Submitted:
26 June 2023
Posted:
26 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and instruments
2.2. Fabrication of manganese dioxide
2.3. Preparation of modified electrodes
3. Results and discussion
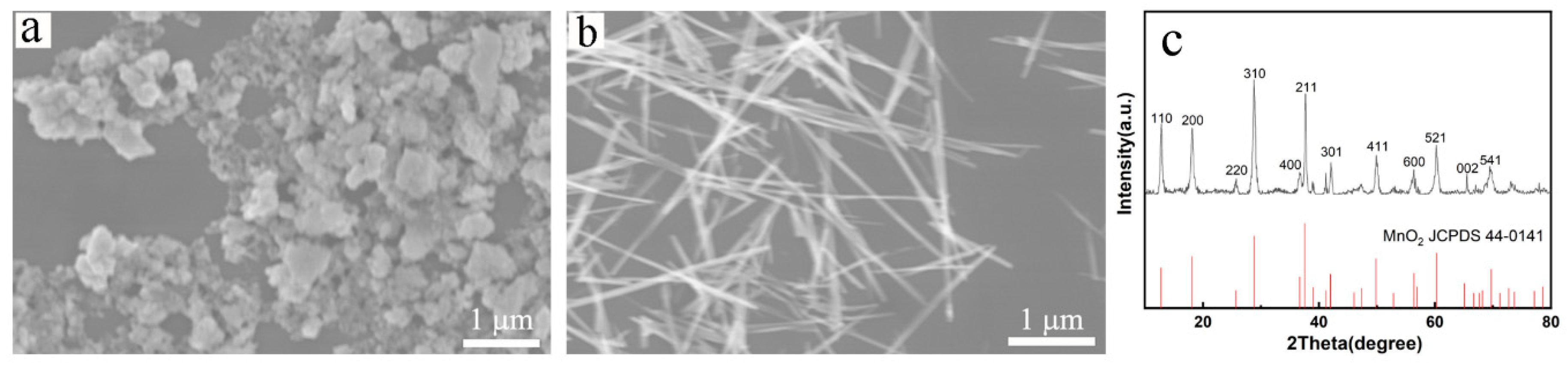
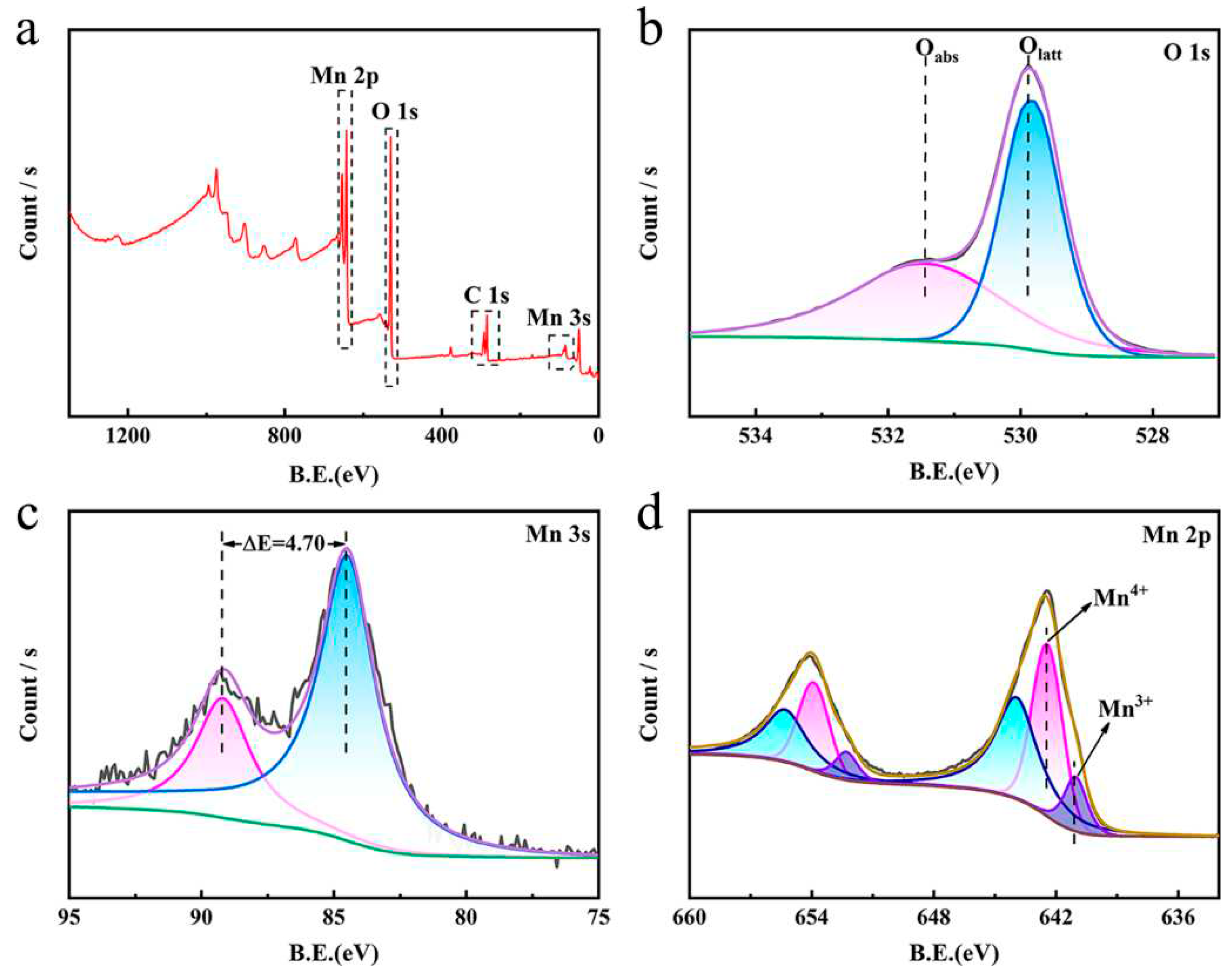
3.1. Characterization of manganese dioxide
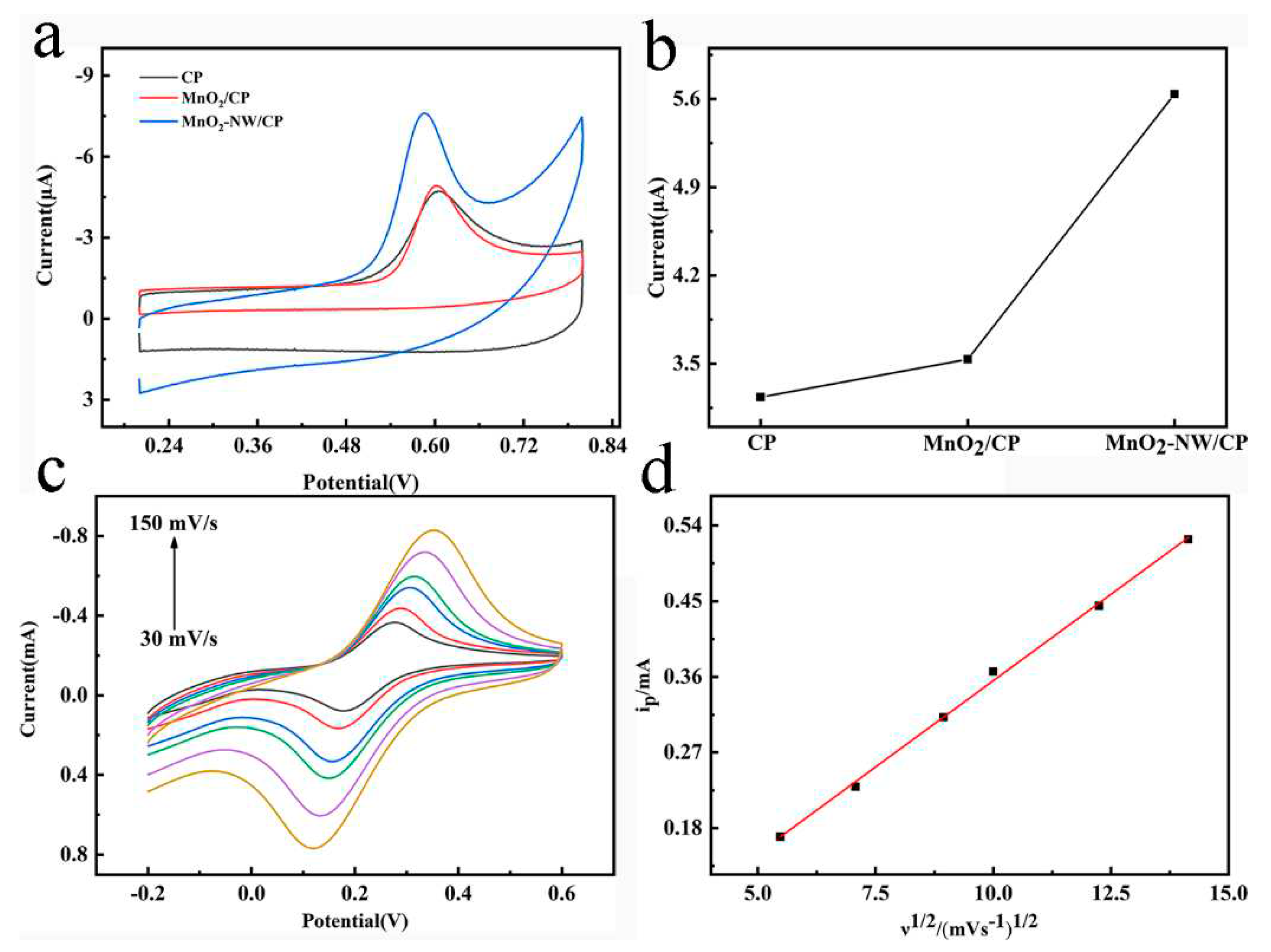
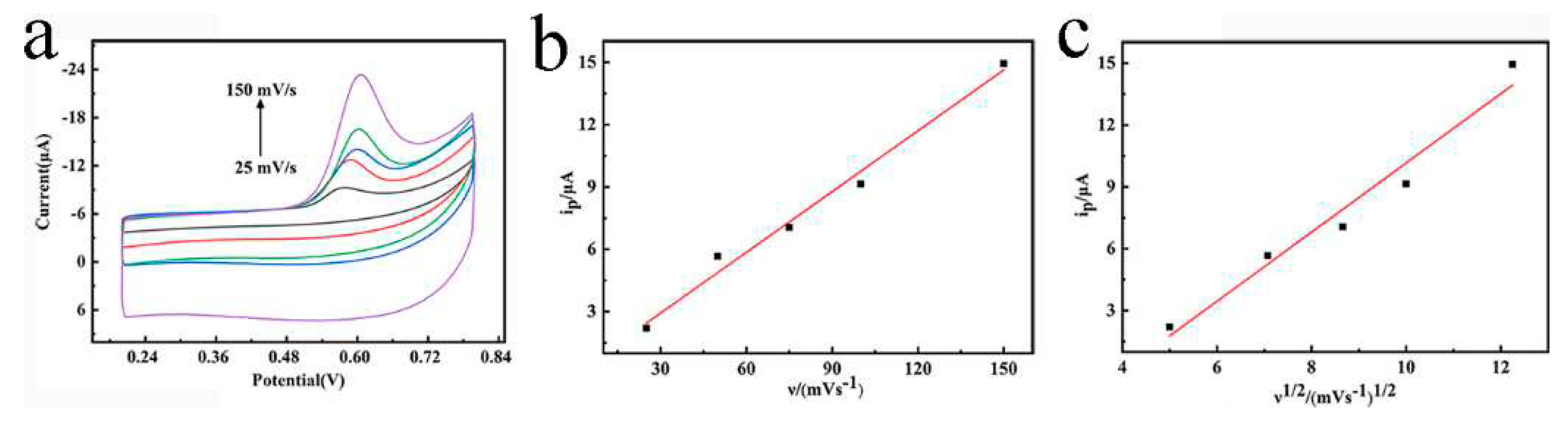
3.2. Electrochemical behavior of TBBPA on MnO2-NW/CP
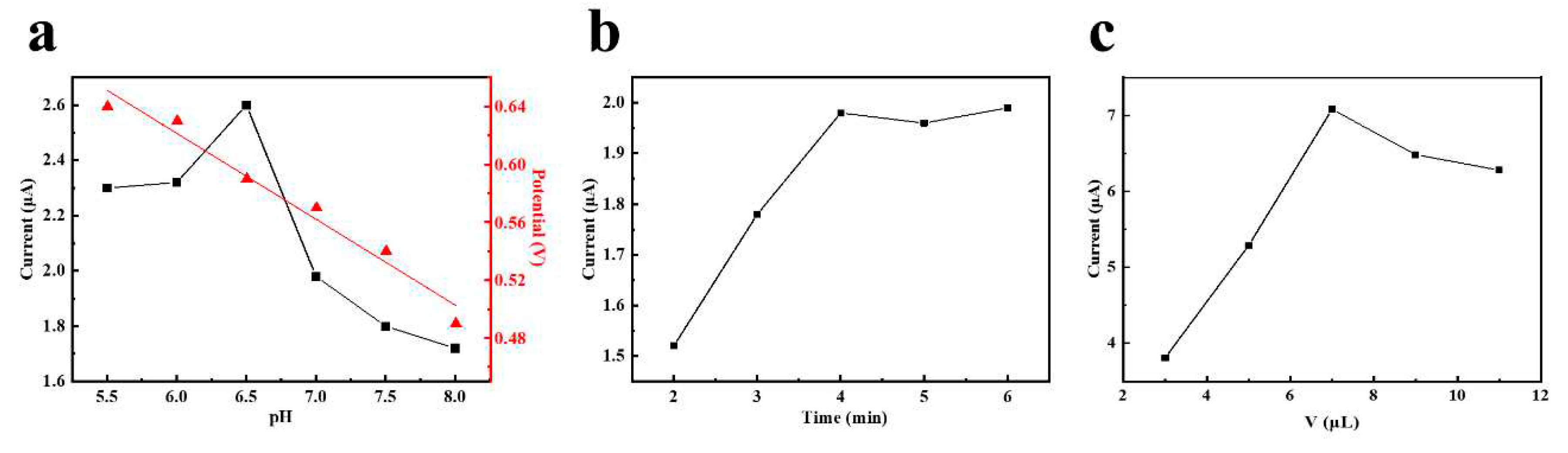
3.3. Optimization of experimental conditions
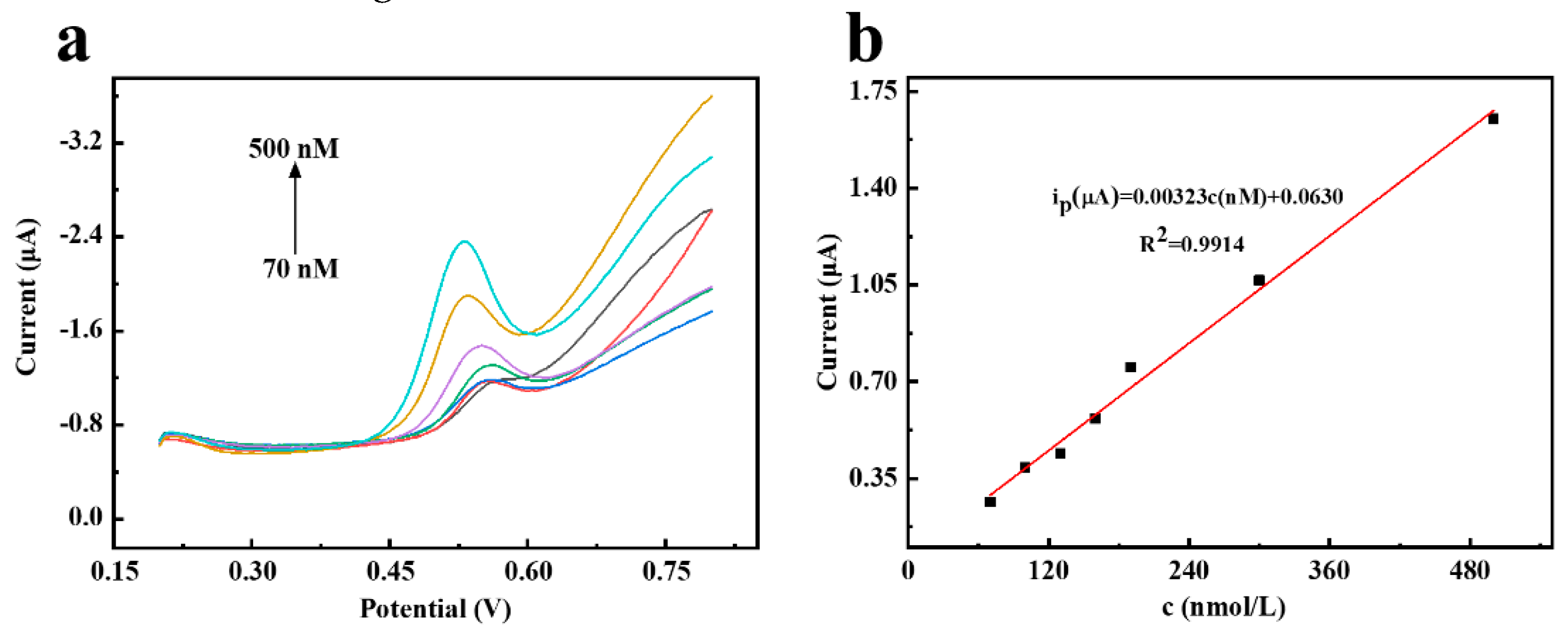
3.4. Electrochemical detection of TBBPA
3.5. Real samples analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, S.; Song, G. J.; Kannan, K.; Moon, H. B. , Occurrence of PBDEs and other alternative brominated flame retardants in sludge from wastewater treatment plants in Korea. Sci. Total Environ. 2014, 470, 1422–1429. [Google Scholar] [CrossRef]
- Shi, Z. X.; Zhang, L.; Li, J. G.; Wu, Y. N. , Legacy and emerging brominated flame retardants in China: A review on food and human milk contamination, human dietary exposure and risk assessment. Chemosphere 2018, 198, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z. X.; Zhang, L.; Zhao, Y. F.; Sun, Z. W.; Zhou, X. Q.; Li, J. G.; Wu, Y. N. , Dietary exposure assessment of Chinese population to tetrabromobisphenol-A, hexabromocyclododecane and decabrominated diphenyl ether: Results of the 5th Chinese Total Diet Study. Environ. Pollut. 2017, 229, 539–547. [Google Scholar] [CrossRef]
- Wluka, A.; Wozniak, A.; Wozniak, E.; Michalowicz, J. , Tetrabromobisphenol A, terabromobisphenol S and other bromophenolic flame retardants cause cytotoxic effects and induce oxidative stress in human peripheral blood mononuclear cells (in vitro study). Chemosphere 2020, 261, 127705. [Google Scholar] [CrossRef] [PubMed]
- Zuiderveen, E. A. R.; Slootweg, J. C.; de Boer, J. , Novel brominated flame retardants—A review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere 2020, 255, 126816. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Saigusa, D.; Tetsu, N.; Yamakuni, T.; Tomioka, Y.; Hishinuma, T. , Neurobehavioral effects of tetrabromobisphenol A, a brominated flame retardant, in mice. Toxicol. Lett. 2009, 189, 78–83. [Google Scholar] [CrossRef]
- Feiteiro, J.; Mariana, M.; Cairrao, E. , Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021, 285, 117475. [Google Scholar] [CrossRef]
- Jagic, K.; Dvorscak, M.; Klincic, D. , Analysis of brominated flame retardants in the aquatic environment: a review. Arh Hig Rada Toksiko 2021, 72, 254–267. [Google Scholar] [CrossRef]
- Gao, W.; Li, G. L.; Liu, H.; Tian, Y.; Li, W. T.; Fa, Y.; Cai, Y. Q.; Zhao, Z. S.; Yu, Y. L.; Qu, G. B.; Jiang, G. B. , Covalent organic frameworks with tunable pore sizes enhanced solid-phase microextraction direct ionization mass spectrometry for ultrasensitive and rapid analysis of tetrabromobisphenol A derivatives. Sci. Total Environ. 2021, 764, 144388. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Hou, X.; Zhang, H.; Zhu, Z.; Jiang, G. Sensitive method for simultaneous determination of TBBPA and its ten derivatives. Talanta 2023, 124750. [Google Scholar] [CrossRef]
- Zeng, L. S.; Cui, H. R.; Chao, J. L.; Huang, K.; Wang, X.; Zhou, Y. K.; Jing, T. , Colorimetric determination of tetrabromobisphenol A based on enzyme-mimicking activity and molecular recognition of metal-organic framework-based molecularly imprinted polymers. Microchem. J. 2020, 187, 142. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Sasaki, K.; Sasaki, Y.; Omata, A.; Ichino, R.; Fujimaki, S. , Photometric Screening of Tetrabromobisphenol A in Resin Using Iron(III) Nitrate/Hexacyanoferrate(III) Mixture as a Colorimetric Reagent. Anal Sci 2021, 37, 1815–1819. [Google Scholar] [CrossRef]
- Fu, H. J.; Wang, Y.; Xiao, Z. L.; Wang, H.; Li, Z. F.; Shen, Y. D.; Lei, H. T.; Sun, Y. M.; Xu, Z. L.; Hammock, B. , A rapid and simple fluorescence enzyme-linked immunosorbent assay for tetrabromobisphenol A in soil samples based on a bifunctional fusion protein. Ecotoxicol. Environ. Saf. 2020, 188, 109904. [Google Scholar] [CrossRef]
- Li, Z. F.; Wang, Y.; Vasylieva, N.; Wan, D. B.; Yin, Z. H.; Dong, J. X.; Hammock, B. , An Ultrasensitive Bioluminescent Enzyme Immunoassay Based on Nanobody/Nanoluciferase Heptamer Fusion for the Detection of Tetrabromobisphenol A in Sediment. Anal. Chem. 2020, 92, 10083–10090. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. J.; Zhang, Z. H.; Cai, R.; Rao, W.; Long, F. , Molecularly imprinted electrochemical sensor based on nickel nanoparticles-graphene nanocomposites modified electrode for determination of tetrabromobisphenol A. Electrochim. Acta 2014, 117, 385–392. [Google Scholar] [CrossRef]
- Zhang, Z. H.; Cai, R.; Long, F.; Wang, J. , Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T. T.; Feng, Y. Q.; Zhou, L. X.; Tao, Y.; Luo, D.; Jing, T.; Shen, X. L.; Zhou, Y. K.; Mei, S. R. , Selective and sensitive detection of tetrabromobisphenol-A in water samples by molecularly imprinted electrochemical sensor. Sens. Actuators B Chem. 2016, 236, 153–162. [Google Scholar] [CrossRef]
- Wang, Y. Y.; Liu, G. S.; Hou, X. D.; Huang, Y. N.; Li, C. Y.; Wu, K. B. , Assembling gold nanorods on a poly-cysteine modified glassy carbon electrode strongly enhance the electrochemical reponse to tetrabromobisphenol A. Microchim Acta 2015, 183, 689–696. [Google Scholar] [CrossRef]
- Zhou, T. T.; Zhao, X. Y.; Xu, Y. H.; Tao, Y.; Luo, D.; Hu, L. Q.; Jing, T.; Zhou, Y. K.; Wang, P.; Mei, S. R. , Electrochemical determination of tetrabromobisphenol A in water samples based on a carbon nanotubes@zeolitic imidazole framework-67 modified electrode. RSC Adv. 2020, 10, 2123–2132. [Google Scholar] [CrossRef]
- Guo, J. H.; Zhou, B. Y.; Li, S. Y.; Tong, Y. Y.; Li, Z.; Liu, M. H.; Li, Y. H.; Qu, T. X.; Zhou, Q. X. , Novel electrochemical sensor from magnetic carbon dots and cetyltrimethylammonium bromide for sensitive measurement of tetrabromobisphenol A in beverages. Chemosphere 2022, 298, 134326. [Google Scholar] [CrossRef]
- Shao, Y. M.; Zhu, Y.; Zheng, R.; Wang, P.; Zhao, Z. Z.; An, J. , Highly sensitive and selective surface molecularly imprinted polymer electrochemical sensor prepared by Au and MXene modified glassy carbon electrode for efficient detection of tetrabromobisphenol A in water. Adv Compo Hybrid Ma 2022, 5, 3104–3116. [Google Scholar] [CrossRef]
- Zhao, Q. , Zhou, H., Wu, W., Wei, X., Jiang, S., Zhou, T., Lu, Q. (2017). Sensitive electrochemical detection of tetrabromobisphenol A based on poly (diallyldimethylammonium chloride) modified graphitic carbon nitride-ionic liquid doped carbon paste electrode. Electrochimica Acta 2017, 254, 214–222. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y. Y.; Zeng, T.; Wan, Q. J.; Yang, N. J. , Morphology-dependent sensing performance of CuO nanomaterials. Anal. Chim. Acta 2021, 1171, 338663. [Google Scholar] [CrossRef]
- Luo, S. X.; Yang, M. Z.; Wu, Y. H.; Li, J.; Qin, J.; Feng, F. , A Low Cost Fe3O4-Activated Biochar Electrode Sensor by Resource Utilization of Excess Sludge for Detecting Tetrabromobisphenol A. Micromachines 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Y.; Zhang, Y. Y.; Li, C.; Li, C.; Zeng, T.; Wan, Q. J.; Li, Y. W.; Ke, Q.; Yang, N. J. , Nanointerfaces of expanded graphite and Fe2O3 nanomaterials for electrochemical monitoring of multiple organic pollutants. Electrochim. Acta 2020, 329, 135118. [Google Scholar] [CrossRef]
- Baral, A.; Satish, L.; Zhang, G. Y.; Ju, S. H.; Ghosh, M. K. , A Review of Recent Progress on Nano MnO2: Synthesis, Surface Modification and Applications. J Inorg Organomet Polym Mater 2020, 31, 899–922. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S. L.; Ji, J.; Huang, H. B. , Amorphous MnO2 surviving calcination: an efficient catalyst for ozone decomposition. Catal. Sci. Technol. 2019, 9, 5090–5099. [Google Scholar] [CrossRef]
- Guan, J. F.; Huang, Z. N.; Zou, J.; Jiang, X. Y.; Peng, D. M.; Yu, J. G. , A sensitive non-enzymatic electrochemical sensor based on acicular manganese dioxide modified graphene nanosheets composite for hydrogen peroxide detection. Ecotoxicol. Environ. Saf. 2020, 190, 110123. [Google Scholar] [CrossRef]
- Huang, Z. N.; Liu, G. C.; Zou, J.; Jiang, X. Y.; Liu, Y. P.; Yu, J. G. , A hybrid composite of recycled popcorn-shaped MnO2 microsphere and Ox-MWCNTs as a sensitive non-enzymatic amperometric H2O2 sensor. Microchem. J. 2020, 158, 105215. [Google Scholar] [CrossRef]
- Yakubu, S.; Xiao, J. X.; Gu, J. P.; Cheng, J.; Wang, J.; Li, X. S.; Zhang, Z. , A competitive electrochemical immunosensor based on bimetallic nanoparticle decorated nanoflower-like MnO2 for enhanced peroxidase-like activity and sensitive detection of Tetrabromobisphenol A. Sens. Actuators B Chem. 2020, 325, 128909. [Google Scholar] [CrossRef]
- Torrinha, A.; Morais, S. , Electrochemical (bio)sensors based on carbon cloth and carbon paper: An overview. Trends Analyt Chem 2021, 142, 116324. [Google Scholar] [CrossRef]
- Li, Y. H.; Xu, Q. Z.; Li, Q. Y.; Wang, H. Q.; Huang, Y. G.; Xu, C. W. , Pd deposited on MWCNTs modified carbon fiber paper as high-efficient electrocatalyst for ethanol electrooxidation. Electrochim. Acta 2014, 147, 151–156. [Google Scholar] [CrossRef]
- Kannan, P.; Maiyalagan, T.; Marsili, E.; Ghosh, S.; Niedziolka-Jonsson, J.; Jonsson-Niedziolka, M. , Hierarchical 3-dimensional nickel-iron nanosheet arrays on carbon fiber paper as a novel electrode for non-enzymatic glucose sensing. Nanoscale 2016, 8, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Y.; Wang, C. X.; Xu, Y. J.; Dai, D. M.; Deng, X. Y.; Gao, H. T. , A Novel Electrochemical Sensor Based on A Glassy Carbon Electrode Modified with GO/MnO2 for Simultaneous Determination of Trace Cu(II) and Pb(II) in Environmental Water. ChemistrySelect 2019, 4, 11862–11871. [Google Scholar] [CrossRef]
- Radulescu, M. C.; Bucur, M. P.; Bucur, B.; Radu, G. L. , Ester flavorants detection in foods with a bienzymatic biosensor based on a stable Prussian blue-copper electrodeposited on carbon paper electrode. Talanta 2019, 199, 541–546. [Google Scholar] [CrossRef]
- Tagsin, P.; Suksangrat, P.; Klangtakai, P.; Srepusharawoot, P.; Ruttanapun, C.; Kumnorkaew, P.; Pimanpang, S.; Amornkitbamrung, V. , Electrochemical mechanisms of activated carbon, alpha-MnO2 and composited activated carbon-alpha-MnO2 films in supercapacitor applications. Appl. Surf. Sci. 2021, 570, 151056. [Google Scholar] [CrossRef]
- Cui, Y.; Song, H. K.; Shi, Y. Y.; Ge, P. X.; Chen, M. D.; Xu, L. L. , Enhancing the Low-Temperature CO Oxidation over CuO-Based alpha-MnO2 Nanowire Catalysts. Nanomaterials 2022, 12, 2083. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, X. X.; Wu, K. B.; Li, C.; Wang, Y. Y. , Electrochemical sensing of terabromobisphenol A at a polymerized ionic liquid film electrode and the enhanced effects of anions. Ionics (Kiel) 2018, 24, 2843–2850. [Google Scholar] [CrossRef]
- Ganesh, A.; Sivakumar, T.; Sankar, G. , Biomass-derived porous carbon-incorporated MnO2 composites thin films for asymmetric supercapacitor: synthesis and electrochemical performance. J. Mater. Sci. Mater. Electron. 2022, 33, 14772–14783. [Google Scholar] [CrossRef]
- Shen, T. Y.; Liu, T. C.; Mo, H. Q.; Yuan, Z. C.; Cui, F.; Jin, Y. X.; Chen, X. J. , Cu-based metal-organic framework HKUST-1 as effective catalyst for highly sensitive determination of ascorbic acid. RSC Adv. 2020, 10, 22881–22890. [Google Scholar] [CrossRef]
- Stradins, J.; Hasanli, B. , Anodic voltammetry of phenol and benzenethiol derivatives.1. Influence of pH on electrooxidation potentials of substituted phenols and evaluation of pK(a) from anodic voltammetry data. J. Electroanal. Chem.
- Shen, J.; Bian, C.; Xia, S. H.; Wu, K. B. , Poly(sulfosalicylic acid)-functionalized gold nanoparticles for the detection of tetrabromobisphenol A at pM concentrations. J. Hazard. Mater. 2020, 388, 121733. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, J. Y.; Lou, Z. Z.; Zeng, L. X.; Zhu, M. S. , Molecularly imprinted photoelectrochemical sensor for detecting tetrabromobisphenol A in indoor dust and water. Microchem. J. 2021, 188, 320. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, S.; Jia, B. Y.; Guo, Y. J.; Zou, Y. M.; Song, N. H.; Xiao, J. X.; Liang, K. L.; Bu, Y. Q.; Zhang, Z. , Indirect competitive-structured electrochemical immunosensor for tetrabromobisphenol A sensing using CTAB-MnO2 nanosheet hybrid as a label for signal amplification. Anal. Bioanal. Chem. 2021, 413, 4217–4226. [Google Scholar] [CrossRef] [PubMed]
| Sensor assembly | Method | LOD | Linear range | Reference | |
| CNTs@ZIF-67/PFDA/AB | DPV | 4.2 nM | 10 ~ 150 nM | [19] | |
| AuNPs-PSSA/GCE | DPV | 25 pM | 0.1 ~ 10 nM | [42] | |
| MI-TiO2/Au/rGO | DPV, CV | 0.51 nM | 1.68 ~ 100 nM | [43] | |
| CTAB-MnO2/Pd | I-t | 0.17 ng/mL | 0 ~ 81 ng/mL | [44] | |
| poly(PPBim-DS)/GCE | DPV | 20 nM | 0.05 ~ 10 µM | [39] | |
| MnO2-NW/CP | DPV | 3.1 nM | 70 ~ 500 nM | This work | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




