus, F. Brief Report: Treatment of Adult T-Cell Leukemia-Lymphoma with Zidovudine and Interferon Alfa. N. Engl. J. Med. 1995, 332, 1749–1751. [Google Scholar] [CrossRef]
- Okamura, J.; Utsunomiya, A.; Tanosaki, R.; Uike, N.; Sonoda, S.; Kannagi, M.; Tomonaga, M.; Harada, M.; Kimura, N.; Masuda, M.; et al. Allogeneic Stem-Cell Transplantation with Reduced Conditioning Intensity as a Novel Immunotherapy and Antiviral Therapy for Adult T-Cell Leukemia/Lymphoma. Blood 2005, 105, 4143–4145. [Google Scholar] [CrossRef]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated Anti-CCR4 Monoclonal Antibody (KW-0761) for Relapsed Adult T-Cell Leukemia-Lymphoma: A Multicenter Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef]
- Shichijo, T.; Nosaka, K.; Tatetsu, H.; Higuchi, Y.; Endo, S.; Inoue, Y.; Toyoda, K.; Kikukawa, Y.; Kawakita, T.; Yasunaga, J.-I.; et al. Beneficial Impact of First-Line Mogamulizumab-Containing Chemotherapy in Adult T-Cell Leukaemia-Lymphoma. Br. J. Haematol. 2022, 198, 983–987. [Google Scholar] [CrossRef]
- Suehiro, Y.; Hasegawa, A.; Iino, T.; Sasada, A.; Watanabe, N.; Matsuoka, M.; Takamori, A.; Tanosaki, R.; Utsunomiya, A.; Choi, I.; et al. Clinical Outcomes of a Novel Therapeutic Vaccine with Tax Peptide-Pulsed Dendritic Cells for Adult T Cell Leukaemia/Lymphoma in a Pilot Study. Br. J. Haematol. 2015, 169, 356–367. [Google Scholar] [CrossRef]
- Nerenberg, M.; Hinrichs, S.H.; Reynolds, R.K.; Khoury, G.; Jay, G. The Tat Gene of Human T-Lymphotropic Virus Type 1 Induces Mesenchymal Tumors in Transgenic Mice. Science 1987, 237, 1324–1329. [Google Scholar] [CrossRef]
- Furuta, Y.; Aizawa, S.; Suda, Y.; Ikawa, Y.; Kishimoto, H.; Asano, Y.; Tada, T.; Hikikoshi, A.; Yoshida, M.; Seiki, M. Thymic Atrophy Characteristic in Transgenic Mice That Harbor PX Genes of Human T-Cell Leukemia Virus Type I. J. Virol. 1989, 63, 3185–3189. [Google Scholar] [CrossRef]
- Hinrichs, S.H.; Nerenberg, M.; Reynolds, R.K.; Khoury, G.; Jay, G. A Transgenic Mouse Model for Human Neurofibromatosis. Science (80-. ). 1987, 237, 1340–1343. [Google Scholar] [CrossRef]
- Green, J.E.; Hinrichs, S.H.; Vogel, J.; Jay, G. Exocrinopathy Resembling Sjögren’s Syndrome in HTLV-1 Tax Transgenic Mice. Nature 1989, 341, 72–74. [Google Scholar] [CrossRef]
- Yoshida, E.M.I.; Takiguchi, M.; Hatanaka, M.; Yamamoto, H. Induction of Inflammatory Arthropathy Resembling Rheumatoid Artbritis. 1983, 253, 4–6.
- Yamamoto, H.; Sekiguchi, T.; Yamamoto, I. Histopathological Observation of Joint Lesions of Extremities in Mice Transferred Genome. Exp. Toxicol. Pathol. Off. J. Gesellschaft fur Toxikologische Pathol. 1993, 45, 233–238. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sekiguchi, T.; Itagaki, K.; Saijo, S.; Iwakura, Y. Inflammatory Polyarthritis in Mice Transgenic for Human T Cell Leukemia Virus Type I. Arthritis Rheum. 1993, 36, 1612–1620. [Google Scholar] [CrossRef]
- Ruddle, N.H.; Li, C.B.; Horne, W.C.; Santiago, P.; Troiano, N.; Jay, G.; Horowitz, M.; Baron, R. Mice Transgenic for HTLV-I LTR-Tax Exhibit Tax Expression in Bone, Skeletal Alterations, and High Bone Turnover. Virology 1993, 197, 196–204. [Google Scholar] [CrossRef]
- Bieberich, C.J.; King, C.M.; Tinkle, B.T.; Jay, G. A Transgenic Model of Transactivation by the Tax Protein of HTLV-I. Virology 1993, 196, 309–318. [Google Scholar] [CrossRef]
- Grossman, W.J.; Kimata, J.T.; Wong, F.H.; Zutter, M.; Ley, T.J.; Ratner, L. Development of Leukemia in Mice Transgenic for the Tax Gene of Human T-Cell Leukemia Virus Type I. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 1057–1061. [Google Scholar] [CrossRef]
- Grossman, W.J.; Ratner, L. Cytokine Expression and Tumorigenicity of Large Granular Lymphocytic Leukemia Cells From Mice Transgenic for the Tax Gene of Human T-Cell Leukemia Virus Type I. Blood 1997, 90, 783–794. [Google Scholar] [CrossRef]
- Gao, L.; Deng, H.; Zhao, H.; Hirbe, A.; Harding, J.; Ratner, L.; Weilbaecher, K. HTLV-1 Tax Transgenic Mice Develop Spontaneous Osteolytic Bone Metastases Prevented by Osteoclast Inhibition. Blood 2005, 106, 4294–4302. [Google Scholar] [CrossRef]
- Hall, A.P.; Irvine, J.; Blyth, K.; Cameron, E.R.; Onions, D.E.; Campbell, M.E. Tumours Derived from HTLV-I Tax Transgenic Mice Are Characterized by Enhanced Levels of Apoptosis and Oncogene Expression. J. Pathol. 1998, 186, 209–214. [Google Scholar] [CrossRef]
- Hasegawa, H.; Sawa, H.; Lewis, M.J.; Orba, Y.; Sheehy, N.; Yamamoto, Y.; Ichinohe, T.; Tsunetsugu-Yokota, Y.; Katano, H.; Takahashi, H.; et al. Thymus-Derived Leukemia-Lymphoma in Mice Transgenic for the Tax Gene of Human T-Lymphotropic Virus Type I. Nat. Med. 2006, 12, 466–472. [Google Scholar] [CrossRef]
- Ohsugi, T.; Kumasaka, T.; Okada, S.; Urano, T. The Tax Protein of HTLV-1 Promotes Oncogenesis in Not Only Immature T Cells but Also Mature T Cells. Nat. Med. 2007, 13, 527–528. [Google Scholar] [CrossRef]
- Shinohara, T. [HTLV-I tax mediated activation of cellular genes in transgenic mice]. Hokkaido Igaku Zasshi. 1991, 66, 534–543. [Google Scholar]
- Kim, S.J.; Winokur, T.S.; Lee, H.D.; Danielpour, D.; Kim, K.Y.; Geiser, A.G.; Chen, L.S.; Sporn, M.B.; Roberts, A.B.; Jay, G. Overexpression of Transforming Growth Factor-Beta in Transgenic Mice Carrying the Human T-Cell Lymphotropic Virus Type I Tax Gene. Mol. Cell. Biol. 1991, 11, 5222–5228. [Google Scholar] [CrossRef]
- Iwakura, Y.; Saijo, S.; Kioka, Y.; Nakayama-Yamada, J.; Itagaki, K.; Tosu, M.; Asano, M.; Kanai, Y.; Kakimoto, K. Autoimmunity Induction by Human T Cell Leukemia Virus Type 1 in Transgenic Mice That Develop Chronic Inflammatory Arthropathy Resembling Rheumatoid Arthritis in Humans. J. Immunol. 1995, 155, 1588–1598. [Google Scholar] [CrossRef]
- Portis, T.; Harding, J.C.; Ratner, L. The Contribution of NF-Kappa B Activity to Spontaneous Proliferation and Resistance to Apoptosis in Human T-Cell Leukemia Virus Type 1 Tax-Induced Tumors. Blood 2001, 98, 1200–1208. [Google Scholar] [CrossRef]
- Kitajima, I.; Shinohara, T.; Bilakovics, J.; Brown, D.A.; Xu, X.; Nerenberg, M. Ablation of Transplanted HTLV-I Tax-Transformed Tumors in Mice by Antisense Inhibition of NF-Kappa B. Science 1992, 258, 1792–1795. [Google Scholar] [CrossRef]
- Kwon, H.; Ogle, L.; Benitez, B.; Bohuslav, J.; Montano, M.; Felsher, D.W.; Greene, W.C. Lethal Cutaneous Disease in Transgenic Mice Conditionally Expressing Type I Human T Cell Leukemia Virus Tax. J. Biol. Chem. 2005, 280, 35713–35722. [Google Scholar] [CrossRef]
- Ratner, L.; Portis, T.; Robek, M.; Harding, J.; Grossman, W. Studies of the Immortalizing Activity of HTLV Type 1 Tax, Using an Infectious Molecular Clone and Transgenic Mice. AIDS Res. Hum. Retroviruses 2000, 16, 1647–1651. [Google Scholar] [CrossRef]
- Portis, T.; Grossman, W.J.; Harding, J.C.; Hess, J.L.; Ratner, L. Analysis of P53 Inactivation in a Human T-Cell Leukemia Virus Type 1 Tax Transgenic Mouse Model. J. Virol. 2001, 75, 2185–2193. [Google Scholar] [CrossRef]
- Mitra-Kaushik, S.; Harding, J.; Hess, J.; Schreiber, R.; Ratner, L. Enhanced Tumorigenesis in HTLV-1 Tax-Transgenic Mice Deficient in Interferon-Gamma. Blood 2004, 104, 3305–3311. [Google Scholar] [CrossRef]
- Xu, Z.; Hurchla, M.A.; Deng, H.; Uluçkan, O.; Bu, F.; Berdy, A.; Eagleton, M.C.; Heller, E.A.; Floyd, D.H.; Dirksen, W.P.; et al. Interferon-Gamma Targets Cancer Cells and Osteoclasts to Prevent Tumor-Associated Bone Loss and Bone Metastases. J. Biol. Chem. 2009, 284, 4658–4666. [Google Scholar] [CrossRef]
- Esser, A.K.; Rauch, D.A.; Xiang, J.; Harding, J.C.; Kohart, N.A.; Ross, M.H.; Su, X.; Wu, K.; Huey, D.; Xu, Y.; et al. HTLV-1 Viral Oncogene HBZ Induces Osteolytic Bone Disease in Transgenic Mice. Oncotarget 2017, 8, 69250–69263. [Google Scholar] [CrossRef]
- Yamamoto-Taguchi, N.; Satou, Y.; Miyazato, P.; Ohshima, K.; Nakagawa, M.; Katagiri, K.; Kinashi, T.; Matsuoka, M. HTLV-1 BZIP Factor Induces Inflammation through Labile Foxp3 Expression. PLoS Pathog. 2013, 9, e1003630. [Google Scholar] [CrossRef]
- Mitagami, Y.; Yasunaga, J.-I.; Kinosada, H.; Ohshima, K.; Matsuoka, M. Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 BZIP Factor Transgenic Mice. PLoS Pathog. 2015, 11, e1005120. [Google Scholar] [CrossRef]
- Yasuma, K.; Yasunaga, J.; Takemoto, K.; Sugata, K.; Mitobe, Y.; Takenouchi, N.; Nakagawa, M.; Suzuki, Y.; Matsuoka, M. HTLV-1 BZIP Factor Impairs Anti-Viral Immunity by Inducing Co-Inhibitory Molecule, T Cell Immunoglobulin and ITIM Domain (TIGIT). PLoS Pathog. 2016, 12, e1005372. [Google Scholar] [CrossRef]
- Kinosada, H.; Yasunaga, J.-I.; Shimura, K.; Miyazato, P.; Onishi, C.; Iyoda, T.; Inaba, K.; Matsuoka, M. HTLV-1 BZIP Factor Enhances T-Cell Proliferation by Impeding the Suppressive Signaling of Co-Inhibitory Receptors. PLoS Pathog. 2017, 13, e1006120. [Google Scholar] [CrossRef]
- Higuchi, Y.; Yasunaga, J.-I.; Mitagami, Y.; Tsukamoto, H.; Nakashima, K.; Ohshima, K.; Matsuoka, M. HTLV-1 Induces T Cell Malignancy and Inflammation by Viral Antisense Factor-Mediated Modulation of the Cytokine Signaling. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 13740–13749. [Google Scholar] [CrossRef]
- Sugata, K.; Yasunaga, J.-I.; Mitobe, Y.; Miura, M.; Miyazato, P.; Kohara, M.; Matsuoka, M. Protective Effect of Cytotoxic T Lymphocytes Targeting HTLV-1 BZIP Factor. Blood 2015, 126, 1095–1105. [Google Scholar] [CrossRef]
- Zhao, T.; Satou, Y.; Matsuoka, M. Development of T Cell Lymphoma in HTLV-1 BZIP Factor and Tax Double Transgenic Mice. Arch. Virol. 2014, 159, 1849–1856. [Google Scholar] [CrossRef]
- Yamazaki, J.; Mizukami, T.; Takizawa, K.; Kuramitsu, M.; Momose, H.; Masumi, A.; Ami, Y.; Hasegawa, H.; Hall, W.W.; Tsujimoto, H.; et al. Identification of Cancer Stem Cells in a Tax-Transgenic (Tax-Tg) Mouse Model of Adult T-Cell Leukemia/Lymphoma. Blood 2009, 114, 2709–2720. [Google Scholar] [CrossRef]
- Kuribayashi, W.; Takizawa, K.; Sugata, K.; Kuramitsu, M.; Momose, H.; Sasaki, E.; Hiradate, Y.; Furuhata, K.; Asada, Y.; Iwama, A.; et al. Impact of the SCF Signaling Pathway on Leukemia Stem Cell-Mediated ATL Initiation and Progression in an HBZ Transgenic Mouse Model. Oncotarget 2016, 7, 51027–51043. [Google Scholar] [CrossRef]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A Severe Combined Immunodeficiency Mutation in the Mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef]
- Araki, R.; Fujimori, A.; Hamatani, K.; Mita, K.; Saito, T.; Mori, M.; Fukumura, R.; Morimyo, M.; Muto, M.; Itoh, M.; et al. Nonsense Mutation at Tyr-4046 in the DNA-Dependent Protein Kinase Catalytic Subunit of Severe Combined Immune Deficiency Mice. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 2438–2443. [Google Scholar] [CrossRef]
- McCune, J.M.; Namikawa, R.; Kaneshima, H.; Shultz, L.D.; Lieberman, M.; Weissman, I.L. The SCID-Hu Mouse: Murine Model for the Analysis of Human Hematolymphoid Differentiation and Function. Science 1988, 241, 1632–1639. [Google Scholar] [CrossRef]
- Mosier, D.E.; Gulizia, R.J.; Baird, S.M.; Wilson, D.B. Transfer of a Functional Human Immune System to Mice with Severe Combined Immunodeficiency. Nature 1988, 335, 256–259. [Google Scholar] [CrossRef]
- Dorshkind, K.; Pollack, S.B.; Bosma, M.J.; Phillips, R.A. Natural Killer (NK) Cells Are Present in Mice with Severe Combined Immunodeficiency (Scid). J. Immunol. 1985, 134, 3798–3801. [Google Scholar]
- Bosma, G.C.; Fried, M.; Custer, R.P.; Carroll, A.; Gibson, D.M.; Bosma, M.J. Evidence of Functional Lymphocytes in Some (Leaky) Scid Mice. J. Exp. Med. 1988, 167, 1016–1033. [Google Scholar] [CrossRef]
- Shultz, L.D.; Schweitzer, P.A.; Christianson, S.W.; Gott, B.; Schweitzer, I.B.; Tennent, B.; McKenna, S.; Mobraaten, L.; Rajan, T. V; Greiner, D.L. Multiple Defects in Innate and Adaptive Immunologic Function in NOD/LtSz-Scid Mice. J. Immunol. 1995, 154, 180–191. [Google Scholar] [CrossRef]
- Hesselton, R.M.; Greiner, D.L.; Mordes, J.P.; Rajan, T. V; Sullivan, J.L.; Shultz, L.D. High Levels of Human Peripheral Blood Mononuclear Cell Engraftment and Enhanced Susceptibility to Human Immunodeficiency Virus Type 1 Infection in NOD/LtSz-Scid/Scid Mice. J. Infect. Dis. 1995, 172, 974–982. [Google Scholar] [CrossRef]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/Gamma(c)(Null) Mouse: An Excellent Recipient Mouse Model for Engraftment of Human Cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human Lymphoid and Myeloid Cell Development in NOD/LtSz-Scid IL2R Gamma Null Mice Engrafted with Mobilized Human Hemopoietic Stem Cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef]
- Okada, S.; Harada, H.; Ito, T.; Saito, T.; Suzu, S. Early Development of Human Hematopoietic and Acquired Immune Systems in New Born NOD/Scid/Jak3null Mice Intrahepatic Engrafted with Cord Blood-Derived CD34 + Cells. Int. J. Hematol. 2008, 88, 476–482. [Google Scholar] [CrossRef]
- Pearson, T.; Shultz, L.D.; Miller, D.; King, M.; Laning, J.; Fodor, W.; Cuthbert, A.; Burzenski, L.; Gott, B.; Lyons, B.; et al. Non-Obese Diabetic-Recombination Activating Gene-1 (NOD-Rag1 Null) Interleukin (IL)-2 Receptor Common Gamma Chain (IL2r Gamma Null) Null Mice: A Radioresistant Model for Human Lymphohaematopoietic Engraftment. Clin. Exp. Immunol. 2008, 154, 270–284. [Google Scholar] [CrossRef]
- Ishihara, S.; Tachibana, N.; Okayama, A.; Murai, K.; Tsuda, K.; Mueller, N. Successful Graft of HTLV-I-Transformed Human T-Cells (MT-2) in Severe Combined Immunodeficiency Mice Treated with Anti-Asialo GM-1 Antibody. Jpn. J. Cancer Res. 1992, 83, 320–323. [Google Scholar] [CrossRef]
- Ohsugi, T.; Ishibashi, K.; Shingu, M.; Nomura, T. Engraftment of HTLV-I-Transformed Human T-Cell Line into SCID Mice with NK Cell Function. J. Vet. Med. Sci. 1994, 56, 601–603. [Google Scholar] [CrossRef]
- Feuer, G.; Zack, J.A.; Harrington, W.J.J.; Valderama, R.; Rosenblatt, J.D.; Wachsman, W.; Baird, S.M.; Chen, I.S. Establishment of Human T-Cell Leukemia Virus Type I T-Cell Lymphomas in Severe Combined Immunodeficient Mice. Blood 1993, 82, 722–731. [Google Scholar] [CrossRef]
- Kondo, A.; Imada, K.; Hattori, T.; Yamabe, H.; Tanaka, T.; Miyasaka, M.; Okuma, M.; Uchiyama, T. A Model of in Vivo Cell Proliferation of Adult T-Cell Leukemia. Blood 1993, 82, 2501–2509. [Google Scholar] [CrossRef]
- Imada, K.; Takaori-Kondo, A.; Uchiyama, T. [Analysis of in vivo cell proliferation of ATL using SCID mice]. Rinsho. Ketsueki. 1995, 36, 573–577. [Google Scholar]
- Watters, K.M.; Dean, J.; Hasegawa, H.; Sawa, H.; Hall, W.; Sheehy, N. Cytokine and Growth Factor Expression by HTLV-1 Lck-Tax Transgenic Cells in SCID Mice. AIDS Res. Hum. Retroviruses 2010, 26, 593–603. [Google Scholar] [CrossRef]
- Yan, P.; Fu, J.; Qu, Z.; Li, S.; Tanaka, T.; Grusby, M.J.; Xiao, G. PDLIM2 Suppresses Human T-Cell Leukemia Virus Type I Tax-Mediated Tumorigenesis by Targeting Tax into the Nuclear Matrix for Proteasomal Degradation. Blood 2009, 113, 4370–4380. [Google Scholar] [CrossRef]
- Takeda, S.; Maeda, M.; Morikawa, S.; Taniguchi, Y.; Yasunaga, J.-I.; Nosaka, K.; Tanaka, Y.; Matsuoka, M. Genetic and Epigenetic Inactivation of Tax Gene in Adult T-Cell Leukemia Cells. Int. J. cancer 2004, 109, 559–567. [Google Scholar] [CrossRef]
- Arnold, J.; Zimmerman, B.; Li, M.; Lairmore, M.D.; Green, P.L. Human T-Cell Leukemia Virus Type-1 Antisense-Encoded Gene, Hbz, Promotes T-Lymphocyte Proliferation. Blood 2008, 112, 3788–3797. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Terashima, K.; Taruishi, M.; Hasegawa, H.; Ito, M.; Tanaka, Y.; Mori, N.; Sata, T.; Koyanagi, Y.; Maeda, M.; et al. Rapid Tumor Formation of Human T-Cell Leukemia Virus Type 1-Infected Cell Lines in Novel NOD-SCID/Gammac(Null) Mice: Suppression by an Inhibitor against NF-KappaB. J. Virol. 2003, 77, 5286–5294. [Google Scholar] [CrossRef]
- Ohsugi, T.; Horie, R.; Kumasaka, T.; Ishida, A.; Ishida, T.; Yamaguchi, K.; Watanabe, T.; Umezawa, K.; Urano, T. In Vivo Antitumor Activity of the NF-KappaB Inhibitor Dehydroxymethylepoxyquinomicin in a Mouse Model of Adult T-Cell Leukemia. Carcinogenesis 2005, 26, 1382–1388. [Google Scholar] [CrossRef]
- Watanabe, M.; Ohsugi, T.; Shoda, M.; Ishida, T.; Aizawa, S.; Maruyama-Nagai, M.; Utsunomiya, A.; Koga, S.; Yamada, Y.; Kamihira, S.; et al. Dual Targeting of Transformed and Untransformed HTLV-1-Infected T Cells by DHMEQ, a Potent and Selective Inhibitor of NF-KappaB, as a Strategy for Chemoprevention and Therapy of Adult T-Cell Leukemia. Blood 2005, 106, 2462–2471. [Google Scholar] [CrossRef]
- Ohsugi, T.; Kumasaka, T.; Ishida, A.; Ishida, T.; Horie, R.; Watanabe, T.; Umezawa, K.; Yamaguchi, K. In Vitro and in Vivo Antitumor Activity of the NF-KappaB Inhibitor DHMEQ in the Human T-Cell Leukemia Virus Type I-Infected Cell Line, HUT-102. Leuk. Res. 2006, 30, 90–97. [Google Scholar] [CrossRef]
- Ohsugi, T.; Kumasaka, T.; Okada, S.; Ishida, T.; Yamaguchi, K.; Horie, R.; Watanabe, T.; Umezawa, K. Dehydroxymethylepoxyquinomicin (DHMEQ) Therapy Reduces Tumor Formation in Mice Inoculated with Tax-Deficient Adult T-Cell Leukemia-Derived Cell Lines. Cancer Lett. 2007, 257, 206–215. [Google Scholar] [CrossRef]
- Satou, Y.; Nosaka, K.; Koya, Y.; Yasunaga, J.-I.; Toyokuni, S.; Matsuoka, M. Proteasome Inhibitor, Bortezomib, Potently Inhibits the Growth of Adult T-Cell Leukemia Cells Both in Vivo and in Vitro. Leukemia 2004, 18, 1357–1363. [Google Scholar] [CrossRef]
- Shu, S.T.; Nadella, M.V.P.; Dirksen, W.P.; Fernandez, S.A.; Thudi, N.K.; Werbeck, J.L.; Lairmore, M.D.; Rosol, T.J. A Novel Bioluminescent Mouse Model and Effective Therapy for Adult T-Cell Leukemia/Lymphoma. Cancer Res. 2007, 67, 11859–11866. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Uchihara, J.; Terashima, K.; Honda, M.; Sata, T.; Ito, M.; Fujii, N.; Uozumi, K.; Tsukasaki, K.; Tomonaga, M.; et al. Efficient Intervention of Growth and Infiltration of Primary Adult T-Cell Leukemia Cells by an HIV Protease Inhibitor, Ritonavir. Blood 2006, 107, 716–724. [Google Scholar] [CrossRef]
- El Hajj, H.; El-Sabban, M.; Hasegawa, H.; Zaatari, G.; Ablain, J.; Saab, S.T.; Janin, A.; Mahfouz, R.; Nasr, R.; Kfoury, Y.; et al. Therapy-Induced Selective Loss of Leukemia-Initiating Activity in Murine Adult T Cell Leukemia. J. Exp. Med. 2010, 207, 2785–2792. [Google Scholar] [CrossRef]
- Ikebe, E.; Kawaguchi, A.; Tezuka, K.; Taguchi, S.; Hirose, S.; Matsumoto, T.; Mitsui, T.; Senba, K.; Nishizono, A.; Hori, M.; et al. Oral Administration of an HSP90 Inhibitor, 17-DMAG, Intervenes Tumor-Cell Infiltration into Multiple Organs and Improves Survival Period for ATL Model Mice. Blood Cancer J. 2013, 3, e132. [Google Scholar] [CrossRef]
- Ju, W.; Zhang, M.; Petrus, M.; Maeda, M.; Pise-Masison, C.A.; Waldmann, T.A. Combination of 9-Aminoacridine with Campath-1H Provides Effective Therapy for a Murine Model of Adult T-Cell Leukemia. Retrovirology 2014, 11, 43. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Ju, W.; Waldmann, T.A. Effective Treatment of a Murine Model of Adult T-Cell Leukemia Using Depsipeptide and Its Combination with Unmodified Daclizumab Directed toward CD25. Blood 2009, 113, 1287–1293. [Google Scholar] [CrossRef]
- Hasegawa, H.; Yamada, Y.; Tsukasaki, K.; Mori, N.; Tsuruda, K.; Sasaki, D.; Usui, T.; Osaka, A.; Atogami, S.; Ishikawa, C.; et al. LBH589, a Deacetylase Inhibitor, Induces Apoptosis in Adult T-Cell Leukemia/Lymphoma Cells via Activation of a Novel RAIDD-Caspase-2 Pathway. Leukemia 2011, 25, 575–587. [Google Scholar] [CrossRef]
- Zimmerman, B.; Sargeant, A.; Landes, K.; Fernandez, S.A.; Chen, C.-S.; Lairmore, M.D. Efficacy of Novel Histone Deacetylase Inhibitor, AR42, in a Mouse Model of, Human T-Lymphotropic Virus Type 1 Adult T Cell Lymphoma. Leuk. Res. 2011, 35, 1491–1497. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Kunami, N.; Katsuya, H.; Nogami, R.; Ishikawa, C.; Yotsumoto, F.; Tanji, H.; Mori, N.; Takeshita, M.; Miyamoto, S.; et al. Targeting Bcl-2 Family Proteins in Adult T-Cell Leukemia/Lymphoma: In Vitro and in Vivo Effects of the Novel Bcl-2 Family Inhibitor ABT-737. Cancer Lett. 2012, 317, 218–225. [Google Scholar] [CrossRef]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.-N.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan Extracted from Cladosiphon Okamuranus Tokida Induces Apoptosis of Human T-Cell Leukemia Virus Type 1-Infected T-Cell Lines and Primary Adult T-Cell Leukemia Cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef]
- Fauzi, Y.R.; Nakahata, S.; Chilmi, S.; Ichikawa, T.; Nueangphuet, P.; Yamaguchi, R.; Nakamura, T.; Shimoda, K.; Morishita, K. Antitumor Effects of Chloroquine/Hydroxychloroquine Mediated by Inhibition of the NF-ΚB Signaling Pathway through Abrogation of Autophagic P47 Degradation in Adult T-Cell Leukemia/Lymphoma Cells. PLoS One 2021, 16, e0256320. [Google Scholar] [CrossRef]
- Aikawa, A.; Kozako, T.; Uchida, Y.; Yoshimitsu, M.; Ishitsuka, K.; Ohsugi, T.; Honda, S.-I. Cell Death Induced by Dorsomorphin in Adult T-Cell Leukemia/Lymphoma Is AMPK-Independent. FEBS J. 2020, 287, 4005–4015. [Google Scholar] [CrossRef]
- Ishikawa, C.; Senba, M.; Mori, N. Importin Β1 Regulates Cell Growth and Survival during Adult T Cell Leukemia/Lymphoma Therapy. Invest. New Drugs 2021, 39, 317–329. [Google Scholar] [CrossRef]
- Zhang, M.; Mathews Griner, L.A.; Ju, W.; Duveau, D.Y.; Guha, R.; Petrus, M.N.; Wen, B.; Maeda, M.; Shinn, P.; Ferrer, M.; et al. Selective Targeting of JAK/STAT Signaling Is Potentiated by Bcl-XL Blockade in IL-2-Dependent Adult T-Cell Leukemia. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 12480–12485. [Google Scholar] [CrossRef]
- Ishikawa, C.; Senba, M.; Mori, N. Anti-Adult T-cell Leukemia/Lymphoma Activity of Cerdulatinib, a Dual SYK/JAK Kinase Inhibitor. Int. J. Oncol. 2018, 53, 1681–1690. [Google Scholar] [CrossRef]
- Ishikawa, C.; Matsuda, T.; Okudaira, T.; Tomita, M.; Kawakami, H.; Tanaka, Y.; Masuda, M.; Ohshiro, K.; Ohta, T.; Mori, N. Bisphosphonate Incadronate Inhibits Growth of Human T-Cell Leukaemia Virus Type I-Infected T-Cell Lines and Primary Adult T-Cell Leukaemia Cells by Interfering with the Mevalonate Pathway. Br. J. Haematol. 2007, 136, 424–432. [Google Scholar] [CrossRef]
- Machijima, Y.; Ishikawa, C.; Sawada, S.; Okudaira, T.; Uchihara, J.; Tanaka, Y.; Taira, N.; Mori, N. Anti-Adult T-Cell Leukemia/Lymphoma Effects of Indole-3-Carbinol. Retrovirology 2009, 6, 7. [Google Scholar] [CrossRef]
- Guimaraes-Correa, A.B.; Crawford, L.B.; Figueiredo, C.R.; Gimenes, K.P.; Pinto, L.A.; Grassi, M.F.R.; Feuer, G.; Travassos, L.R.; Caires, A.C.F.; Rodrigues, E.G.; et al. C7a, a Biphosphinic Cyclopalladated Compound, Efficiently Controls the Development of a Patient-Derived Xenograft Model of Adult T Cell Leukemia/Lymphoma. Viruses 2011, 3, 1041–1058. [Google Scholar] [CrossRef]
- Ishikawa, C.; Jomori, T.; Tanaka, J.; Senba, M.; Mori, N. Peridinin, a Carotenoid, Inhibits Proliferation and Survival of HTLV-1-Infected T-Cell Lines. Int. J. Oncol. 2016, 49, 1713–1721. [Google Scholar] [CrossRef]
- Ishikawa, C.; Senba, M.; Mori, N. Butein Inhibits NF-ΚB, AP-1 and Akt Activation in Adult T-Cell Leukemia/Lymphoma. Int. J. Oncol. 2017, 51, 633–643. [Google Scholar] [CrossRef]
- Fatfat, M.; Fakhoury, I.; Habli, Z.; Mismar, R.; Gali-Muhtasib, H. Thymoquinone Enhances the Anticancer Activity of Doxorubicin against Adult T-Cell Leukemia in Vitro and in Vivo through ROS-Dependent Mechanisms. Life Sci. 2019, 232, 116628. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Orba, Y.; Kimura, T.; Iha, H.; Ogata, M.; Tsuji, T.; Ainai, A.; Sata, T.; Okamoto, T.; Hall, W.W.; et al. Inhibition of the SDF-1alpha-CXCR4 Axis by the CXCR4 Antagonist AMD3100 Suppresses the Migration of Cultured Cells from ATL Patients and Murine Lymphoblastoid Cells from HTLV-I Tax Transgenic Mice. Blood 2009, 114, 2961–2968. [Google Scholar] [CrossRef]
- Tary-Lehmann, M.; Saxon, A.; Lehmann, P. V The Human Immune System in Hu-PBL-SCID Mice. Immunol. Today 1995, 16, 529–533. [Google Scholar] [CrossRef]
- King, M.A.; Covassin, L.; Brehm, M.A.; Racki, W.; Pearson, T.; Leif, J.; Laning, J.; Fodor, W.; Foreman, O.; Burzenski, L.; et al. Human Peripheral Blood Leucocyte Non-Obese Diabetic-Severe Combined Immunodeficiency Interleukin-2 Receptor Gamma Chain Gene Mouse Model of Xenogeneic Graft-versus-Host-like Disease and the Role of Host Major Histocompatibility Complex. Clin. Exp. Immunol. 2009, 157, 104–118. [Google Scholar] [CrossRef]
- Lan, P.; Tonomura, N.; Shimizu, A.; Wang, S.; Yang, Y.-G. Reconstitution of a Functional Human Immune System in Immunodeficient Mice through Combined Human Fetal Thymus/Liver and CD34+ Cell Transplantation. Blood 2006, 108, 487–492. [Google Scholar] [CrossRef]
- Melkus, M.W.; Estes, J.D.; Padgett-Thomas, A.; Gatlin, J.; Denton, P.W.; Othieno, F.A.; Wege, A.K.; Haase, A.T.; Garcia, J.V. Humanized Mice Mount Specific Adaptive and Innate Immune Responses to EBV and TSST-1. Nat. Med. 2006, 12, 1316–1322. [Google Scholar] [CrossRef]
- Tonomura, N.; Habiro, K.; Shimizu, A.; Sykes, M.; Yang, Y.-G. Antigen-Specific Human T-Cell Responses and T Cell-Dependent Production of Human Antibodies in a Humanized Mouse Model. Blood 2008, 111, 4293–4296. [Google Scholar] [CrossRef]
- Danner, R.; Chaudhari, S.N.; Rosenberger, J.; Surls, J.; Richie, T.L.; Brumeanu, T.-D.; Casares, S. Expression of HLA Class II Molecules in Humanized NOD.Rag1KO.IL2RgcKO Mice Is Critical for Development and Function of Human T and B Cells. PLoS One 2011, 6, e19826. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, T.; Katano, I.; Ito, R.; Ito, M.; Harigae, H.; Ishii, N.; Sugamura, K. Induction of Human Humoral Immune Responses in a Novel HLA-DR-Expressing Transgenic NOD/Shi-Scid/Γcnull Mouse. Int. Immunol. 2012, 24, 243–252. [Google Scholar] [CrossRef]
- Strowig, T.; Gurer, C.; Ploss, A.; Liu, Y.-F.; Arrey, F.; Sashihara, J.; Koo, G.; Rice, C.M.; Young, J.W.; Chadburn, A.; et al. Priming of Protective T Cell Responses against Virus-Induced Tumors in Mice with Human Immune System Components. J. Exp. Med. 2009, 206, 1423–1434. [Google Scholar] [CrossRef]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; et al. Generation of Functional Human T-Cell Subsets with HLA-Restricted Immune Responses in HLA Class I Expressing NOD/SCID/IL2r Gamma(Null) Humanized Mice. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 13022–13027. [Google Scholar] [CrossRef]
- Willinger, T.; Rongvaux, A.; Takizawa, H.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Auerbach, W.; Eynon, E.E.; Stevens, S.; Manz, M.G.; et al. Human IL-3/GM-CSF Knock-in Mice Support Human Alveolar Macrophage Development and Human Immune Responses in the Lung. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 2390–2395. [Google Scholar] [CrossRef]
- Billerbeck, E.; Barry, W.T.; Mu, K.; Dorner, M.; Rice, C.M.; Ploss, A. Development of Human CD4+FoxP3+ Regulatory T Cells in Human Stem Cell Factor-, Granulocyte-Macrophage Colony-Stimulating Factor-, and Interleukin-3-Expressing NOD-SCID IL2Rγ(Null) Humanized Mice. Blood 2011, 117, 3076–3086. [Google Scholar] [CrossRef]
- Huntington, N.D.; Legrand, N.; Alves, N.L.; Jaron, B.; Weijer, K.; Plet, A.; Corcuff, E.; Mortier, E.; Jacques, Y.; Spits, H.; et al. IL-15 Trans-Presentation Promotes Human NK Cell Development and Differentiation in Vivo. J. Exp. Med. 2009, 206, 25–34. [Google Scholar] [CrossRef]
- Chen, Q.; Khoury, M.; Chen, J. Expression of Human Cytokines Dramatically Improves Reconstitution of Specific Human-Blood Lineage Cells in Humanized Mice. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 21783–21788. [Google Scholar] [CrossRef]
- Katano, I.; Takahashi, T.; Ito, R.; Kamisako, T.; Mizusawa, T.; Ka, Y.; Ogura, T.; Suemizu, H.; Kawakami, Y.; Ito, M. Predominant Development of Mature and Functional Human NK Cells in a Novel Human IL-2-Producing Transgenic NOG Mouse. J. Immunol. 2015, 194, 3513–3525. [Google Scholar] [CrossRef]
- Takahashi, T.; Katano, I.; Ito, R.; Goto, M.; Abe, H.; Mizuno, S.; Kawai, K.; Sugiyama, F.; Ito, M. Enhanced Antibody Responses in a Novel NOG Transgenic Mouse with Restored Lymph Node Organogenesis. Front. Immunol. 2017, 8, 2017. [Google Scholar] [CrossRef]
- Miyazato, P.; Yasunaga, J.; Taniguchi, Y.; Koyanagi, Y.; Mitsuya, H.; Matsuoka, M. De Novo Human T-Cell Leukemia Virus Type 1 Infection of Human Lymphocytes in NOD-SCID, Common Gamma-Chain Knockout Mice. J. Virol. 2006, 80, 10683–10691. [Google Scholar] [CrossRef]
- Takajo, I.; Umeki, K.; Morishita, K.; Yamamoto, I.; Kubuki, Y.; Hatakeyama, K.; Kataoka, H.; Okayama, A. Engraftment of Peripheral Blood Mononuclear Cells from Human T-Lymphotropic Virus Type 1 Carriers in NOD/SCID/Gammac(Null) (NOG) Mice. Int. J. cancer 2007, 121, 2205–2211. [Google Scholar] [CrossRef]
- Villaudy, J.; Wencker, M.; Gadot, N.; Gillet, N.A.; Scoazec, J.-Y.; Gazzolo, L.; Manz, M.G.; Bangham, C.R.M.; Dodon, M.D. HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2−/− Gamma C−/− Mice. PLoS Pathog. 2011, 7, e1002231. [Google Scholar] [CrossRef]
- Tezuka, K.; Xun, R.; Tei, M.; Ueno, T.; Tanaka, M.; Takenouchi, N.; Fujisawa, J. An Animal Model of Adult T-Cell Leukemia: Humanized Mice with HTLV-1-Specific Immunity. Blood 2014, 123, 346–355. [Google Scholar] [CrossRef]
- Espíndola, O. de M.; Siteur-van Rijnstra, E.; Frankin, E.; Weijer, K.; van der Velden, Y.U.; Berkhout, B.; Blom, B.; Villaudy, J. Early Effects of HTLV-1 Infection on the Activation, Exhaustion, and Differentiation of T-Cells in Humanized NSG Mice. Cells 2021, 10. [Google Scholar] [CrossRef]
- Xiang, J.; Rauch, D.A.; Huey, D.D.; Panfil, A.R.; Cheng, X.; Esser, A.K.; Su, X.; Harding, J.C.; Xu, Y.; Fox, G.C.; et al. HTLV-1 Viral Oncogene HBZ Drives Bone Destruction in Adult T Cell Leukemia. JCI insight 2019, 4. [Google Scholar] [CrossRef]
- Hiyoshi, M.; Okuma, K.; Tateyama, S.; Takizawa, K.; Saito, M.; Kuramitsu, M.; Araki, K.; Morishita, K.; Okada, S.; Yamamoto, N.; et al. Furin-Dependent CCL17-Fused Recombinant Toxin Controls HTLV-1 Infection by Targeting and Eliminating Infected CCR4-Expressing Cells in Vitro and in Vivo. Retrovirology 2015, 12, 73. [Google Scholar] [CrossRef]
- Inocencio, N.M.; Moehring, J.M.; Moehring, T.J. Furin Activates Pseudomonas Exotoxin A by Specific Cleavage in Vivo and in Vitro. J. Biol. Chem. 1994, 269, 31831–31835. [Google Scholar] [CrossRef]
- Percher, F.; Curis, C.; Pérès, E.; Artesi, M.; Rosewick, N.; Jeannin, P.; Gessain, A.; Gout, O.; Mahieux, R.; Ceccaldi, P.-E.; et al. HTLV-1-Induced Leukotriene B4 Secretion by T Cells Promotes T Cell Recruitment and Virus Propagation. Nat. Commun. 2017, 8, 15890. [Google Scholar] [CrossRef]
- Tezuka, K.; Okuma, K.; Kuramitsu, M.; Matsuoka, S.; Tanaka, R.; Tanaka, Y.; Hamaguchi, I. Control of Human T-Cell Leukemia Virus Type 1 (HTLV-1) Infection by Eliminating Envelope Protein-Positive Cells with Recombinant Vesicular Stomatitis Viruses Encoding HTLV-1 Primary Receptor. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Karkhanis, V.; Hu, Y.-J.; Baiocchi, R.A.; Imbalzano, A.N.; Sif, S. Versatility of PRMT5-Induced Methylation in Growth Control and Development. Trends Biochem. Sci. 2011, 36, 633–641. [Google Scholar] [CrossRef]
- Ernzen, K.; Melvin, C.; Yu, L.; Phelps, C.; Niewiesk, S.; Green, P.L.; Panfil, A.R. The PRMT5 Inhibitor EPZ015666 Is Effective against HTLV-1-Transformed T-Cell Lines in Vitro and in Vivo. Front. Microbiol. 2023, 14, 1101544. [Google Scholar] [CrossRef]
- Pérès, E.; Blin, J.; Ricci, E.P.; Artesi, M.; Hahaut, V.; Van den Broeke, A.; Corbin, A.; Gazzolo, L.; Ratner, L.; Jalinot, P.; et al. PDZ Domain-Binding Motif of Tax Sustains T-Cell Proliferation in HTLV-1-Infected Humanized Mice. PLoS Pathog. 2018, 14, e1006933. [Google Scholar] [CrossRef]
- Maksimova, V.; Smith, S.; Seth, J.; Phelps, C.; Niewiesk, S.; Satou, Y.; Green, P.L.; Panfil, A.R. HTLV-1 Intragenic Viral Enhancer Influences Immortalization Phenotype in Vitro, but Is Dispensable for Persistence and Disease Development in Animal Models. Front. Immunol. 2022, 13, 954077. [Google Scholar] [CrossRef]
| Strain | Genotype | Characteristics | Limitation | Reference |
|---|---|---|---|---|
| SCID | scid | T cell and B cell defect | T cell and B cell leakage Radiosensitive |
[59] |
| NOD/SCID | nod/scid | T cell and B cell defect Decreased NK cell, DC and macrophage activity Complement deficiency |
T cell and B cell leakage Radiosensitive Spontaneous lymphoma |
[65] |
| NOG | nod/scid/IL-2Rγnull | T cell, B cell and NK cell defect Decreased DC and macrophage activity Complement deficiency |
Radiosensitive | [67] |
| NSG | nod/scid/IL-2Rγnull | T cell, B cell and NK cell defect Decreased DC and macrophage activity Complement deficiency |
Radiosensitive | [68] |
| NOJ | nod/scid/jak-3null | T cell, B cell and NK cell defect Decreased DC and macrophage activity Complement deficiency |
Radiosensitive | [69] |
| NRG | nod/rag-1null/IL-2Rγnull | T cell, B cell and NK cell defect Decreased DC and macrophage activity Complement deficiency |
- | [70] |
| Drug name | Target | Mouse | Efficacy | Reference |
|---|---|---|---|---|
| Bay 11-7082 | NF-κB inhibitor | NOG mouse | Inhibits tumor growth and invasion | [80] |
| DHMEQ | NF-κB inhibitor | NK(-)SCID mouse SCID mouse |
Inhibits tumor growth and invasion | [81,82,83,84] |
| Ritonavir | HIV protease inhibitor | NOG mouse | Inhibits tumor growth and invasion | [87] |
| Fucoidan | Survivin inhibitor | SCID mouse | Partially inhibits tumor growth | [95] |
| Incadronate | Mevalonate pathway inhibitor | SCID mouse | Reduces tumor formation | [101] |
| PS-341 | Proteasome inhibitor | SCID mouse | Inhibits tumor growth | [85] |
| PS-341 Zoledronic acid |
Proteasome inhibitor Osteoclast inhibitor |
NOD/SCID mouse | Inhibits tumor growth | [86] |
| 9-aminoacridine (9AA) Campath-1H |
Increase p53 transcription activity NF-κB activation inhibitor Humanized anti-CD52 antibody |
NOD/SCID mouse | Inhibits tumor growth Extends survival |
[90] |
| Depsipeptide Daclizumab |
HDAC inhibitor Anti-IL-2Rα antibody |
NOD/SCID mouse | Extends survival | [91] |
| LBH589 | HDAC inhibitor | SCID mouse | Induces tumor cell apoptosis Extends survival |
[92] |
| AR-42 | HDAC inhibitor | NOD/SCID mouse | Extends survival | [93] |
| ABT-737 | Bcl-2, Bcl-X(L), and Bcl-w inhibitor | SCID mouse | Inhibits tumor growth | [94] |
| 17-DMAG | HSP90 inhibitor | NOG mouse | Inhibits tumor invasion Extends survival |
[89] |
| As(2)O(3) IFN-α |
Proteolysis of Tax Antiviral |
SCID mouse | Inhibits tumor cell immortality | [88] |
| C7a | Antitumor effect | NSG mouse | Extends survival | [103] |
| Indole-3-carbinol | Antitumor effect | SCID mouse | Inhibits tumor growth | [102] |
| AMD3100 | CXCR4 antagonist | SCID mouse | Inhibits tumor cell infiltration into liver and lung tissue | [107] |
| Chloroquine Hydroxychloroquine |
Autophagy inhibitor | NOG mouse | Inhibits tumor growth Extends survival |
[96] |
| Dorsomorphin | AMPK inhibitor | NOD/SCID mouse | Inhibits tumor growth | [97] |
| Ivermectin | IPOα/β1 inhibitor | SCID mouse | Inhibits tumor growth | [98] |
| Ruxolitinib Navitoclax |
JAK inhibitor Bcl-2/Bcl-xL inhibitor |
NSG mouse | Inhibits tumor growth | [99] |
| Cerdulatinib | Dual SYK/JAK inhibitor | SCID mouse | Inhibits tumor growth | [100] |
| Peridinin | Antitumor effect | SCID mouse | Inhibits tumor growth | [104] |
| Butein | Antitumor effect | SCID mouse | Inhibits tumor growth | [105] |
| Thymoquinone Doxorubicin |
Antitumor effect Anticancer drug |
NOD/SCID mouse | Inhibits tumor growth | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





