1. Introduction
Chirality is a concept coined for the first time in 1893 by Lord Kelvin, it is defined as the absence of a reflection symmetry of a certain object. Therefore, a chiral molecule is defined such that it is not possible to create an overlap between it and its mirror image through a rotation operation [
1,
2,
3]. A chiral molecule and its mirror image are called
enantiomers in the nomenclature of chemistry. Both enantiomers share almost all the same physical and chemical properties such as melting point, boiling point, density, and viscosity, yet differ in optical activity. When polarized light is passing through an enantiomer the enantiomer will be called D (dextrorotatory), R (rectus) or “right-handed” if the polarized light coming out is rotating clockwise. An enantiomer will be called L (levorotatory), S (sinister) or “left-handed” if the polarized light coming out is counterclockwise [
4,
5,
6,
7].
The biochemical properties of enantiomers differ following the stereo-selectivity of enzymes and receptors means that they will react differently with each of the enantiomers or not react at all with one of the enantiomers [
8,
9]. There are 20 amino acids that make up the building blocks from which proteins are formed in the human body. Amino acids are formed in the body or are consumed through food in the form of their L enantiomer, therefore all biological processes can only exist using L-type amino acids. Consumption of D-type amino acids will have no effect and the body will eliminate them. Similarly, the sugars that the body consumes such as glucose, fructose, and lactose for anabolic, catabolic, and energy-producing biological processes are of the D-sugar stereoselctive form so that the enzymes and proteins can only function through them. Consuming L-sugars will have no effect at all, and the body will remove them same as in the case of D-type amino acids. Another two famous examples that show how the enantiomeric structure affects the way the body reacts to them: the Limonene molecule whose D enantiomer is the more common and is the main ingredient in citrus peel oil, which has the smell of oranges and is used as a flavoring agent in food production. On the other hand, the L-enantiomer of Limonene is found in peppermint oils and has a milder smell reminiscent of lemon. The significance of chirality gained widespread recognition in the 19th century, notably exemplified by the tragic case of the drug thalidomide. This drug contained both enantiomers, with L-Thalidomide being effective against pregnancy-related nausea and D-Thalidomide causing devastating birth defects in infants. Consequently, the separation of enantiomers became crucial to ensure the safe use of drugs and prevent such catastrophic consequences [
10].
In recent decades, isothermal titration calorimetry (ITC) became a very prominent technique in the biochemistry world [
11]. The usual ITC is a calorimetry technique used to study biomolecular interactions, for example, protein-ligand bonds, lipid systems, and nuclear acid identification. ITC is also widely used in drug design and enzyme kinetics studies [
12]. It allows us to directly measure the enthalpy change ( H), the bond affinity constant (K), and the stoichiometric coefficient (
n) for biomolecular interactions. The graph that is obtained at the end of the measurement shows the Y-Axis as heat change (which appears as peaks on the graph) that occurs during the injections (in units of mCal/second, mJ/second or mW/second) as a function of time (X-axis), and this is the raw graph of the measurement. The raw graph is analyzed by integrating the area of the peaks and normalizing the value so that the units on the Y-axis appear in units of energy per mole of injectant (the analysis of the results is done automatically for biological and biochemical measurements of bond interactions between ligand and protein due to the well-known graph shape of a titration curve of a sigmoidal graph) as a function of the molar ratio of the ligand: protein (X-axis). Analysis of the graph gives the change in enthalpy ( H) whose value is equal to the largest Y value from the baseline. The stoichiometric coefficient (
n) of the reaction whose value is equal to the molar ratio when the Y axis is at its median value, and the bonding affinity (known as K constant) whose value is equal to the slope of the graph at that point where the value of the stoichiometric coefficient was obtained. By utilizing the relationship ΔG = ΔH - TΔS = -RTlnK, researchers can extract information about changes in both entropy (ΔS) and Gibbs free energy (ΔG). Furthermore, ITC allows for measurements at different temperatures, enabling the determination of heat capacity changes at constant pressure (ΔCp = ΔΔH/ΔT). This additional information on heat capacity further enhances the understanding of biomolecular processes [
13,
14].
The principle of operation of ITC is to measure the change in heat generated as a result of titration at a constant temperature. The instrument includes a small volume syringe (up to ~300mL) containing a solution at a high concentration of a particular substance (titrant). The sample cell with a larger volume than that of the syringe (up to ~1.4mL) with a solution of another substance (titrand) at a lower concentration than the concentration of the solution in the syringe. The reference cell, with the same volume of the sample cell, contains the solvent of the sample solution (water, ethanol, buffer, etc). The sample cell and the reference cell are protected by an adiabatic shield that keeps the temperature constant and prevents the entry and exit of heat into and out of the system. When titration occurs, most of the time it involves a chemical reaction, which leads to heat changes that cause a change in temperature even if it is a very small change. To keep both the sample cell and the reference cell at the same temperature, an installed thermostat with heat regulators apply voltage to compare the temperature of the sample cell to the reference cell [
15]. The voltage applied to the time unit is the feedback of the temperature change and it is equal to the heat change caused due to the titration and it is expressed as energy
vs. time [
16,
17,
18,
19].
The isothermal titration calorimetry technique boasts an impressive level of sensitivity, capable of measuring chemical reactions even with tiny heat changes, such as those encountered in chiral interactions[
20]. When a chemical interaction takes place, it is often accompanied by a notable change in enthalpy, attributed to the formation or decomposition of chemical bonds. Leveraging the ITC technique, researchers can delve into a wide range of chemical reactions and precisely measure their thermodynamic properties
. The remarkable ITC sensitivity of approximately 0.1 µCal/sec, enables the detection and quantification of even subtle heat changes. This attribute proves invaluable in investigating intricate chiral interactions where the heat changes can be relatively small. By utilizing ITC, scientists can gain insights into the thermodynamics of chiral interactions and unravel the underlying mechanisms governing their behavior
. Beyond chiral interactions, the versatility of the ITC technique extends to a broad spectrum of chemical reactions. Whether it involves the formation of new bonds or the breakdown of existing ones, ITC empowers researchers to explore and quantify the associated enthalpy changes. This capacity enables a comprehensive understanding of chemical reactions, unveiling the thermodynamic intricacies that drive them. In summary, the high sensitivity of the ITC technique allows for the precise measurement of chemical reactions, even those with minimal heat changes, making it a valuable tool in the study of chiral interactions and a wide range of other chemical processes. Its ability to unravel the thermodynamic properties of these reactions contributes to advancing our understanding of fundamental chemical principles
. Shvalb
et al. showed that ITC can measure chiral interactions of different amino acids on the surface of chiral crystals, demonstrating selective adsorption [
21,
22,
23]. Recently, Werber et al. used ITC to measure homo- and hetero-chiral interactions in chiral solutions; moreover, they were able to measure chiral interactions in organometallic complexes, biological systems and nano-systems [
24].
Here, we used ITC to investigate the racemic dilution heat Hrac) of different side chain groups of amino acids in water – Alanine (aliphatic), Phenylalanine (aromatic), Aspartic acid (acidic), Histidine (basic), Asparagine (amidic), Methionine (contains sulfur) and Serine (contains hydroxyl). These amino acids were chosen in order to examine the dependence of the racemic dilution heat on the nature of the side chain. As previous studies showed that chiral interactions are accompanied by a small heat change, ITC is the perfect instrument for measuring such interactions. In contrast to studies that emphasize the optical activity of chiral molecules, this approach provides a thermodynamic perspective for chiral interactions.
2. Materials and Methods
The following analytical grade chemicals were purchased from Sigma-Aldrich or Tokyo Chemical Industry and used without further purification: L/D-alanine (>98%), L/D-serine (>98%), L/D-methionine (>98%), L/D-histidine (>99%), L/D-phenylalanine (>98%), L/D-asparagine (>98%) and L/D-aspartic acid (>98%).
2.1. Solution Preparation
The following L-amino acids – Ala, Ser, Met, His, Phe, Asn and Asp – were weighed to prepare solutions in double-distilled water at respective concentrations of 1M, 1M, 200mM, 250mM, 125mM, 150mM, 25mM. The same procedure was performed to prepare solutions of the corresponding D-amino acids (five-fold diluted).
2.2. Experimental
ITC measurements were performed using a VP-ITC calorimeter (MicroCal). The racemic dilution enthalpies were determined at 20°C, 30°C and 45°C. The sample cell was loaded with 1.42mL of D-amino acid solution in double-distilled water, and the 300 μL syringe was filled with a solution of L-amino acid. Each consecutive 5 μL injection lasted 8.5sec, with an interval of 400sec between injections (56 injections in total). The cell was stirred constantly at a rate of 300rpm, and the reference power that had been chosen for the measurements was 20μCal/sec. Before measurement, the solutions were degassed under vacuum using ThermoVac (MicroCal) for 5 min at 25°C to remove air bubbles. Results were analyzed using Origin software.
3. Results
ITC was used to investigate the racemization heat of amino acids with different types of side chains. We utilized small steps of titration of concentrated L amino acid solutions, each to a five-fold more dilute solution of the corresponding D enantiomer until racemization. The total heat absorbed or released by the titration is known to comprise several components: racemization ( H
racemization) as known as hetrochiral interactions, homochiral interactions ( H
LL, H
DD), pH change ( H
pH), and dilution ( H
dilution). The equation we got for the total heat is:
We neglect the homochiral heat of interaction following our previous experiments which that show that it is negligible. Preliminary experiment showed that the pH change upon titration of water to D amino acid solution involves a negligible heat change.The equation can thus be minimized:
The racemic dilution heat Htotal provided by titration of L amino acid solution to the D enantiomer solution until racemization. The graph was integrated for each amino acid, and both syringe and cell moles to get quantitative values.
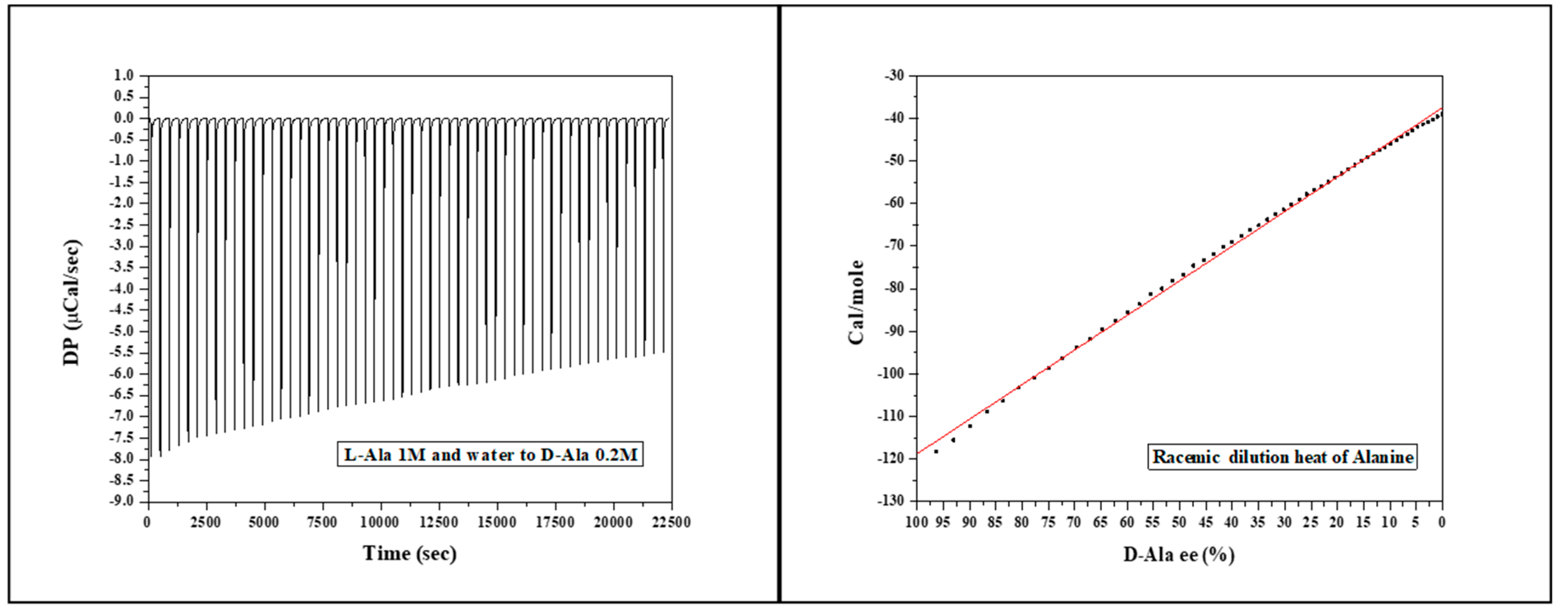
In
Figure 1a, the heat flow for all injections of 1M L-Ala to 0.2M D-Ala at 30°C is shown, and the ITC peaks are all exothermic.
Figure 1b shows the molar heat of racemic dilution of Alanine at 30°C as a function of D-Ala enantiomeric excess. The graph displays a highly linear (R
2 > 0.99) trend of decreasing heat of racemic dilution as a result of the formation of the racemic mixture from -118 to -39Cal.
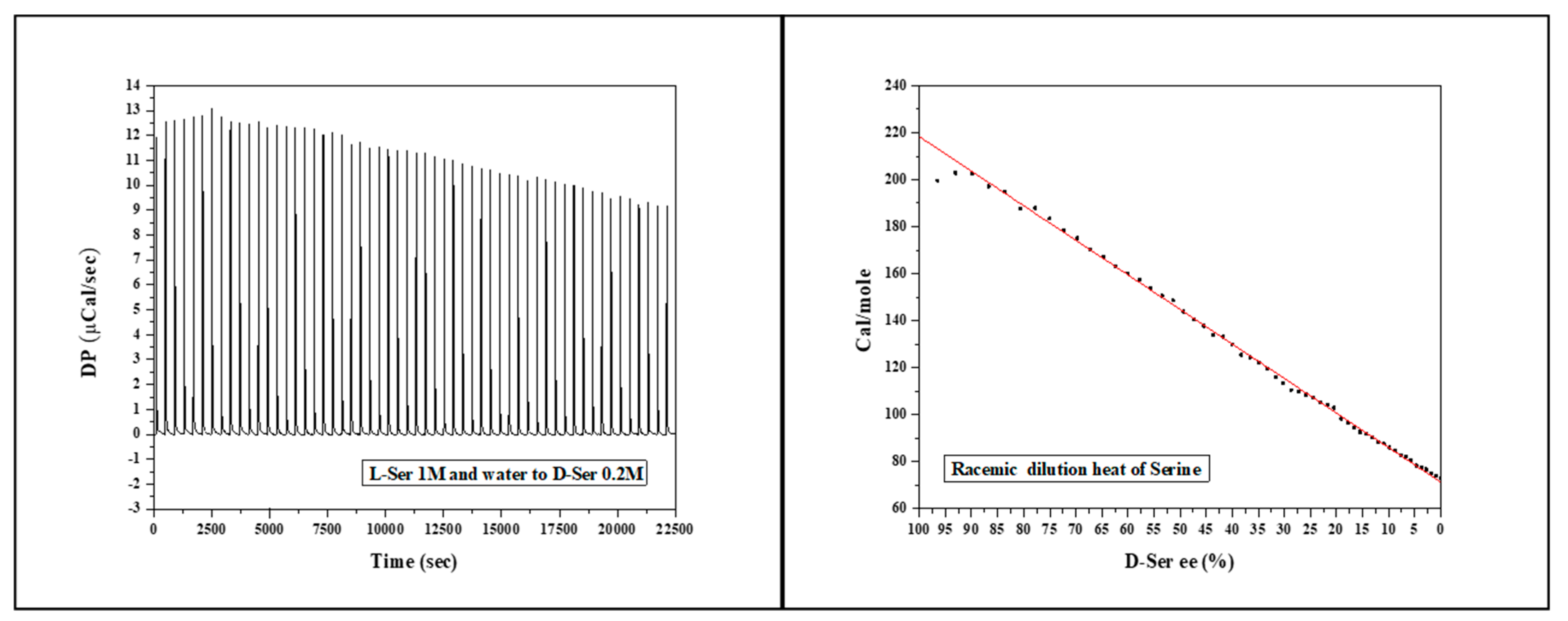
Figure 2a shows the heat flow for injection of 1M L-Ser to 0.2M D-Ser at 30°C. In contrast to Alanine racemic dilution, in this case, the ITC peaks are endothermic. The heat of the racemic mixture decreases from 200Cal to 73Cal. It can be deduced that the racemic dilution for amino acids can be both exothermic and endothermic.
Figure 2b presents the molar heat change for Serine racemic dilution at 30°C as a function of D-Ser enantiomeric excess. Similar to Alanine racemization,the heat change is highly linear.
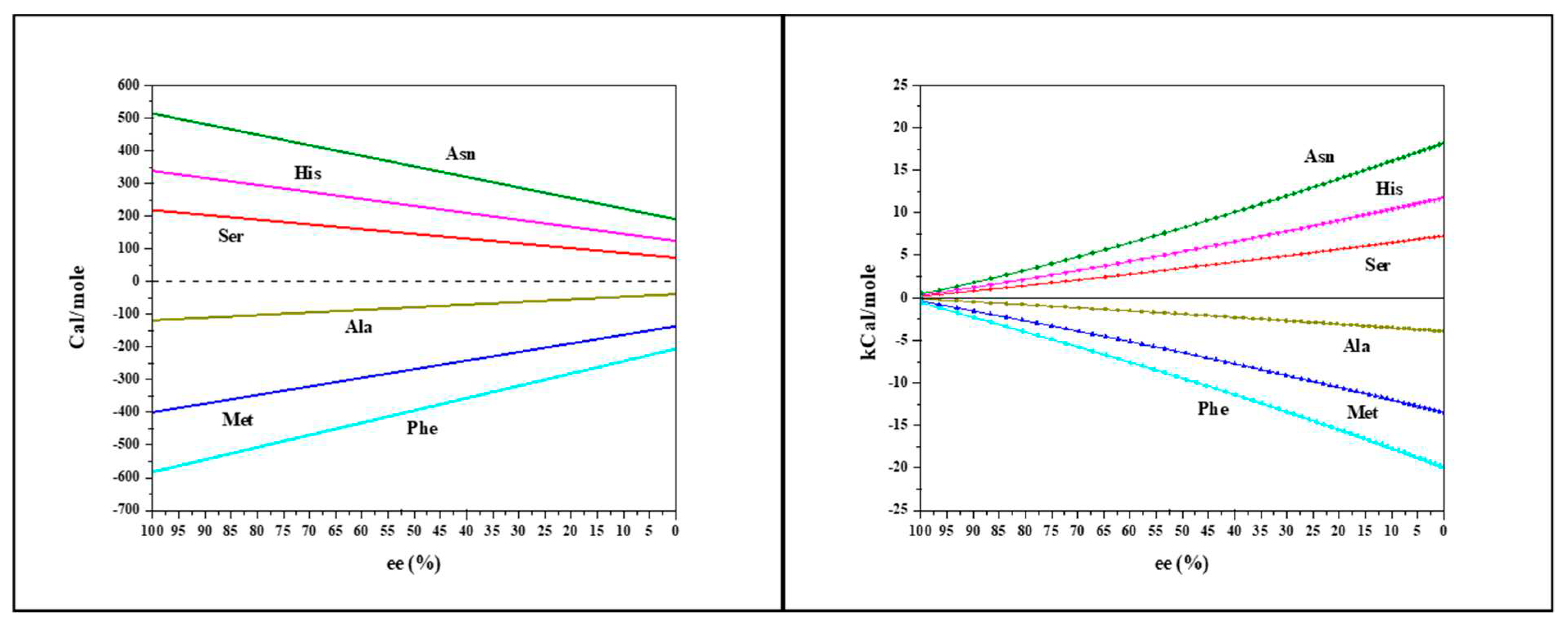
The molar heat change at 30°C as a function of enantiomeric excess is shown in Figure 3.
Figure 3a presents the heat change per injection. Asparagine, Histidine and Serine exhibit endothermic behavior (Asn shows the greatest heat change), while Alanine, Methionine and Phenylalanine exhibit exothermic behavior (Phe shows the greatest change).
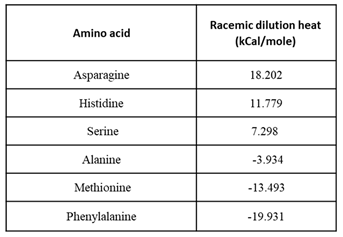
Figure 3b illustrates the molar total heat for different amino acidsas a function of the enantiomeric excess. The numerical racemization heat values are summarized in
Table 1.
Aspartic acid gave ambiguous results; each injection led to both endothermic and negative heat change (Figure S1). This can be attributed to the low solubility of Asp in water, which limits the concentration, such that the heat change is below the ITC threshold. There could also two reactions that take place during the titration. The numerical racemization heat values are summarized in
Table 1.
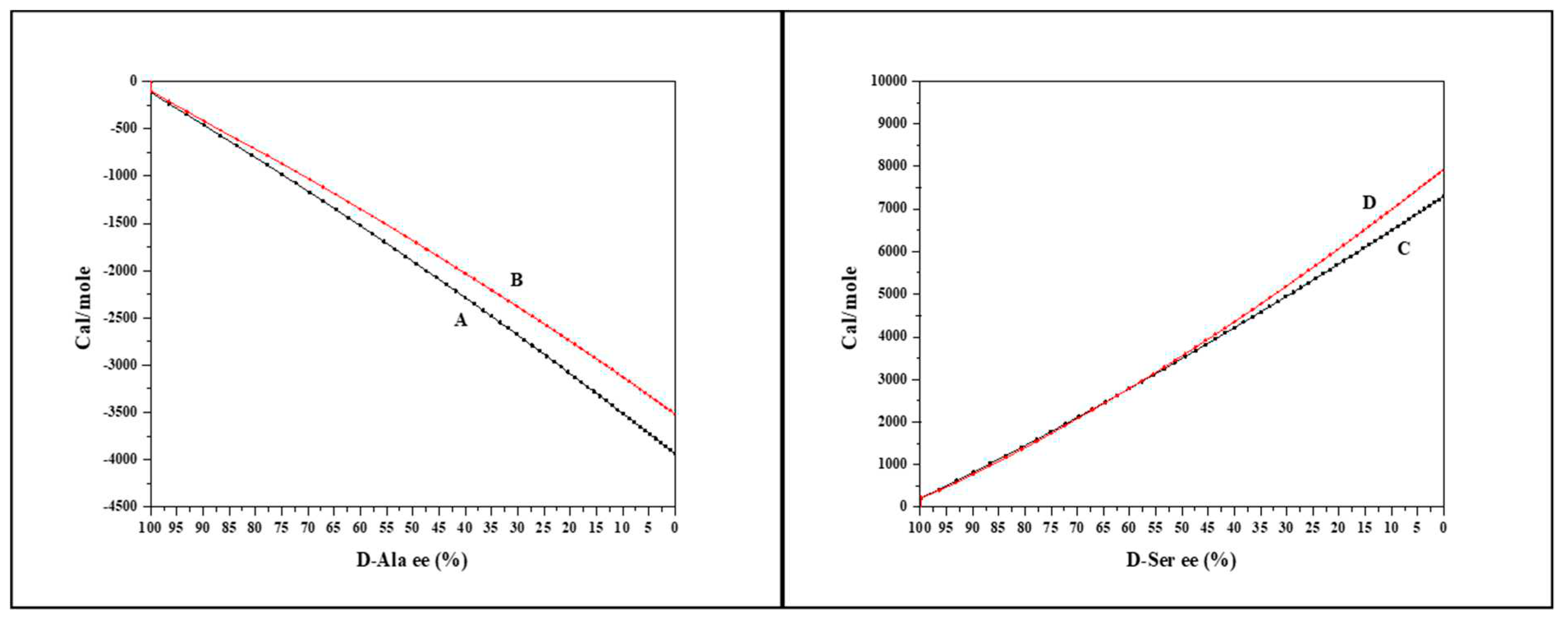
The effect of concentration and temperature on the racemic dilution heat for Alanine (exothermic racemic dilution) and Serine (endothermic racemic dilution) were examined.
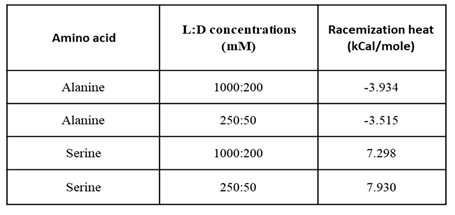
To examine the effect of concentration, we diluted four-fold both enantiomers of Ala and Ser.
Figure 4a shows the total heat of Ala racemic dilution at the different concentrations. The diluted Ala racemic dilution shows exothermic heat change and lower quality.
Figure 4b shows the same behavior for-dilute Serine except for the endothermic heat change, which means that the racemic dilution enthalpy sign is conserved. When syringe and cell moles are normalized, the racemic dilution heat for dilute and concentrated amino acids converges.
From the racemic dilution values in Table2, we can see that the racemic dilution heat is largely independent on concentration. High concentrations are favored due to the better quality.
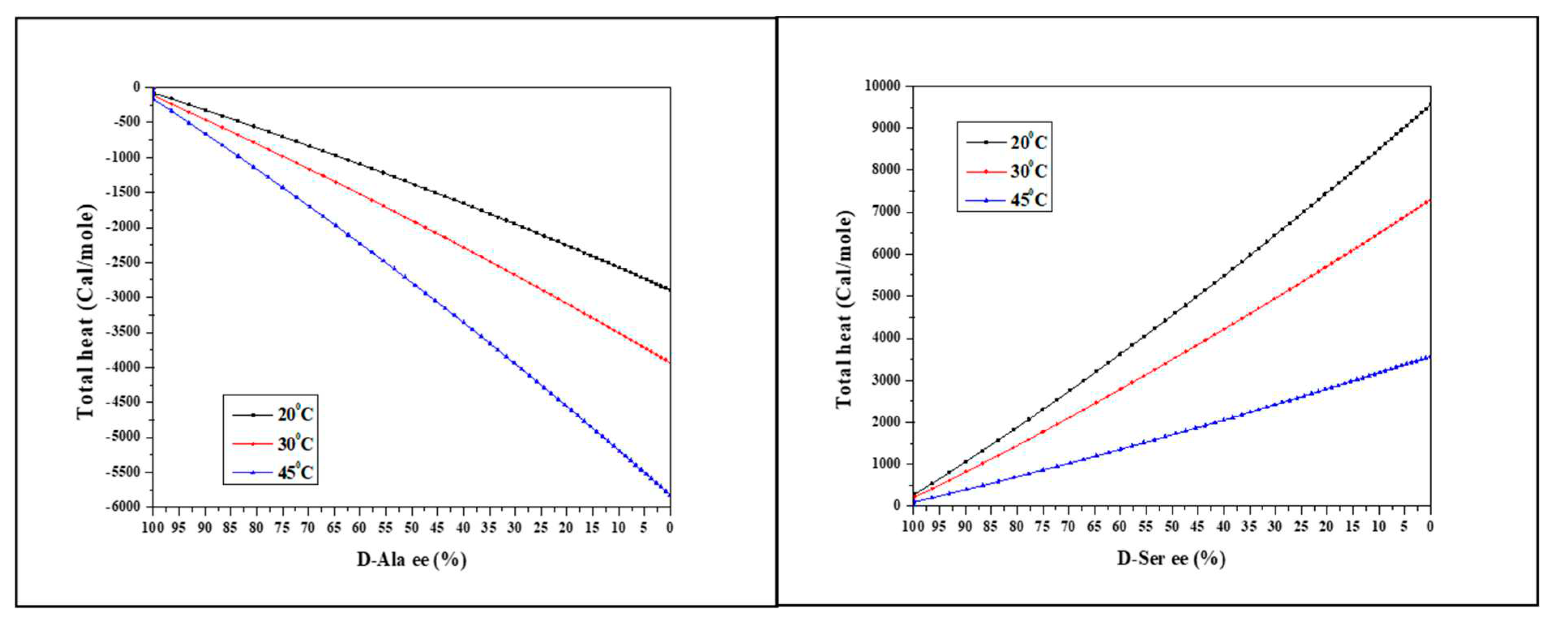
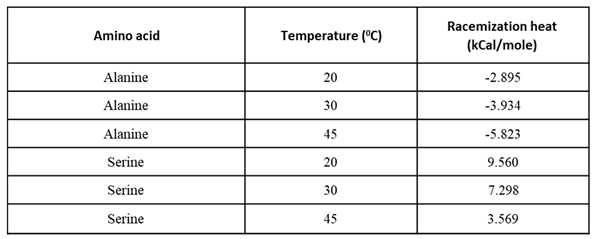
To examine the effect of temperature, we measured the racemic dilution heat of Ala and Ser at 20, 30 and 45°C.
Figure 5a shows the ITC total heat of Alanine racemization at different temperatures. More heat is released at higher temperatures.
Figure 5b displays the opposite trend for Serine racemization – less heat is adsorbed at higher temperatures.
From
Table 3, the temperature can be used as a resolution enhancer when the nature of the reaction is known. As the nature of Aspartic acid racemic dilution heat at 30°C is not known, we tried to racemize Asp at “extreme” temperatures of 5 and 60°C to figure out the appearance of both endothermic and exothermic contributions. The 5°C measurement shows clear endothermic heat changes (Figure S2), while the 60°C measurement shows clear exothermic heat changes (Figure S3).
4. Discussion
It seems that when the nature of the reaction is known, the resolution of the obtained measurements can be improved. Because of that, it was decided to try to measure the nature of the racemization of the aspartic acid at 300C for which we received endothermic and exothermic peaks at low intensities and low resolution when we do not know the reason why both types of peaks appear. The idea was to measure the racemization of the aspartic acid at “extreme” temperatures in order to obtain one of two possibilities: the first possibility is that one temperature will improve the resolution of the measurement while the other temperature will damage it, and this it will be possible to conclude whether the racemization for the aspartic acid is endothermic or exothermic. A second possibility is that each of the temperatures will give a different nature of the racemization, and by this, it will be possible to conclude that for aspartic acid at 300C two reactions occur simultaneously during the racemization. From the results obtained and shown a distinct endothermic change was observed at 50C (Figure S2), albeit at a low intensity, and a distinct exothermic change at 600C (Figure S3), and from this it can be concluded that for aspartic acid at 300C, two reactions occur simultaneously that offset each other energetically. In the initial conclusions, we were not able to find why the heat of racemization of some of the amino acids is endothermic and for others it is exothermic, but the results we obtained for the aspartic acid at the extreme temperatures can shed some light on this reason. It can be noticed that the amino acids for which endothermic heat changes were obtained (histidine, serine, and asparagine) have the possibility of forming a hexagonal ring structure where the closing of the ring is made possible through the formation of intramolecular hydrogen bonds of the amino acid. The hydrogen bond to form the intramolecular ring in histidine is between the amino hydrogen and the non-bonding pair of electrons of the nitrogen on the ring in the residue, in serine it is between the non-bonding pair of electrons of the carboxylic oxygen and the hydrogen of the hydroxyl in the residue, and in asparagine it is between the amino hydrogen and the non-bonding electron pair of the nitrogen in the affluent remnant. The creation of the intramolecular ring stabilizes it energetically, and in order to create the mixture in this way, energy is required to break the intramolecular hydrogen bond of the amino acid in order to dissolve it in water. The creation of the racemic mixture for the different amino acids was done in double distilled water and at a neutral pH, so the carboxylic hydrogen is released and the oxygen it was bound to is negatively charged, and the amino part is positively charged. The situation described earlier does not allow phenylalanine to form a hexagonal ring through hydrogen bonds, therefore the heat changes in the formation of the racemic mixture are exothermic for it. For alanine and methionine there is absolutely no possibility of creating intramolecular hydrogen bonds, therefore also for them the heat changes in the formation of the racemic mixtures are exothermic. For the aspartic acid, both endothermic and exothermic heat changes were obtained at 300C, and according to what was said above, this amino acid can form a stabilizing hexagonal ring bond through a hydrogen bond between the amino hydrogen and the non-bonding electron pair of the oxygen in the residue. Since at 300C both endothermic and exothermic heat changes are obtained in each injection, it can be concluded that at this temperature the system is in a state of energy equilibrium between the energy required to break the hydrogen bond that forms the ring bond of the aspartic acid and the energy released as a result of dissolving in water. A confirmation of this can be seen according to Figures S2 and S3 which at 50C the system does not have enough energy to break the ring hydrogen bond and as a result, endothermic heat changes are obtained at this temperature. On the other hand, at 600C the system has more than enough energy to break this bond and the resulting heat changes at this temperature are exothermic as a result from the energy released from the aspartic acid dissolving in water.
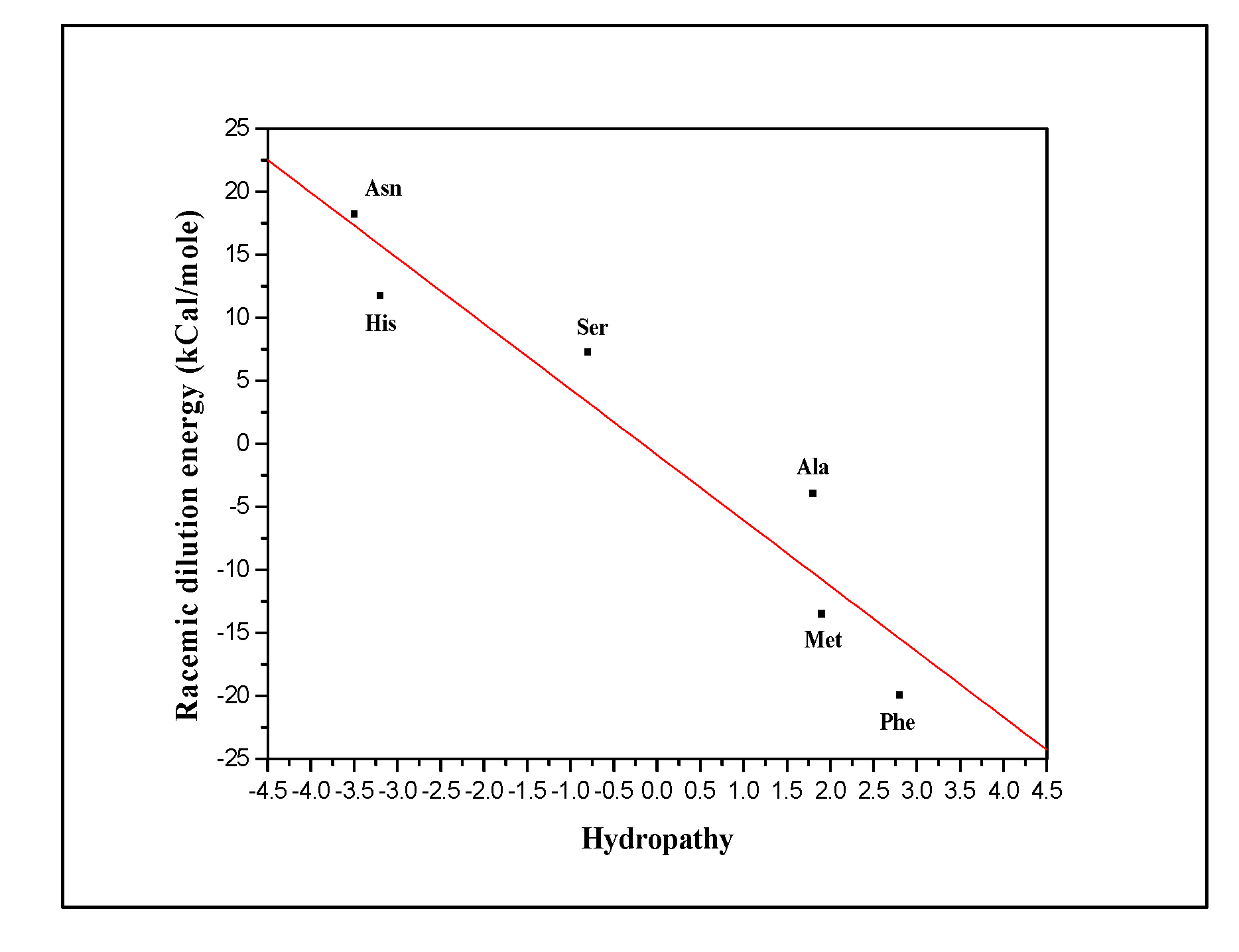
To explain our results, we tried to find the relation between the amino acids and the racemic dilution heat values and found a correlation between the racemic dilution energy and the hydropathy of the amino acid. The hydropathy index is the change in the free energy of a molecule when it moves from a hydrophobic to a hydrophilic environment, which shows how hydrophobic (or hydrophilic) the molecule is. Every molecule has a hydropathy index and it depends on the origin and target environments, as well as on physical conditions such as pressure and temperature. Negative values are obtained for hydrophilic molecules, while positive values are obtained for hydrophobic ones. Molecules with more hydrophilic groups such as hydroxyl, amine, amide and other polar groups are more hydrophilic with a more negative hydropathy value, while those with more hydrophobic groups like carbon chain, aromatic rings and non-polar groups are more hydrophobic with a more positive hydropathy value.
From
Figure 6 we see a fairly linear relationship (R
2 = 0.885) between the heats of racemization obtained for the tested amino acids and the hydropathy index of those amino acids. Alanine, Methionine and Phenylalanine for which exothermic racemic dilution heat was observed are considered hydrophobic amino acids (hydropathy values of 1.8, 1.9 and 2.8, respectively); Phenylalanine, the most hydrophobic among them, has the most exothermic racemic dilution. On the other hand, Asparagine, Histidine and Serine for which endothermic racemic dilution heat was observed are considered hydrophilic (hydropathy values of -3.5, -3.2 and -0.8, respectively); Asparagine, the most hydrophilic amino acid, exhibits the most endothermic racemic dilution. As written before, the reason why endothermic racemization values were obtained for some of the amino acids tested (asparagine, serine and histidine) was due to the ability of those amino acids to form intramolecular hydrogen bonds to form a hexagonal ring that stabilizes the amino acid, and in order to break this bond to form the racemic mixture require energy. On the other hand, for some of the amino acids tested, negative racemization values were obtained (alanine, methionine and phenylalanine) since there are no intramolecular hydrogen bonds for which energy is required to break down. The amino acids for which negative racemization values were obtained fall under the category of the hydrophobic amino acids, so it can be assumed that the values of the racemization values obtained for the hydrophobic amino acids are affected by hydrophobic bonds. Hydrophobic bonds are bonds formed when hydrophobic molecules are in a hydrophilic medium, which aim to reduce the energy to a minimum, hydrophobic molecules bind to each other, and as a result less water molecules surround them. In order to prove the claim that the exothermic heats of racemization are affected by hydrophobic bonds, two statements were established: 1. In order to create hydrophobic bonds, it is necessary to provide energy in order to break the bonds between the hydrophobic molecule and the water molecules that surround it, 2. The hydrophobic bonds depend on the structure of the molecule when aromatic molecules form less hydrophobic bonds than aliphatic molecules. From the above it appears that for phenylalanine an exothermic heat of racemization with the greatest value was obtained because this amino acid forms the least amount of hydrophobic bonds, therefore the heat of racemization of this amino acid derives mainly from the racemic management of the two enantiomers in water. What was said earlier can be reinforced by the heat of racemization obtained for alanine, which is an aliphatic hydrophobic molecule, and therefore creates more hydrophobic bonds than phenylalanine, and this is accompanied by a greater consuming of energy, and ultimately to lower exothermic heats of racemization. Methionine, which is an amino acid with a longer carbon chain than alanine and is not aromatic like phenylalanine, can form more hydrophobic bonds than phenylalanine but less than alanine, and this is reflected in the intermediate values of the exothermic racemization heats obtained in the measurements.
In conclusion, we found that chiral interactions hardly take part in the formation of the racemic mixture, the heat change depends only on amino acid-solvent interactions. To make sure that the chiral interactions does not take part in the formation of the racemic mixture and that the heat change depends only on amino acid-solvent interactions, we also did comparative measurements. In the comparative measurements, we titrated L enantiomer of each tested amino acid to L enantiomer in the cell. In those measurements, the concentrations were the same as those for the measurements of the racemic dilution. The only difference between the racemic dilution measurement and the comparative measurement we mentioned before is that the racemic dilution measurement contains the addition of the chiral interactions, therefore subtract the comparative measurement heat changes from the racemic dilution measurement heat changes gives us the chiral interactions heat changes. When we are comparing the comparative measurements and the racemic dilution measurements, we are getting very close heat change values (Figures S4 and S5), and when we are calculating the chiral interactions, we are getting very small values. From the above, we can conclude that the racemic dilution heat change is almost unaffected by the chiral interactions and depends mainly on the amino acid-solvent interactions during the dilution and it’s correlation to the hydropathy of the amino acid.
Acknowledgments
Matan Oliel acknowledges the Bar-Ilan President’s Ph.D. Scholar and the Institute for Nanotechnology and Advanced Materials at Bar-Ilan University for Ph.D. Scholarship.
Conflicts of Interest
The authors declare no conflict of interest
References
- Synthesis, A. 3, 3 0 -Diaryl-BINOL Phosphoric Acids as Enantioselective Extractants of Benzylic Primary Amines. 2011;43(March 2010):34-43. [CrossRef]
- Berthod, A. Chiral Recognition in Separation Methods: Mechanisms and Applications.; 2010. [CrossRef]
- Brock CP, Schweizer WB, Dunitd JD. the Validity of Wallach’s Rule: On the Density and Stability. CarbonN Y, 1991.
- May, R. The physical identity of enantiomers. p a r t ii.* (. Published online 1936:586-593.
- Chirality, I. natural light has not yet been found having any preferred direction of polarization [ Velluz 1965 ]. both in solution and in the solid amorphous state, and that the optical rotatory power of turpentine. Media, 1558. [Google Scholar]
-
Biot , Chromatic Polarization , and the Theory of Fits. Vol 64.; 2011.
- 7. Harris AB, Kamien RD, Lubensky TC. Molecular chirality and chiral parameters. Rev Mod Phys, 1999; 71(5), 1745–1757. [CrossRef]
- Bonner, WA. Chirality and life. Orig Life Evol Biosph, 1995; 25(1-3), 175–190. [Google Scholar] [CrossRef]
- 9. Srividya N, Lange I, Lange BM. Determinants of Enantiospecificity in Limonene Synthases. Biochemistry, 2020; 59(17), 1661–1664. [CrossRef]
- 10. Eriksson T, Bjöurkman S, Roth B, Fyge Å, Höuglund P. Stereospecific determination, chiral inversion in vitro and pharmacokinetics in humans of the enantiomers of thalidomide. Chirality, 1995; 7(1), 44–52. [CrossRef]
- 11. Freyer MW, Lewis EA. Isothermal Titration Calorimetry: Experimental Design, Data Analysis, and Probing Macromolecule/Ligand Binding and Kinetic Interactions. Methods Cell Biol, 2008; 84(07), 79–113. [CrossRef]
- 12. Matos MJ, Vilar S, García-Morales V, et al. Insight into the functional and structural properties of 3-arylcoumarin as an interesting scaffold in monoamine oxidase B inhibition. ChemMedChem, 2014; 9(7), 1488–1500. [CrossRef]
- Saboury, AA. Application of a new method for data analysis of isothermal titration calorimetry in the interaction between human serum albumin and Ni2+. J Chem Thermodyn, 2003; 35(12), 1975–1981. [Google Scholar] [CrossRef]
- Pierce MM, Raman CS, Nall BT. Isothermal Titration Calorimetry of Protein–Protein Interactions.Michael. 1999;221:213-221.
- Ladbury, JE. Application of isothermal titration calorimetry in the biological sciences: Things are heating up! Biotechniques, 2004; 37(6), 885–887. [Google Scholar] [CrossRef]
- Kobori T, Iwamoto S, Takeyasu K, Ohtani T. Biopolymers Volume 85 / Number 4 295. Biopolymers, 2007; 85(4), 392–406. [CrossRef]
- Synthesis, A. Intrinsic Asymmetries of Amino Acid Enantiomers and Their Peptides: A Possible Role in the Origin of Biochirality. 2011;43(March 2010):34-43. 20 March. [CrossRef]
- 18. Scolnik Y, Portnaya I, Cogan U, et al. Subtle differences in structural transitions between poly-l- and poly-d-amino acids of equal length in water. Phys Chem Chem Phys, 2006; 8(3), 333–339. [CrossRef]
- 19. Kardos J, Yamamoto K, Hasegawa K, Naiki H, Goto Y. Direct measurement of the thermodynamic parameters of amyloid formation by isothermal titration calorimetry. J Biol Chem, 2004; 279(53), 55308–55314. [CrossRef]
- Synthesis, A. Chiral Configuration of the Hydration Layers of D- and L-Alanine in Water Implied from Dilution Calorimetry. 2011;43(March 2010):34-43. 20 March. [CrossRef]
- 21. Shval A, Mastai Y. Isothermal titration calorimetry as a new tool to investigate chiral interactions at crystal surfaces. Chem Commun, 2011; 47(20), 5735–5737. [CrossRef]
- 22. Shinitzky M, Shvalb A, Elitzur AC, Mastai Y. Entrapped energy in chiral solutions: Quantification and information capacity. J Phys Chem B, 2007; 111(37), 11004–11008. [CrossRef]
- 23. Dryzun C, Mastai Y, Shvalb A, Avnir D. Chiral silicate zeolites. J Mater Chem, 2009; 19(14), 2062–2069. [CrossRef]
- Werber L, Mastai Y. Isothermal titration calorimetry for chiral chemistry. Chirality, 2018; 30(5), 619–631. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).