1. Introduction
There are about 40 different species of sesame plant belonging to the Pedaliaceae family and the
Sesamum genus. Sesame, which has been cultivated since ancient times, has many wild and cultivated varieties [
1]. In addition to its use as a food additive, it is also widely used in traditional medicine due to its properties of demulcent, mildly laxative, emollient [
2], antifungal [
3], antioxidant [
4], analgesic [
5], anticancer and antiproliferative [
6], vitamin B and E supplier [
7], serum cholesterol (lipid) and blood pressure-lowering potential [
8,
9].
Many studies on biochemical and biological activities have been carried out on the seed and oil of the sesame plant [
7,
10,
11,
12]. It is a very valuable agricultural crop, especially in its seeds with high amino acid content, thanks to its protein content of up to 28% and oil content of up to 55% [
13]. It has also been proven by studies that this seed oil contains saturated fatty acids (14%), monounsaturated fatty acids (39%) and, polyunsaturated fatty acids (46%) [
1]. The most important bioactive components of sesame are sesamin, which protects the liver from oxidative destruction, and sesamolin, which is a kind of insecticide. These two components are the major lignans of sesame seed oil [
14]. Sesamin is also a noncompetitive inhibitor, which plays an important role on its anti-inflammatory effect [
15]. In addition to these two basic components, sesame contains two more components as sesamol and tocopherol [
16].
Although the use of other vertebrates, including humans, in many scientific studies in the field of health brings convenience, as in every study, it also brings ethical problems. The most effective solution for the ethical problems that may occur was the use of
in vitro cell culture method [
17,
18]. Cell culture is the method of providing human body conditions to the cells under the appropriate temperature, humidity and carbon dioxide environment in a medium containing various cell types isolated from plants or mammals, and nutrients such as amino acids, vitamins, inorganic salts, serum and glucose. By imitating human body conditions, cells are allowed to reproduce in an artificial environment, and it helps to lay the foundations of many studies, from researching metabolomics, transcriptomics, proteomics and other cellular processes at the level of basic sciences to drug development and understanding drug action pathways, and gene therapy principles through cell culture studies [
19,
20].
L929 fibroblast cell cultures originating from the mouse C3H/An connective tissue have been used in studies such as investigation of the
in vitro oligo-hydroxyalkanoates effect on the growing, the anti-inflammatory and antioxidant properties of the chondroitin sulfate, the cytochemical properties of the fibroblast-preadipocyte relationships, and the acute activation of glucose uptake, in addition to virus studies. The cells contained in this culture are also HPRT+ and APRT+ [
21,
22,
23,
24]. A549 lung epithelial cell cultures, which have the ability to synthesize lecithin by using the cytidine diphosphocholine pathway, have been used in studies such as inhibition of cancer cells, antiproliferation and identification of its mechanisms, and investigation of the effects of insulin and insulin-like growth factor on apoptosis and cell proliferation [
25,
26]. MCF-7 breast epithelial cell cultures, which are capable of expressing the WNT7B oncogene, have been used in studies on the inhibition of telomerase activity and the effect of estrogen in the apoptotic pathway, as well as in studies on inhibition and antiproliferation of cell division and understanding these mechanisms [
27,
28,
29]. Although cisplatin is used alone, it is mostly used in combination with other cytostatic drugs. Cisplatin destroys cells that can cause certain types of cancer such as testicular tumors, ovarian tumors, lung tumors, etc. It can be used as a control group in cell culture studies to determine the effectiveness of antiproliferation [
30,
31,
32,
33].
By examining the gene expressions in tissues and/or cells at the level of mRNA in terms of form and quantity, transcriptomic analyzes provide great contributions to the understanding of the importance of these genes for the organism, as well as learning the functions of genes [
34,
35]. In these analyzes, on the other hand, is the simultaneous study of mRNA transcripts generated by transcription from the cell genome. It aims to measure the expression level of a selected subset or all of the genes, based on the amount of RNA present in a sample [
36,
37]. With these analyzes, it is possible to identify genes related to disease and physiological processes, to reveal the structural-functional interactions between genes, to reveal the roles of genes in development, to reveal their expression profiles, and to compare different organisms on the genetic basis [
38,
39].
In this context, the current work purposed to investigate the wound healing and antiproliferative properties of the plant extract of
in vitro grown
S. orientale L. cv. “Gökova” using L929 fibroblast, MCF-7 breast and, A549 lung epithelial cell lines. Another aim of the study, in MCF-7 and A549 cell lines treated with the plant extract or cisplatin, was to examine at the transcriptional level of CRISPR-associated system 3 (CAS3) endonuclease [
40], CRISPR-associated system 9 (CAS9) endonuclease [
41], and B-cell lymphoma XL (anti-apoptotic, BCL-XL) [
42] genes expressions, which we think are effective in metabolic processes such as cell growth and division, control of apoptotic pathways and cellular cycle. This study is derived from a part of PhD student Sevil Yeniocak's doctoral thesis.
2. Results
2.1. Wound healing properties of sesame plant extract
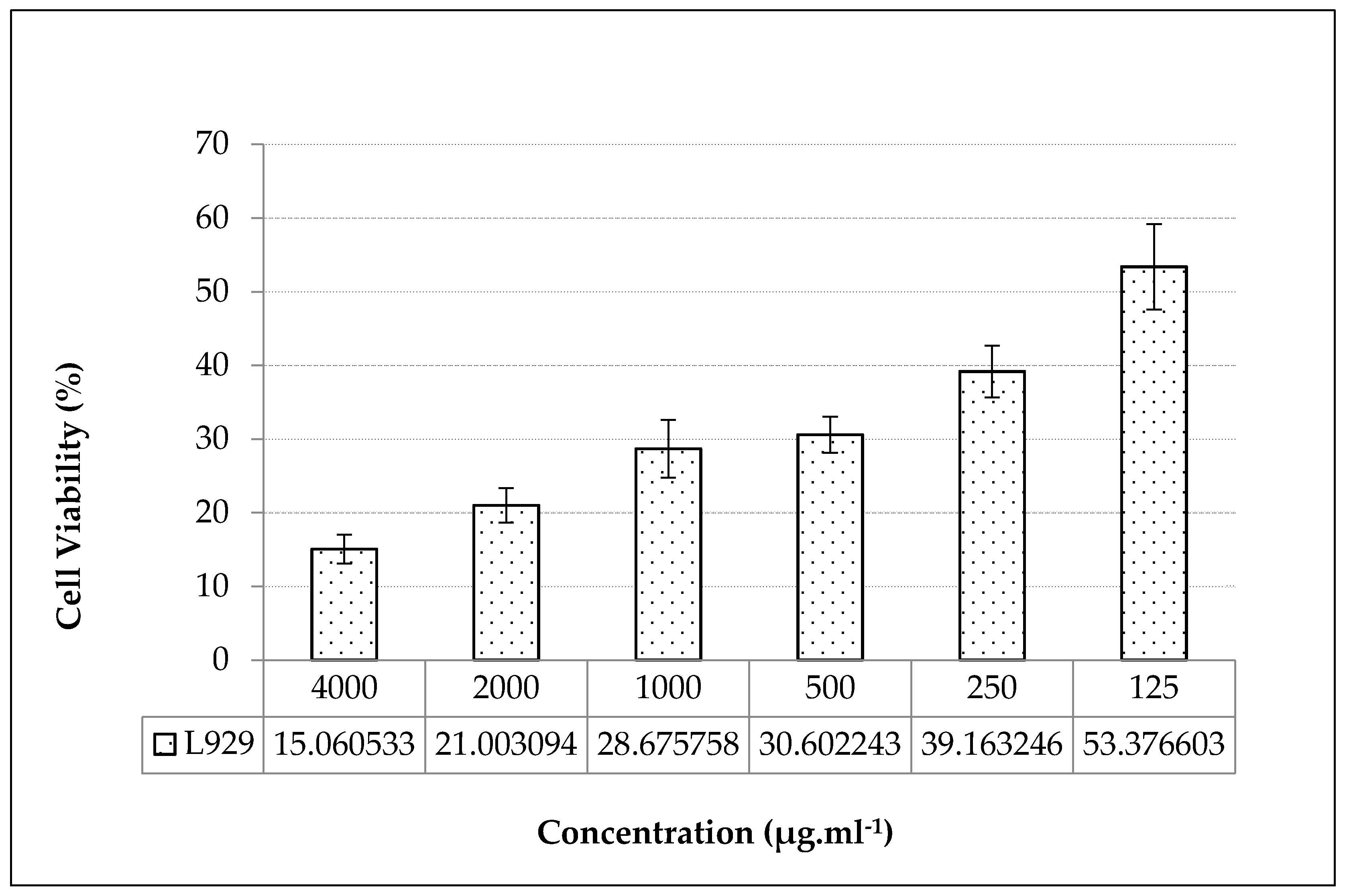
In order to determine the subcytotoxic doses of the applied plant extract in wound healing trials, we primarily determined the cytotoxicity of the extract. The cytotoxic effects of
in vitro grown
S. orientale L. cv. “Gökova” plant extract were performed by MTT assay using L929 fibroblast cell line. According to the MTT test result, the IC
50 value of the plant extract was calculated as 154,70±7,16 µg.ml
-1 (
Figure 1).
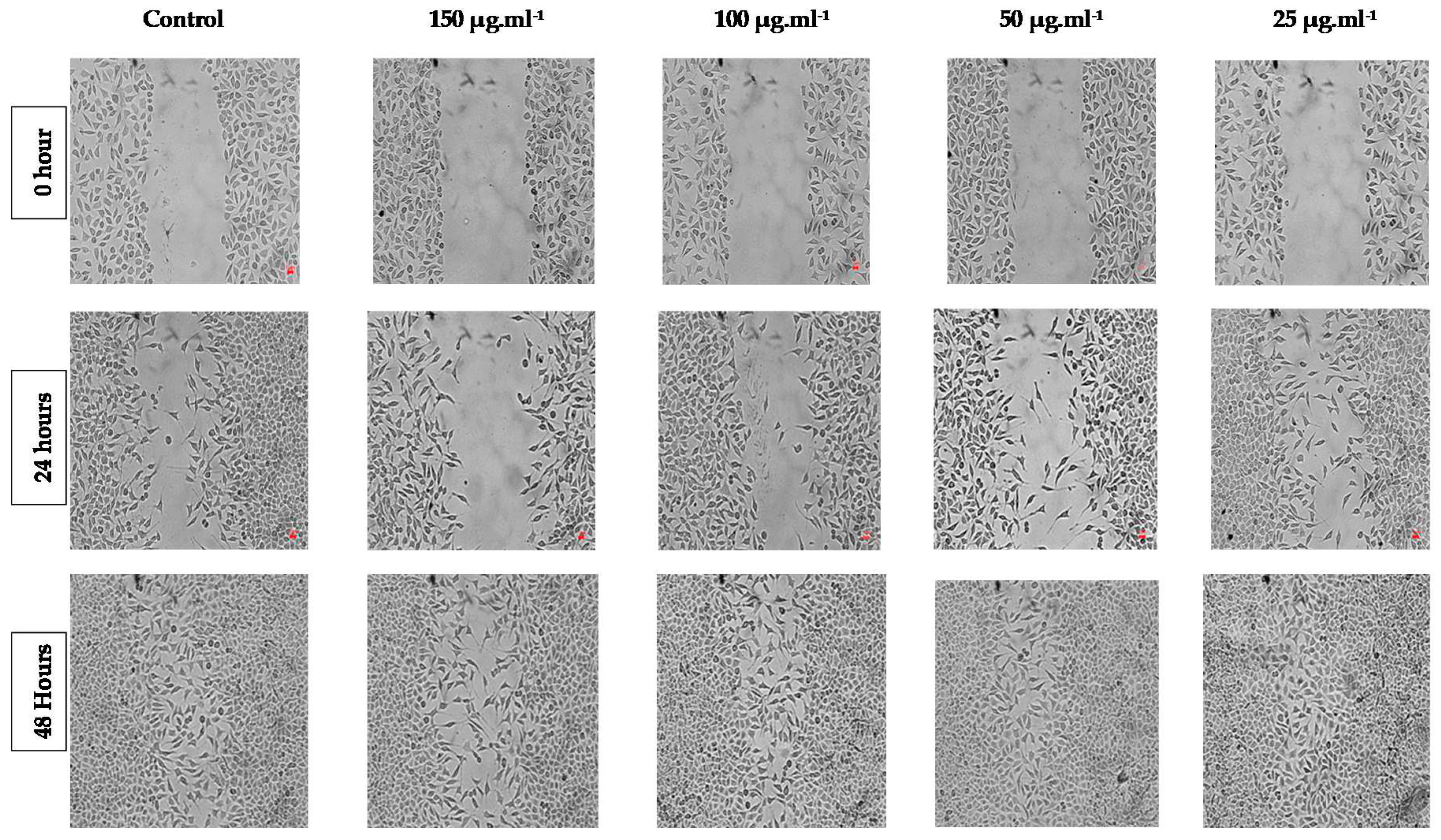
The wound healing activity of the plant extract applied at subcytotoxic doses of IC
50 was determined by
in vitro scratch test performed by incubation of the plant extract for 24-48 hours periods on L929 fibroblast cells. According to the results of the
in vitro scratch test, it had a positive effect on the healing of the wounds formed on L929 fibroblast cell cultures, after 48 hours in decreasing subdoses of the plant extracts. In plant extract applications at reduced subdoses of IC
50, wound healing was quite evident at 48 hours after 25 µg.ml
-1 plant extract treatments (
Figure 2).
2.2. Anticancer properties of sesame plant extract
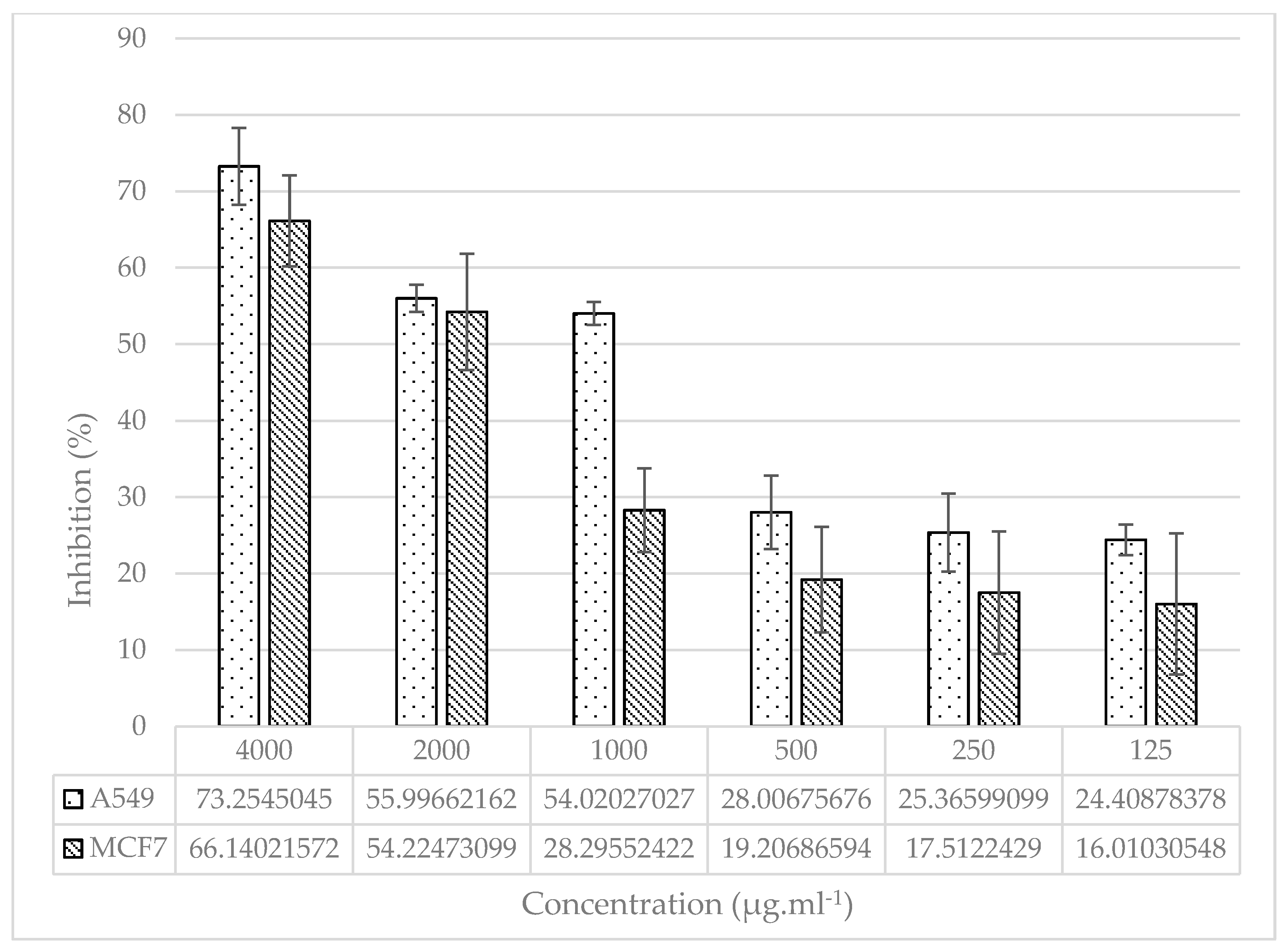
In the A549 lung epithelium cell line treated with the different concentration of the
in vitro grown
S. orientale L. cv. “Gökova” plant extract, the cell viability inhibition percentage was variable, manifesting a concentration related effect. While at the highest concentration of the treated extract (4000 µg.ml
-1), the inhibition was 73.25 ± 5.03 %, at the lowest concentration of the treated extract (125 µg.ml
-1), it was 24.41 ± 1.99 % (
Figure 3).
Additionally, in the MCF-7 mammary epithelium cell line treated with the different concentration of the plant extract, the percentage of cell viability inhibition was 16.01 ± 9.23 % at 125 µg.ml
-1, and 66.14 ± 5.95 % at 4000 µg.ml
-1 (
Figure 3).
When the plant extract effect on both cell lines was compared in the treatment of similar concentrations, it was observed that the extract caused a significant inhibition on the A549 cell line at all treatment doses (especially at 1000 µg.ml
-1 concentration) (
Figure 3).
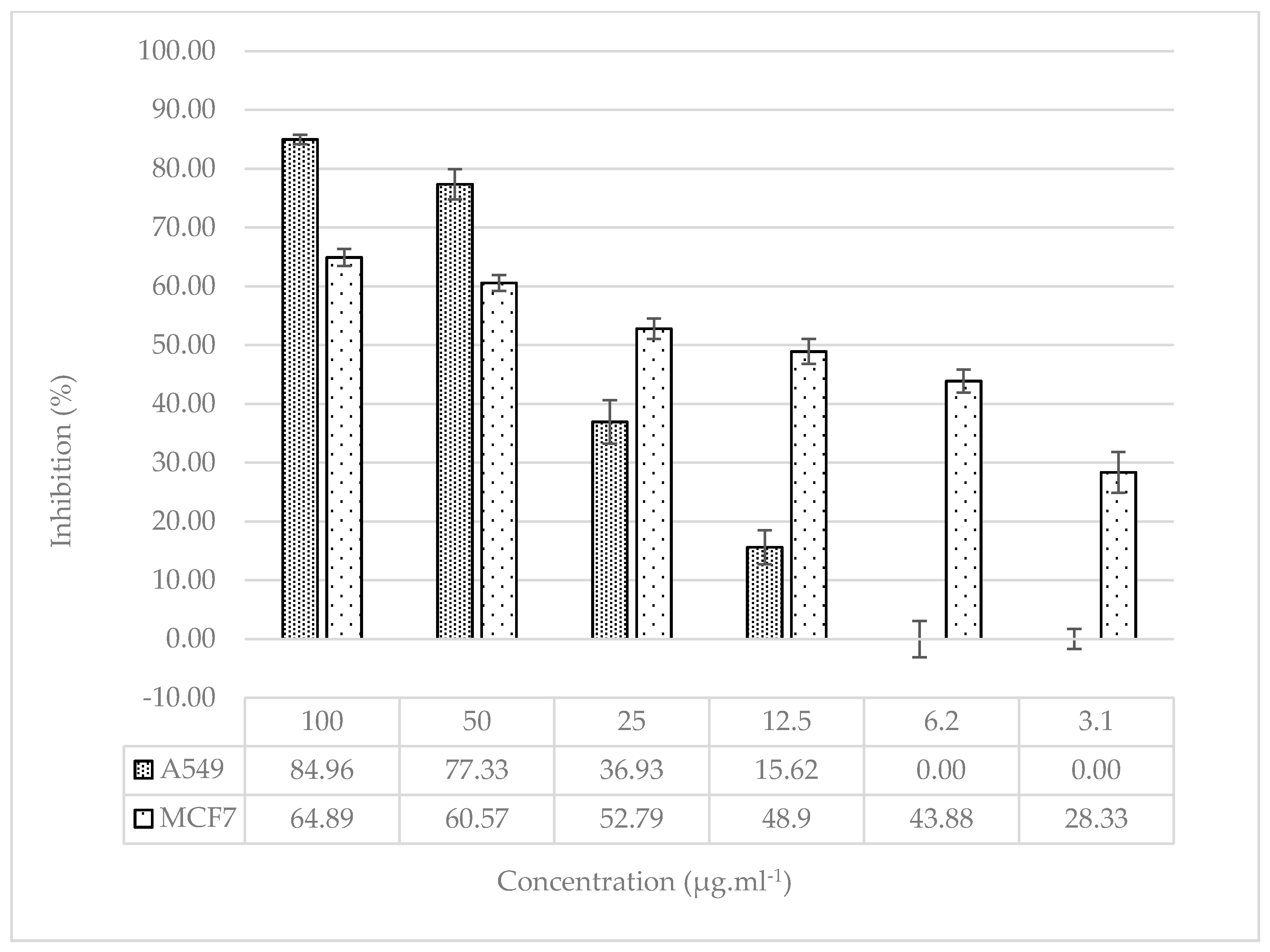
In order to compare the significance of the antiproliferative activity of the plant extract treatment, cisplatin was treated at concentrations of 3.1 µg.ml
-1 to 100 µg.ml
-1 in both cell lines as control group. In the A549 lung epithelium cell line treated with the different concentration of cisplatin, the percentage of cell viability inhibition was ranged from 0.0 ± 1.7 % (3.1 µg.ml
-1) to 84.96 ± 0.81 % (100 µg.ml
-1), IC
50 = 43 ± 2.15. Furthermore, in the MCF-7 mammary epithelium cell line treated with the different concentration of cisplatin, the percentage of cell viability inhibition was ranged from 28.33 ± 3.49 % (3.1 µg.ml
-1) to 64.89 ± 1.46 % (100 µg.ml
-1), IC
50 = 12.5 ± 1.79 (
Figure 4).
2.3. Transcriptomic analyzes in treated cell lines
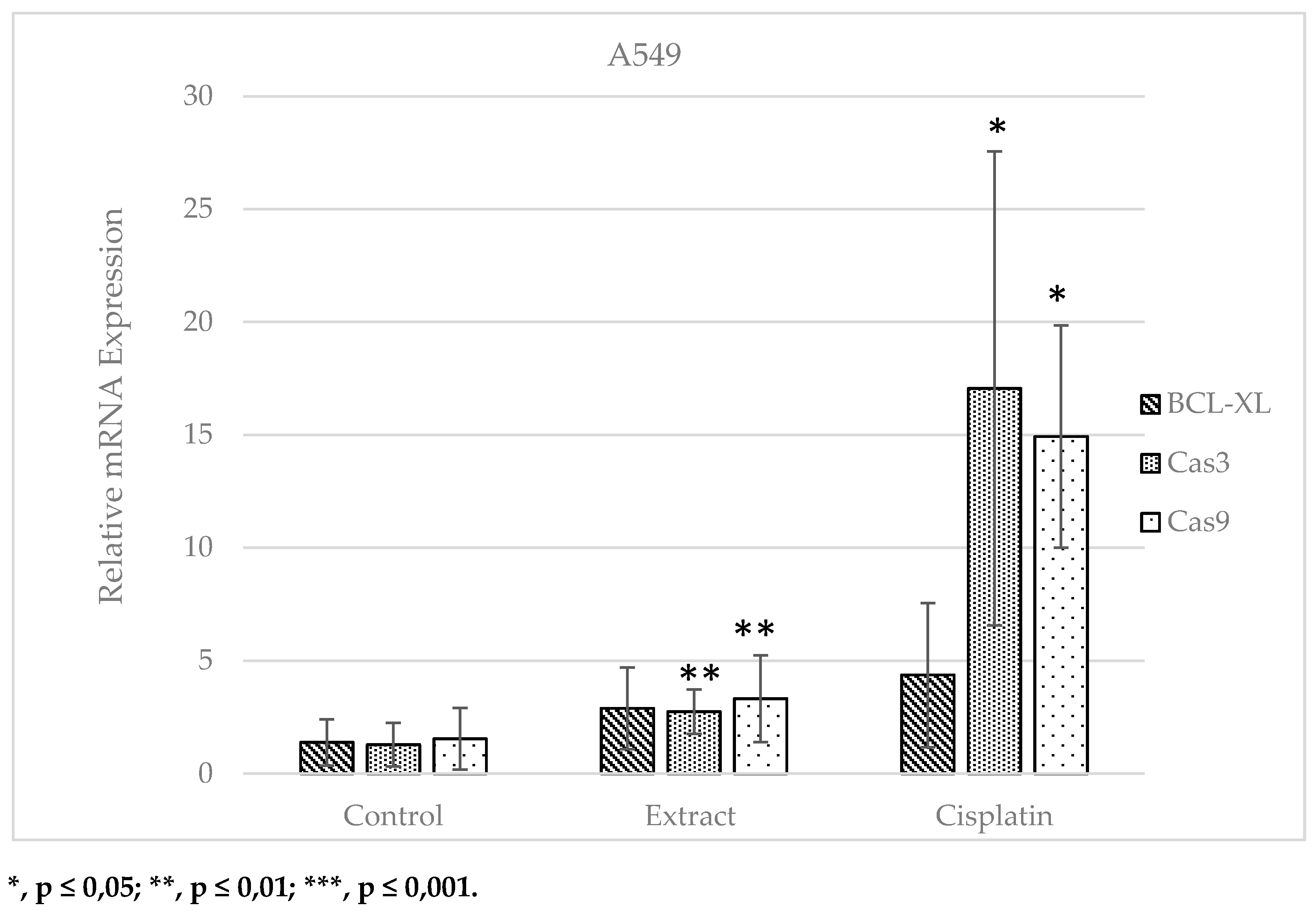
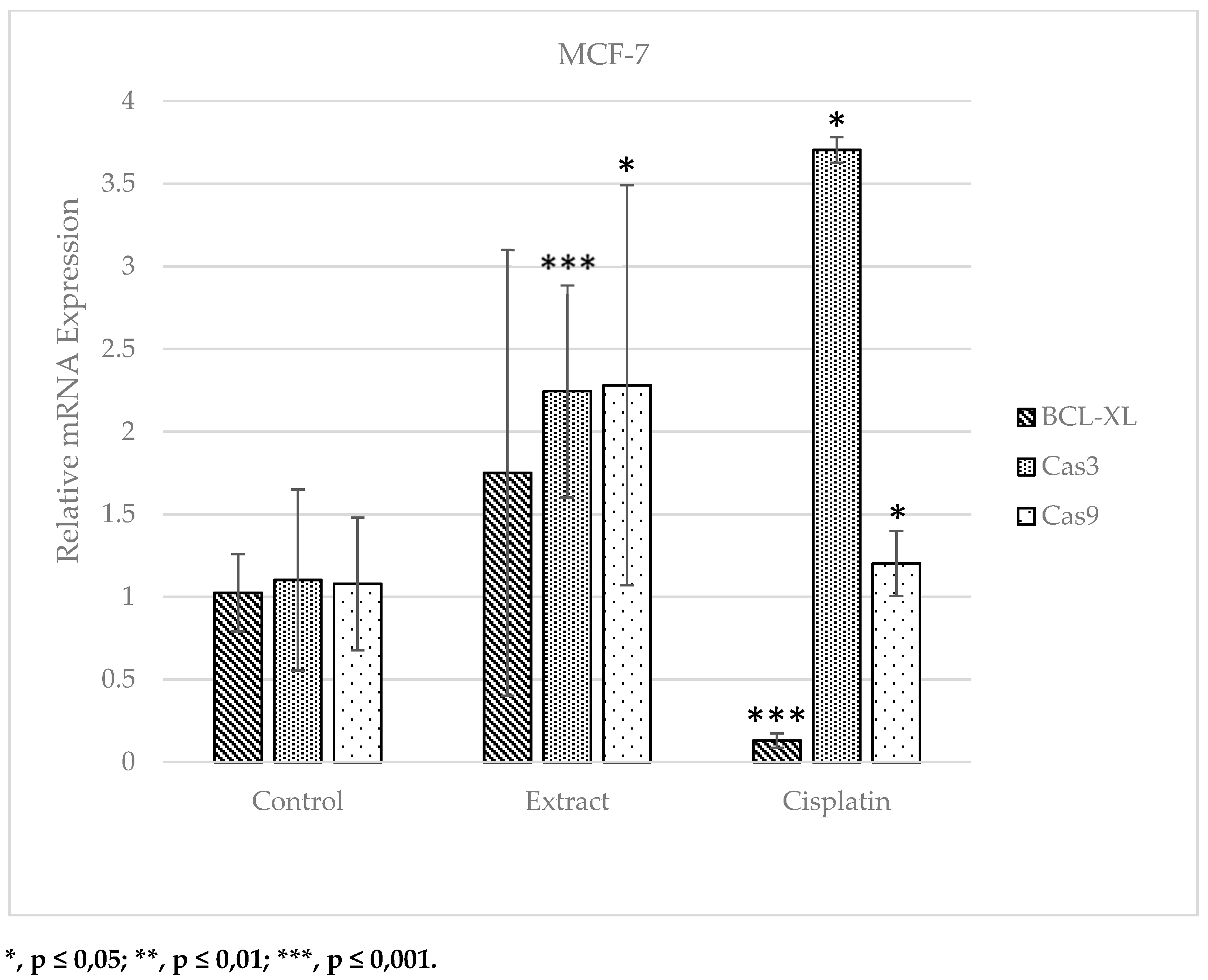
Graphs were created by calculating the mean of the Ct values (with standard errors and standard deviations) obtained after Real-Time PCR carried out to specified the expression levels of BCL-XL, Cas3 and Cas9 genes at the mRNA level in A549 and MCF-7 cell lines.
When the data for the A549 cell line were examined, an increase was monitored in the mRNA expression level of the
BCL-XL gene, which is negatively correlated with the apoptotic pathway, in the extract and cisplatin groups, respectively, compared to the control group, whereas this rise was not statistically remarkable. A statistically remarkable enhancement was observed in the mRNA expression levels of
Cas3 and
Cas9 genes, which are positively correlated with the apoptotic pathway, in the extract and cisplatin applied groups compared to the control group (
Figure 5).
When the data for the MCF-7 cell line were analyzed, the
BCL-XL gene, whose mRNA expression level was suppressed in the apoptotic pathway, did not show a significant change in the cell lines treated with extract when compared to the control group. However, it showed a statistically considerable decrease in the cisplatin applied group. A significant increase was detected in the extract and cisplatin applied groups compared to the control group in
Cas3 and
Cas9 genes, whose mRNA expression level was induced in the apoptotic pathway. The fact that the treatment of breast cancer cells (MCF-7) with 922.73 µg.ml
-1 S. oriantale plant extract for 6 hours induced the expression of
Cas9 gene more than cisplatin treatment, which is used as an anti-cancer drug and causes serious side effects, is a very remarkable result in this study (
Figure 6).
3. Discussion
3.1. The plant extract in decreasing subcytotoxic doses is effective on wound healing
Almost all medicinal plants have always played important role as pharmacologically for every country in the world. These plants are influenced to cure different illnesses and deactivate possible epidemics, to add flavor to meals and to conserve foods [
43,
44]. These plants are considered valuable sources of conventional drugs and the most synthetic drugs are produced from them. Bioactive components produced by these plants are largely responsible for the different metabolic patways of plant species used throughout the world [
45,
46].
studying the influences of medicinal plant species on health is important for the invention or creation of novel drugs. In this context, in the current study, the wound healing and anticancer properties of sesame plant, whose seeds and oil are an important plant in traditional medicine, were investigated. The subcytotoxic doses (IC
50, 154,70±7,16 µg.ml
-1) of the treated
in vitro grown
S. orientale L. cv. “Gökova” plant extract provided the healing of artificially created wounds on L929 fibroblast cell cultures within 48 hours (
Figure 2). Wound is defined as the deterioration of the anatomical and functional integrity of healthy tissues [
47]. Wound healing is the systematic, biochemical and cellular cases induced by trauma, resulting in the production of new tissue. The main principle of wound healing is to decrease the damage of tissues, to ensure sufficient tissue oxygenation and perfusion, as well as appropriate nutrition and moistening of the tissue [
48,
49]. In 2019 Bolla
et al. investigated the wound healing characteristics of the methanolic extract of
Aristolochia saccate on L929 cell line. In this study, they determined 93.52% wound closure 48 hours after the plant extract treatment [
50]. In the same year, Alsawalha
et al. used the L929 fibroblast cell line to study the wound healing potential of
Dioscorea villosa extract. In this study, they found that 125 μg.ml
-1 of plant extract induced 88.58% cell migration in L929 fibroblast cell [
51]. Similarly, in 2022, Danna et al. used the L929 fibroblast cell line to determine the wound healing potential of
Peucedanum ostruthium leaf and rhizome extracts. In this study, L929 fibroblast cells represented parallel wound closure stimulation with both extracts (IC
50, 801 for leaf extrct; IC
50, 385 for rhizome extract) [
52]. As in our study, these studies in the literature have shown that these plants used in traditional medicine may have wound healing potential and these results also support our study.
3.2. The plant extract in increasing doses is effective on antiproliferation
Although there are many synthetic drugs designed from raw materials obtained from plant extracts, the diversity of diseases that people are exposed to and the different responses of people to diseases increase the importance of drug studies. It is estimated that acceptable therapy is available for only one-third of known human diseases. For this reason, it is important for future studies to reveal the biological characteristics of species of medical importance [
53,
54]. The use of herbal products in cancer treatment can prevent health problems that may occur relatively. Therefore, studies on the chemical components of various plant sources used as anticancer agents have increased in recent years [
55]. For example, curcumin from soybeans, polyphenols from green tea, resveratrol from grapes, lycopene from tomatoes, and crocetin from saffron are compounds that are effective in cancer treatment [
56,
57,
58].
In the current study, the anticancer properties of
in vitro grown
S. orientale L. cv. “Gökova” plant extract were also investigated using MCF-7 mammary epithelium and A549 lung epithelium cell lines. The antiproliferative effect of the extract was also increased with increasing doses for both lines. In the MCF-7 cell line, cell division inhibition was determined as ~66.14% for the highest dose of plant extract applied. In 2020, Nivethitha
et al. investigated the anticancer properties of
S. indicum plant extract in MCF-7 cell line, similar to our study. In the study, they found that the anticancer activity of the plant extract (IC
50, 148.76 µg.ml
-1) was quite good [
59]. Siao
et al. investigated the anti-proliferation properties and action mechanism of
S. indicum seeds, in a study they conducted in 2015 and they used MCF-7 cell lines as in our work. Their results showed that the cell viability inhibition the dose-dependently (10 and 50 μM sesamin decreased the cell viability by 18 and 30% respectively) increased [
60]. All these studies and the results obtained from some other studies in the literature are in line with the results obtained from our study [
61,
62].
On the other hand, in the A549 lung epithelium cell line, cell division inhibition percentage was determined as ~73.25% for the highest dose of plant extract applied. In a 2017 study by Watanabe
et al., it was proven that sesamol indicated anticancer properties against A549 cell lines at 50 µM concentration and 6 hours of treatment. The study showed that this effect of sesamin makes it a potential anticancer agent because lignan has the ability to prevent DNA damage. It has also been reported that sesamin's ability to decrease COX-2 gene expression in the A549 cell line inactivates the inflammatory response that reduces restenosis due to inhibition of the PI3K-Akt pathway [
63,
64]. All these results are also in matching with the results obtained in our work.
3.3. CRISPR-associated system 9 and 3 positively correlated with apoptotic pathway
The development and cell differentiation is a biological process regulated and controlled by genes. When this regulation is disrupted as a result of genetic change, the end point is the emergence of malignant appearance. For this reason, it may be useful to look at gene expression levels that are thought to be effective in metabolic processes in order to understand cancer and the mechanisms that cause cancer [
40,
41,
65]. In this context, in our study, the expressions of CRISPR-associated system 3 (
CAS3) endonuclease, CRISPR-associated system 9 (
CAS9) endonuclease, and B-cell lymphoma XL (
BCL-XL, anti-apoptotic) genes, which are responsible for the apoptotic pathway, were investigated before and after
in vitro grown
S. orientale L. cv. “Gökova” plant extract treatment in MCF-7 breast cancer and A549 lung epithelium cell lines.
Han
et al. in 1999, investigated the antiproliferative properties of curcumin in BKS-2 immature B lymphoma cell line. They found that the curcumin decreased the
BCL-XL expression as well as the tumor suppressor gene
p53 in B cells [
66]. In addition to inhibiting apoptosis,
BCL-XL has also been reported to be a key regulator of tumor progression, cell migration and other important cellular functions [
67]. In the current study, the anti-apoptotic
BCL-XL expression is suppressed when the cell receives apoptosis signal. Therefore, the expression level is expected to decrease in the extract and cisplatin administered groups compared to the control group [
68]. According to the results obtained, it is thought that the
BCL-XL gene, whose expression was not suppressed after the extract and cisplatin application, is not related to the apoptotic pathway in A549 cancer cells. The expected effect was observed in MCF-7 cells treated with cisplatin. There was no significant effect of “Gökova” sesame plant extract on the
BCL-XL gene.
In our study, it was also determined that the
Cas3 and
Cas9 expression levels, which are known to be positively correlated with the apoptotic pathway, were significantly increased in the extract and cisplatin applied groups compared to the control group. This showed that both cisplatin and plant extract could be associated with cell death by suppressing A549 lung epithelium cell proliferation and inducing apoptosis. It can be said by looking at
Cas3 mRNA expression levels that cisplatin used in chemotherapy promotes lung cancer cells to apoptosis at a higher rate compared to the study material, “Gökova” sesame plant extract. However, the high toxic effect of cisplatin [
69] is known through studies, and the serious side effects caused by this situation show that the extract obtained from “Gökova” sesame plant extract can be used instead of cisplatin. When
Cas9 mRNA expression levels on MCF-7 cells were examined, it was observed that the plant extract induced apoptosis more than cisplatin. This shows that “Gökova” sesame plant extract can also be preferred instead of cisplatin, which is already known to have high toxic properties. A study by Özkan
et al. in 2021 confirmed that small extracellular vesicles of
Allium sativum induce caspase-mediated apoptosis and inhibit the proliferation of cancer cells. In this work, the A549 cell line was used and a significant increase in some proapoptotic genes for cancer cells was also noted in parallel with Cas3 gene expression levels. After the extract application, Cas9 gene expression increased 2.5 times [
70]. These results are also in agreement with the results obtained from the present work.
4. Materials and Methods
4.1. Plant Material and In vitro Culture establishment
The
S. orientale cv. “Gökova” seeds grown in the collection gardens (
Figure 7A, B) were derived from Muğla Metropolitan Municipality, Agricultural Services Unit, Local Seed Bank (the cooperation protocol no: 10452259-030.02-1240). For
in vitro culture initiation, surface sterilization of seeds was used by modifying the methods previously developed by Kaya
et al. and Ozudogru
et al. [
71,
72]. In the method, the seeds were washed with EtOH (70%) for 5 minutes, H
2O
2 (10%) for 10 minutes, sodium hypochlorite (NaOCl, Domestos
®, 20% and 10%) twice for 5 minutes, then rinsed thoroughly with sterile distilled water until detergent residues were removed. The “Gokova” sesame seeds, which became free of contaminants after sterilization, were carried on MS [
73] supplemented with 1 mg.l
-1 kinetin, 20 g.l
-1 sucrose, and 7 g.l
-1 agar (pH 5.8) at standard culture conditions [23 ± 2 °C, 16/8 hours light cycle (50 μmol.m
-2.s
-1 white cool fluorescent light)] [
74].
In vitro germinated
S. orientale cv. “Gökova” micro-shoots were subcultured at 4-week periods and mass propagated to obtain the plant material required for the cell extracts (
Figure 7C).
4.2. Extraction protocol of In vitro Grown Sesame Micro-shoots
The
in vitro grown micro-shoots of
S. orientale cv. “Gökova” were left to dry in a fan drying etuv at 35 °C for overnight. Dried plant materials were milled in a grinder with the help of liquid nitrogen. The ground plant was suspended with 99% ethyl alcohol (Merck KGaA, Darmstadt, Germany) as 10 times and subjected to ultrasonic extraction by vortexing. In the extraction process, after each 30-minute cycle, the suspension was precipitated at 4000 rpm for 4 minutes via thermoregulated centrifuge and the supernatant was filtered with the help of filter paper and transferred to the beaker. This process was repeated twice. The obtained extract was collected in an Erlenmeyer flask and a fume hood was used to remove the alcohol from the environment. Thus, crude extract was obtained for use in cell lines. The extract stock solution was prepared with Dimethyl sulfoxide (DMSO) at a concentration of 200 mg.ml
-1. Test concentrations to be used in the investigation of
in vitro cytotoxic effects, wound healing and antiprolative effects on cancer cell lines were prepared by diluting with Dulbecco’s Minimal Essential Medium (DMEM) from this stock [
75].
4.3. Cell lines and passage of cell lines
L929 fibroblast (ATCC
® CCL-1), A549 lung epithelium (ATCC
® CCL-185) and MCF-7 mammary epithelial (ATCC
® HTB-22) used as cell lines in the current work were commercially available. The cell lines were amplified in DMEM medium supplemented with 1% antibiotic (penicillin-streptomycin) solution and 10% fetal bovine serum (FBS) at 37 °C in a 5% CO
2 incubator [
76].
Each cell line was grown as a monolayer culture in T 25 flasks at 37°C in an oven with 5% CO
2 under 98% humidity, and the flask contained medium was poured when the cells reached approximately 80% density. Cellular debris and serum were removed by washing the surface with sterile D-PBS. In order to separate the monolayer cells from the surface, trypsin EDTA was applied in an amount to cover the cell surface and incubated for about five minutes. The detachment of cells from the surface was followed under an inverted microscope. In order to inhibit the efficacy of trypsin on cell lines with impaired adher-ence, DMEM containing at least five times the trypsin volume was added. The cells in the flask were pipetted, the cell suspension was taken into a sterile 15 ml falcon and centrifuged at 1200 rpm for 4 minutes. After centrifugal precipitation, the supernatant was removed and the pellet was dissolved in fresh medium (1 ml), inoculated into T 75 flasks and left for incubation [
75,
76].
4.4. Cytotoxicity Analysis by MTT Method
In vitro cytotoxic effects of
S. orientale cv. “Gökova” plant extract were determined by 3-4,5-dimethyl-thiazolyl-2,5-diphenyltetrazolium bromide (MTT) test [
77]. The L929 fibroblast cells was used for MTT. The cell lines were grown as monolayer culture on a T 75 flask at 37 °C in an oven with 5% CO
2 under 98% humidity and the medium was removed when the cells reached approximately 80% density. The flask surface was treated with Dulbecco's phosphate-buffered saline (D-PBS) and trypsin was added and incubated for 5 minutes. The cells separated from the surface by the effect of trypsin were suspended with serum-containing DMEM, placed in a sterile 15 ml falcon and centrifuged at 1200 rpm for 4 minutes. After centrifugal precipitation, the supernatant was transferred and the pellet was dissolved in fresh medium (1 ml), and the obtained cell suspension was counted on a thoma slide with Trypan Blue dye. The 3T3 cell suspension was inoculated into 96-well microplates with 200 µl of 1 × 104 cells in each well and incubated for 24 hours at 37 °C, 5% CO
2, and 98% humidity.
After incubation, the culture medium in the plates was emptied and changed with 200 µl of different concentrations of the plant extract in DMEM, and only 200 µl of medium was added to the cell control well. After 24 hours of incubation of the active substance applied plates, the wells were emptied completely and 100 µl of D-PBS was added to each well twice and washed. After washing, 100 µL of fresh medium and 10 µL of MTT were transferred to 1/10 wells and incubated for 3 hours at 37 °C in an oven with 5% CO2 under 98% humidity.
After this treatment, the wells were emptied and 100 µl of DMSO was added, incubated in a shaker for 25 minutes at room temperature and absorbance was taken at 540 nm. The cell viability in the control well was accepted as 100% and the viability percentage was calculated in the other wells. The inhibitory concentration 50 (IC
50, the concentration in which 50% of the cells die) values were calculated statistically in the exel program [
77].
The percentage of viable cells was determined by the following formula:
Sampleabs: Absorbance of S. orientale cv. “Gökova” applied wells
Control𝑎𝑏𝑠: Absorbance of the control well
4.5. In vitro Scratch Test
Wound healing activity of
S. orientale cv. “Gökova” plant extract was determined by
in vitro scratch test. L929 cells taken from the flask and counted were inoculated as 75 × 104 cells in a 60 mm cell culture dish. A wound was created with a 10 µl micropipette tip on the surface of the cells, which filled nearly 100% of the surface of the cell culture dish in which they were attached for approximately 24-48 hours. While creating the wound, the pipette tip was drawn on the cell culture dish from one end to the other at a constant speed in one go, and the cell lines were cleaned once with D-PBS and the ruptured cells were removed. The cell lines were treated with 2.5 ml of convenient medium [1% antibiotic solution with high glucose ratio and DMEM containing 10% Fetal Bovine Serum (FBS)] containing sub-cytotoxic doses of extract at different concentrations. As the control group, complete medium and solvent with extract solution were prepared at the same concentrations as the sample and transferred to petri dishes. Then, wound line was visualized with phase contrast microscope at 0, 4, 24, and 48 hours, compared with the control group, and wound healing activity was determined [
78].
4.6. Determination of the Antiproliferative Effect of Plant Extract in A549 Lung and MCF-7 Mammary Cell Lines by MTT Method
The cells were grown in 1% antibiotic (penicillin-streptomycin) solution with a high glucose ratio and DMEM containing 10% FBS at 37 °C in 5% CO
2. Then, the cell lines were transferred to 96 plates with 1.104 cells in each well and incubated for 24 hours at 37 °C in 5% CO
2. After this step, the mixtures containing fresh medium containing different doses of the active substance were poured into the wells and incubated again for 24 hours under the same conditions. A row for each concentration was determined on the plates, and the wells in the plates, which were worked with 8 replicates of the same concentration of active substance and 4 repetitions of solvent, were completely emptied after 24 hours of incubation, and 100 µl of D-PBS was added to each well and washed. After washing, 100 µl of fresh medium and 10 µl of MTT were added and incubated for 3 hours at 37 °C in an oven with 5% CO
2 under 98% humidity. After the incubation, the wells were emptied and 100 µl of DMSO was added, incubated in a shaker for 25 minutes at room temperature and absorbance was taken at 540 nm. The cell viability (the formula has been identified in section 4.4.) in the cell control well was accepted as 100%, and % inhibition was calculated according to the viability values in other wells. The inhibitory concentration 50 (IC
50, the concentration in which 50% of the cells die) values were calculated statistically in the EXCEL program [
79].
4.7. Molecular Analyzes - Determination of Gene Expressions at Transcriptomic Levels
The RNA was extracted from A549 and MCF-7 cell lines using Thermo Scientific™ GeneJET RNA Purification Kit (Cat. No. K0732). The quality and the quantity of the isolated RNAs were determined by spectrophotometer. DNA contamination was eliminated using the Thermo Scientific™ DNase I Solution (1 unit/µl), RNase-free (Cat. No. 89836) Kit. For cDNA synthesis, the total RNAs were reverse transcribed using the OneScript
® Plus cDNA Synthesis Kit (Cat. No. G236) and the oligo-dT primers contained in this kit. Amplification of reverse transcribed RNAs was determined by Real-Time PCR using the Ampliqon RealQ Plus 2 × Master Mix Green Kit (Cat. No. A323402) and in the presence of primers for the three genes of interest. The related genes and the sequences of the forward and reverse primers of these genes are given in
Table 1. The thermal cycle used in the reaction is 30 seconds of denaturation at 95 °C, followed by 30 seconds of annealing at 55-58 °C and 30 seconds of extension at 72 °C. Real-Time PCR was analyzed by repeating six times. All groups were interpreted by normalizing the expression of beta-actin gene, a housekeeping gene, and using the 2-∆∆Ct (2^(-delta delta Ct)) method. A graph was created by calculating the mean of the obtained values (with standard deviation and standard errors) [
80].
The signification of expression levels of the five target genes obtained after qRT-PCR compared to the control group was statistically analyzed by T-Test method. Among the data obtained as a result of the T-Test, those with p ≤ 0.05 were considered statistically significant.
5. Conclusions
The current work aimed to investigate the wound healing and anticancer properties of the in vitro grown S. orientale L. cv. “Gökova” plant extract using L929 fibroblast, A549 lung epithelial, and MCF-7 breast epithelial cell lines. Wounds, especially chronic ones, affect many patients and reduce their quality of life. In this context, it can be said that the plant extract can be effective on wound healing at doses treated with L292 fibroblast cells in our study. On the other hand, in A549 lung epithelial and MCF-7 cell lines treated with the plant extract was to investigate for its anticancer properties and it was also determined that the extract inhibited cell viability at increasing doses. After animal experiments and clinical applications to be carried out in the future, this plant extract may have the potential to be used in wound healing and/or anticancer drugs. In our study, the expressions of CRISPR-related system 3 (CAS3) endonuclease, CRISPR-related system 9 (CAS9) endonuclease and B-cell lymphoma XL (anti-apoptotic, BCL-XL) genes in A549 and MCF-7 cell lines treated with plant extract and cisplatin were also evaluated. The significant increase in these gene expressions indicated that the applied extracts were effective in metabolic processes such as cell growth and division, control of apoptotic pathways and cellular cycle and it can be also said that the applied plant extract has much less toxic compared to cisplatin and it has good anticancer properties. In conclusion, in the light of the data obtained from this study, the in vitro grown S. orientale L. cv. “Gökova” plant extract can be tested in other cancer cell lines such as colon, liver, cervical, prostate, blood and skin cancer cell lines, in the future. This plant extract also has the potential to make useful contributions to the development of anticancer drugs with animal experiments and clinical applications to be made in the future.
Author Contributions
Conceptualization, E.K., N.S., and A.U.; methodology, E.K., N.S., and A.U.; validation, S.Y., S.G. and I.D.; investigation, S.Y., S.G., and I.D.; data curation, S.Y. and S.G.; writing—original draft preparation, E.K., S.G., and N.S.; writing—review and editing, E.K., N.S., S.Y., S.G., A.U., and I.D.; visualization, S.Y. and S.G; supervision, E.K., N.S., and A.U; project administration, E.K., N.S, and A.U.
Funding
This research received no external funding.
Data Availability Statement
The research data supporting this publication are provided within this paper.
Acknowledgments
We would like to thank the employees of Muğla Metropolitan Municipality, Department of Agricultural Services, Muğla Local Seed Center for plant material support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anilakumar, K.R.; Pal, A.; Khanum, F.; Bawa, A.S. Nutritional, medicinal and industrial uses of sesame (Sesamum indicum L.) seeds - An overview. Agric. Conspec. Sci. 2010, 75, 159–168, https://hrcak.srce.hr/66001. [Google Scholar]
- Zhang, H.; Miao, H.; Wang, L.; Qu, L.; Liu, H.; Wang, Q.; Yue, M. Genome sequencing of the important oilseed crop Sesamum indicum L. Genome Biol. 2013, 14, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lavaee, F.; Moshaverinia, M.; Malek Hosseini, S.A.; Jamshidzade, A.; Zarei, M.; Jafarian, H.; Haddadi, P.; Badiee, P. Antifungal effect of sesame medicinal herb on Candida Species. Braz. J. Pharm. Sci. 2019, 55, e17479. [Google Scholar] [CrossRef]
- Sirato-Yasumoto, S.; Katsuta, M.; Okuyama, Y.; Takahashi, Y.; Ide, T. Effect of sesame seeds rich in sesamin and sesamolin on fatty acid oxidation in rat liver. J. Agri. Food Chem. 2001, 49, 2647–2651. [Google Scholar] [CrossRef]
- Rokonuzzaman, N.L. Investigation of the analgesic and antioxidant activity from an ethanolic extract of seeds of Sesamum indicum. Pak. J. Biol. Sci. 2009, 12, 595–598. [Google Scholar] [CrossRef]
- Smith, D.E.; Salerno, J.W. Selective growth inhibition of a human malignant melanoma cell line by sesame oil in vitro. Prostaglandins Leukot. Essent. Fatty Acids 1992, 46, 145–150. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Pettersson, D.; Appelqvist, L.A. Sesamin (a compound from sesame oil) increases tocopherol levels in rats fed ad libitum. Lipids 1995, 30, 499–505. [Google Scholar] [CrossRef]
- Ogawa, H.; Sasagawa, S.; Murakami, T.; Yoshizumi, H. Sesame lignans modulate cholesterol metabolism in the stroke-prone spontaneously hypertensive rat. Clin. Exp. Pharmacol. Physiol. Suppl. 1995, 1, 10–12. [Google Scholar] [CrossRef]
- Hirata, F.; Fujita, K.; Ishikura, Y.; Hosoda, K.; Ishikawa, T.; Nakamura, H. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis, 1996, 122, 135–136. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Andersson, R.E.; Kamal-Eldin, A. HPLC analysis of sesaminol glucosides in sesame seeds. J. Agric. Food Chem. 2006, 54, 633–638. [Google Scholar] [CrossRef]
- Uzun, B.; Arslan, C.; Furat, S. Variation in fatty acid compositions, oil content and oil yield in germplasm collection of sesame (Sesamum indicum L.). J. Am. Oil. Chem. Soc. 2008, 85, 1135–1142. [Google Scholar] [CrossRef]
- Williamson, K.S.; Morris, J.B.; Pye, Q.N.; Kamat, C.D.; Hensley, K. A survey of sesamin and composition of tocopherol variability from seeds of eleven diverse sesame (Sesamum indicum L.) genotypes using HPLC-PAD-ECD. Phytochem. Anal. 2008, 19, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Rai, A.K.; Kumari, R.; Bhat, K.V. Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacogn. Rev. 2014, 8, 147–155. [Google Scholar] [CrossRef]
- Jeng, K.C.G.; Hou, R.C.W. Sesamin and Sesamolin: Nature's Therapeutic Lignans. Curr. Enzyme Inhib. 2005, 1, 11–20. [Google Scholar] [CrossRef]
- Shimizu, S.; Akimoto, K.; Shinmen, Y.; Kawashima, H.; Sugano, M.; Yamada, H. Sesamin is a potent and specific inhibitor of delta 5 desaturase in polyunsaturated fatty acid biosynthesis. Lipids 1991, 26, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Marchand, P.A.; Zajicek, J.; Lewis, N.G. Oxygen insertion in Sesamum indicum furanofuran lignans. Diastereoselective syntheses of enzyme substrate analogues. Can. J. Chem. 1997, 75, 840–849. [Google Scholar] [CrossRef]
- Hughes, J. Xenografting: ethical issues. J. Med. Ethics 1998, 24, 18–24. [Google Scholar] [CrossRef]
- Lowe, K.C.; Davey, M.R.; Power, J.B. Perfluorochemicals: their applications and benefits to cell culture. Trends Biotechnol. 1998, 16, 272–277. [Google Scholar] [CrossRef]
- Čuperlović-Culf, M.; Barnett, D.A.; Culf, A.S.; Chute, I. Cell culture metabolomics: applications and future directions. Drug Discov. 2010, 15, 610–621. [Google Scholar] [CrossRef]
- Friedmann, T.; Roblin, R. Gene Therapy for Human Genetic Disease?: Proposals for genetic manipulation in humans raise difficult scientific and ethical problems. Sci. 1972, 175, 949–955. [Google Scholar] [CrossRef]
- Sun, J.; Dai, Z.; Zhao, Y.; Chen, G.Q. In vitro effect of oligohydroxyalkanoates on the growth of mouse fibroblast cell line L929. Biomaterials 2007, 28, 3896–903. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Moldovan, L.; Moisei, M.; Trif, M. Liposomal formulation of chondroitin sulfate enhances its antioxidant and anti-inflammatory potential in L929 fibroblast cell line. J. Liposome Res. 2013, 23, 145–53. [Google Scholar] [CrossRef] [PubMed]
- Jeney, F.; Bazsó-Dombi, E.; Oravecz, K.; Szabó, J.; Nagy, I.Z. Cytochemical studies on the fibroblast-preadipocyte relationships in cultured fibroblast cell lines. Acta Histochem. 2000, 102, 381–389. [Google Scholar] [CrossRef]
- Roelofs, B.; Tidball, A.; Lindborg, A.E.; TenHarmsel, A.; Vander Kooy, T.O.; Louters, L.L. Acute activation of glucose uptake by glucose deprivation in L929 fibroblast cells. Biochimie. 2006, 88, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Juszczak, M.; Karpińska, M.M.; Langner, E.; Walczak, K.; Lemieszek, M.K.; Skrzypek, A.; Niewiadomy, A.; Rzeski, W. Synthesis of 2-(2,4-dihydroxyphenyl)thieno-1,3-thiazin-4-ones, their lipophilicity and anticancer activity in vitro. Mol Divers. 2015, 19, 725–736. [Google Scholar] [CrossRef]
- Kodama, Y.; Baxter, R.C.; Martin, J.L. Insulin-like growth factor-I inhibits cell growth in the a549 non-small lung cancer cell line. Am. J. Respir. Cell Mol. Biol. 2002, 27, 336–344. [Google Scholar] [CrossRef]
- Comsa, S.; Cimpean, A.M.; Raica, M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Wang, T.T.; Phang, J.M. Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res. 1995, 55, 2487–2489. [Google Scholar] [PubMed]
- Ramachandran, C.; Fonseca, H.B.; Jhabvala, P.; Escalon, E.A.; Melnick, S.J. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002, 184, 1–6. [Google Scholar] [CrossRef]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef]
- Kondagunta, G.V.; Bacik, J.; Donadio, A.; Bajorin, D.; Marion, S.; Sheinfeld, J.; Bosl, G.J.; Motzer, R.J. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005, 23, 6549–6555. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Miyagi, Y.; Kawanishi, K.; Yamada, S.; Miyagi, Y.; Kodama, J.; Yoshinouchi, M.; Kudo, T. Effect of cisplatin on cell death and DNA crosslinking in rat mammary. Adenocarcinoma in vitro. Acta Med Okayama 1999, 53, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.; McLaughlin, K.; Kelland, L.R.; Brown, R. Cisplatin-DNA damage recognition proteins in human tumour extracts. Br J Cancer. 1993, 67, 742–748. [Google Scholar] [CrossRef]
- Crist, A.M.; Hinkle, K.M.; Wang, X.; Moloney, C.M.; Matchett, B.J.; Labuzan, S.A.; Frankenhauser, I.; Azu, N.O.; Liesinger, A.M.; Lesser, E.R.; Serie, D.J.; Quicksall, Z.S.; Patel, T.A.; Carnwath, T.P.; DeTure, M.; Tang, X.; Petersen, R.C.; Duara, R.; Graff-Radford, N.R.; Allen, M.; Carrasquillo, M.M.; Li, H.; Ross, O.A.; Ertekin-Taner, N.; Dickson, D.W.; Asmann, Y.W.; Carter, R.E.; Murray, M.E. Transcriptomic analysis to identify genes associated with selective hippocampal vulnerability in Alzheimer’s disease. Nat. Commun. 2021, 12, 2311. [Google Scholar] [CrossRef] [PubMed]
- Manechini, J.R.V.; Santos, P.H.D.S.; Romanel, E.; Brito, M.D.S.; Scarpari, M.S.; Jackson, S.; Pinto, L.R.; Vicentini, R. Transcriptomic Analysis of Changes in Gene Expression During Flowering Induction in Sugarcane Under Controlled Photoperiodic Conditions. Front. Plant Sci. 2021, 12, 635784. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; Mardinoglu, A.; Zhang, C.; von Feilitzen, K.; Mulder, J.; Sjöstedt, E.; Hober, A.; Oksvold, P.; Zwahlen, M.; Ponten, F.; Lindskog, C.; Sivertsson, Å.; Fagerberg, L.; Brodin, P. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Sci. 2019, 366, eaax9198. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; Asplund, A.; Sjöstedt, E.; Lundberg, E.; Szigyarto, C.A.; Skogs, M.; Takanen, J.O.; Berling, H.; Tegel, H.; Mulder, J.; Nilsson, P.; Schwenk, J.M.; Lindskog, C.; Danielsson, F.; Mardinoglu, A.; Sivertsson, A.; von Feilitzen, K.; Forsberg, M.; Zwahlen, M.; Olsson, I.; Navani, S.; Huss, M.; Nielsen, J.; Ponten, F.; Uhlén, M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Wolf, J.B. Principles of transcriptome analysis and gene expression quantification: an RNA-seq tutorial. Mol. Ecol. Resour. 2013, 13, 559–72. [Google Scholar] [CrossRef]
- Morozova, O.; Hirst, M.; Marra, M.A. Applications of new sequencing technologies for transcriptome analysis. Annu. Rev. Genomics Hum. Genet. 2009, 10, 135–51. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H.; Horvath, P.; Moineau, S.; Mojica, F.J.; Terns, R.M.; Terns, M.P.; White, M.F.; Yakunin, A.F.; Garrett, R.A.; van der Oost, J.; Backofen, R.; Koonin, E.V. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Jinek, M.; Jiang, F.; Taylor, D.W.; Sternberg, S.H.; Kaya, E.; Ma, E.; Anders, C.; Hauer, M.; Zhou, K.; Lin, S.; Kaplan, M.; Iavarone, A.T.; Charpentier, E.; Nogales, E.; Doudna, J.A. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Sci. 2014, 343, 1247997. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.S.W.; Soetedjo, A.A.P.; Lau, H.H.; Hui Jin Ng, N.; Ghosh, S.; Nguyen, L.; Krishnan, V.G.; Choi, H.; Roca, X.; Hoon, S.; Kee Keong Teo, A. BCL-xL/BCL2L1 is a critical anti-apoptotic protein that promotes the survival of differentiating pancreatic cells from human pluripotent stem cells. Cell Death Dis. 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, N.R.; Soejarto, D.D. Global importance of medicinal plants. The conservation of medicinal plants 1991, 26, 25–51. [Google Scholar]
- Aslam, M.S.; Ahmad, M.S. Worldwide importance of medicinal plants: Current and historical perspectives. Recent Adv Biol Med. 2016, 2, 909. [Google Scholar] [CrossRef]
- Kıvrak, Ş.; Göktürk, T.; Kıvrak, İ.; Kaya, E.; Karababa, E. Investigation of Phenolic Profiles and Antioxidant Activities of Some Salvia Species Commonly Grown In Southwest Anatolia Using UPLC-ESI-MS/MS. Food Sci Technol. 2019, 2, 423–431. [Google Scholar] [CrossRef]
- Mohammed, A.H. Importance of medicinal plants. Research in Pharmacy and Health Sciences 2019, 5, 124–125. [Google Scholar] [CrossRef]
- Kujath, P.; Michelsen, A. Wounds - from physiology to wound dressing. Dtsch Arztebl Int. 2008, 105, 239–248. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J Dent Res. 2010, 89, 219–29. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open biology 2020, 10, 200223. [Google Scholar] [CrossRef]
- Bolla, S.R.; Mohammed Al-Subaie, A.; Yousuf Al-Jindan, R.; Papayya Balakrishna, J.; Kanchi Ravi, P.; Veeraraghavan, V.P.; Arumugam Pillai, A.; Gollapalli, S.S.R.; Palpath Joseph, J.; Surapaneni, K.M. In vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression. Heliyon 2019, 5, e01648. [Google Scholar] [CrossRef]
- Alsawalha, M.; Al-Subaie, A.M.; Al-Jindan, R.Y.; Bolla, S.R.; Salahuddin, M.; Veeraraghavan, V.P. Dioscorea villosa Leaf Extract Enhances in vitro Wound Healing and Expression of Extra Cellular Matrix Factors Transforming Growth Factor-Beta 1 and Collagen-1 in L929 Cell Lines. Pharmacogn Mag. 2019, 15, 483–494. [Google Scholar] [CrossRef]
- Danna, C.; Bazzicalupo, M.; Ingegneri, M.; Smeriglio, A.; Trombetta, D.; Burlando, B.; Cornara, L. Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum ostruthium (L. ) W. D. J. Koch. Molecules 2022, 27, 4271. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Mahasneh, A.M. Antiproliferative Activity of Plant Extracts Used Against Cancer in Traditional Medicine. Sci Pharm. 2010, 78, 33–46. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Gutheil, W.G.; Reed, G.; Ray, A.; Anant, S.; Dhar, A. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol. 2012, 13, 173–179. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006, 71, 1397–421. [Google Scholar] [CrossRef]
- Lee, B.M.; Park, K.K. Beneficial and adverse effects of chemopreventive agents. Mutat Res. 2003, 523, 265–278. [Google Scholar] [CrossRef]
- Nivethitha, R.; Thangavelu, L.; Geetha, R.V.; Anitha, R.; Kumar, R.S.; Raghunandhakumar, S. In Vitro Anticancer Effect of Sesamum Indicum Extract. J Complement Med Res. 2020, 11, 99–105. [Google Scholar]
- Siao, A.C.; Hou, C.W.; Kao, Y.H.; Jeng, K.C. Effect of sesamin on apoptosis and cell cycle arrest in human breast cancer mcf-7 cells. Asian Pac J Cancer Prev. 2015, 16, 3779–3783. [Google Scholar] [CrossRef]
- Akl, M.R.; Ayoub, N.M.; Abuasal, B.S.; Kaddoumi, A.; Sylvester, P.W. Sesamin synergistically potentiates the anticancer effects of γ-tocotrienol in mammary cancer cell lines. Fitoterapia. 2013, 84, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Matsuzaki, Y.; Koyama, M.; Hitomi, T.; Kawanaka, M.; Enoki-Konishi, M.; Okuyama, Y.; Takayasu, J.; Nishino, H.; Nishikawa, A.; Osawa, T.; Sakai, T. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007, 98, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Iizumi, Y.; Iizuka-Ohashi, M.; Sowa, Y.; Sakai, T. The Pleiotropic Regulation of Cyclin D1 by Newly Identified Sesaminol-Binding Protein ANT2. Oncogenesis 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Fang, Q.; Zhu, Y.; Wang, Q.; Song, M.; Gao, G.; Zhou, Z. Suppression of Cyclooxygenase-2 Increases Chemosensitivity to Sesamin through the Akt-PI3K Signalling Pathway in Lung Cancer Cells. Int J Mol Med. 2019, 43, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, G.; El-Kadi, A.O. Buthionine Sulfoximine, an inhibitor of glutathione biosynthesis, induces expression of soluble epoxide hydrolase and markers of cellular hypertrophy in a rat cardiomyoblast cell line: roles of the NF-κB and MAPK signaling pathways. Free Radic Bio Med. 2015, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Chung, S.T.; Robertson, D.A.; Ranjan, D.; Bondada, S. Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin Immunol. 1999, 93, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Gabellini, C.; Trisciuoglio, D.; Del Bufalo, D. Non-canonical roles of Bcl-2 and Bcl-xL proteins: relevance of BH4 domain. Carcinog. 2017, 38, 579–587. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, J.; Hessler, P.; Tahir, S.K.; Chen, J.; Jin, S.; Souers, A.J.; Leverson, J.D.; Lam, L.T. Genomic analysis and selective small molecule inhibition identifies BCL-XL as a critical survival factor in a subset of colorectal cancer. Mol Cancer 2015, 14, 126–135. [Google Scholar] [CrossRef]
- Mohamadi, N.; Kazemi, S.M.; Mohammadian, M.; Toofani Milani, A.; Moradi, Y.; Yasemi, M.; Ebrahimi far, M.; Mazloumi Tabrizi, M.; Ebrahimi Shahmabadi, H.; Akbarzadeh Khiyavi, A. Toxicity of Cisplatin-Loaded Poly Butyl Cyanoacrylate Nanoparticles in a Brain Cancer Cell Line: Anionic Polymerization Results. Asian Pac J Cancer Prev. 2017, 18, 629–632. [Google Scholar] [CrossRef]
- Özkan, İ.; Koçak, P.; Yıldırım, M.; Ünsal, N.; Yılmaz, H.; Telci, D.; Şahin, F. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Ozudogru, E.A.; Kaya, E.; Kirdok, E.; Issever Ozturk, S. In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cell. Dev. Biol. - Plant 2011, 47, 309–320. [Google Scholar] [CrossRef]
- Kaya, E.; Souza, F.; Yilmaz-Gokdogan, E.; Ceylan, M.; Jenderek, M. Cryopreservation of Citrus Seed via Dehydration Followedby Immersion in Liquid Nitrogen. Turk. J. Biol. 2017, 41, 242–248. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Yeniocak, S.; Kaya, U.; Dal, B.; Şaman, Z.; Galatalı, S.; Kaya, E. Cryopreservation of Sesamum orientale L. local cultivar ‘Gökova Sesame’ germplasm. Trends Cell Biol. 2022, 15, 63–68. [Google Scholar]
- Abdul Ghafoor, N.; Galatali, S.; Yeniocak, S.; Kaya, E.; Saraç, N.; Uğur, A. Investigating anticancer potency of in vitro propagated endemic Thymus cilicicus Boiss. & Bal. extract on human lung, breast, and prostate cancer cell lines. Biol. 2022, 77, 3229–3239. [Google Scholar] [CrossRef]
- Baygar, T. , Saraç N. Antimicrobial activity of clementine peel essential oil with its cytotoxic and in vitro wound healing potential on NIH-3T3 fibroblast cells. Mugla J. Sci. Technol. 2018, 4, 143–147. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gebäck, T.; Schulz, M.M.; Koumoutsakos, P.; Detmar, M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques 2009, 46, 265–274. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: the MTT assay. In Cancer Cell Culture (Methods and Protocols), Cree, I., Eds.; Humana Press: New York, 2011; Volume 731, pp. 237–245. [Google Scholar] [CrossRef]
- Çiçek, S.; Ağar, H.; Galatalı, S.; Kaya, E. Transcriptomic Analysis of AREB1 and AREB2 Genes Playing Important Roles in Drought Stress Tolerance in Tomato under in vitro Drought Stress. Environ Anal Ecol Stud. 2023, 10, 1203–1209. [Google Scholar]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013, 3, 71–85. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).