Submitted:
26 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Options of Different Concentrations of Proline to Improve RWC and Growth Recovery of Drought-Stressed Oilseed Rape
2.2. Impact of Proline Application on Morphometric Parameters of Oilseed Rape Seedlings Exposed to Drought
2.3. Impact of Proline Application on RWC of Prolonged Drought-Stressed Oilseed Rape leaves
2.4. Impact of Proline Application on Chlorophyll Content of Drought-Stressed Oilseed Rape Leaves
2.5. Impact of Proline Application on Ethylene Emission of Drought-Stressed Oilseed Rape Leaves
2.6. Impact of Proline Application on H2O2 Levels of Drought-Stressed Oilseed Rape Leaves
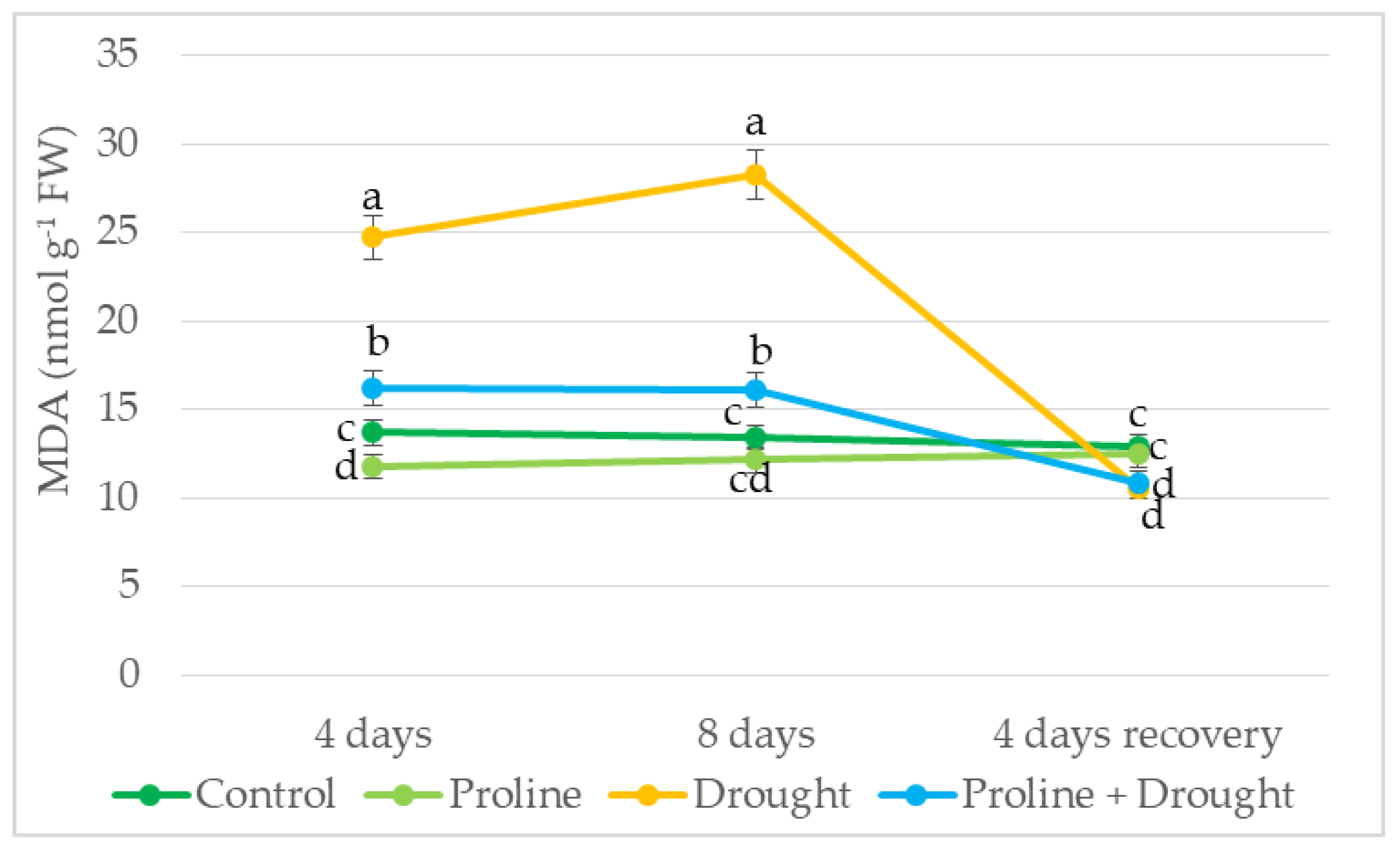
2.7. Impact of Proline Application on MDA Content of Drought-Stressed Oilseed Rape Leaves
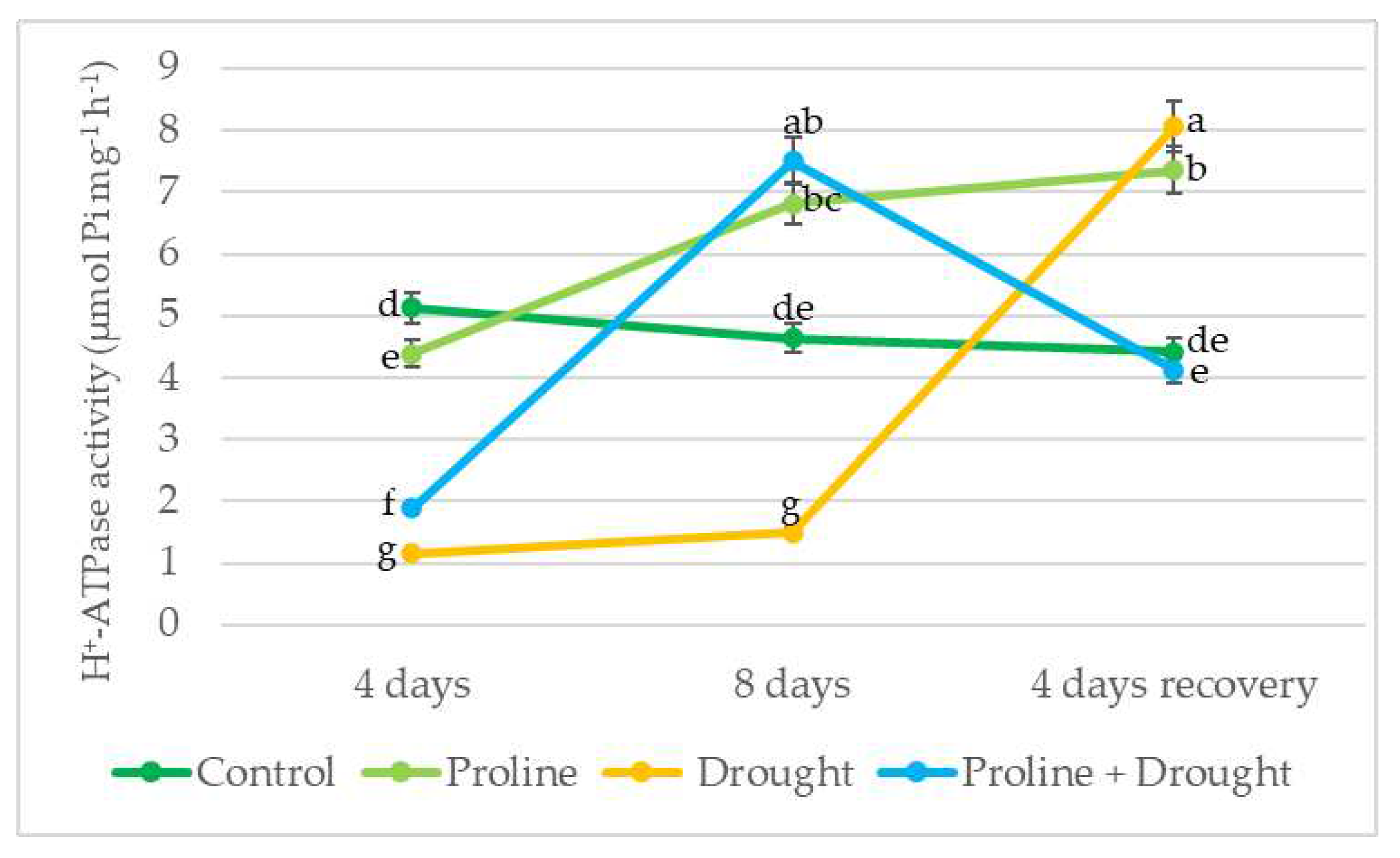
2.8. Efects of Exogenous Proline on PM ATPase activity of Rapeseed Seedlings Exposed to Drought
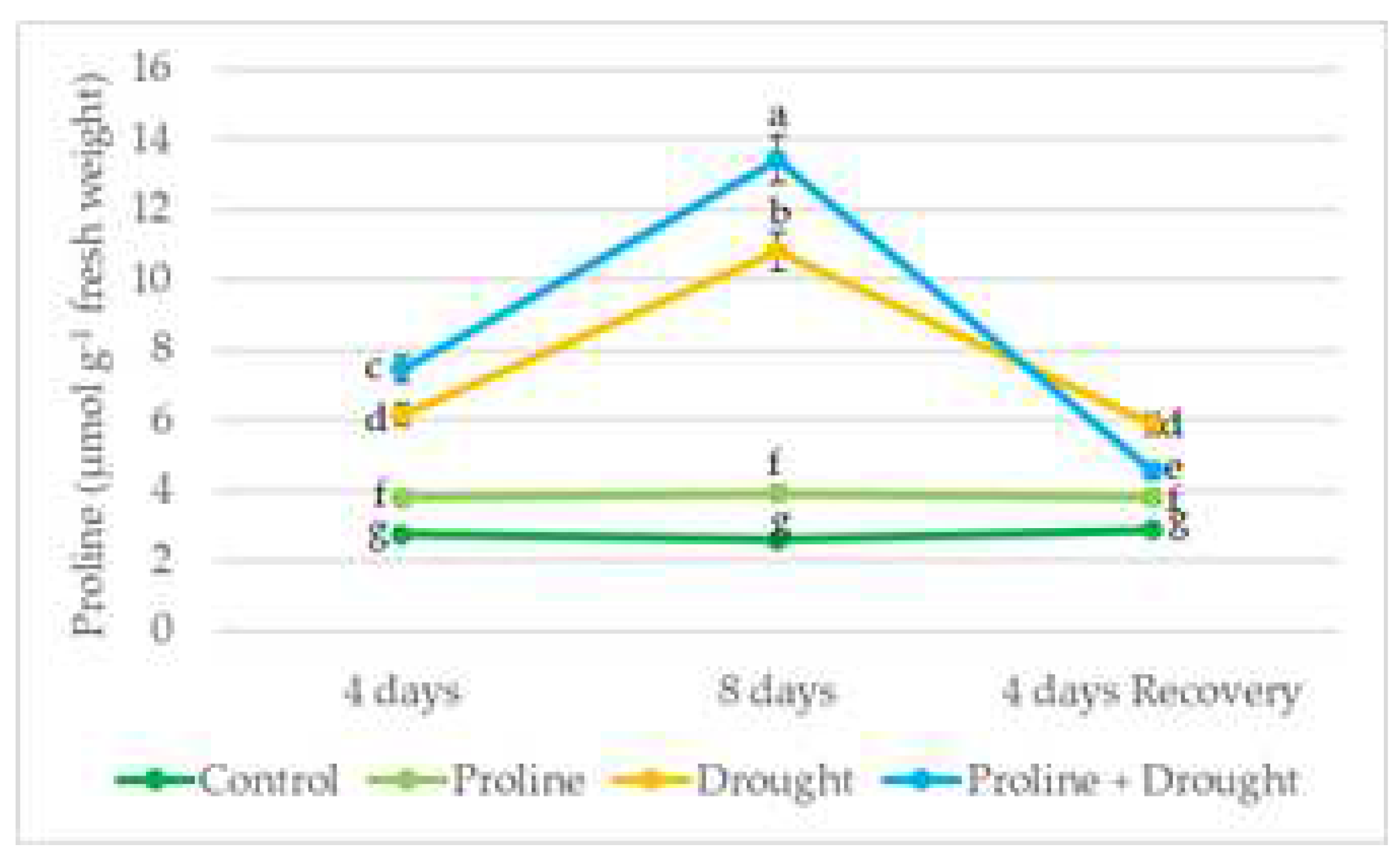
2.9. Efect of Exogenous Proline on Endogenuos Proline Content of Rapeseed Seedlings Exposed to Drought
2.10. Efect of Exogenous Proline on Survival of Plants
3. Discussion
4. Materials and Methods
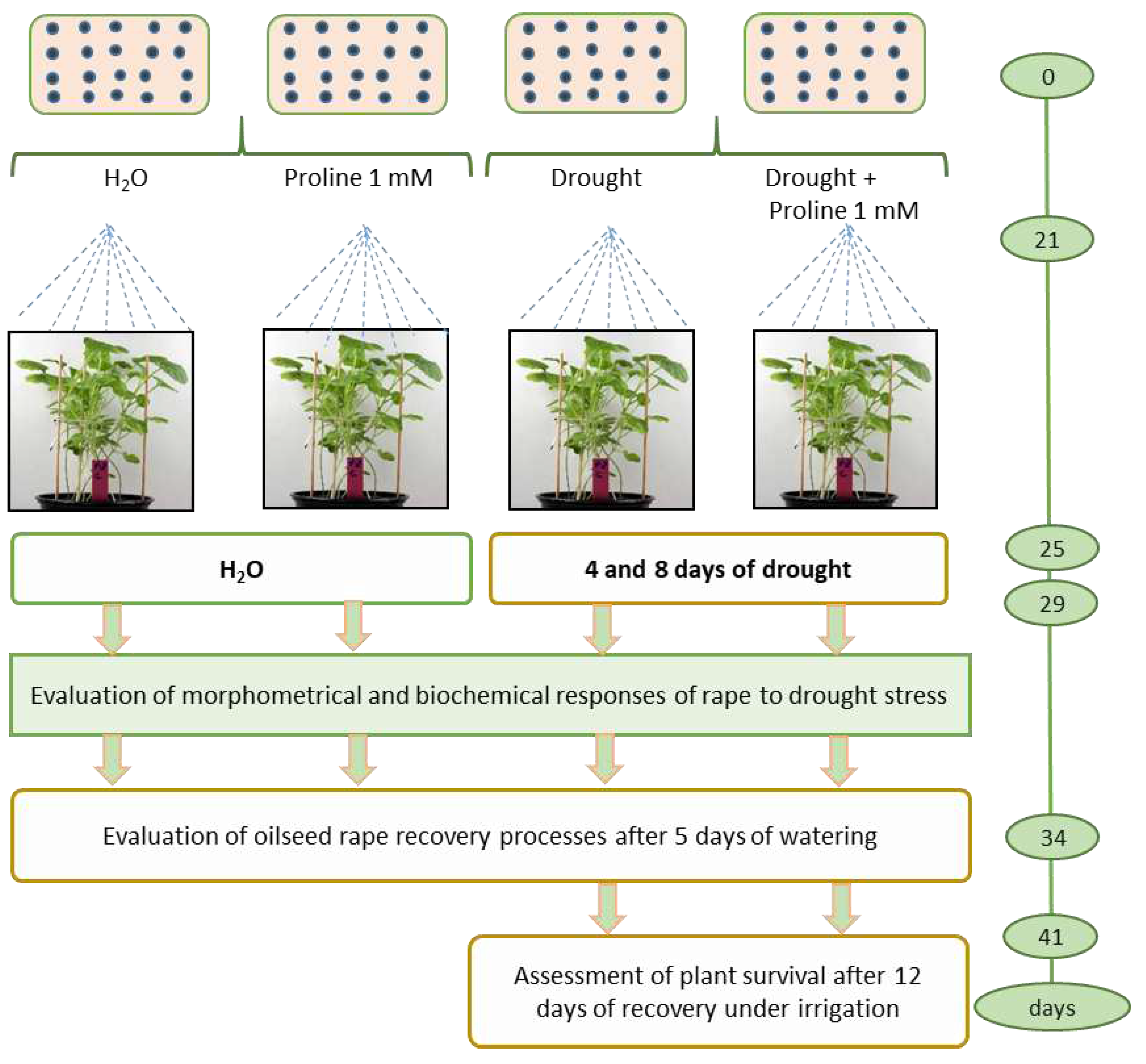
4.1. Plant Material and Growth Conditions
4.2. Treatments
4.2.1. L-proline aqueous solutions (Roth) was used for seedling spraying at the 3–4 leaf stage (BBCH-scale 13-14 [57].4.2.2. Drought treatment for the drought stress control studies. During the simulated drought, irrigation was stopped to allow gradual drying of the soil. Soil moisture was estimated using a soil moisture meter (Biogrod, China).
4.3. Determination of the Active Proline Concentration
4.4. Experimental Design of Drought Stress Control Studies
4.5. Sampling
4.6. Morphometrical Measurements
4.7. Recovery Evaluation
4.8. Survival Evaluation
4.9. Relative Water Content (RWC)
4.10. Assessment of Biochemical Parameters
4.10.1. Photosynthetic Pigments.
4.10.2. Ethylene.
4.10.3. Hydrogen Peroxide (H2O2).
4.10.4. MDA content.
4.10.5. H+-ATPase activity assay.
4.10.6. Proline.
4.11. Statistical analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Della Marta, P. M.; Haylock, M. R.; Luterbacher, J.; Wanner, H. Doubled length of western European summer heat waves since 1880. J. Geophys. Res. Atmos. 2007, 112, D15. [Google Scholar] [CrossRef]
- Peña-Ortiz, C.; Barriopedro, D.; García-Herrera, R. Multidecadal Variability of the Summer Length in Europe, J. of Climat. 2015, 28, 5375–5388. [Google Scholar] [CrossRef]
- Basarin, B.; Lukić, T.; Matzarakis, A. Review of Biometeorology of Heatwaves and Warm Extremes in Europe. Atmosphere 2020, 11, 1276. [Google Scholar] [CrossRef]
- Petrov, P.; Petrova, A.; Dimitrov, I.; Tashev, T.; Olsovska, K.; Brestic, M.; Misheva, S. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J. Agron. Crop Sci. 2018, 204, 219–227. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol Biochem. 2017, 118, 427–437. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: a multifunctional amino acid. Trends Plant Sci. 2009, 15, 89–97. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, O.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–166. [Google Scholar]
- Farooq, M.; Nawaz, A.; Chaudhry, M.; Indrasti, R.; Rehman, A. Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma-aminobutyric acid. J. Agron. Crop Sci. 2017, 203, 464–472. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kumar, V.; Burritt, D.J.; Fujita, M.; Mäkelä, P. Osmoprotectant-mediated abiotic stress tolerance in plants. In Proline Metabolism and Its Functions in Development and Stress Tolerance; Springer Nature: Cham, Switzerland, 2019; pp. 41–72. [Google Scholar]
- Caverzan, A.; Casassola, A.; Patussi Brammer, S. Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Abiotic and biotic stress in plants—recent advances and future perspectives; Shanker, A.K., Shanker, C., Eds., Eds.; InTech, 2016; pp. 463–480. [Google Scholar]
- Orsini, F.; Pennisi, G.; Mancarella, S.; Al Nayef, M. , Sanoubar R.; Nicola, S.; Gianquinto, G. Hydroponic lettuce yields are improved under salt stress by utilizing white plastic film and exogenous applications of proline. Sci. Hortic. 2018, 233, 283–293. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Wu, K.; Zhang, Q.; Feng, Y.; Miao, Y.; Yan, Z. Exogenous Application of Chitosan Alleviate Salinity Stress in Lettuce (Lactuca sativa L.). Horticulturae 2021, 7, 342. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants : A Review. Int J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Bukhari, S.A.H.; Peerzada, A.M.; Javed, M.H.; Dawood, M.; Hussain, N.; Ahmad, S. Growth and Development Dynamics in Agronomic Crops under Environmental Stress. In Agronomic Crops; Springer: Berlin/Heidelberg, Germany, 2019; pp. 83–114. [Google Scholar]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on cereal, legume, tuber and root crops production. Agric. Water Manag. 2020, 179, 18–33. [Google Scholar] [CrossRef]
- Cartea, E.; De Haro-Bailón, A.; Padilla, G.; Obregón-Cano, S.; del Rio-Celestino, M.; Ordás, A. Seed Oil Quality of Brassica napus and Brassica rapa Germplasm from Northwestern Spain. Foods 2019, 8, 292. [Google Scholar] [CrossRef]
- Kordrostami, M.; Mafakheri, M. Rapeseed: Biology and Physiological Responses to Drought Stress. In The Plant Family Brassicaceae; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 263–276. [Google Scholar]
- Batool, M.; El-Badri, A.M.; Hassan, M.U.; Haiyun, Y.; Chunyun, W.; Zhenkun, Y.; Jie, K.; Wang, B.; Zhou, G. Drought Stress in Brassica napus: Effects, Tolerance Mechanisms, and Management Strategies. J. Plant Growth Regul. 2022, 1–25. [Google Scholar] [CrossRef]
- Pullens, J.W.M.; Sharif, B.; Trnka, M.; Balek, J.; Semenov, M.A.; Olesen, J.E. Risk factors for European winter oilseed rape production under climate change. Agric. For. Meteorol. 2019, 272, 30–39. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant responses to water stress. Ann. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- El Sabagh, A.; Hossain, A.; Barutcular, C.; Gormus, O.; Ahmad, Z.; Hussain, S.; Islam, M.; Alharby, H.; Bamagoos, A.; Kumar, N.; et al. Effects of drought stress on the quality of major oilseed crops: Implications and possible mitigation strategies—A review. Appl. Ecol. Environ. Res.. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Ni, F.; Rizwan, M.; Fahad, S.; Hu, L. Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiol. Plant. 2019, 41, 25. [Google Scholar] [CrossRef]
- Signorelli, S.; Dewi, J.R.; Considine, M.J. Soil Water Content Directly Affects Bud Burst Rate in Single-Node Cuttings of Perennial Plants. Agronomy 2022, 12, 360. [Google Scholar] [CrossRef]
- Radzikowska, D.; Sulewska, H.; Bandurska, H.; Ratajczak, K.; Szymańska, G.; Kowalczewski, P.Ł.; Głowicka-Wołoszyn, R. Analysis of Physiological Status in Response to Water Deficit of Spelt (Triticum aestivum ssp. spelta) Cultivars in Reference to Common Wheat (Triticum aestivum ssp. vulgare). Agronomy 2022, 12, 1822. [Google Scholar]
- Forlani, G.; Trovato, M.; Funck, D.; Signorelli, S. Regulation of Proline Accumulation and Its Molecular and Physiological Fctions in Stress Defence. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P.S.A., Eds.; Springer International Publishing: Cham, 2019; pp. 73–97. [Google Scholar]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Akram, N.A.; Iqbal, M.; Muhammad, A.; Ashraf, M.; Al-Qurainy, F.; Shafiq, S. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. In Protoplasma.; 2018; Volume 255, pp. 163–174. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Anee, T. I.; Khan, M. I. R.; Fujita, M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. South African Journal of Botany 2018, 115, 50–57. [Google Scholar] [CrossRef]
- Sharif, P.; Seyedsalehi, M.; Paladino, O.; Van Damme, P.; Sillanpää, M.; Sharifi, A. Effect of drought and salinity stresses on morphological and physiological characteristics of canola. Int. J. Environ. Sci. Technol. 2018, 15, 1859–1866. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Li, J.J.; Zeng, L.; Cheng, Y.; Lu, G.Y.; Fu, G.P.; Ma, H.Q.; Liu, Q.Y.; Zhang, X.K.; Zou, X.L.; Li, C.H. Exogenous melatonin alleviates damage from drought stress in Brassica napus L. (rapeseed) seedlings. Acta Physiol. Plant. 2018, 40, 3. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M.; Athar, H.U. R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot 2007, 39, 1133–44. 57. [Google Scholar]
- Kamran, M.; Shahbaz, M.; Ashraf, M.; Akram, N. A. Alleviation of drought-induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak. J. Bot, 2009, 41, 621–632. [Google Scholar]
- Aslam, M.M.; Farhat, F.; Siddiqui, M.A.; Yasmeen, S.; Khan, M.T.; Sial, M.A.; Khan, I.A. Exploration of physiological and biochemical processes of canola with exogenously applied fertilizers and plant growth regulators under drought stress. PLoS One. 2021, 16. [Google Scholar] [CrossRef]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically Triggered Heat and Drought Stress Tolerance in Rice by Proline Application. J Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- Valluru, R.; Davies, W.J.; Reynolds, M.P.; Dodd, I.C. Foliar Abscisic acid-to-ethylene accumulation and response regulate shoot growth sensitivity to mild drought in wheat. Front. Plant Sci. 2016, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M. ; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Todorova, D.; Sergiev, I.; Katerova, Z.; Shopova, E.; Dimitrova, L.; Brankova, L. Assessment of the Biochemical Responses of Wheat Seedlings to Soil Drought after Application of Selective Herbicide. Plants 2021, 10, 733. [Google Scholar] [CrossRef]
- Signorelli, S.; Coitiño, E. .L.; Borsani,O.; Monza, J. Molecular mechanisms for the reaction between OH radicals and proline: insights on the role as reactive oxygen species scavenger in plant stress. J.of Physical Chemistr. B 2014, 118, 37–47. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F., Haider; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; Varshney, R.K. Assessment of proline function in higher plants under extreme temperature. Plant Biol J 2023, 25, 379–395. [Google Scholar] [CrossRef]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How Reactive Oxygen Species and Proline Face Stress Together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Dawood, M.G.; Sh Sadak, M。. Physiological role of glycinebetaine in alleviating the deleterious effects of drought stress on canola plants (Brassica napus L.). Middle East J Agric Res. 2014, 3, 943–954. [Google Scholar]
- Gong, H.J.; Chen, K.M.; Chen, G.C.; Wang, S.M.; Zhang, C.L. Drought stress stimulates p-nitrophenyl phosphate hydrolysis rate of the plasma membrane H+-ATPase from wheat leaves. Plant Growth Regul. 2003, 40, 139–145. [Google Scholar] [CrossRef]
- Feng, X.; Liu, W.; Zeng, F.; Chen, Z.; Zhang, G.; Wu, F. K+ uptake, H+-ATPase pumping activity and Ca2+ efflux mechanism are involved in drought tolerance of barley. Environ. Exp. Bot. 2016, 129, 57–66. [Google Scholar] [CrossRef]
- Michalak, A.; Wdowikowska, A.; Janicka, M. Plant Plasma Membrane Proton Pump: One Protein with Multiple Functions. Cells. 2022, 11, 4052. [Google Scholar] [CrossRef]
- Chang, N.; Ziwen, Z.; Yeyun, L.; Xianchen, Z. Exogenously applied Spd and Spm enhance drought tolerance in tea plants by increasing fatty acid desaturation and plasma membrane H+-ATPase activity. Plant Physiol. Biochem. 2022, 170, 225–233. [Google Scholar]
- Mi, C.; Wang, Q.; Zhao, Y.A.; Zhang, C.L.; Sun, C.; Liu, Z.G.; Lin, L.B. Changes in the Differentially Expressed Proteins and Total Fatty Acid Contents in Winter Rapeseed (Brassica rapa L.) Leaves under Drought Stress. Russ J Plant Physiol 2022, 69, 31. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Jiang, D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.). Envir. and Experiment. Bot. 2019, 166, 103804. [Google Scholar] [CrossRef]
- Kauer, G.; Asthir, B. Proline: a key player in plant abiotic stress tolerance. Biol. Plantarum. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants. In BBCH Monograph; Meier, U., Ed. Quedlinburg: Julius Kühn-Institut, 2018; pp. 85–8. [Google Scholar]
- Fiebelkorn, D.; Rahman, M. Development of a protocol for frost-tolerance evaluation in rapeseed/canola (Brassica napus L.). The Crop Journal 2016, 4, 147–152. [Google Scholar] [CrossRef]
- Weng, M.; Cui, L.; Liu, F.; Zhang, M.; Shan, L.; Yang, S.; Deng, X.-P. Effects of Drought stress on antioxidant enzymes in seedlings of different wheat genotypes. Pak. J. Bot. 2015, 47, 49–56. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Child, R.D.; Chauvaux, N.; John, K.; Van Onckelen, H.A.; Ulvskov, P. Ethylene biosynthesis in oilseed rape pods in relation to pod shatter. J. Exp. Bot. 1998, 49, 829–838. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid method for the quantification of microgram quantities of proteins utilising the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Darginavičienė, J.; Pašakinskienė, I.; Maksimov, G.; Rognli, O.A.; Jurkonienė, S.; Šveikauskas, V.; Bareikienė, N. Changes in plasmalemma K+ Mg2+-ATPase dephosphorylating activity and H+ transport in relation to freezing tolerance and seasonal growth of Festuca pratensis Huds. J. Plant Physiol. 2008, 165, 825–832. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
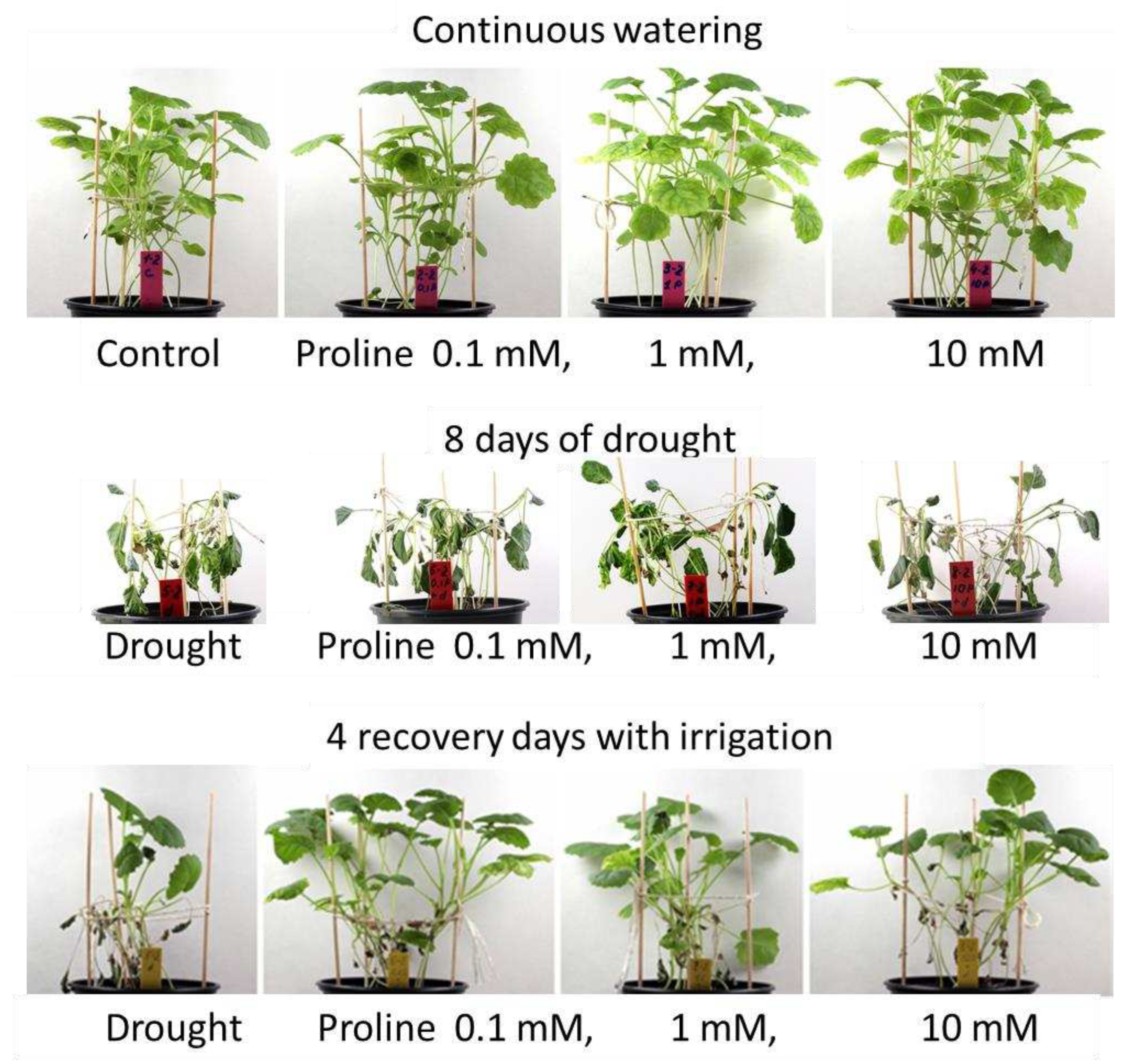
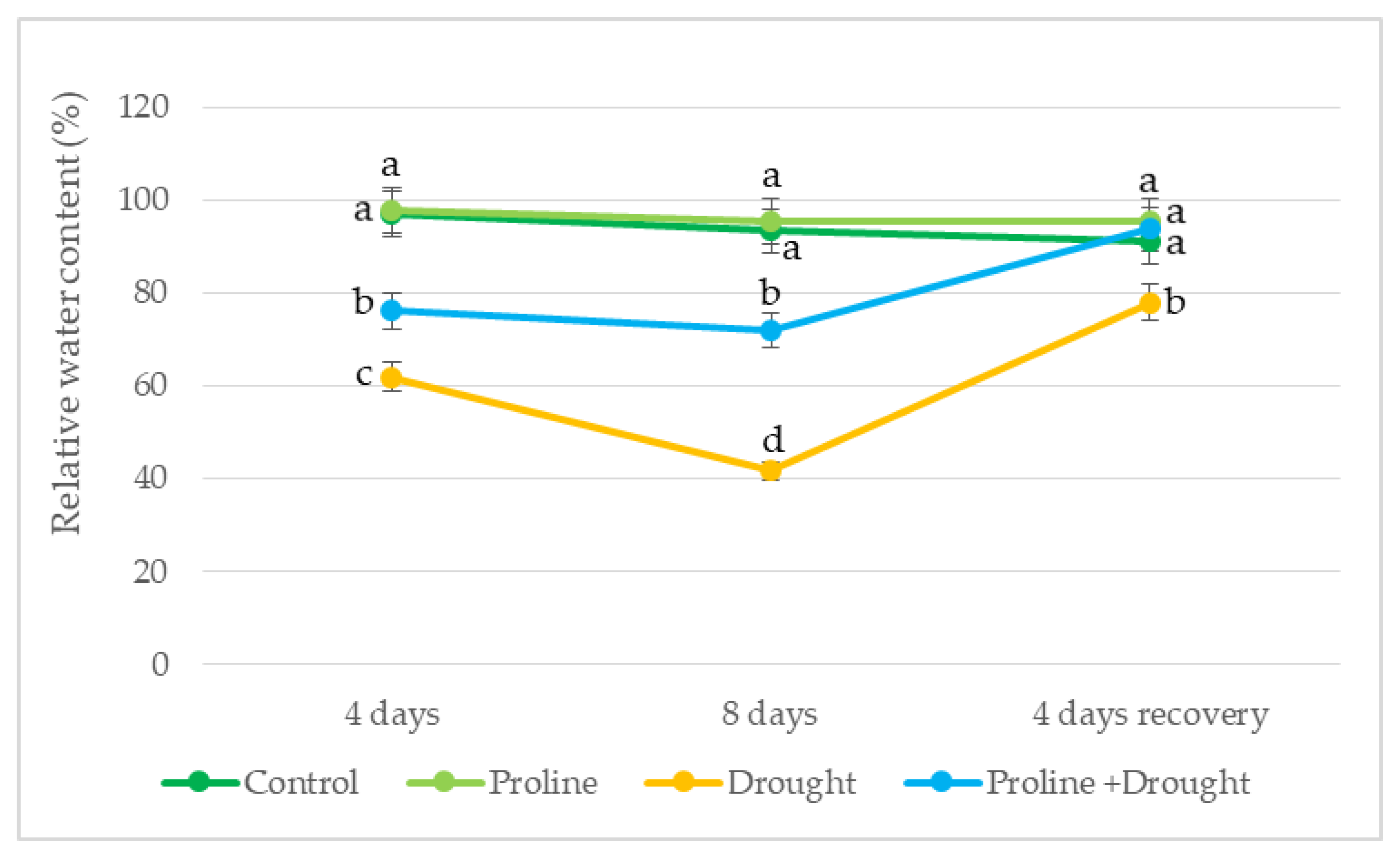
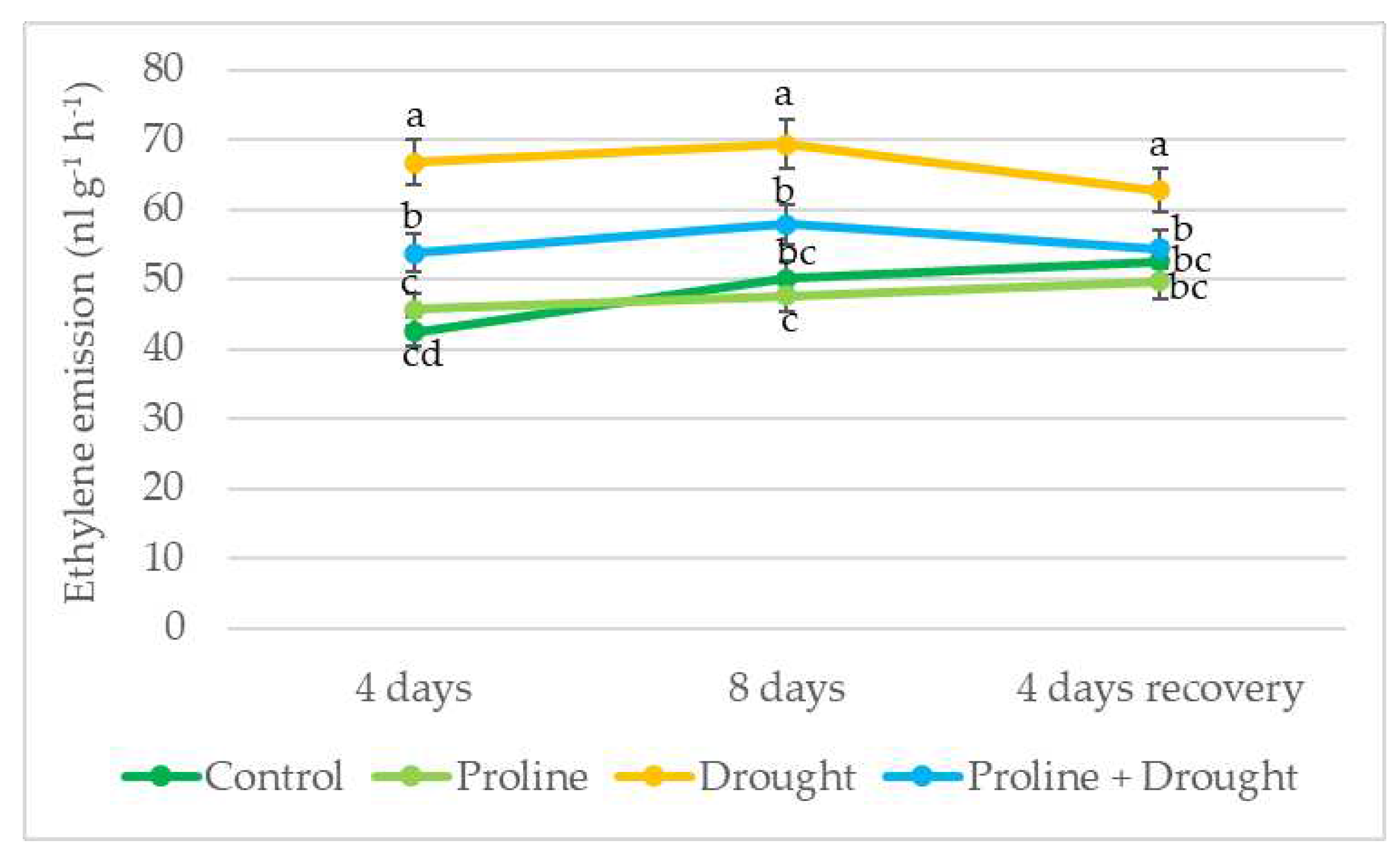
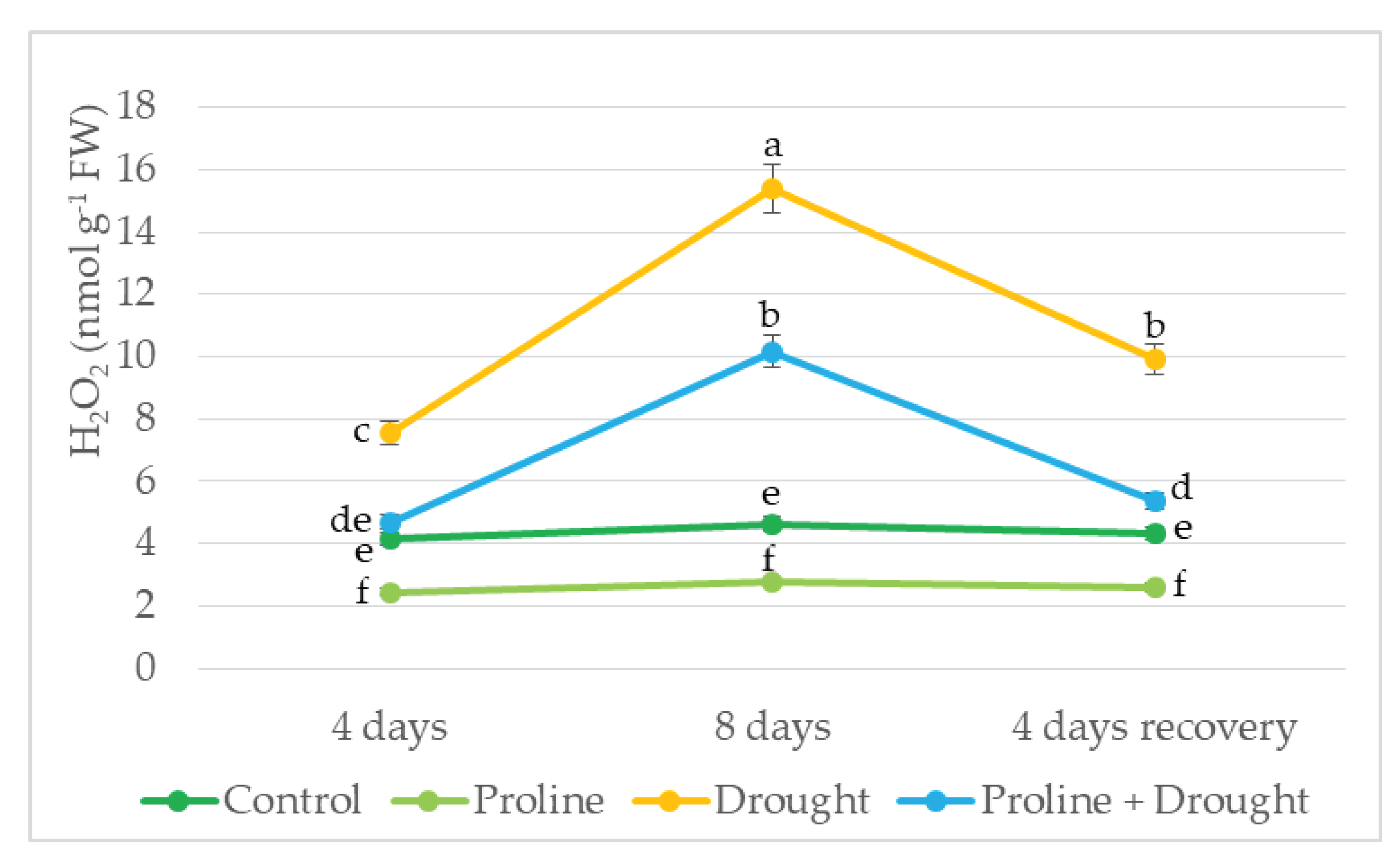
| Treatment (3 ml) | RWC, % | |
|---|---|---|
| 4 days | 8 days | |
| Control, H2O | 82.3 a | 82.5a |
| Proline 0.1 mM | 81.5 a | 81.7a |
| Proline 1 mM | 82.3 a | 80.7a |
| Proline 10 mM | 84.0 a | 85.6 a |
| Drought | 70.1 c | 63.2 d |
| Proline 0.1 mM + Drought | 74.7 b | 58.8 e |
| Proline 1 mM + Drought | 73.6 b | 70.7 c |
| Proline 10 mM + Drought | 70.9 c | 59.8 f |
| Treatment (12.5 ml) | ||
| Control, H2O | 84.2 a | 83.5 a |
| Proline 0.1 mM | 85.2 a | 87.2 a |
| Proline 1 mM | 80.7 a | 87.3 a |
| Proline 10 mM | 80.1 a | 86.8 a |
| Drought | 63.1 d | 51.9 e |
| Proline 0.1 mM + Drought | 73.7 b | 60.1 d |
| Proline 1 mM + Drought | 74.6 b | 72.6 bc |
| Proline 10 mM + Drought | 70.4 c | 58.7 d |
| Treatment (3 ml) | Average Weight (g) | |
|---|---|---|
| Fresh | Dry | |
| Control, H2O | 0.91 ab | 0.051 a |
| Proline 0.1 mM | 0.88 b | 0.052 a |
| Proline 1 mM | 1.01 a | 0.055 a |
| Proline 10 mM | 1.24 a | 0.052 a |
| Drought | 0.84 c | 0.041 c |
| Proline 0.1 mM + Drought | 0.75 cd | 0.045 ab |
| Proline 1 mM + Drought | 0.78 c | 0.049 ab |
| Proline 10 mM + Drought | 0.97 ab | 0.044 b |
| reatment (12.5 ml) | ||
| Control, H2O | 1.13 a | 0.057 a |
| Proline 0.1 mM | 1.26 a | 0.056 a |
| Proline 1 mM | 1.47 a | 0.059 a |
| Proline 10 mM | 1.35 a | 0.057 a |
| Drought | 1.02 b | 0.043 c |
| Proline 0.1 mM + Drought | 1.07 ab | 0.043 c |
| Proline 1 mM + Drought | 1.18 a | 0.055 ab |
| Proline 10 mM + Drought | 1.11 a | 0.051 b |
| Treatment | Average Length (cm) | Average Weight (g) | |||||||
| Fresh | Dry | ||||||||
| 4 days | 8 days | 4 days recovery | 4 days | 8 days | 4 days recovery | 4 days | 8 days | 4 days recovery | |
| Control H2O | 15.26 a | 17.27 a | 18.00 b | 0.68 b | 0.74 b | 0.77 b | 0.045 a | 0.045 a | 0.046 a |
| Proline | 15.76 a | 17.93 a | 19.03 a | 0.74 a | 0.87 a | 0.89 a | 0.048 a | 0.048 a | 0.049 a |
| Drought | 14.64 b | 14.74 c | 15.71 c | 0.51 c | 0.45 d | 0.46 c | 0.030 c | 0.041 b | 0.038 b |
| Proline + Drought | 14.89 ab | 15.29 b | 17.75 b | 0.67 b | 0.49 c | 0.70 b | 0.036 b | 0.047 a | 0.039 b |
| Treatment | Chlorophyll contents (mg g–1 FW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chlorophyll a | Chlorphyll b | Chlorophyll a+b | |||||||
| 4 days | 8 days | 4 days recovery | 4 days | 8 days | 4 days recovery | 4 days | 8 days | 4 days recovery | |
| Control, H2O | 0.98 a | 0.99 a | 0.85 a | 0.24 a | 0.23 a | 0.24 a | 1.22 a | 1.22 a | 1.09 a |
| Proline | 0.97 a | 1.02 a | 0.85 a | 0.25 a | 0.24 a | 0.25 a | 1.22 a | 1.23 a | 1.11 a |
| Drought | 0.78 c | 0.57 c | 0.67 b | 0.21 a | 0.12 b | 0.18 b | 0.98 c | 0.69 c | 0.85 b |
| Proline + Drought | 0.93 b | 0.62 b | 0.89 a | 0.21 a | 0.13 b | 0.22 a | 1.13 b | 0.75 b | 1.10 a |
| Treatment | Number of survived plants (%) |
|---|---|
| Control H2O | 100.00 a |
| Proline | 100.00 a |
| Drought | 19.79±2.21 b |
| Proline + Drought | 44.10 ±3.15 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





