1. Introduction
Scallop (
Patinopecten yessoensis), as an important economic shellfish variety bred in northern China, has become a leading industry to drive the development of local fishery economy [
1,
2]. Based on the dietary consumption habits of Chinese residents, vitality and freshness are the criteria to judge the quality of aquatic products as well as the factor of the first importance when purchasing aquatic products, hence endowing fresh products with higher economic value for their plump, tender and delicious meat quality together with their plentiful and diversified nutrition [
3]. Before being out into the market, Scallop will go through four stages, that is, catching, clean-up and temporary breeding, anhydrous storage and sales [
4]. Therefore, issues such as optimizing and improving the transportation methods of aquatic products, enhancing the survival rate, reducing nutrient loss and maintaining the original flavor quality are urgent to be solved in industrial upgrading.
Ecological ice temperature anhydrous live preservation technology is a burgeoning method to drive aquatic animals into a dormant state at low temperature 4 which can reduce their metabolic rate and conduct live preservation transportation under low temperature without water [
5,
6]. While being compared with traditional hydrous transportation, it manages to improve transportation efficiency and lower logistics cost, but the influence of stress factors such as low temperature cold acclimatization and hypoxic exposure and desiccation in waterless transportation on the physiological state and flavor quality of shellfish should be taken into consideration [
7]. Hongbo Mi et al., after storing the scallop at low temperature without water for 96h, they found that the muscle and liver tissue were somewhat damaged during the process of live preservation, but did not lead to irreparable injury [
8]. Hengzong Lin et al. Held the view that before the anhydrous live preservation, if the Pacific oysters were trained in coldness at the ecological ice temperature, the effect of temperature stress on the oxidative immune system and the metabolism of energy substances could be reduced [
9]. Lixin Yan et al. simulated the vitality of scallop during circulation by virtue of analyzing the energy content such as adenosine triphosphate, and found that the vitality of scallop decreased for 24 h [
10]. High temperature stress caused by increasing seawater temperature significantly affects the energy metabolism [
11]. However, there is no systematic report on the stress regulation mechanism caused by stress such as chilling stress and anhydrous storage. In this study, aiming to study the effects of environmental stress on the body of scallop, the effect of environmental stress in cold acclimatization and anhydrous storage and transportation in the temporary breeding stage was studied.
2. Materials and Methods
2.1. Experimental raw materials
The scallop (Patinopecten yessoensis) cultured in net cages in Nanguang Island, Shandong, China, with shell length of (76.30 ± 6.48) mm, shell height of (18.97 ± 1.86) mm, and wet weight of (59.63 ± 12.76) g were selected as raw materials. In April 2022, immediately after being caught, they were sealed with foam boxes (ice pack cooling) to Shandong Provincial Key Laboratory of Agricultural Products Storage, Transportation and Preservation Technology. Those arrived in the laboratory in the living anhydrous hypoxia stress state were immediately placed in artificial circulation seawater for clean-up and bred temporarily at the water temperature of (15 ± 2) ℃, and salinity of 32 ‰. After temporary breeding, after 7d, those with strong vitality and wonderful health state were selected to carry out the experiment. This experiment was approved by the Animal Ethics Committee of Guangdong Ocean University, and the approval code is 1122003004.
2.2. Experimental design and methods
After temporary breeding in the laboratory, healthy and dynamic Scallop were selected for gradient cooling and cold acclimatization to its ecological ice temperature. That is, by setting the cooling rate of the temporary breeding system at 2℃ / h, after lowering the temperature for every 1h, they were maintained at constant temperature for 3h. Temporary breeding temperature of 15℃ was lowered to dormant temperature at 4℃ through 24 h, and then they were removed for anhydrous gas conditioning packaging. A dozen of Scallop was set in group with a (335 * 220 * 175) mm foam box which was placed with a biological ice bag at the bottom. Meanwhile, the temperature insulation bag was put between the ice bag and the Scallop. The packed Scallop were stored and transported in the low temperature vibration chamber at 4℃ for 72h, after which they were awaken in water, that is to place the Scallop after anhydrous storage and transportation into into the temporary breeding system. Meanwhile, by the system program, the temperature was increased by the ecological ice temperature at a rate of 2℃ / h. After heating for every 1h, they were maintained at constant temperature for 3 h until it turned to 15 ℃, after which relevant index detection was performed.
2.3. Analysis of the test
2.3.1. Living health evaluation
Edge shrinkage The edge shrinkage rate was referred to the method of Li Yaxuan etc [
12]. And the edge shrinkage rate = edge shrinkage distance / shell height× 100%
Stimulus response time The pointed tweezers were used to stimulate the pallium of the scallop alive, and the contraction response time was recorded with a stopwatch.
Heart rate The measurement method was slightly modified according to that of Bakhmet et al [
13]. First, the scallop shell was cleaned, the infrared sensor was stuck outside the scallop shell near the heart, the scallop was placed in artificial seawater at 15℃artificial for stewing, which would be then detected after the antennas of the pallium were fully displayed. The Power Lab instrument parameter range (2~5 V) was set with low pass (1~10 Hz) and ac coupling. The LabChart software was utilized to record the changes of scallop heartbeat stability, and intercept the area of the 2 auricular appendages and one ventricular waveform, thus obtaining complete and smooth waveform map. And then the stable waveform in 10 min detection time was calculated, and 5 scallops were checked each time, totaling 3 times in parallel.
2.3.2. Nucleotides and the associated compounds
The adductor muscle and striated muscle were extracted and mashed by adding 5% PCA solution in ice bath. The PH was adjusted to 2-3.5, 4000 rpm for centrifugation of 5min, so as to obtain the supernatant, which was then filtered with 0.45 μ m membrane filter, and analyzed by HPLC after adding phosphate buffer. Chromatographic conditions: column symmetry C18, (4.60 mm 150 mm, 5 μ m); Mobile phase A: 0.05 mol/L KH2PO4-K2HPO4 (pH 6.78), mobile phase B chromatographic methanol; Detection wavelength: 259 nm; Sample intake: 20 μ L; flow rate: 1 mL/min; column temperature: 40℃; gradient elution.
Nucleotide energy charge AEC (adenylate energy charge) value is an indicator reflecting the degree of environmental stress on animals, which can reflect the freshness of scallops. AEC(%) = (2ATP+ADP)/(2(ATP+ADP+AMP))×100%.
2.3.3. Proteomics
The adductor muscle at different treatment stages were selected for proteomic analysis. Samples then underwent protein extraction, enzymatic peptide digestion, liquid chromatography-mass spectrometry (LC-MS) data collection, database retrieval, and bioinformatics analysis. Chromatography-mass spectrometry detection conditions: mobile phase A: formic acid aqueous solution, mobile phase B: 0.1% (v / v) formic acid solution. Peptide fragments were dissolved by liquid chromatography in mobile phase A and separated using an ultra-efficient liquid phase system. Liquid phase gradient setting: 0~120 min, 8%~100% mobile phase B, flow rate: 300 nL / min.
2.3.4. Metabonomics
The adductor muscle at different treatment stages were selected for metabolomics analysis. After shock crushing and cold sonication, the samples were centrifuged at 12,000 rmp for 10 minutes at 4℃, and the supernatant was removed for machine testing. Column: C18 column (Zorbax Eclipse C18(1.8μm*2.1mm * 100mm), chromatographic separation conditions: column temperature 30℃, flow rate 0.3 mL/min; mobile phase composition A: 0.1% formic acid solution, B: pure acetonitrile, gradient elution.
Positive mode: heater temperature 325 °C; sheath gas flow: 45 arb(arbitrary units); aux gas flow: 15 arb; sweep gas flow: 1 arb; electrospray voltage: 3.5 KV; capillary temperature: 330 °C; S-Lens RF Level: 55%.
Negative mode: heater temperature 325 °C; sheath gas flow: 45 arb; aux gas flow: 15 arb; sweep gas flow: 1 arb; electrospray voltage: 3.5 KV; capillary temperature: 330 °C; S-Lens RF Level: 55%.
Scanning mode: full scan (Full Scan, m/z 100~1500) and data-dependent mass spectrometry (dd-MS2, TopN = 10); resolution: 120,000 (MS1) & 60,000 (MS2). Collision Mode: High Energy Collision Dissociation (HCD).
2.4. Data processing
The experimental results were expressed as "mean ± standard deviation (X±SD)", the experimental data were processed by Origin and SPSS software and analyzed by univariate analysis, and the significance level was set as P <0.05.
3. Results
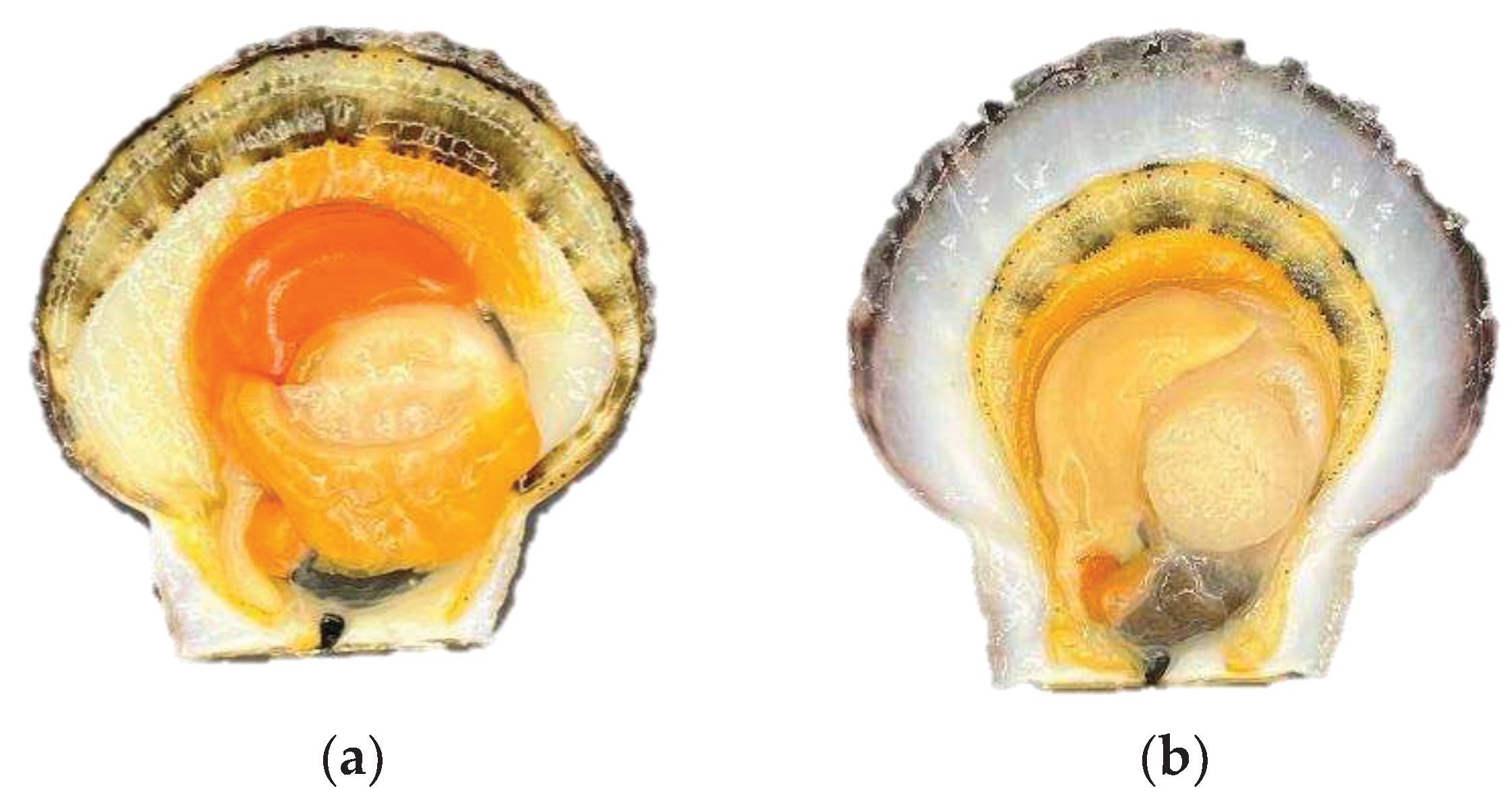
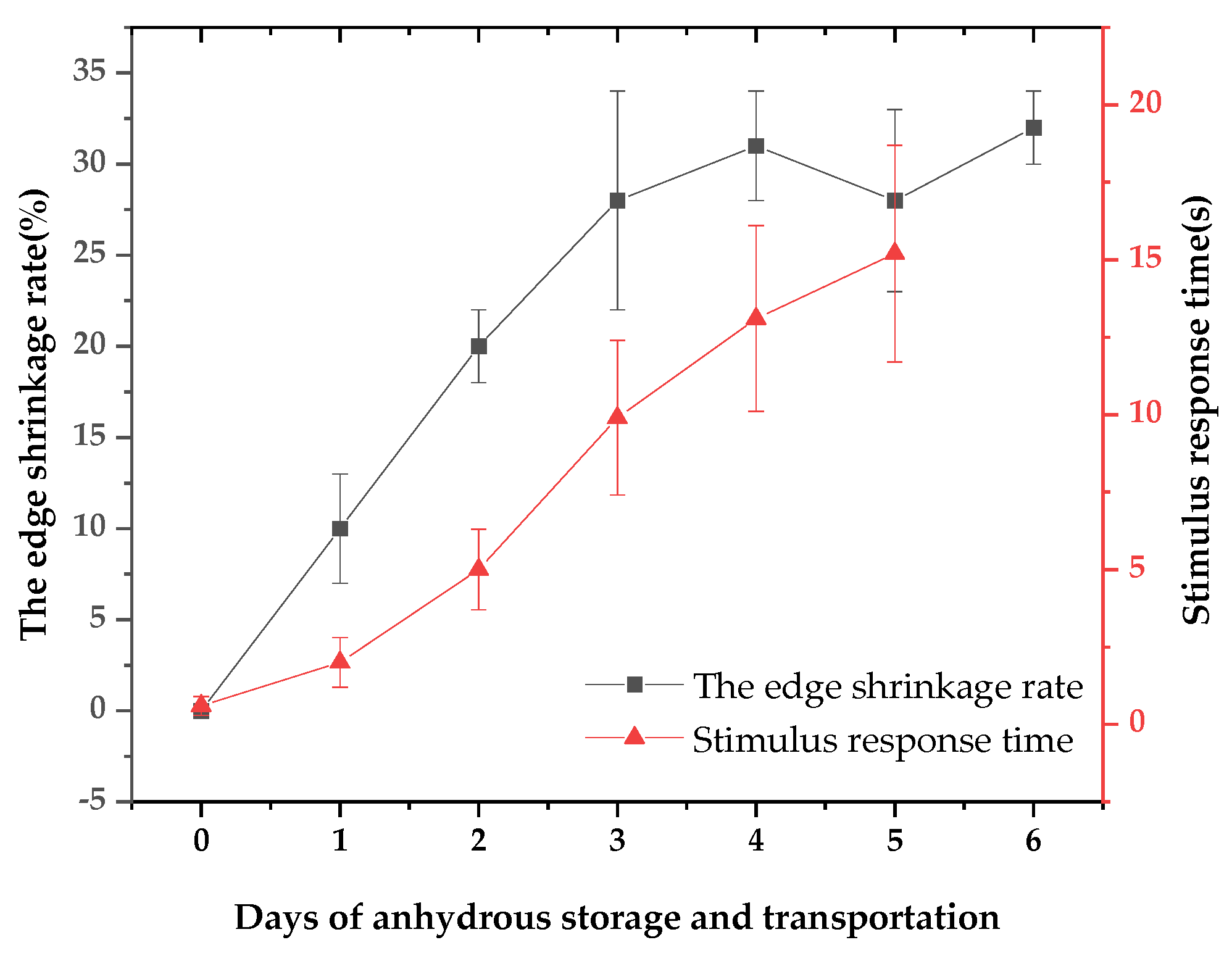
3.1. The apparent vitality of Patinopecten yessoensis decreased during anhydrous storage and transportation
The apparent vitality of
Patinopecten yessoensis was evaluated by edge shrinkage rate and stimulus response time. During the period of purification and temporary breeding and cold acclimatization, the pallium of living scallop was full and had no shrinkage, and the tentacles fully extended to the shell. However, during the storage and transportation, with the time going by, the edge shrinkage phenomenon appeared and the rate gradually increased. By the third day of anhydrous storage and transportation, the rate reached 28.05% (
Figure 1). Meanwhile, it was revealed by the Pearson correlation analysis with the pallium stimulus response time that the correlation coefficient was 0.914, showing a significant positive correlation (p=0.011 <0.05), that is, the worse the contraction phenomenon, the worse the living state, and the longer the stimulus response time (
Figure 2).
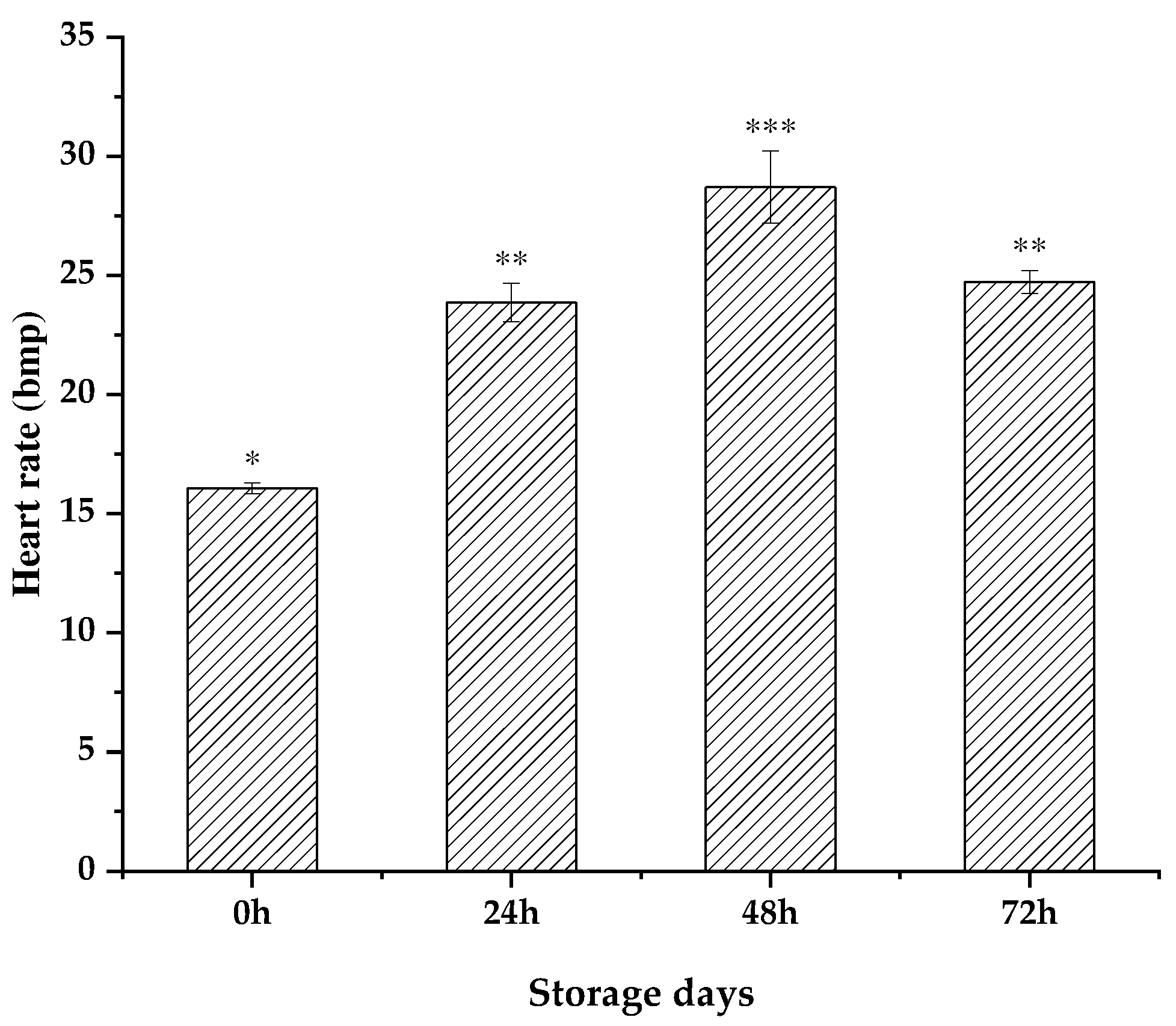
3.2. The Patinopecten yessoensis vitality dropped during the anhydrous storage and transportation process
The infrared sensing was employed to monitored the heart rate activity of scallops without doing any damage, and the heart rate cycle and intensity were recorded. The bmp value was the number of heart beats per minute. By the preliminary research of the research group, it was found that during the 15℃ temporary breeding period, the average heart rate of scallops was 18.39 times / min. The the gradient cooling was used to monitor the heart rate in real time. With the decrease of temperature, the heart rate gradually decreased, and the heart rate dropped to 7.39 times / min at the cooling endpoint of 4℃ [
14]. After 15℃ of rehydration, the heart rate was significantly higher than that of the control group (p <0.05) (
Figure 3). Among them, the heart rate values of 24h, 48h and 72h were 23.86±0.80bpm, 28.70±1.51bpm, 24.72±0.48bmp respectively, which was 60.41% higher than the control group, and the survival rate of scallops decreased after 72h, and the heart rate measurement was terminated.
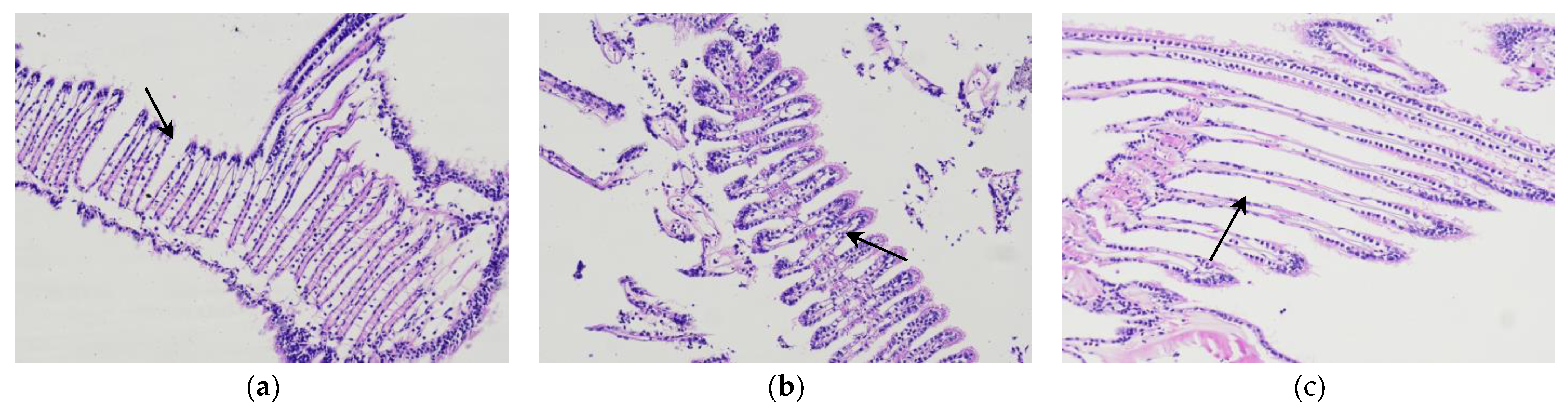
3.3. Changes in microorganizational structure of the gills
In the contrast of microscopic tissue sections of the gills after temporary breeding, cold acclimatization and water storage for 3d (
Figure 4), it can be shown in the Figure that the gills filaments in the clean-up and temporary breeding period were uniform and neat in arrangement. After gradient cooling cold acclimatization treatment, they became shorter and thicker. After being transferred from water environment into anhydrous storage environment, the scallops closed the shells to retain moisture, thus reducing the oxygen intake. As from oxygen breathing to oxygen respiration, low oxygen stress led filaments to increase space and extend in order to increase the contact area with air exchange. Duan et al. Figure d out in the research that Japanese Penaeus orientalis’ respiration burst in an anhydrous environment for 3h, showed respiration weakened at 10h, and eventually the gill cavity movement slowed down, leading to the death of the body [
15].
3.4. Nucleotide-lineage compounds
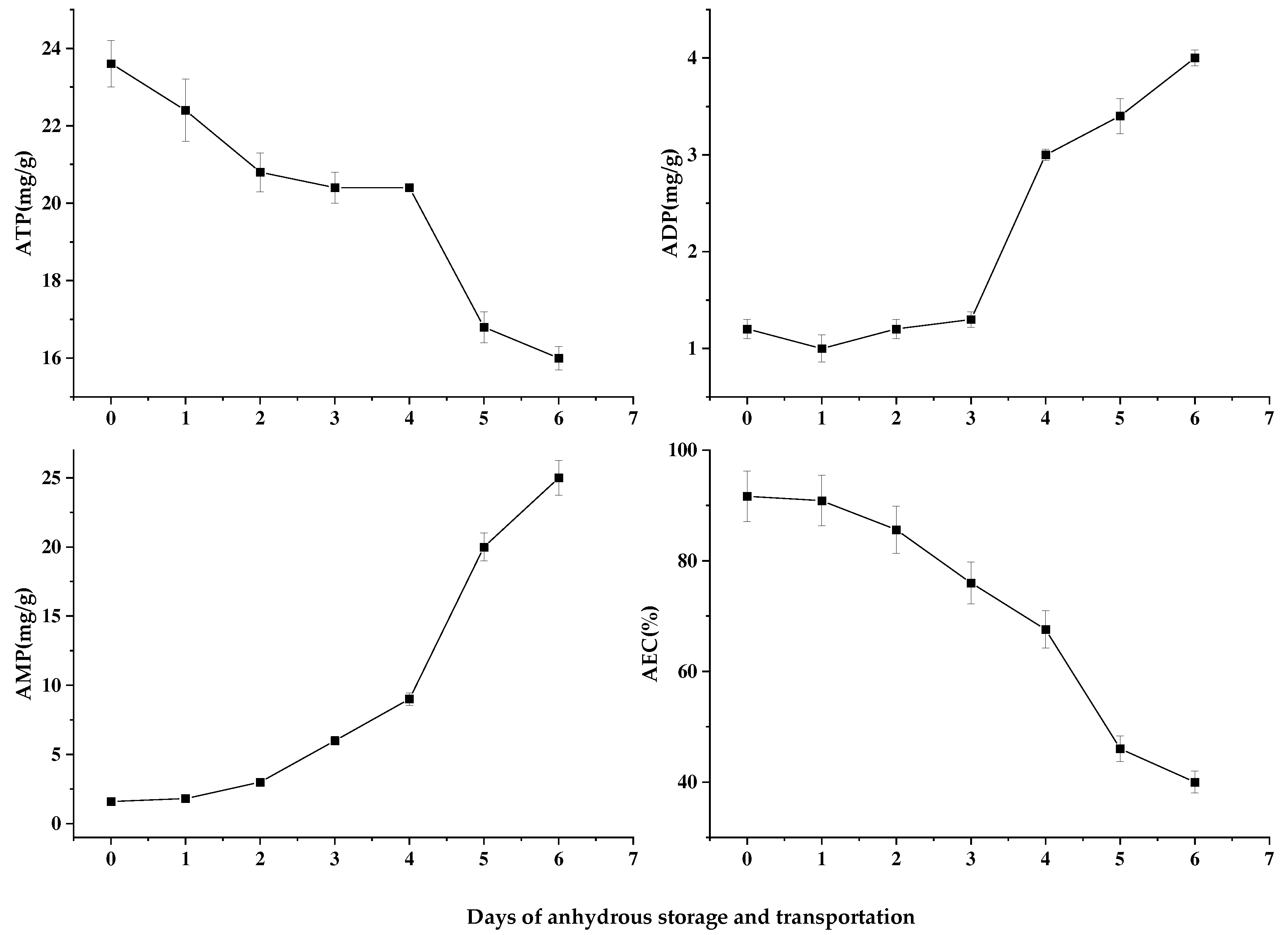
ATP provides the energy necessary to maintain normal life activities, and the change in its content can better reflect the life status of shellfish [
16]. In the process of anhydrous storage, the ATP content of Patinopecten yessoensis decreases by ladder (
Figure 5). However, when the Patinopecten yessoensis changed from hydrous to anhydrous storage environment after cold acclimation, its breathing mode was changed from aerobic to anaerobic, and the release of energy was reduced, so the ATP showed a decreasing trend [
17]. During the 2-4d of anhydrous storage and transportation, scallops were in the stress state for a long time, and started their own stress response mechanism to mobilize energy materials to maintain the body balance, so the ATP content maintained a stable state in this stage. However, in the later period of anhydrous storage and transportation, with the gradual depletion of glycogen and other substances, the rapid ATP degradation showed a steep decline. Meanwhile, the decomposition of ATP led to an increase of ADP content. ADP was the substrate of adenylate kinase reaction, producing 1 ATP and 1 AMP from 2 ADP. The change of ADP and AMP content were shown in
Figure 5. With the extension of anhydrous storage and transportation, the body cannot maintain the relative stability of ATP content in anoxic environment, that is, the occurrence of ADP and AMP accumulation.
Nucleotide-energy AEC is widely used in the vitality and quality evaluation of live fish and shellfish. By studying, Maguire et al. revealed that AEC value in scallop muscle could effectively reflect the stress intensity and self state at that time, and the AEC value was divided into three stages to show the vitality of shellfish in different periods: 80% -90% stood for good state and reproduction available; 50% -70% represented slow growing but recoverable reproduction; and less than 50% meant irreversible [
18,
19]. At 4-5d, the AEC value was 67.59% and 46.02%(
Figure 5), respectively. Therefore, to ensure the good growth state of scallops after rehydration, the storage and transportation time shall be controlled within 4d.
3.5. Environmental stress induced changes in the proteome
In order to obtain the differential protein expression changes of scallops in three different stages: fresh temporary breeding storage (XHZ), cold acclimation (LXHZ) and anhydrous live preservation storage (BHZ), the non-labeled quantitative proteomics was used in the study. The data were searched by UniProt database, identifying 4,548 peptides, 4,134 unique peptides and 856 proteins (
Table 1). Based on the above data, a systematic bioinformatics analysis of quantitatively informative proteins was carried out, including protein annotation, significant difference analysis, annotation clustering based on significant difference and protein interaction network analysis, so as to provide reference directions for the in-depth study of the proteome.
3.5.1. GO annotation
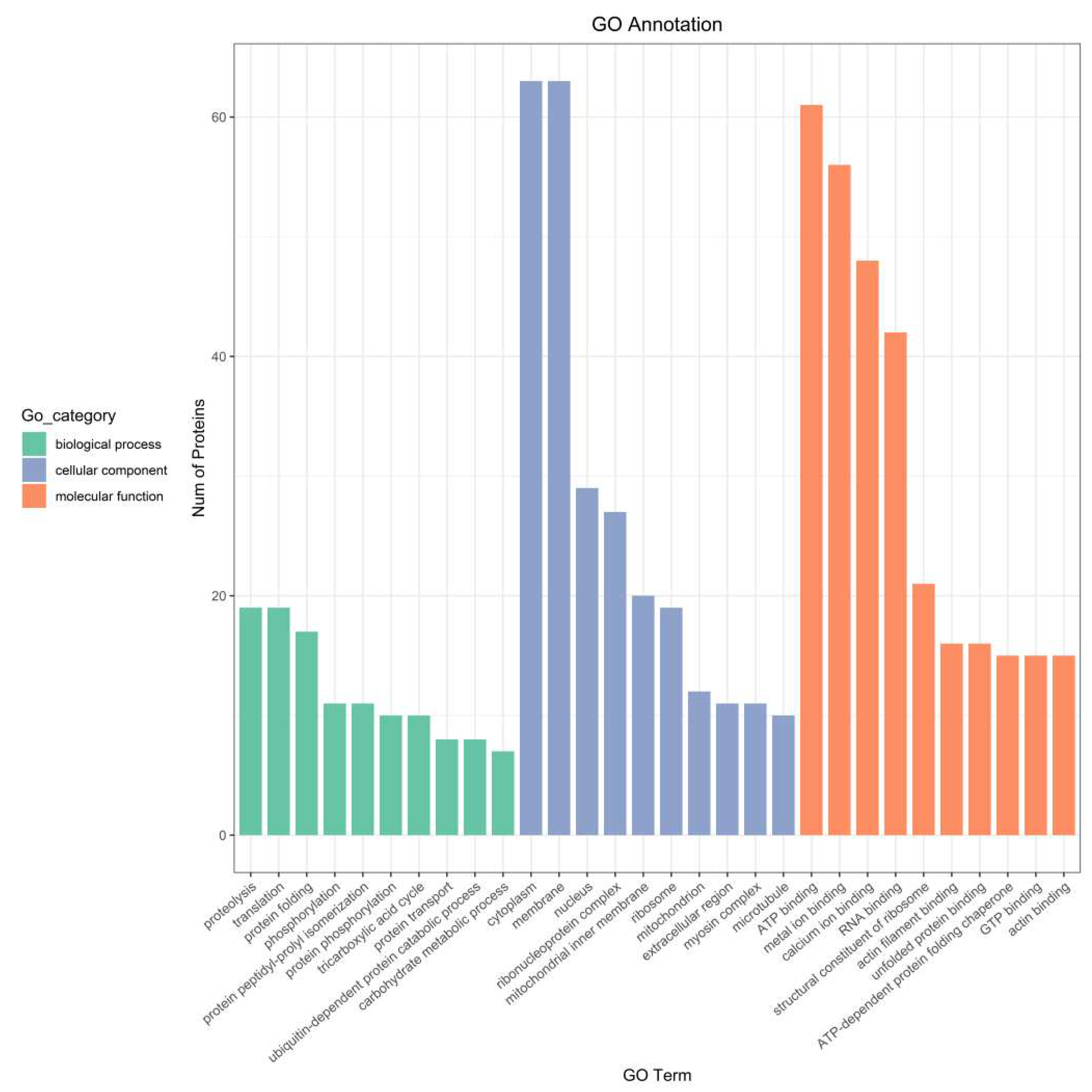
GO (GeneOntology) refers to an internationally standardized classification system for gene function description, which is divided into three categories, namely, the Cellular Component which is used to describe subcellular structure, location and macromolecular complexes, the Molecular Function which is used to describe the function of individual gene products, and the Biological Process which is used to describe the biological process in which the gene encoded products participate. According to the GO annotation results, the number of proteins corresponding to different GO entries was counted, and the annotation results of top10 in each major class of the GO database were drawn. As seen in Fig (
Figure 6), their biological functions were mainly focused on Proteolysis, Translation, Protein folding, Phosphorylation And other aspects. Mainly involved in cytoplasm in terms of cellular components, membrane, ribosome, mitochondrion and other structure. In terms of molecular functions were ATP binding, metal ion binding, and structural constituent of ribosome et al.
3.5.2. COG annotation
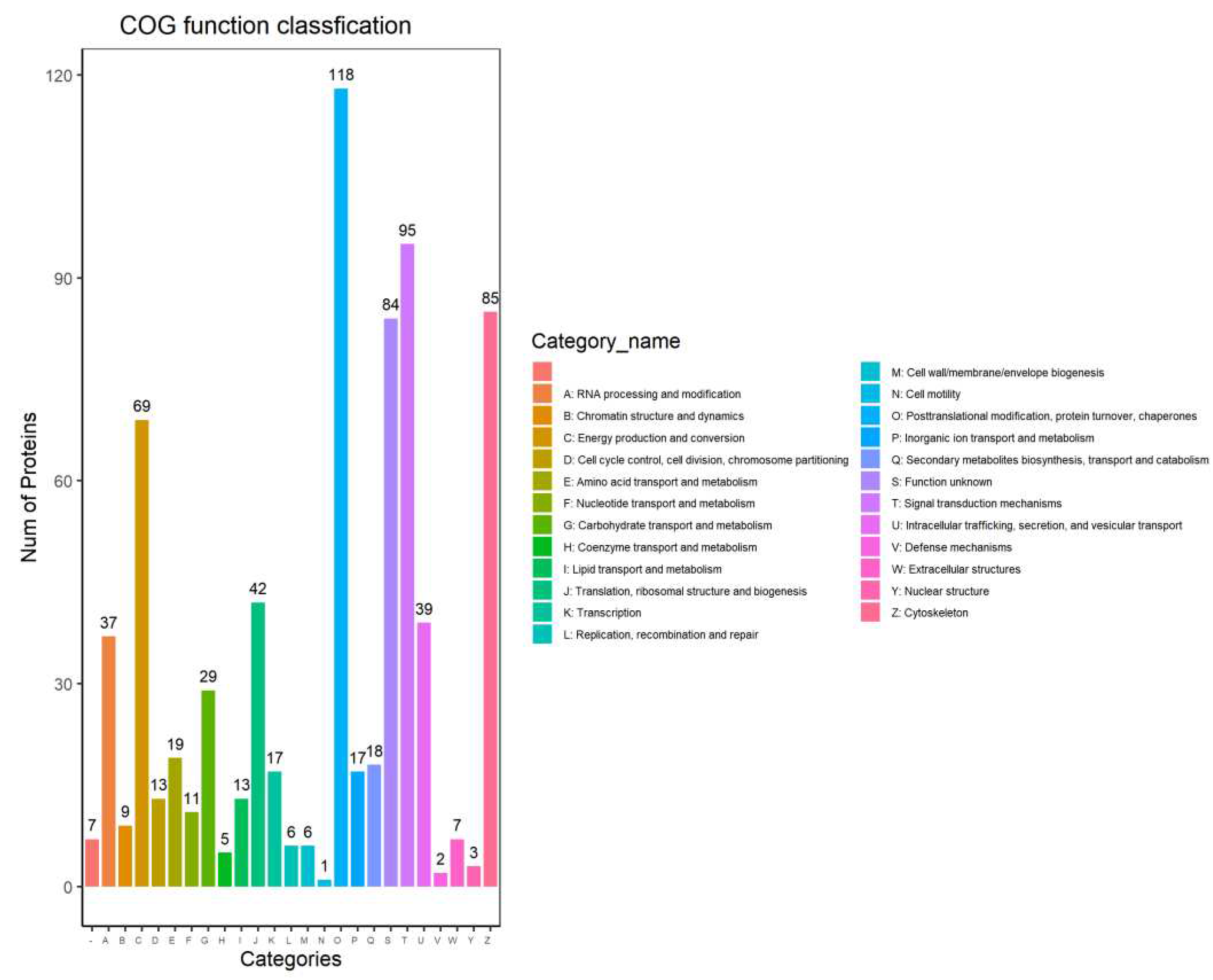
COG (Cluster of Orthologous Groups of proteins) refers to the protein database created and maintained by NCBI, which was constructed based on the phylogenetic relationship classification of the coding proteins of the complete genomes of bacteria, algae and eukaryotes. Through the alignment, a certain protein sequence can be annotated to a certain COG, and each cluster of COG is composed of direct homologous sequence, so that one can speculate on the function of the sequence. The COG database was divided into 26 categories according to its functions, and the COG database annotation bar charts were drawn according to the annotation results with results showing that the identified proteins were mainly concentrated in Posttranslational modification, protein turnover, chaperones; Signal transduction mechanisms; Cytoskeleton; Energy production and conversion and other processes (
Figure 7).
3.5.3. Analysis of variance
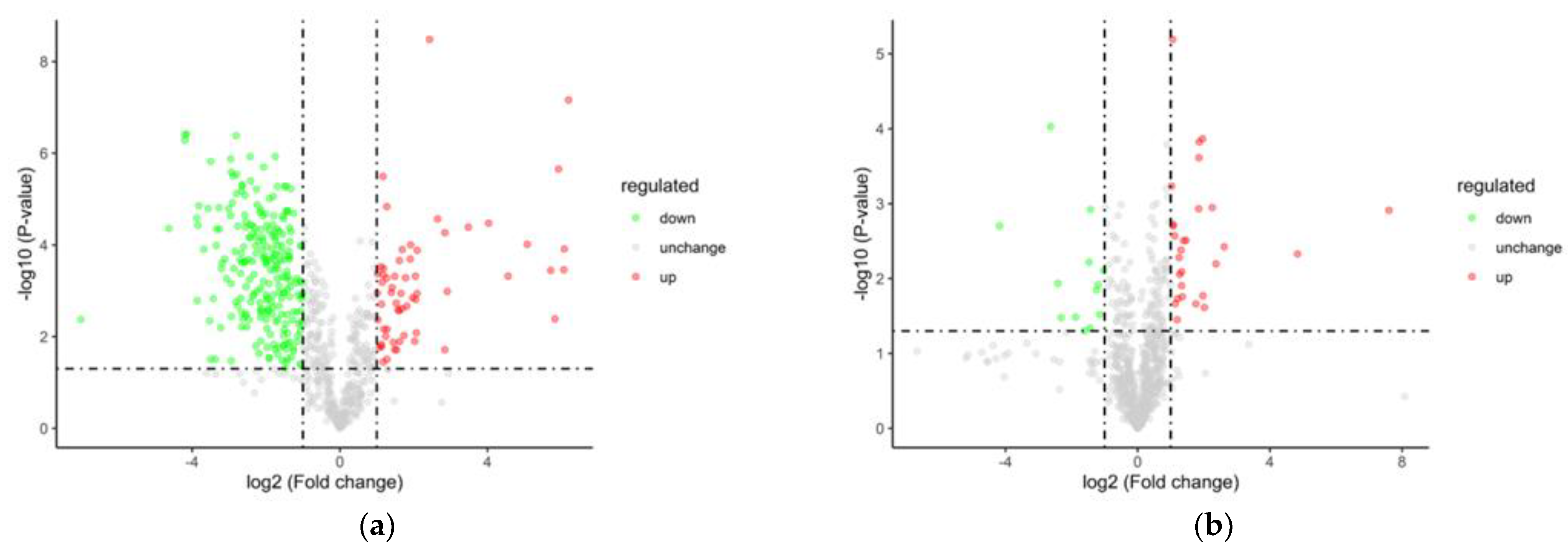
The fresh temporary breeding (XHZ), cold acclimation (LXHZ), and anhydrous live preservation storage and transportation (BHZ) were used for between-group comparison, in which the ratio of all biological replicates in the comparison samples was taken as the multiple of difference (Fold Change, FC), and the protein was increased when FC≥2(logFC≥1)while when FC≤0.5(logFC≤-1, protein showed downregulation of expression. Among them, the cold acclimation group upregulated 60 and 237 differentially expressed proteins compared with the transient group. It was speculated that scallops inhibited the enzyme activity by cold acclimation, so the number of downregulated expressed proteins was more. However, the unwatered storage group raised 28 differential proteins and lowered 13 differential proteins compared with the cold acclimation group (
Table 2),
Figure 8 shows the volcano plot of differential protein.
3.5.4. GO annotation and enrichment analysis of significantly differential proteins
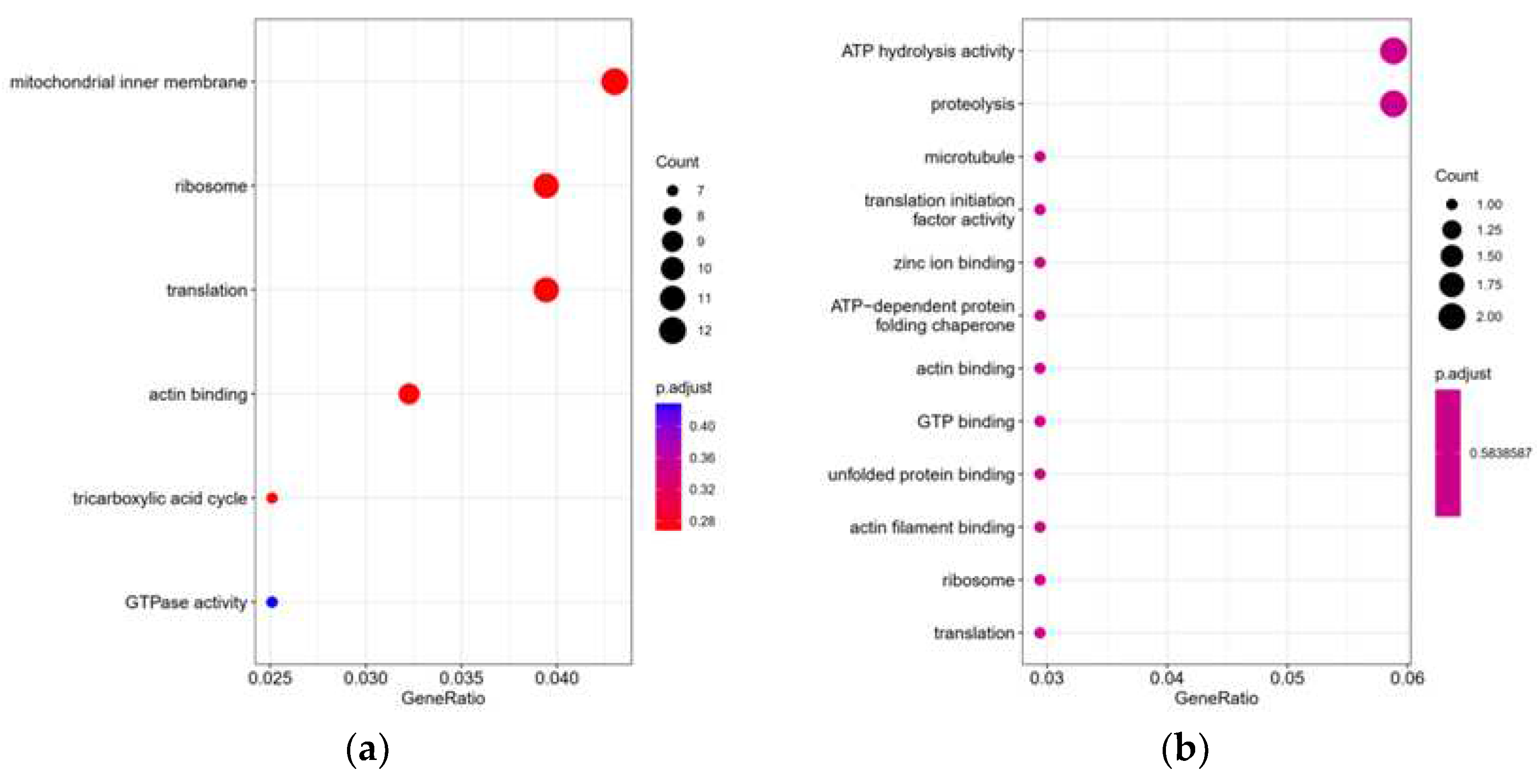
GO function significance enrichment analysis gave GO function entries significantly enriched in the differential proteins compared to all identified protein backgrounds, thus giving which biological functions the differential proteins are significantly associated with (p <0.05). Based on the enrichment results, the bubbles of the enriched GO entries are drawn (
Figure 9). The results found that after gradient cooling, the differential proteins were mainly enriched in mitochondrial inner membrane, ribosome, translation, actin bing and other biological functions and pathways, while the transition from cold acclimation to no water storage stage, the body mainly had significant changes in ATP hydrolysis activity and proteolysis.
3.6. Environmental stress induced changes in the metabolome
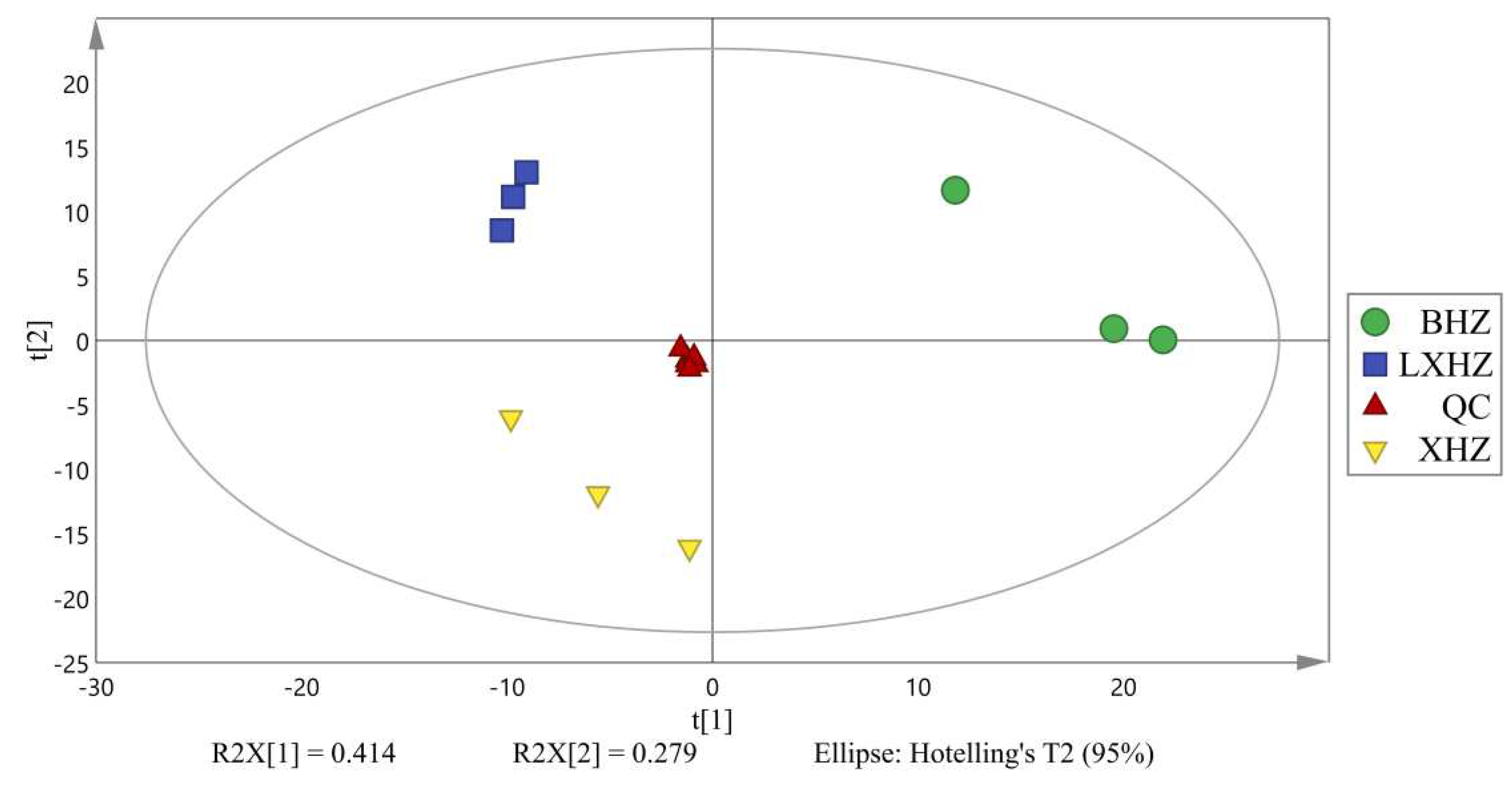
The complex metabolic reactions and their regulation in organisms are not conducted alone, and often formed complex pathways and networks by different genes and proteins, whose mutual influence and mutual regulation eventually lead to systematic changes of the metabolome. Global metabolites in and out of the cell were analyzed qualitatively or semi-quantitatively by non-target metabolomics to explore the effects of cold acclimation stress and anhydrous storage on scallop metabolic processes. Before doing the difference analysis, the principal component analysis (PCA) of the grouped samples for the difference comparison was conduced first to observe the variation size between the difference groups and between the samples within the group.
Figure 10 shows that the three experimental samples were very significant, almost all of them are within the 95% confidence interval, indicating that cold acclimation and unless storage and transportation had a significant impact on scallop metabolism and can be used for subsequent analysis.
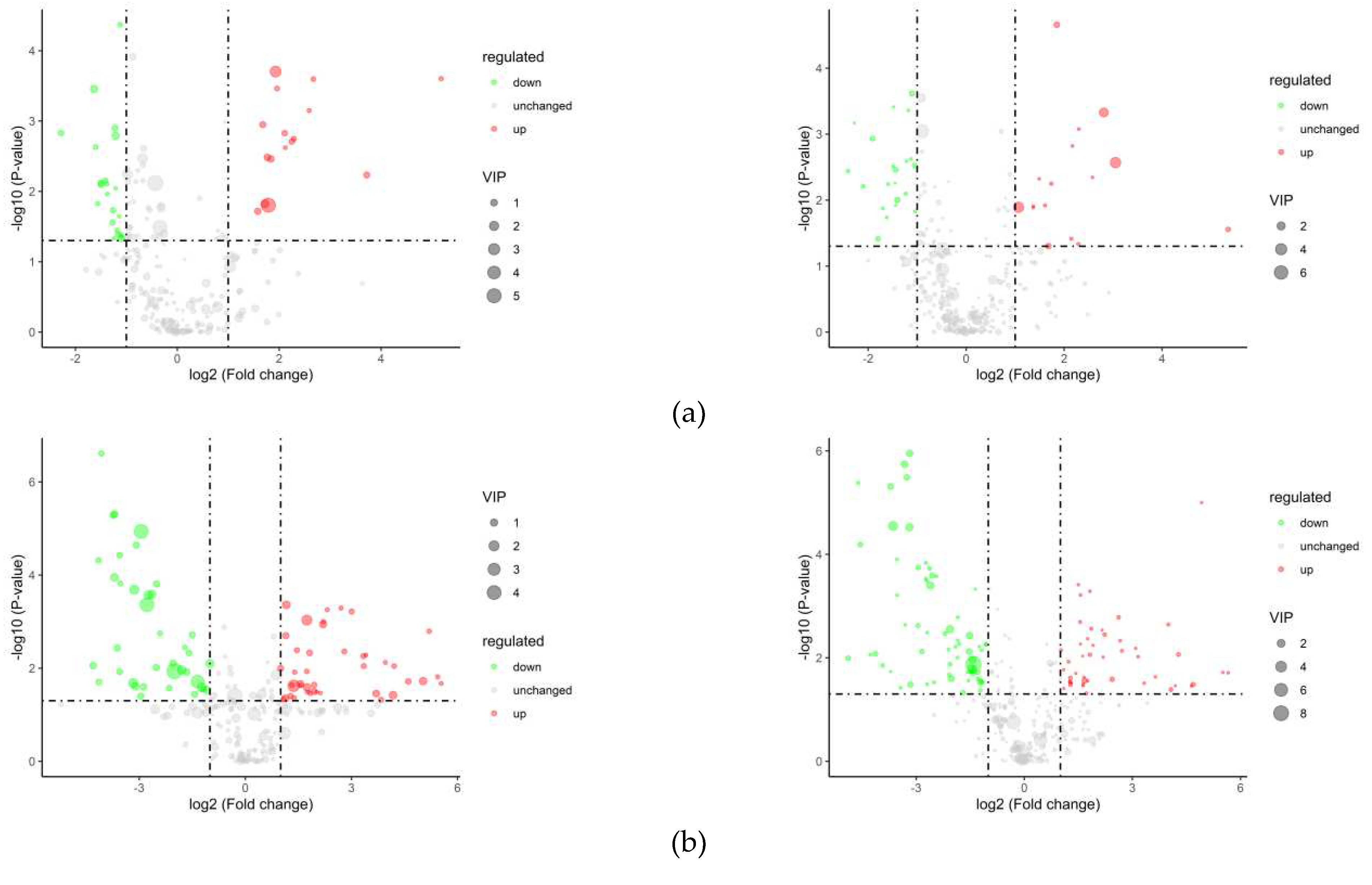
3.6.1. Differential metabolite screening
Figure 11 shows the volcano plots of differential metabolite in positive and negative ion models. Comparing the differential metabolites between the transient breeding group and the cold acclimation group, 68 differential metabolites were obtained, including Adenylosuccinic acid, Adenylosuccinic acid, Guanosine, and Dodecyl sulfate, among which 30 were upregulated products and 38 were downregulated. The cold acclimation group was stored and transported without water for 3d, which produced 179 differential metabolites, including DL-Arginine, myristic acid, Adenylosuccinic acid, Tetradecanoyl-L-Carnitine, Oleoyl-L-Carnitine, Palmitic acid, of which 85 were upregulated products and 94 were downregulated.
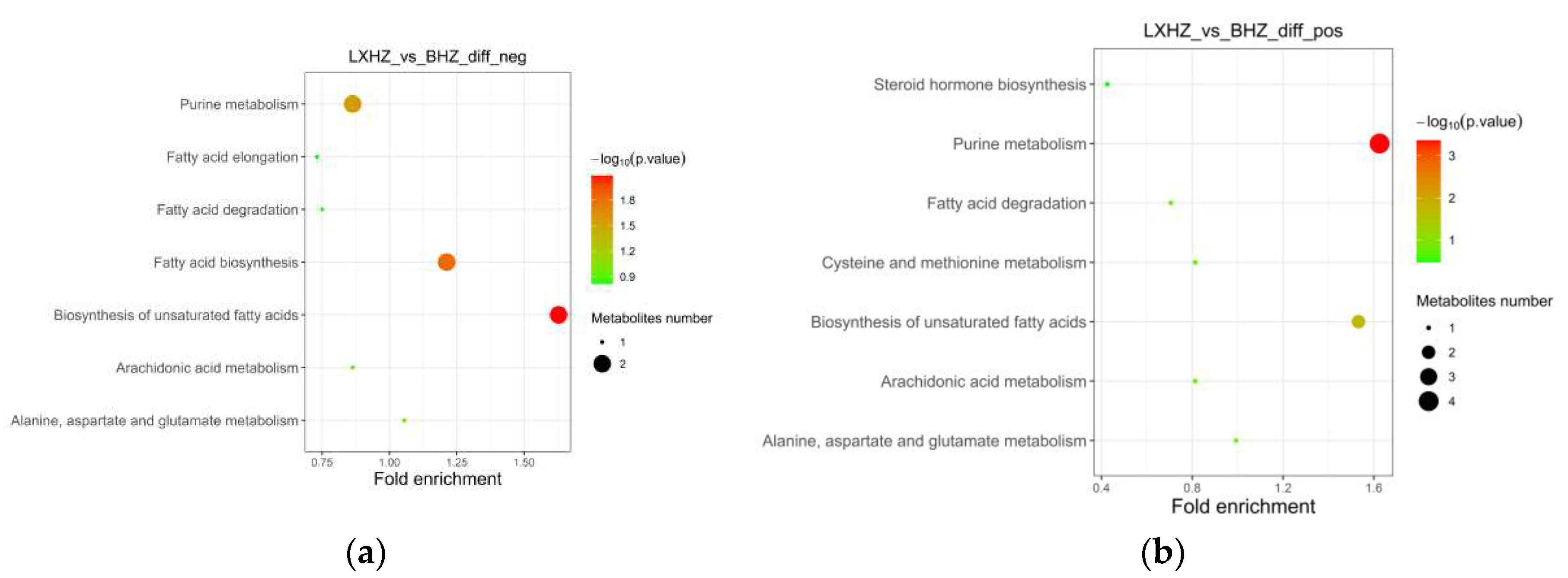
3.6.2. Functional annotation and enrichment analysis of the differential metabolite KEGG
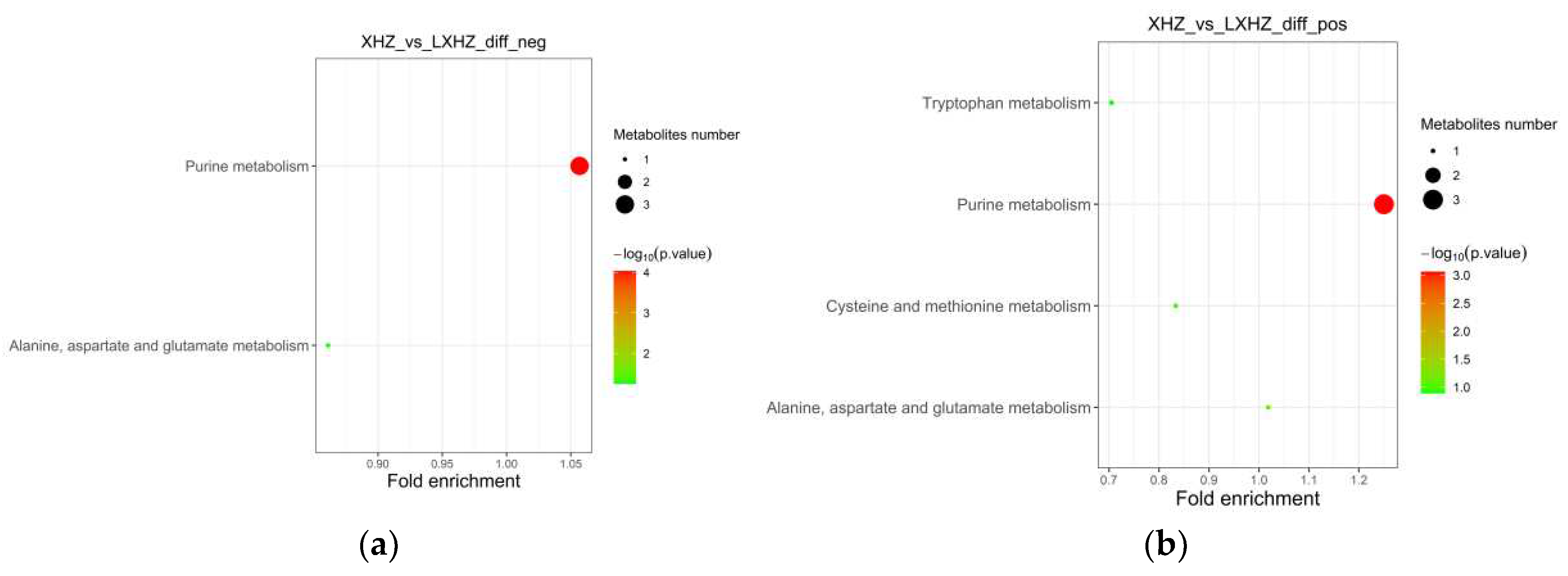
The differential metabolites were annotated through the KEGG database and classified by the pathway based on the corresponding pathway, and the size of the scatter in the Figure indicated the number of differential metabolites enriched on the pathway. As can be seen in
Figure 12 and
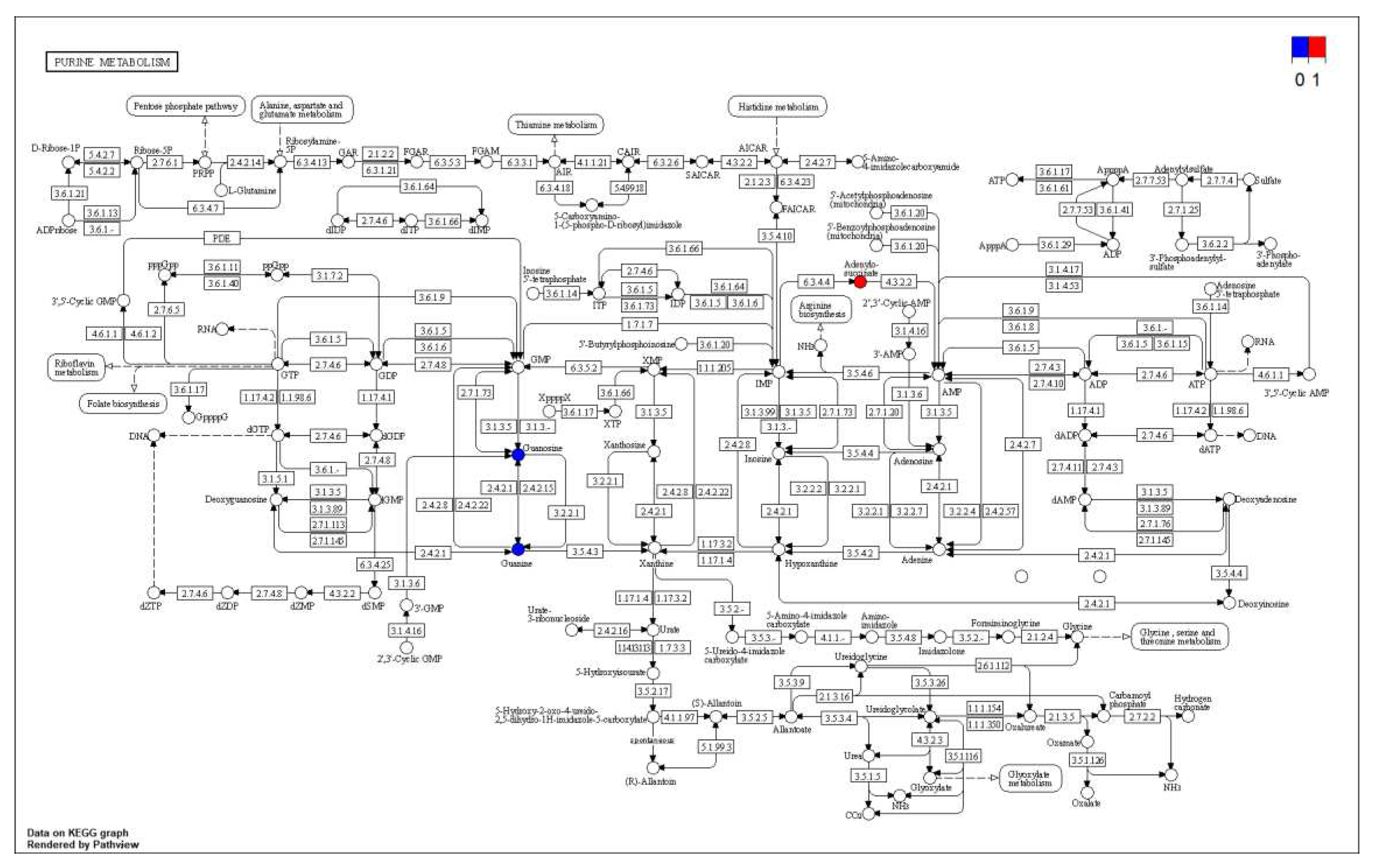
Figure 13, the differential metabolites produced by the transient group by cold acclimation treatment mainly belonged to the Purine metabolism pathway of KEGG. The cold acclimation promoted the purine metabolism of scallops, and the differential metabolites were labeled to the pathway map showing the content of Adenylosuccinate and IMP, which were 3.60 times and 3.37 times that of the transient control, respectively, while the content of Guanosine decreased significantly.
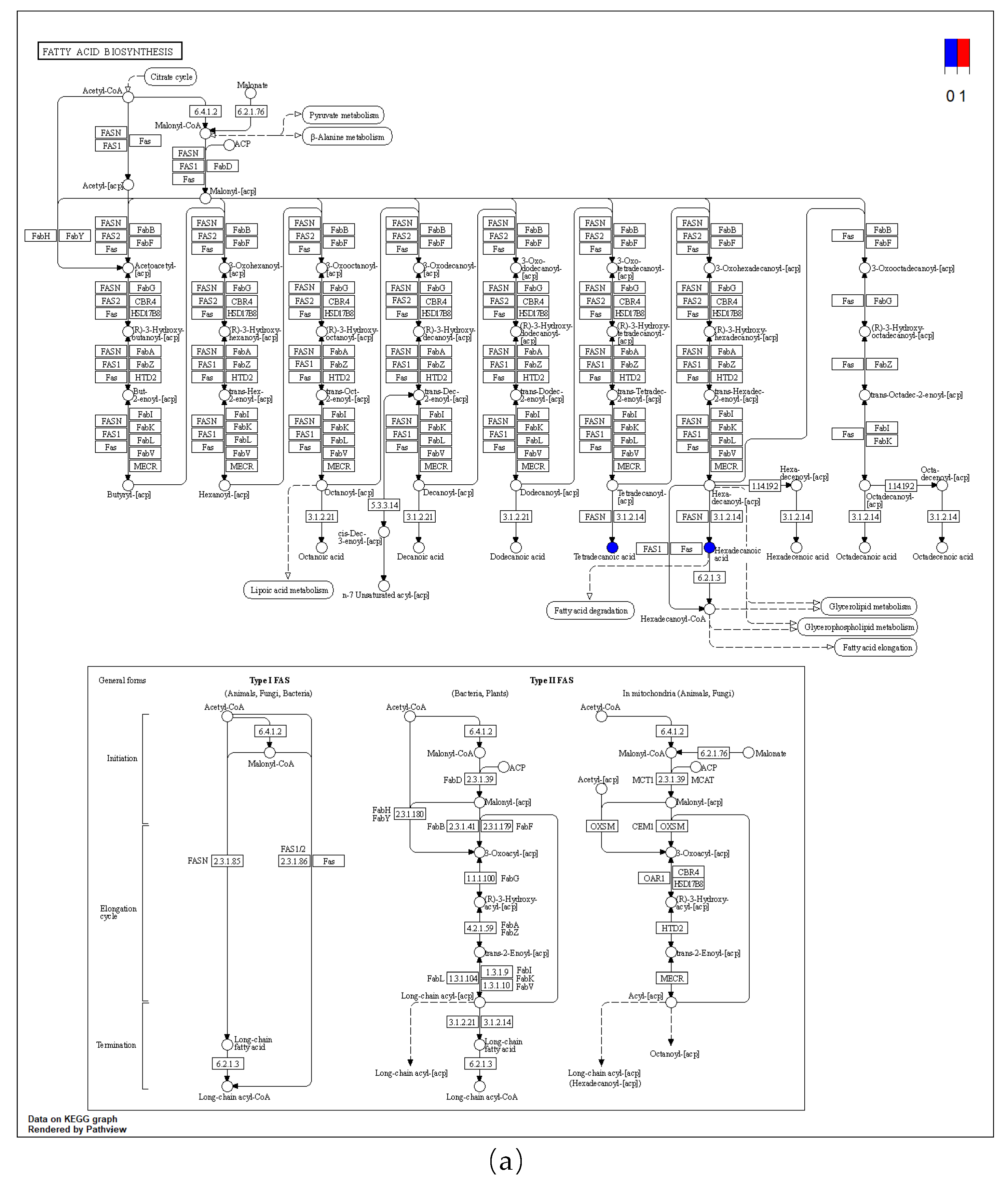
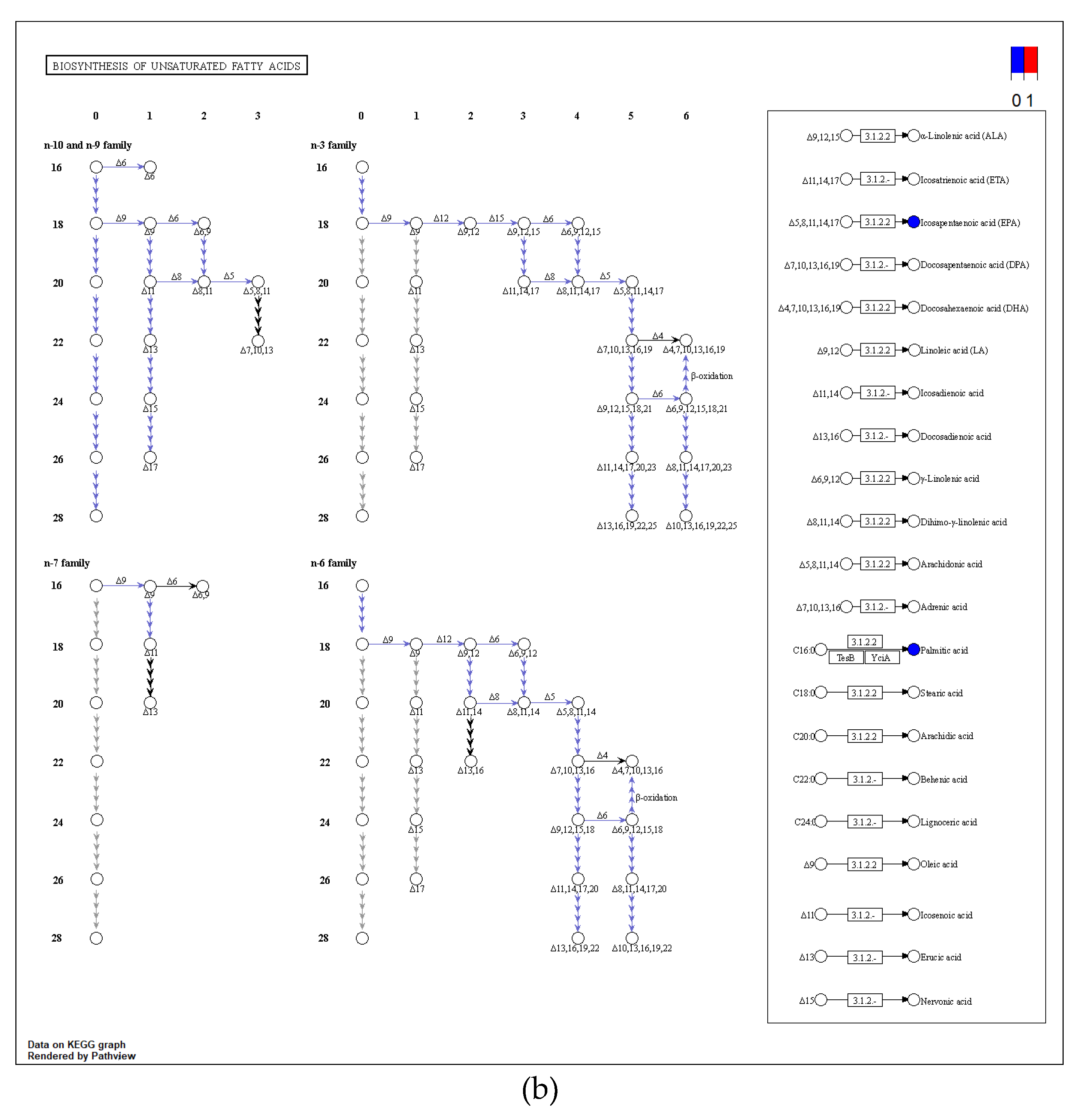
The cold acclimation group was stored and transported without water for 3d, and the differential metabolites generated at this stage mainly belong to the biosynthesis of unsaturated fatty acids, fatty acid biosynthesis and purine metabolism pathways of KEGG (
Figure 14 and
Figure 15). It was then speculated that low temperature and anhydrous storage and transportation inhibited the metabolism of saturated and unsaturated fatty acids. After making their differential metabolites visible in the pathway map, its metabolites Tetradecanoic acid, Hexadecanoic acid, Icosapentaenoic acid (EPA) and Palmitic acid were significantly reduced.
4. Discussion and Conclusions
The flavor quality of scallop is closely related to its physiological state. In the living market, opening rate, closure sensitivity and mantle shrinkage often reflect the quality of the scallop [
20]. Biochemical indicators such as pH, glycogen, ATP-related substances, and K values are also widely used in shellfish activity evaluation methods [
21,
22]. The apparent vitality of Patinopecten yessoensis evaluated by the rate of edge shrinkage and stimulus response time was consistent with the results of Yaxuan Li et al. The degree of the pallium was positively correlated with the stimulus response time. The greater the degree of edge shrinkage, the longer the stimulus response time, that is, the worse the vitality state.
When stressed by adverse external environment, living animals can produce stress and cause changes in heart rate, and non-implantable heart rate measurement is more and more widely used in Marine organisms. Mat et al. detected the rhythmic activity of Pacific oysters by means of adhesion sensing. Dong et al. studied the temperature and heat resistance of limpers at different temperatures through heart rate assessment methods. Chen et al. also used the method of heart rate assessment to evaluate the temperature and heat resistance of abalone, which shows that the method of heart rate assessment is becoming more and more mature in the study of shellfish.
It is well recognized that shellfish usually upregulate the expression of a large number of stress proteins to cope with protein damage from environmental stress, and this process requires expenditure of large amounts of energy [
23,
24,
25,
26,
27,
28]. Energy material is often the most direct reaction to the physiological state of the scallop. ATP gradually decreased in the process of anhydrous storage and transportation of Patinopecten yessoensis, which was also consistent with the results of Q. Zhang et al [
29,
30]. In T.Y.Xu's study, 7 different metabolites were produced after wet storage and transportation, and the pathway analysis showed that the tricarboxylic acid cycle was the most susceptible pathway [
31]. Energy metabolism involves the degradation and synthesis of high-energy phosphates [
32], and both GMP and IMP in this study may be related to this process. The results of proteomic GO annotation also showed that the differential proteins were mainly characterized in tricarboxylic acid cycle, mitochondrion and ATP, which consistent with metabolome.
Author Contributions
Conceptualization, X.M. Qin and P.H. Jiang; methodology, X.Y. Chang; software, validation and formal analysis, D.J. Chen; investigation and resources, X.P. Fan; data curation and writing—original draft preparation, P.H. Jiang; writing—review and editing, C.F. Zhang; visualization, supervision, project administration, and funding acquisition, X.M. Qin. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2021YFD2100504) and the Special expert program for Taishan scholars (ts20190956).
Data Availability Statement
Data is contained within the article.
Acknowledgments
We are grateful to Qingdao STD Standard Testing CO., Ltd. for assisting in MS analysis and bioinformatics analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, Z.; Li, D.; Wang, X.; Wang, Q.; Li, H.; Teng, W.; Liu, X.; Zhou, Z. Reason of massive mortality of Japanese scallop Pationopectin yessoenisis in raft cultivation in coastal Changhai county. Fisheries Science 2019, 38, 420–427. [Google Scholar]
- Wang, Q.C. Introduction of Pationopectin yessoenisis and the prospect of increasing culture in north China. Fisheries Science, 1984, 4, 24–27. [Google Scholar]
- Guan, B.B.; Chen, B.; Cheng, X.H. Research on characterization and evaluation methods of aquatic products. Fujian Analysis and Testing 2019, 28, 15–21. [Google Scholar] [CrossRef]
- Pan, L.L.; Lin, C.X.; Zhang, G.C.; et al. The influence of purification of temporary breeding and low temperature water transportation on the quality of scallop. The Journal of Agricultural Engineering 2017, 33, 301–307. [Google Scholar] [CrossRef]
- Nie, B.X.; Zhang, Y..H.; Sun, X.D.; et al. Key technologies for the transportation of live fish and their processes. Fishery Modernization 2014, 41, 34–39. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Q. Progress in Understanding Environmental Stress and Physiological Regulation Mechanism in Aquatic Animals during Live Transportation. Food Science 2021, 42, 319–325. [Google Scholar]
- LPan, L.; Jiang, J.L.; Zhang, N.; et al. Effects of different temperatures on quality of live bay scallop argopectenirradians packed by oxygenation. Fisherish Science 2019, 38, 182–187. [Google Scholar]
- Mi, H.B.; Zhang, T.; Jiang, Q.; et al. Effect of low temperature retention on muscle texture and liver biochemical properties of scallop. Journal of Food Science and Technology 2022, 40, 141–150. [Google Scholar]
- Lin, H.Z.; Gao, J.L.; Liang, Z.Y.; et al. The effect of cold stress mode on current oxidative stress and energy consumption of Pacific oysters. Journal of Ocean University of Guangdong 2022, 42, 95–103. [Google Scholar]
- Yan, L.X.; Tian, Y.Y.; Jiang, M.H.; et al. Changes of vitality and flavor characteristics of shrimp scallop in waterless transportation - wet storage sales. Aquatic Sciences 2022, 41, 44–51. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, X.B.; Zhou, K.; et al. Effect of high temperature stress on glycogen metabolism in gills of Yesso scallop Patinopecten yessoensis. Fish and Shellfish Immunology 2023, 138(9 May 2023). 9.
- Li, Y.X.; Liu, J.R.; Zhou, J.; et al. Effects of tied-up relaying on stress-reduction and storage-stability of live dived Patinopecten yessoensis. Journal of Fisheries of China 2022, 46, 605–615. [Google Scholar]
- Bakhmet, I.; Aristov, D.; Marchenko, J.; et al. Handling the heat: changes in the heart rate of two congeneric blue mussel species and their hybrids in response to water temperature. J Sea Res 2022, 185, 102218. [Google Scholar] [CrossRef]
- Chang, X.Y.; Jiang, P.H.; Deng, J.; Fan, X.P.; Qin, X.M. The influence of chilling stress induced dormancy on the life characteristics and nutritional quality indexes of shrimp scallop. South China Fisheries Science 2023, 19, 129–139. [Google Scholar]
- Duan, Y.; Zhang, J.; Dong, H.; et al. Effect of desiccation and resubmersion on the oxidative stress response of the kuruma shrimp Marsupenaeus japonicus. Fish and Shellfish Immunology 2016, 91–99. [Google Scholar] [CrossRef]
- Lee, A.-C.; Lee K-TJ JO, M.S. Technology. The enzyme activities of opine and lactate dehydrogenases in the gills, mantle, foot, and adductor of the hard clam Meretrix lusoria. Journal of Marine Science and Technology 2011, 19, 4. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The Importance of ATP-related Compounds for the Freshness and Flavor of Post-mortem Fish and Shellfish Muscle: A Review. C R C Critical Reviews in Food Technology 2015, 57, 1787–1798. [Google Scholar] [CrossRef]
- Hiltz, D.F.; Dyer, W.J. Octopine in postmortem adductor muscle of the sea scallop (Placopecten magellanicus). Journal of the Fisheries Research Board of Canada 1971, 28, 869–874. [Google Scholar] [CrossRef]
- Atkinson D, E. Energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef]
- Duncan, P.F. Post-harvest physiology of the scallop Pecten maximus(L.)[D].Glasgow:University of Glasgow 1993.
- Vallé, M.; Malle, P.; Bouquelet, S. Evaluation of fish decomposition by liquid chromatographic assay of ATP degradation product. Journal of AOAC international 1998, 81, 571–578. [Google Scholar] [CrossRef]
- Pacheco-Aguilar, R.; Marquez-Ríos, E.; Lugo-Sánchez, M.E.; et al. Postmortem changes in the adductor muscle of Pacific lions-paw scallop (Nodipecten subnodosus) during ice storage. Food chemistry 2008, 106, 253–259. [Google Scholar] [CrossRef]
- Sanders, B. M.; Hope, C.; Pascoe, V.M.; et al. Characterization of the Stress Protein in Response Two with Different Species of Collisella Limpets Tolerances Temperature. Physiological Zoology 2012, 64, 1471–1489. [Google Scholar] [CrossRef]
- Tomanek, L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species' biogeographical distribution ranges and metabolic costs. Journal of Experimental Biology 2010, 213, 971–9. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, L.; Somero, G.N. Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. Journal of Experimental Biology 2002, 205 Pt 5, 677–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Chen, H.; Wang, M.; Zhou, Z.; Qiu, L.; Wang, L.; Song, L. . The transcriptional response of the Pacific oyster Crassostrea gigas under simultaneous bacterial and heat stresses. Developmental and Comparative Immunology: Ontogeny, Phylogeny, Aging 2019, 94. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, Q.; Liu, C.; Wang, L.; Zhou, Z.; Gong, C.; Zhang, A.; Zhang, H.; Qiu, L.; Song, L. The transcriptional response of the Pacific oyster Crassostrea gigas against acute heat stress. Fish Shellfish Immunol. 2017, 68, 132–143. [Google Scholar] [CrossRef]
- Zhang, S.; Han, G.; Dong, Y. , Temporal patterns of cardiac performance and genes encoding heat shock proteins and metabolic sensors of an intertidal limpet Cellana toreuma during sublethal heat stress. J. Therm. Biol. 2014, 41, 31–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, M.H.; Tian, Y.Y.; et al. A Relevance of Adductor Protein Solubility to ATP in Yesso Scallop Patinopecten yessoensis. Fisheries Science 2020, 39, 476–482. [Google Scholar] [CrossRef]
- Jinyang, L.; Junrong, L.; Yuanyong, T.; et al. Effects of post-harvest handling on biochemical metabolism of bottom cultured live scallop (Patinopecten yessoensis). Journal of Fisheries of China 2017, 41, 81–87. [Google Scholar]
- Xu, T.Y.; Tian, Y.Y.; Li, Y.X.; et al. UPLC-MS based metabolomic study of live scallop Mizuhopecten yessoensis during quality determination period. Journal of Dalian Ocean University 2021, 4, 637–645. [Google Scholar]
- Díaz-Enrich, M.J.; Ramos-Martínez, J.I.; Ibarguren, I. Implication of guanosine 3’,5’-cyclic monophosphate, adenosine 3’,5’-cyclic monophosphate, adenosine 5’-mono-, di- and triphosphate and fructose-2,6-bisphosphate in the regulation of the glycolytic pathway in hypoxic/anoxic mussel, Mytilus galloprovincialis. Molecular and cellular biochemistry 2002, 240, 111–118. [Google Scholar]
Figure 1.
Living edge shrinkage of Patinopecten yessoensis in different period (a) Temporary breeding and cold acclimatization; (b) Anhydrous storage and transportation 3d.
Figure 1.
Living edge shrinkage of Patinopecten yessoensis in different period (a) Temporary breeding and cold acclimatization; (b) Anhydrous storage and transportation 3d.
Figure 2.
Changes of apparent vitality index during anhydrous storage and transportation.
Figure 2.
Changes of apparent vitality index during anhydrous storage and transportation.
Figure 3.
Heart rate change of Patinopecten yessoensis after rehydration at different storage times.
Figure 3.
Heart rate change of Patinopecten yessoensis after rehydration at different storage times.
Figure 4.
Microstructure of Patinopecten yessoensis gills at different stages (200), (a) temporary breeding stage; (b) cold acclimation stage; (c) no water storage 3d.
Figure 4.
Microstructure of Patinopecten yessoensis gills at different stages (200), (a) temporary breeding stage; (b) cold acclimation stage; (c) no water storage 3d.
Figure 5.
Changes in nucleotide series of Patinopecten yessoensis during anhydrous storage and transportation.
Figure 5.
Changes in nucleotide series of Patinopecten yessoensis during anhydrous storage and transportation.
Figure 6.
Bar graph of the GO annotation results.
Figure 6.
Bar graph of the GO annotation results.
Figure 7.
Bar graph of the COG annotation results.
Figure 7.
Bar graph of the COG annotation results.
Figure 8.
The volcano plot of the differential proteins (a) LXHZ vs XHZ; (b) BHZ vs LXHZ.
Figure 8.
The volcano plot of the differential proteins (a) LXHZ vs XHZ; (b) BHZ vs LXHZ.
Figure 9.
GO enriched bubble diagram of differential proteins Figure (a) LXHZ vs XHZ; (b) BHZ vs LXHZ.
Figure 9.
GO enriched bubble diagram of differential proteins Figure (a) LXHZ vs XHZ; (b) BHZ vs LXHZ.
Figure 10.
PCA diagram of mass spectrometry data of three scallop samples and mix samples (XHZ- temporary breeding group, LXHZ-cold acclimation group, BHZ-anhydrous storage and transportation group, QC-quality control mixed samples).
Figure 10.
PCA diagram of mass spectrometry data of three scallop samples and mix samples (XHZ- temporary breeding group, LXHZ-cold acclimation group, BHZ-anhydrous storage and transportation group, QC-quality control mixed samples).
Figure 11.
Volcano plots of the differential metabolite in positive and negative ion models (a) LXHZ vs XHZ; (b) BHZ vs LXHZ.
Figure 11.
Volcano plots of the differential metabolite in positive and negative ion models (a) LXHZ vs XHZ; (b) BHZ vs LXHZ.
Figure 12.
KEGG enrichment pathway of cold-acclimated differential metabolites (a). Negative ion mass spectrum; (b) Positive ion mass spectrum.
Figure 12.
KEGG enrichment pathway of cold-acclimated differential metabolites (a). Negative ion mass spectrum; (b) Positive ion mass spectrum.
Figure 13.
Metabolic pathway map of Purine metabolism.
Figure 13.
Metabolic pathway map of Purine metabolism.
Figure 14.
KEGG enrichment pathway of anhydrous storage and transportation differential metabolites (a) Negative ion mass spectrum; (b) Positive ion mass spectrum.
Figure 14.
KEGG enrichment pathway of anhydrous storage and transportation differential metabolites (a) Negative ion mass spectrum; (b) Positive ion mass spectrum.
Figure 15.
Metabolic pathway map (a) fatty acid biosynthesis; (b) biosynthesis of unsaturated fatty acids.
Figure 15.
Metabolic pathway map (a) fatty acid biosynthesis; (b) biosynthesis of unsaturated fatty acids.
Table 1.
Protein quantitative results statistics.
Table 1.
Protein quantitative results statistics.
| Database |
Peptides |
Unique peptides |
Protein groups |
Patinopecten yessoensis
(Mizuhopecten yessoensis_6573) |
4548 |
4134 |
856 |
Table 2.
Differential protein quantity statistics.
Table 2.
Differential protein quantity statistics.
| Comparisons |
Up |
Down |
All |
| XHZ_vs_LXHZ |
60 |
237 |
297 |
| LXHZ_vs_BHZ |
28 |
13 |
41 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).