* Correspondence; painfreel@yu.ac.kr
1. Introduction
Aging causes fatty infiltration of skeletal muscles, which decreases physical performance and quality of life. Sarcopenia, defined as age-related muscle loss and functional decline, is the progressive loss of skeletal muscle mass and strength with advancing age.[
1] Diagnosis of sarcopenia was made by quantitative assessment of skeletal muscle mass and quality assessment using estimation of physical performance.[
2,
3] Generally, loss of muscle strength is caused by muscle atrophy and fatty changes, which lead to decreased functional performance. Therefore, the quantitative measurement of muscle mass and fatty infiltration can be a preliminary assessment in the evaluation of sarcopenia.
Proper activation of the core muscle stabilizes the performance of the extremities.[
4] Moreover, the core muscles, including the multifidus muscle, stabilize the segmental lumbar spine.[
5] In addition, atrophy of the multifidus muscle aggravates further compression of osteoporotic spinal compression fractures.[
6,
7] Therefore, patients with further atrophy of the back muscle tend to present with more severe back pain than those with less severe back muscle atrophy.[
8,
9] Hence, core muscle function is essential for maintaining the spine’s stability in older people.
Based on these clinical significances, quantitative assessment of the multifidus muscle can be beneficial for elderly patients to develop a treatment strategy for back pain and to prevent further progression of comorbidities.
The structural and functional decline of skeletal muscles in older people affects the extremities and the axial back core muscles. Moreover, spinal disc degeneration and decreased disc height affect back muscle degeneration.[
10] Therefore, we estimated the relationship between disc degeneration, physical performance, and quantitative decline of the multifidus muscles at the segmental level of the lumbar spine.
2. Materials and Methods
Patients who visited our university hospital spine center between August 2021 and October 2022 were prospectively recruited. Patients aged over 60 years, who underwent lumbar spine magnetic resonance imaging (MRI), were able to walk 10 m independently with or without cane support and were capable of functional outcome evaluation were included in the study. Patients with a history of lumbar spine surgery, severe degenerative scoliosis, spinal infection, communication disorders, diseases that severely limit physical activity, including encephalopathy or severe musculoskeletal disorders, and who were unable to perform functional evaluation due to pain were excluded. This study was reviewed and approved by the Institutional Review of our hospital (OOOO 2021-08-053). All Subjects included in this study provide written informed consent.
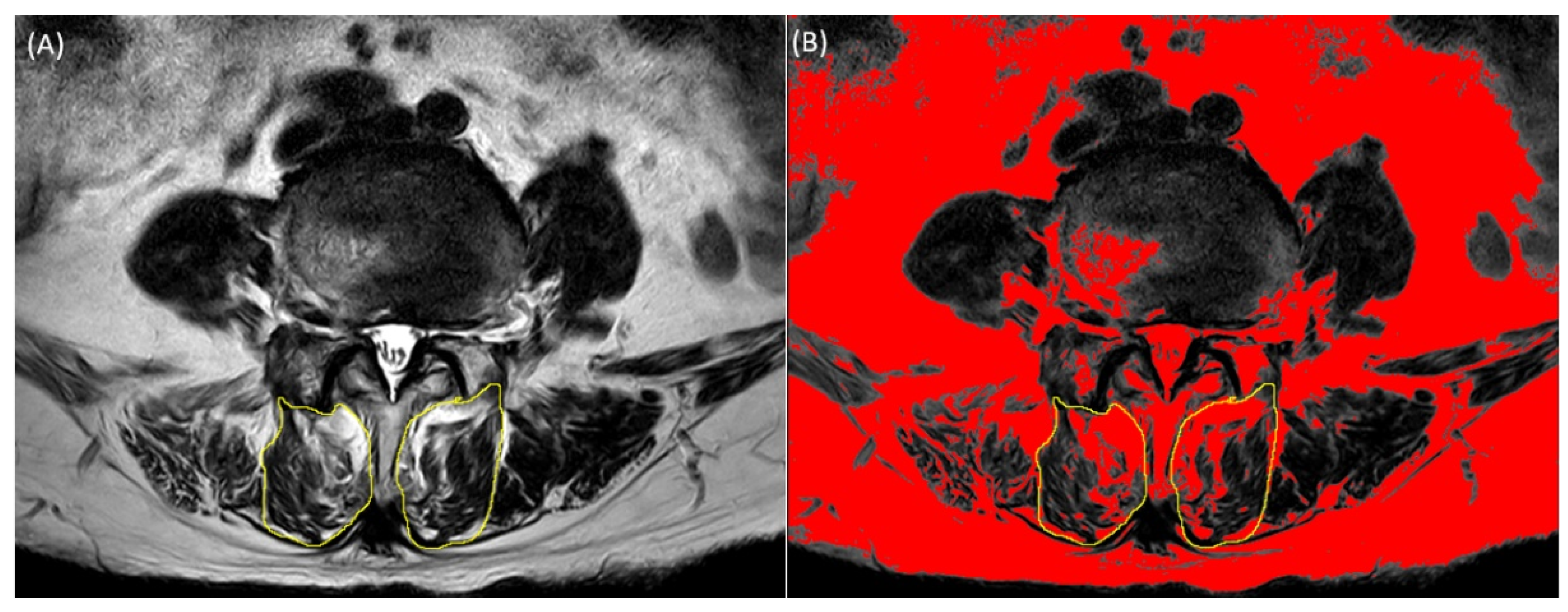
Lumbar spine magnetic resonance imaging was performed using a 3.0-T magnetic resonance unit (Siemens, Germany). Axial T2-weighted MRI (repetition time: 3400–3700 ms, echo time: 90–111 ms) was used to measure the cross-sectional area (CSA) and fatty infiltration of the multifidus muscles at the level of the L2-S1 intervertebral disc. We used image-processing software (ImageJ; National Institutes of Health, Bethesda, MD, USA) to measure the CSA and fatty infiltration of the multifidus muscles. DICOM files extracted from lumbar spine MRI were mounted using the ImageJ program. The CSA of the multifidus muscle was measured by manually outlining the border of each muscle. The fatty infiltration area was measured using the pseudo-coloring technique by converting the 32-bit image to a pseudo-color 8-bit image, followed by threshold analysis, in which the optimal threshold of fat tissue was set manually. Fatty infiltration on axial T2-weighted MR images was converted to red color. The red proportion of CSA of the multifidus muscle was calculated. Functional muscle mass excluding fat tissue, defined as functional CSA (fCSA), was calculated by subtracting the fat area from the total multifidus CSA (
Figure 1). The ratio between the total CSA and fCSA was calculated by dividing the fCSA by the total CSA. In this study, we presented the CSA to fCSA ratio as a percentage by multiplying 100 with the value obtained by dividing CSA by fCSA. In this study, we presented the CSA to fCSA ratio as a percentage by multiplying 100 with the value obtained by dividing CSA by fCSA.
The Pfirrmann grading system is a widely used classification system for intervertebral disc degeneration, which grades disc degeneration from I to V using T2 weighted MR images. Disc degeneration is classified according to the signal intensity, distinction between the annulus and nucleus, and height of the intervertebral disc.[
11] Grade I is a condition in which the disc structure is homogeneous and has a bright and hyperintense white signal intensity with normal disc height. Grade II occurs when the structure of the disc remains hyperintense, with distinct annulus and nucleus margins, and normal height, but the structure is inhomogeneous. Grade III has an inhomogeneous disc structure with intermediate gray signal intensity and unclear nucleus and annulus distinction; however, the disc height is normal or slightly decreased. Grade IV has an inhomogeneous disc structure with hypointense dark gray signal intensity, no distinction between the nucleus and annulus, and a normal or moderately decreased disc height. Grade V is classified when the disc is inhomogeneous, has a hypointense black signal intensity, no distinction between the nucleus and annulus, and a collapsed disc space. We used the Pfirrmann classification to classify the degree of degeneration of the intervertebral disc of L2-S1.
The Short Physical Performance Battery (SPPB) is a commonly used tool for testing physical performance in elderly populations, with high reliability and validity.[
12] The SPPB test consists of three subtests: standing balance, time required to walk 4 m, and time taken to perform five sit-to-stand exercises on a chair consecutively. For balance testing, the participants were asked to stand for at least 10 s, if possible, in three positions. First, by positioning their feet as close as possible, then in a semi-tandem posture, in which one foot is placed behind the other, and in a tandem posture, in which one foot is postured directly behind the other touching it. For the first two positions, 0 or 1 point was given depending on whether it could be maintained for more than 10 s. For the last position, one point was given if the participant was able to hold for 3–10 s and two points if it could be maintained for more than 10 s. The total score is calculated as the sum of the three positions. As a result, a score of at least 0–4 was given for balance testing. For the 4 m walking test, the time required to walk 4 m at the usual pace was measured. The time to stand up from a chair five times consecutively was measured as quickly as possible. Scores of 1–4 based on the time spent were measured for the last two of the three tests. A total score of 0–12 was obtained as the sum of all three tests.
The Berg Balance Scale (BBS) is a well-established and valid method for predicting static and dynamic activities of everyday living. [
13] The test included 14 static and dynamic activities of daily living, including sit-to-stand, independent standing, tandem stepping, and one-leg standing. Each item was evaluated on a scale of 0–4, depending on the degree of function, with a total score of 56 points. A higher score indicates higher functional ability.
Grip strength is a widely used measure of geriatric conditions including frailty. Although grip strength measures the strength of hand muscles, it has been widely used in the elderly to assess the generalized effect of the musculoskeletal system in clinical practice.[
14] The Southampton protocol was used for the consistency of hand grip measurement, in which hand grip was measured using the hand dynamometer alternately from right to left hand three times in total, with the forearm comfortably placed on the chair arm in a sitting position.[
15] Subsequently, the average strength of the measurements was determined.
The functional reach test (FRT) measures the difference between the maximal forward reach and the patient’s arm length in an independent standing posture with the arm elevated to shoulder height. FRT is a precise, inexpensive, and reliable method to measure postural stability.[
16,
17] This study aimed to predict postural instability during daily activities using the FRT.
Finally, the psoas index, which is a widely used index for predicting sarcopenia, was measured. The Psoas index is correlated with back muscle degeneration.[
18] The psoas muscle index was obtained by dividing the bilateral psoas muscle CSA at the L4-5 level by the square of the height.
Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA), and continuous variables were presented as mean ± standard deviation. ANOVA analysis was used to evaluate whether differences existed between lumbar spine levels of total multifidus CSA, fCSA, and the CSA to fCSA ratio. Tukey’s Honest Significant Difference (HSD) test was conducted as a post-hoc analysis to assess significant differences among lumbar spine levels within each group. In addition, one-way analysis of variance was used to evaluate the CSA of the multifidus, fCSA, and CSA to fCSA ratio, classified according to the Pfirrmann Classification of lumbar discs at each level. Finally, Pearson’s correlation analysis was used to evaluate how each lumbar spine level multifidus parameter was correlated with functional outcome measurements (SPPB, BBS, Grip strength, and FRT) and psoas index. Statistical significance was set at p < 0.05.
3. Results
A total of fifty-seven patients (mean age 73.89 ± 6.09; 23 male patients) visiting our hospital were prospectively recruited. The demographic characteristics of the participants are presented in
Table 1.
Values are presented as mean (standard deviation) unless otherwise stated. Abbreviations: BMI, body mass index; SPPB, short physical performance battery; BBS, Berg Balance Scale; FRT, Functional Reach Test. Psoas index: bilateral psoas muscle CSA/height2.
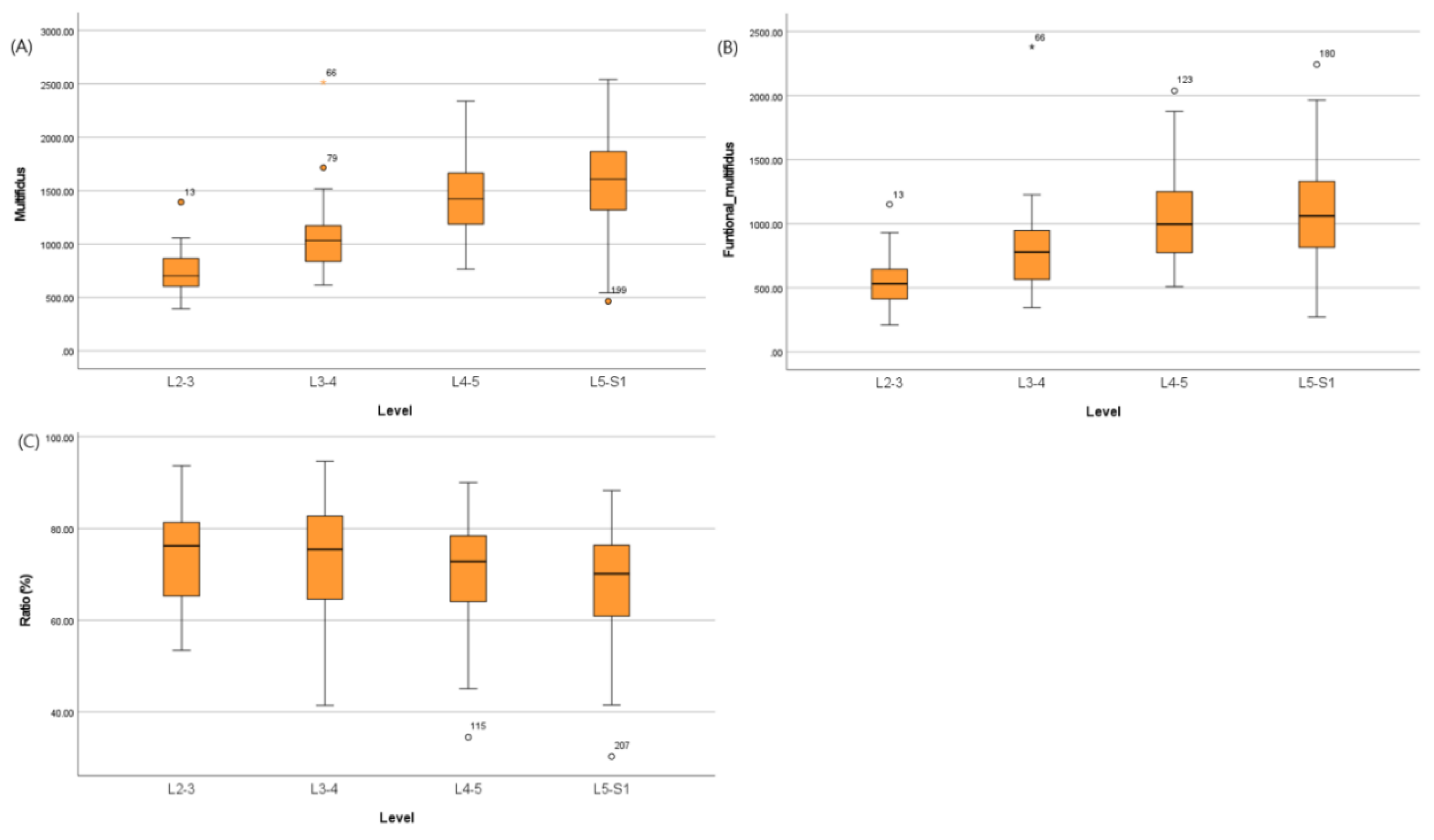
The CSA of the multifidus, fCSA, and CSA to fCSA ratio are presented in
Table 2 and
Figure 2. The CSA of the multifidus, fCSA, and CSA to fCSA ratio showed statistically significant differences between lumbar spine levels within each group (p<0.001, p<0.001, and p=0.022, respectively). However, in the case of the CSA to fCSA ratio values, it was confirmed that there was a relatively constant value among the spine levels when examined through post hoc analysis.
Using one-way ANOVA analysis, the relationship between the degree of disc degeneration and the multifidus, fCSA, and CSA to fCSA ratio were investigated (
Table S1). The degree of disc degeneration and CSA of the multifidus and fCSA and the CSA to fCSA ratio were not statistically significant. However, the CSA to fCSA ratio of the L5-S1 level showed significantly higher fatty infiltration according to the degree of disc degeneration (p<0.001). L5-S1 level was more affected by disc degeneration than the other spinal levels.
Finally, the correlation between the physical functional measurements and multifidus CSA, fCSA, and the CSA to fCSA ratio was analyzed using Pearson correlation analysis. Moreover, the correlation between the psoas index and multifidus parameters was analyzed (
Table 3). Grip strength and the psoas index showed moderate to strong significant correlations with each factor concerning the multifidus muscles at all levels of the lumbar spine. Moreover, all physical functional tests, except BBS, showed a significant correlation with the CSA to fCSA ratio.
4. Discussion
The multifidus muscle strongly correlated with the psoas index and functional performance measurements. Grip strength had a more significant relationship than other measurements. The CSA to fCSA Ratio was evenly distributed on each spinal level compared to the CSA and fCSA of the multifidus muscle. Disc degeneration and the Pfirrmann Classification did not affect multifidus degeneration except at the L5-S1 level. We speculated that multivariant factors, including disc degeneration, affected the multifidus fatty infiltration at the L5-S1 level. We speculated that in older adults, the aging process exerts a more substantial influence on the fatty infiltration of back muscles compared to disc degeneration.
Atrophy and functional decline of the skeletal muscles are closely related to aging. Muscle decline that occurs with aging is associated with the accumulation of adipose tissue. Unloading, disuse, and sex steroid deficiency, such as estrogen deficiency in women and androgen deprivation therapy in men, are associated with increased adipogenesis in the elderly. Adipogenesis is known to lower the sensitivity of skeletal muscles to insulin. As insulin acts as a skeletal anabolic factor [
19], the vicious cycle that ensues leads to muscle atrophy. Therefore, aging and adipogenesis lead to the deterioration of muscle mass, strength, and physical function.
Core muscles, including the multifidus, play an important role in back stabilization. Therefore, atrophy and fatty infiltration of these muscles are associated with back pain and disc degeneration [
20,
21]. In addition, during degeneration, the back muscles, including the multifidus, undergo histological changes. The elderly have a higher proportion of type II muscle fibers compared to healthy individuals[
22], and have transformed from oxidative to glycolytic fibers [
22,
23]. Type 2 and glycolytic fibers are susceptible to fatigue, which may lead to decreased endurance and functional disability.
Previous studies claim that disc degeneration is related to core muscle degeneration.[
10,
11] Intervertebral discs are responsible for two-thirds of axial spinal loading. Also, radiculopathy caused by disc herniation can lead to multifidus atrophy. However, another study showed no relationship between trunk muscle mass and pain intensity.[
24] Therefore, the relationship between multifidus atrophy, back pain, and disc degeneration remains controversial. In this study, we found little correlation between disc and multifidus muscle degeneration. Only the CSA to fCSA ratio of L5-S1 level was statistically significant (p<0.001). Therefore, researchers should consider the effect of age or sarcopenia on back muscle atrophy in addition to disc degeneration.
In the present study, quantitative measurement of the CSA and fCSA of multifidus muscle for each level between L2-S1 increased toward the caudal side. This result is thought to be due to anatomical characteristics. However, in the case of the CSA to fCSA ratio, each level showed a relatively constant value. Moreover, the CSA to fCSA ratio and Psoas index showed a significant relationship. Functional disability measurements showed a significant relationship with this CSA to fCSA ratio. As a result, the CSA to fCSA ratio index was related to the structural and functional measurements of sarcopenia. Therefore, the CSA to fCSA ratio of the back muscles can be a potential index for sarcopenia estimation.
The SPPB, BBS, Grip strength, and FRT were used to measure functional disability in this study. The SPPB is an objective index for measuring daily life performance, including balance and gait, with high reliability and validity.[
12] The BBS is a method for measuring static and dynamic balance related to activities of daily living. The BBS is an indicator that specifically reflects the balance among functional abilities. The FRT was used to measure postural stability. Grip strength was used as an index to represent the musculoskeletal system of the entire body to measure the overall geriatric condition.[
14] These functional disability measurements showed relatively consistent correlations at each level of the multifidus muscle parameters. However, no significant correlation was observed between BBS scores. This may be because the BBS evaluates balance itself intensively rather than the overall functional disability, and balance can be affected by the integration of various factors, including proprioceptive, vestibular, and visual sensory systems [
25,
26,
27].
Grip strength strongly correlated with all parameters of the multifidus muscle. Several previous studies have used grip strength as a predictor of functional disability in the elderly. Therefore, grip strength is commonly used as a representative measurement of overall geriatric condition [
14,
28]. In addition to the elderly, grip strength in middle-aged individuals have also been used as a predictor of disability in old age. In other words, grip strength is a cause and result of geriatric conditions [
29,
30], while other measurements reflect the consequential consequences of geriatric conditions. Therefore, grip strength reflects geriatric condition better than other measurements. Hence, grip strength may be an optimal functional evaluation parameter for back muscle degeneration.
In previous studies, exercise-induced co-contraction of the deep trunk muscles, including the multifidus muscle, reduced back pain, and functional disability [
31]. The lumbar stabilization exercise program for 12 weeks increased multifidus CSA. [
32]. Exercise programs can induce reversible changes in the CSA and reduce pain. Therefore, proper exercise prescription for elderly patients is important, based on objective and quantitative evaluation of the back muscles.
However, this study had several limitations. First, a relatively small number of patients were recruited. Second, we analyzed the multifidus muscle among the various back muscles involved in spinal stabilization. However, the multifidus plays a crucial role in spinal segmental stabilization among the back muscles.[
33] Therefore, we believe that the analysis of the multifidus muscle holds significant clinical relevance.
5. Conclusions
The ratio of fatty infiltration in the multifidus muscle showed a relatively constant value for each spinal level and had a significant and higher correlation with the psoas index and functional disability measures, including SPPB, FRT, and grip strength. Therefore, in elderly patients, back muscle fatty infiltration is significantly influenced by aging rather than disc degeneration. Thus, evaluation and treatment for back muscle atrophy and fatty infiltration can be considered within the same aging category, similar to the decline in muscle mass that occurs in older individuals. Moreover, grip strength showed a high correlation with all parameters of the multifidus muscle. As a result, grip strength can be used as an optimal functional evaluation parameter in studies related to back muscles degeneration.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, D.G.L.; methodology, J.H.Y.; formal analysis, J,H,Y.; investigation, D.G.L.; data curation, D.G.L and , J,H,Y.; writing—original draft preparation, J,H,Y.; writing—review and editing, D.G.L.; visualization, D.G.L and , J,H,Y; supervision, D.G.L.; funding acquisition, D.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a 2022 Yeungnam University research grant.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of NAME OF INSTITUTE (protocol code YUMC 2021-08-053 and approval date 22 OCT 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on a reasonable request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Joint bone spine 2019, 86, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. Journal of the American Medical Directors Association 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: revised European consensus on definition and diagnosis. Age and ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-Y. Effects of core muscle stability training on the weight distribution and stability of the elderly. Journal of physical therapy science 2015, 27, 3163–3165. [Google Scholar] [CrossRef] [PubMed]
- Kliziene, I.; Sipaviciene, S.; Klizas, S.; Imbrasiene, D. Effects of core stability exercises on multifidus muscles in healthy women and women with chronic low-back pain. Journal of back and musculoskeletal rehabilitation 2015, 28, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.; Kim, S.W.; Yu, D. Paraspinal muscle fatty degeneration as a predictor of progressive vertebral collapse in osteoporotic vertebral compression fractures. The Spine Journal 2022, 22, 313–320. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, M.; Serrano Sosa, M.; Cattell, R.; Fan, W.; Li, M.; Chen, J.; Gao, M.; Zhou, Q.; Li, S. Fatty infiltration of paraspinal muscles is associated with bone mineral density of the lumbar spine. Archives of osteoporosis 2019, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.D.; Woodham, M.A.; Woodham, A.W. The role of the lumbar multifidus in chronic low back pain: a review. PM&R 2010, 2, 142–146. [Google Scholar]
- Wallwork, T.L.; Stanton, W.R.; Freke, M.; Hides, J.A. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Manual therapy 2009, 14, 496–500. [Google Scholar] [CrossRef]
- Sun, D.; Liu, P.; Cheng, J.; Ma, Z.; Liu, J.; Qin, T. Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. BMC musculoskeletal disorders 2017, 18, 1–7. [Google Scholar] [CrossRef]
- Faur, C.; Patrascu, J.M.; Haragus, H.; Anglitoiu, B. Correlation between multifidus fatty atrophy and lumbar disc degeneration in low back pain. BMC musculoskeletal disorders 2019, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.F.; Curcio, C.-L.; Alvarado, B.; Zunzunegui, M.V.; Guralnik, J. Validity and reliability of the Short Physical Performance Battery (SPPB): a pilot study on mobility in the Colombian Andes. Colombia medica 2013, 44, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Neuls, P.D.; Clark, T.L.; Van Heuklon, N.C.; Proctor, J.E.; Kilker, B.J.; Bieber, M.E.; Donlan, A.V.; Carr-Jules, S.A.; Neidel, W.H.; Newton, R.A. Usefulness of the Berg Balance Scale to predict falls in the elderly. Journal of geriatric physical therapy 2011, 34, 3–10. [Google Scholar] [PubMed]
- Mehmet, H.; Yang, A.W.; Robinson, S.R. Measurement of hand grip strength in the elderly: A scoping review with recommendations. Journal of bodywork and movement therapies 2020, 24, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Santos, A.; Amaral, T. Differences in handgrip strength protocols to identify sarcopenia and frailty-a systematic review. BMC geriatrics 2017, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: a new clinical measure of balance. Journal of gerontology 1990, 45, M192–M197. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.; Johnson, A.M.; Holmes, J.; Stephenson, F.; Spaulding, S. Predictive validity of the UPDRS postural stability score and the Functional Reach Test, when compared with ecologically valid reaching tasks. Parkinsonism & related disorders 2010, 16, 409–411. [Google Scholar]
- Lee, D.; Kang, M. Correlation between psoas muscle index and degeneration of spinal back muscle in patients with back pain. In Proceedings of the Healthcare; 2021; p. 1189. [Google Scholar]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Frontiers in endocrinology 2016, 7, 69. [Google Scholar] [CrossRef]
- Kader, D.; Wardlaw, D.; Smith, F. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clinical radiology 2000, 55, 145–149. [Google Scholar] [CrossRef]
- Kjaer, P.; Bendix, T.; Sorensen, J.S.; Korsholm, L.; Leboeuf-Yde, C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC medicine 2007, 5, 1–10. [Google Scholar] [CrossRef]
- Shahidi, B.; Hubbard, J.C.; Gibbons, M.C.; Ruoss, S.; Zlomislic, V.; Allen, R.T.; Garfin, S.R.; Ward, S.R. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. Journal of Orthopaedic Research 2017, 35, 2700–2706. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.-F.; Richardson, C.A.; Kippers, V.; Parnianpour, M. Relationship between muscle fiber composition and functional capacity of back muscles in healthy subjects and patients with back pain. Journal of Orthopaedic & Sports Physical Therapy 1998, 27, 389–402. [Google Scholar]
- Rezazadeh, F.; Taheri, N.; Okhravi, S.M.; Hosseini, S.M. The relationship between cross-sectional area of multifidus muscle and disability index in patients with chronic non-specific low back pain. Musculoskeletal Science and Practice 2019, 42, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.; Guskiewicz, K.M.; Petschauer, M.A.; Prentice, W.E. Balance and joint stability: the relative contributions of proprioception and muscular strength. Journal of sport rehabilitation 2000, 9, 315–328. [Google Scholar] [CrossRef]
- Lin, H.W.; Bhattacharyya, N. Balance disorders in the elderly: epidemiology and functional impact. The Laryngoscope 2012, 122, 1858–1861. [Google Scholar] [CrossRef] [PubMed]
- Redfern, M.S.; Yardley, L.; Bronstein, A.M. Visual influences on balance. Journal of anxiety disorders 2001, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Taekema, D.G.; Gussekloo, J.; Maier, A.B.; Westendorp, R.G.; de Craen, A.J. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age and ageing 2010, 39, 331–337. [Google Scholar] [CrossRef]
- Kobayashi, K.; Imagama, S.; Ando, K.; Tsushima, M.; Machino, M.; Ota, K.; Tanaka, S.; Morozumi, M.; Kanbara, S.; Ishiguro, N. Weakness of grip strength reflects future locomotive syndrome and progression of locomotive risk stage: a 10-year longitudinal cohort study. Modern Rheumatology 2020, 30, 573–579. [Google Scholar] [CrossRef]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. Jama 1999, 281, 558–560. [Google Scholar] [CrossRef]
- Richardson, C.; Jull, G. Muscle control–pain control. What exercises would you prescribe? Manual therapy 1995, 1, 2–10. [Google Scholar] [CrossRef]
- Maraschin, M.; Ferrari, S.; Cacciatori, C. The effect of functional stabilization training on the cross sectional area of the deep stabilizers muscles in healthcare workers with chronic low back pain: a pilot study/Effetto di un programma di stabilizzazione funzionale sul trofismo dei muscoli stabilizzatori profondi in operatori sociosanitari con lombalgia cronica: uno studio pilota. Scienza Riabilitativa 2014, 16, 12–22. [Google Scholar]
- Clark, N.; Voight, M.L.; Campbell, A.M.; Pierce, S.; Sells, P.; Cook, R.; Henley, C.; Schiller, L. The relationship between segmental rolling ability and lumbar multifidus activation time. International Journal of Sports Physical Therapy 2017, 12, 921. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).