Introduction
The Global Polio Eradication Initiative (GPEI) was adopted in 1988, and since then, the number of cases due to polioviruses has decreased by over 99.9%. In 2019, the World Health Organization (WHO) reported 143 cases of paralytic poliomyelitis due to wild polioviruses worldwide [
1]. In 2020, the remaining endemic areas with wild poliovirus circulation were limited to Pakistan, Nigeria, and Afghanistan [
2]. Although GPEI has substantially reduced the polio case count, however is facing difficulties in eradicating poliovirus via interrupting the last reservoirs of transmission in Pakistan and Afghanistan and the risk of transportation of wild polioviruses from the endemic areas into polio-free countries, causing outbreaks of poliomyelitis [
3].
The Oral Polio Vaccine (OPV) has successfully produced substantial results in eliminating the wild poliovirus type 2 in 1999, possibly type 3 in 2013, and significantly reducing cases caused by wild poliovirus type 1 [
4,
5]. The OPV has been the vaccine of choice in polio eradication, especially in developing countries, due to its ease of administration, its low price, and its ability to produce mucosal immunity; however, the vaccine has limitations such as low immunogenicity in some tropical countries [
6,
7]. Moreover, the live virus in OPV may cause paralysis in vaccine recipients (Vaccine Associated Paralytic Poliomyelitis or VAPP), and it can genetically recover neurovirulence. It can be converted into Circulating Vaccine Derived Poliovirus cVDPV, thus causing paralysis [
8,
9].
In contrast, studies have demonstrated that the immunity induced by inactivated poliovirus vaccine (IPV) is better than that induced by the OPV.IPV protects against paralytic poliomyelitis, especially in developing countries [
4,
10,
11]. While it is known that immunization with IPV alone provides minimal mucosal immunity against viral shedding compared with that induced by OPV, the combined use of IPV and OPV or the use of IPV alone in settings when the coverage with the OPV is high has been recommended [
12,
13]. Studies in India showed that administering a single dose of IPV substantially boosted humoral and mucosal immunity among children already primed by the OPV [
14,
15]. However, this phenomenon has not been prospectively studied in Pakistan.
We compared the mucosal and humoral response to poliovirus vaccines administered to previously OPV-immunized children to assess the immunity gap among children at risk of high poliovirus transmission.
Methodology
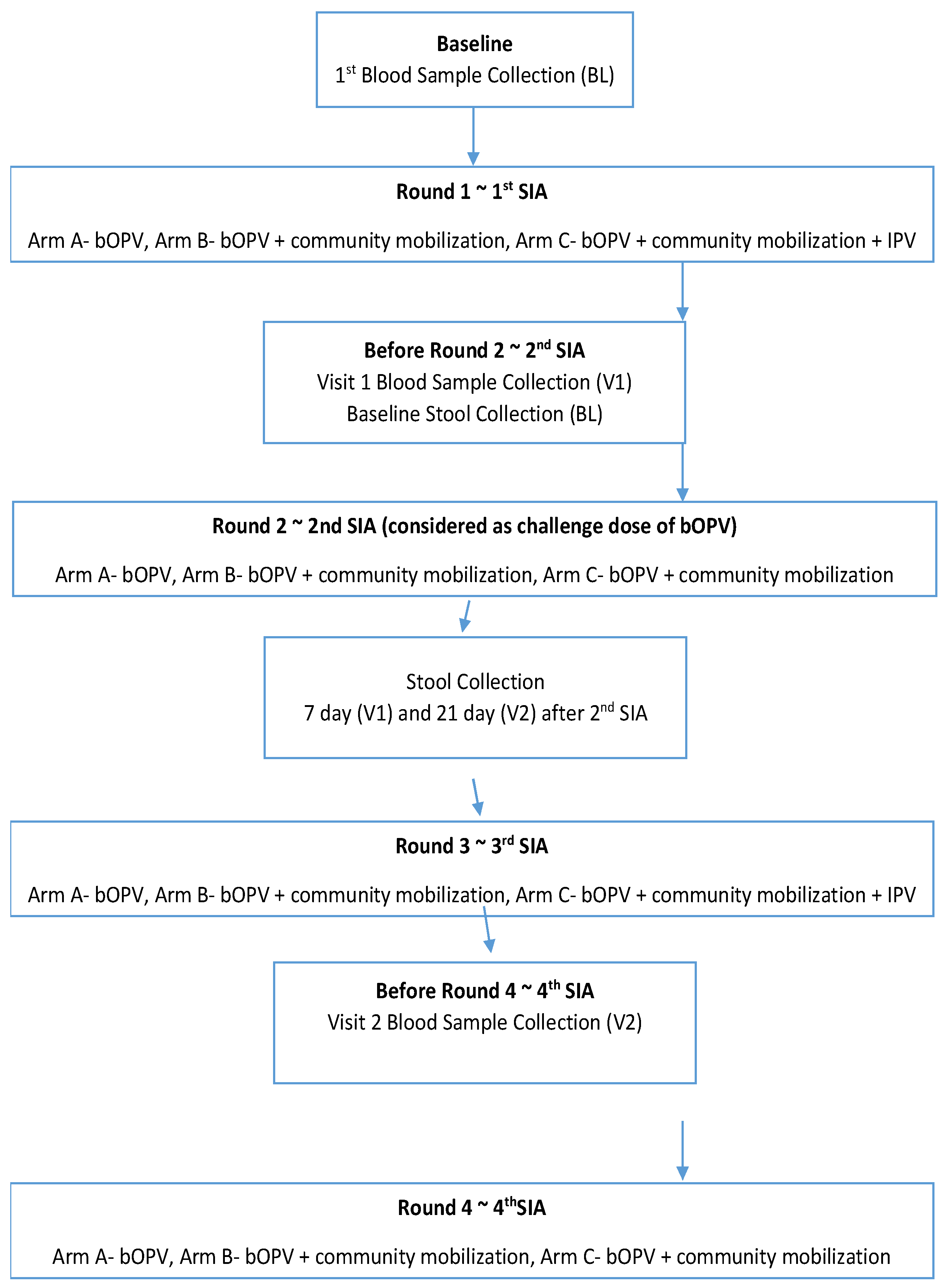
The paper presents the results of the humoral and intestinal immunity findings from a community-based three-arm cluster randomized controlled trial among healthy children under five years of age living in Pakistan's three districts (Karachi, Kashmore and Bajaur) with a high risk of polio [
16]. We randomly allocated clusters using a computer algorithm restricted randomization in blocks of 20 (1:1:1) to receive routine polio program (bOPV) activities (control, arm A), additional interventions with community outreach and mobilization using an enhanced communication package and provision of short-term preventive maternal and child health services and routine immunization (health camps), including bOPV (arm B), or all interventions of arm B with an additional provision of IPV delivered at the maternal and child health camps (arm C ~ bOPV and IPV). Apart from the data collection on vaccine coverage through an independent team, we collected blood and stool samples to estimate the humoral and intestinal immunity among children for all three-study groups. The trial was conducted between June 2013 and May 2014. A total of 387 clusters were randomized (131 to arm A, 127 to arm B, and 129 to arm C); however, 360 clusters remained in the trial until the end (116 in arm A, 122 in arm B, and 122 in arm C)
We calculated separate sample sizes for humoral and intestinal immunity. For the sample size of humoral immunity, we assumed Baseline seroprevalence as 90%, the immunogenicity of bOPV as 50%, the immunogenicity of combined bOPV and IPV as 90%, coverage in the bOPV arm as 90%, coverage in combined bOPV and IPV arm as 90%, power of 80%, expected seroprevalence in bOPV arm as 95%, anticipated seroprevalence in combined bOPV and IPV arm as 98%, dropout rate as 20% and implied design effect of 2. Based on these assumptions, the total sample size per study arm was 590. Since we had three arms, the total sample size per site was 1770, and the total sample size for the trial to estimate humoral immunity was 5310 blood samples per visit.
For intestinal immunity, we assumed baseline seroprevalence as 90%, the immunogenicity of bOPV as 80%, the immunogenicity of combined bOPV and IPV as 90%, coverage in the bOPV arm as 90%, coverage in combined bOPV and IPV arm as 90%, power of 80%, expected poliovirus shedding on day 7 in bOPV arm as 15%, expected poliovirus shedding on day 7 in combined bOPV and IPV arm as 15%, dropout rate as 20% and implied design effect of 2. Based on these assumptions, the total sample size per study arm was 570; since we had three arms, the total sample size per site was 1710, and the total sample size for the trial to estimate humoral immunity was 5130 blood samples per visit.
For the immunity assessment, a subset of subjects was selected randomly from the database of the children before the start of the intervention by the study team, and written consent was taken from their parents. Three milliliters (3 ml) of whole blood were collected by a trained phlebotomist from each subject at three-time points, i.e., at baseline before the first, second, and third rounds of immunization day (SIA) (
Figure 1).
Blood samples were centrifuged, and the separated serum was transported to the Nutrition Research Laboratory (NRL) at the Aga Khan University (AKU) in Karachi under cold chain conditions. These were stored at -20 C until shipment to the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA; where neutralizing antibodies were determined by the method recommended by the World Health Organization [
17] at the Enterovirus Laboratory. Serial dilutions of serum (starting at 1:8 and ending at 1:1024) were incubated with 100 TCID50 of poliovirus types 1, 2, and 3 at 36 _C for three h before 1–2 _ 104 HEp-2 (Cincinnati) cells were added to each well. The HEp-2 (Cincinnati) cell line is susceptible to polioviruses. We assigned unobserved titer values of less than eight if they were less than the starting dilution and more than or equal to 1448 if they were more than the final dilution.
The stool was collected from the baseline survey sample through a random selection technique. These samples were collected before the 2
nd SIA, after 7 and 21 days of the 2nd SIA (Figure X). For stool collection, stool containers were provided to the families with ice packs collected by the study team for processing. These samples were transported to the NRL at the AKU under cold chain conditions. At NRL, three aliquots of stool samples were prepared; two were transported to the Polio Reference Laboratory at NIH Pakistan under strict cold chain maintenance, whereas one aliquot was saved at NRL as a backup. At NIH, WHO standard procedures and guidelines were used to detect the presence of poliovirus and shedding in stool specimens. [
18].
The seroprevalence against polio antibodies was assessed at baseline (before the first SIA), six weeks after the baseline (before the second SIA), and 18 weeks after the baseline (before the fourth SIA). Further, seroconversion (boosting) was assessed six weeks after the baseline (before the second SIA) and 18 weeks after the baseline (before the fourth SIA). Seropositivity was defined as reciprocal titers of poliovirus neutralizing antibodies ≥8; seroconversion was defined as the change from seronegative to seropositive (from reciprocal titer of <8 to ≥8); and boosting was defined as ≥4-fold increase in titers. In this study, ‘‘immune response” combines both boosting and seroconversion. The immune response analysis was restricted to infants with a baseline serological titer of ≤362 to ensure that a 4-fold boosting response could be achieved since the highest titer tested was 1:1448 [
16]. The intestinal immunity was expressed as the composite shedding index of stool viral titers at Baseline days 7 and 21 after challenging with bOPV.
Safety assessments were solicited, severe adverse events were recorded by the study team on prescribed forms for seven days after each vaccination visit, and unsolicited severe events, serious adverse events (defined as adverse events that led to death or were life-threatening, those that necessitated hospital admission, or those that caused persistent or substantial disability), or critical medical events (clinical events not qualifying as serious adverse events but requiring medical intervention) for up to 1 after the final vaccination. Any serious adverse event or important medical event was reported immediately to the investigator and the data safety monitoring board.
The ethics review committee of Aga Khan University, Pakistan and the National Bioethics Committee, Pakistan granted approval of the trial. Individual level consent was taken from the parent of the participating child. This trial is registered with ClinicalTrials.gov, number NCT01908114.
Results
Table 1 shows the serum samples collected at each visit during the trial. Overall, the study team collected 5982 serum samples at baseline, 4905 serum samples at visit 1, and 4564 serum samples at visit 3. The regional data showed that the Karachi team collected 1793 serum samples at baseline, 1565 serum samples at visit 1, and 1323 serum samples at visit 2, the Bajaur team collected 2005 serum samples at baseline, 1532 serum samples at visit 1, and 1352 serum samples at visit two and the Kashmore teams collected 2184 serum samples at baseline, 1806 samples at visit 1 and 1889 serum samples at visit 2.
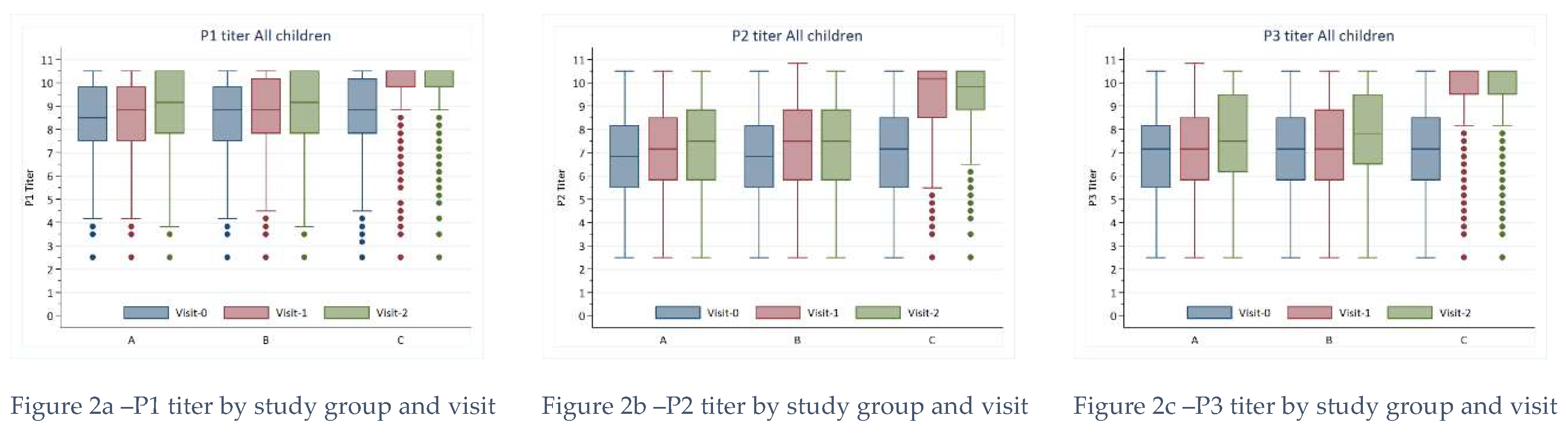
Serum titers were highest for P1 at Baseline compared to titers for P2 and P3. All titers were among those in Group C at Baseline compared to Groups A and B. Further, All titers increased over time for every group. Still, rises were noticeably higher among those in Group C, and the differences compared to Groups A and B were statistically significant at both visit one and visit 2. The most notable change in serum titers for wild-type polioviruses occurred for P1 among children receiving the IPV vaccine (Group C).
The linear predictions from our mixed effects regression model indicate that the mean P1 titer increased by 2.46 (95% CI: 2.35 - 2.56) from baseline to visit 1 for this group (
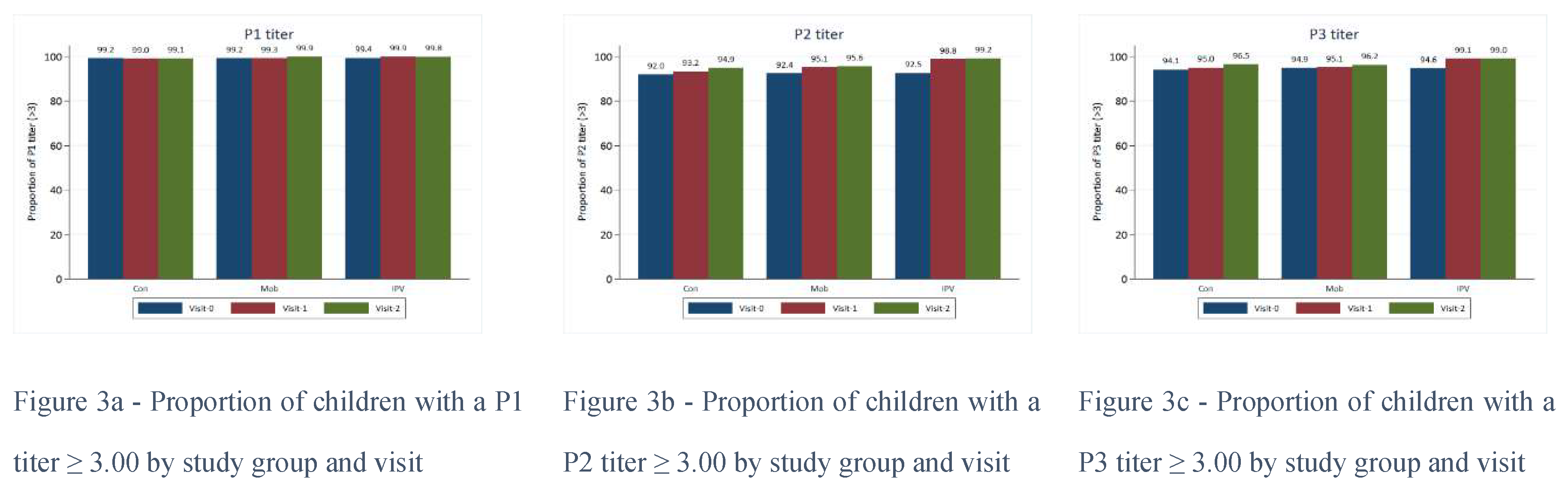
Figure 2). However, almost all (>99%) children tested had a P1 of 3.00 or greater at baseline.
Proportions were similarly high for P2 and P3 titers at baseline (>90%), and these proportions increased over time regardless of the study group. Serum titers for P1, P2, and P3 were higher for those in Group C vs. Groups A and B by visit 1 for all age groups (
Figure 3). P1, P2, and P3 titers were generally higher amongst those who had received a routine OPV dose than those without; this was true for all study groups and visits.
To assess mucosal immunity, the study team collected stool samples for the detection and excretion of the virus at Day 0, Day 7, and Day 21 of the challenge dose (
Figure 1). The study team collected 4210 stool samples on Day 0 (1324 in Karachi, 1371 in Bajaur, and 1515 in Kashmore), 4084 stool samples on Day 7 (1143 in Karachi, 1134 in Bajaur and 1807 in Kashmore), and 4185 stool samples on day 21 (1219 in Karachi, 1191 in Bajaur and 1748 in Kashmore) (
Table 2)
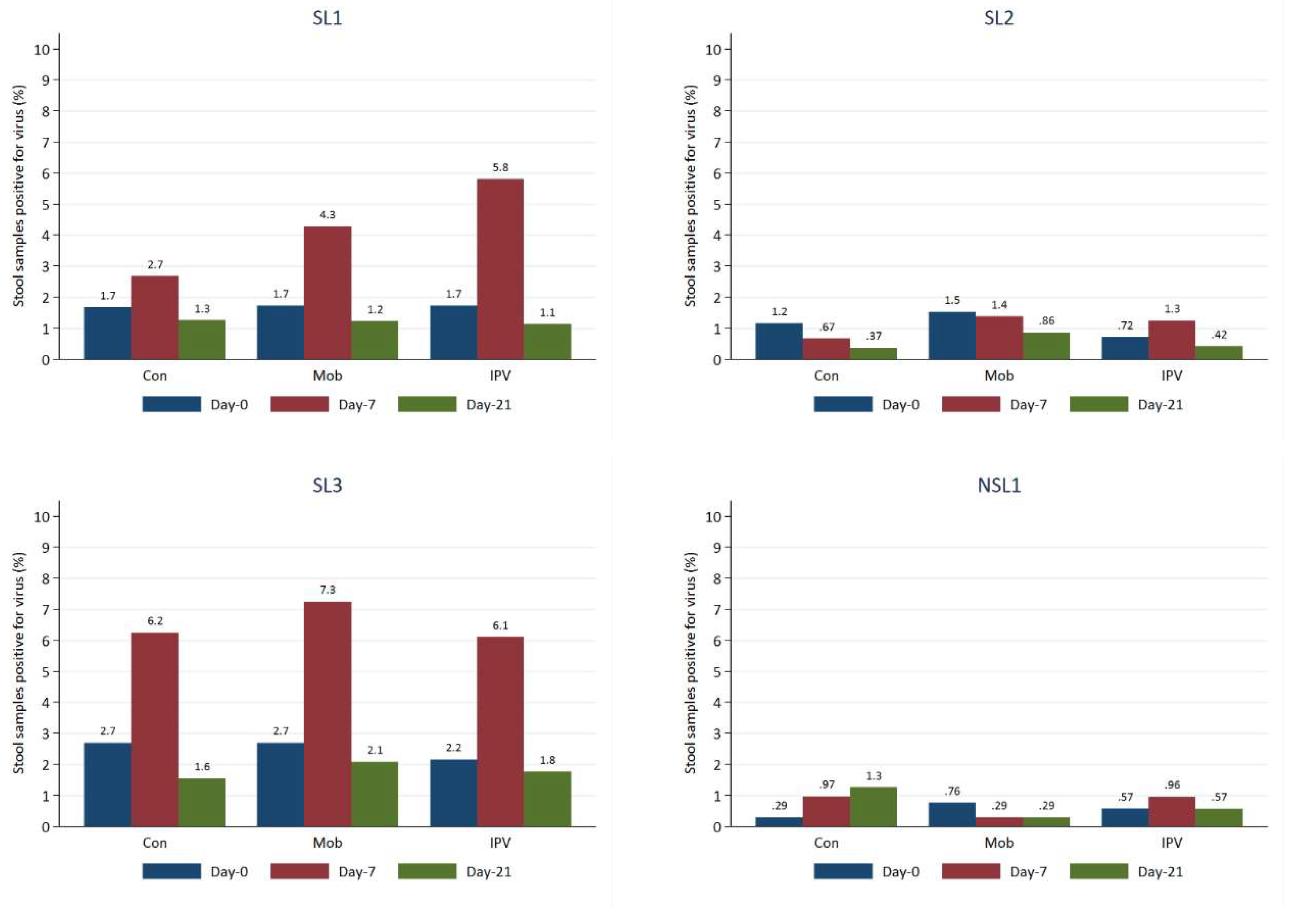
The most frequently detected viruses in the stool samples at baseline were SL3, SL2, and SL1. Our mixed effects model predicted approximately 2-3% of stool samples to be positive for SL3 and SL1 and 1-2% positive for SL2.
Proportions of stool samples positive increased from baseline for a visit 2 for both SL1 and SL3, with the most significant rise occurring for Group C (those receiving IPV), to around 6%, though by visit 2, the proportions positive for SL1 and SL3 dropped to just below those of the samples taken at Baseline (
Figure 4 and 5). NSL1 was detected in stool infrequently in <1% of cases at baseline; proportions remained low over time for all study groups. NPEV and VDPV2 were virtually absent from the stool samples, with only 1-2 cases detected. Viral detections of every strain were typically highest at visit 1 for all age groups, but in most cases, levels fell below those at baseline by the second visit. Viral strains were generally detected more frequently by visiting two among those who had received at least one routine dose of OPV than those who had not.
Discussion
The present study findings show that the serum titers were highest among Group C (IPV+OPV) at the baseline for P1, where its increase over time was also more prominent. Titers for P2 and P3 were statistically significantly higher amongst those who had received a routine OPV dose versus those who had not; this was true for all study groups and visits. Stool samples were positive for SL3 and SL1 (2-3%) and SL2 (1-2%), with the most remarkable rise from baseline for SL1 and SL3 in Group C visit 2.
This trial was designed to answer several important questions to aid in the next phase of polio eradication and help national bodies select the most appropriate vaccine schedule and doses [
19]. Our data also supplement the new and evolving evidence base and is among the first studies in Pakistan to describe the superiority of combined bOPV and IPV on intestinal and humoral immunity against poliovirus. However, the exact OPV vaccination history of the subjects was not known. Furthermore, it was also impossible to attain a reliable estimate of OPV doses children received through SIAs. Hence, we assumed that most children must have received most of the OPV doses offered through SIAs based on the data received from the polio program. Moreover, the baseline titers of poliovirus-neutralizing antibodies were high, which may have affected immune response to subsequent OPV doses.
In conjunction with our study, literature demonstrated several other studies in different populations assessing the increase in humoral and intestinal immunity in children using the IPV+OPV combination. Asturias and colleagues [
20] recorded 80% and 100% seroconversion in Latin American infants after three doses of bOPV combined with zero, one, or two doses of IPV and the induction of intestinal immunity against type 2 poliovirus. Similarly, a Chile study [
21] reported humoral and intestinal immunogenicity from sequential bOPV–IPV. A multicenter trial in Oman evaluated those supplemental doses of IPV had excellent immunogenicity and increased the titer of antibodies against poliovirus type 3. In contrast, additional doses of oral vaccines do not have these effects [
22].
In line with the well-documented differences in responses to OPV between developed and developing countries [
23], various studies were done in Asian countries in this context. Improved humoral immunogenicity was found in Indian infants after a birth dose of bOPV followed by bOPV and one dose of IPV in the EPI schedule [
24]. A study from Bangladesh also concluded that better immunogenicity was obtained from sequential fractional doses of IPV and bOPV schedules [
25]. In Srilankan children, 10–12 years of age, 16%, 9%, and 76% of subjects were shown to excrete poliovirus after challenge in the IPV, fractional IPV (fIPV) and No IPV study arms, respectively [
26]. Thus, confirming that a single fIPV dose boosted mucosal immunity to a similar degree as a single full dose of IPV. A study of Pakistani 9–12 months old infants [
27] found that bOPV+ IPV helped close the immunity gap better than bOPV alone against polioviruses, specifically in chronically malnourished infants. Another study showed that IPV could close immunity gaps when given OPV in the vulnerable Pakistani population [
28].
The current gold standard proxy to approximate intestinal immunity is resistance from shedding followed by an oral challenge [
29]. A study [
30] established that the humoral response provided by the currently available IPV was more significant than earlier formulations yet did not enhance intestinal immunity, being lesser than that obtained with OPV. The resistance to intestinal excretion depends on the challenging dose of the vaccine and so is not absolute. In another study, 67 children who got tOPV were followed up for ten years, where declining serum antibody titers indicated decreasing resistance to intestinal excretion [
31], recognizing that intestinal immunity is temporary [
32,
33]. The study concluded that a weak association exists between pre-challenge antibody titers following IPV or bOPV/IPV immunization and differences in intestinal immunity, which is inadequate in predicting polio type 2 intestinal immunity. [
34]
Overall, the current findings are consistent with previous studies representing a limited role of IPV in boosting mucosal antibodies and inhibiting poliovirus shedding in individuals with no prior exposure to live viruses [
13,
20,
21,
36,
37,
38,
39]. In Cuba, >90% of infants shed any poliovirus after the tOPV challenge, irrespective of whether they had received zero, 2, or 3 prior IPV doses [
39]. The capacity of IPV to generate a primary mucosal immune response with the ability to inhibit live polio replication and thus control poliovirus transmission remains indeterminate. A Phase 2 clinical trial including Panamanian infants determined that IPV-induced serum neutralization does not essentially improve intestinal mucosal immunity or limit viral shedding with a monovalent type 2 OPV challenge. [
40]; but they may lessen the quantity and duration of shedding. [
41,
42]
Studies focusing on poliovirus-specific immunoglobulin A have demonstrated that exposure to live poliovirus through OPV or the environment is essential in inducing mucosal responses to IPV [
37]. A systematic review has pointed out that when IPV is delivered without OPV, it statistically significantly fails to reduce the odds of fecal shedding following challenge dose with live attenuated polioviruses [
13]. Likewise, in two clinical trials from India, a single dose of IPV given to OPV-immunized children substantially boosted protection against poliovirus shedding after a subsequent OPV challenge [
14,
15]. Macklin and colleagues also observed a boost in mucosal protection induced by IPV in OPV-primed individuals, establishing that IPV works in a serotype-specific model [
43]. Moreover, a meta-analysis showed post-vaccination dependence of shedding on several vaccine doses and pre-challenge titers [
44].
However, literature also revealed that three doses of IPV without bOPV induced more significant quantities of virus shedding than those given fewer IPV doses but with bOPV, highlighting that cross-protection can also affect viral shedding [
45]. Evidence from bOPV/IPV integrated trials confirmed a potentially substantial role of IPV in the stimulation of mucosal immunity. In Latin America, a subset of infants who received bOPV at 6, 10, and 14 weeks and an additional dose of IPV at 14 weeks showed a higher type 2–specific stool neutralization at mOPV2 challenge along with lower viral shedding as compared to their peers who received only bOPV [
20,
36]. It is noteworthy that since IPV offers a limited mucosal immune response than OPV [
12,
21], there remains a possibility for poliovirus circulation in populations immunized solely with IPV without causing poliomyelitis, as was reported from Israel [
46].
The impact of IPV on intestinal mucosal immune responses is poorly understood. Improvement in understanding the relationship between the occurrence of mucosal immunity and serum antibodies is significant in characterizing the risk of virus spread in populations immunized with IPV only to plan suitable response strategies to control poliovirus shedding and curtail outbreaks. Further, an innovation of introducing E. coli labile toxin with double attenuating mutations (dmLT) in boosting immunologic responses to IPV at mucosal sites was evaluated. A 4-fold increase in serotype-specific neutralizing antibody (SNA) titers were seen for all three serotypes in 84% of subjects receiving fractional-dose inactivated polio vaccine (fIPV-dmLT) vs. 50% of participants receiving IPV alone. Hence, demonstrating the benefit of fIPV-dmLT over IPV alone. [
47]
The study revealed that [
33] population surveys of serum-neutralizing antibodies to poliovirus could be valuable in high-risk areas using both OPV and IPV to protect against poliomyelitis and as an indicator of intestinal immunity against infection.
Conclusion
In populations with high Oral Polio Vaccine (OPV) failure rates, administering an Inactivated Polio Vaccine (IPV) booster after a minimum of two OPV doses may effectively bridge immunity gaps. The IPV alone offers limited benefits to humoral immunity and doesn't provide intestinal immunity to prevent the infection and propagation of live poliovirus among unexposed populations. Future research is needed to discern the link between mucosal immunity and serum antibodies in characterizing the risk of virus spread in populations immunized with IPV to plan suitable response strategies to control poliovirus shedding and curtail outbreaks.
Author Contributions
ZAB conceived the study, developed the protocol along with MAH and SBS, and secured funding. MAH, IH, RT, ZH, SA and SBS oversaw field operations. IA managed the study data. SC and IA did the statistical analyses. SC interpreted the data. All authors contributed to revising the paper and approved the final version.
Funding
The study was supported by the Bill & Melinda Gates Foundation [grant number: INV OPP1211074]. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data Sharing
The corresponding author has full access to all study data and can provide access upon email request.
Acknowledgments
We want to thank all participants who consented to participate in the study. We would also like to acknowledge the local authorities for their support during the field operation. We also extend our gratitude to Mr. Muhammad Umer from Aga Khan University, Pakistan for his assistance.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in conflict with the subject matter or materials discussed in the manuscript.
References
- Cases of wild poliovirus by country and year. Available at: http://polioeradication.org/wp-content/uploads/2020/01/Weekly-GPEI-Polio-Analyses-wpv-20191231.pdf. [accessed 30/03/2020].
- Global Polio Eradication Initiative, Endemic Countries Available at: http://polioeradication.org/where-we-work/polio-endemic-countries/ [accessed 30/03/2020].
- Khan F, Datta SD, Quddus A, et al. Progress Toward Polio Eradication — Worldwide, January 2016–March 2018. MMWR Morb Mortal Wkly Rep 2018; 67:524–528. [CrossRef]
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future microbiology. 2015 May;10(5):791-808. [CrossRef]
- World Health Organization, https://www.who.int/biologicals/areas/vaccines/polio/opv/en/ [accessed 30/03/2020].
- Grassly NC, Fraser C, Wenger J, et al. new strategies for the elimination of polio from India. Science 2006; 314:1150–3. [CrossRef]
- John TJ, Jayabal P. Oral polio vaccination of children in the tropics: I. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol 1972; 96:263–9. [CrossRef]
- Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccinederived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol 2005; 59:587–635.
- Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 2014;210(Suppl. 1): S380–9.
- Robertson SE, Traverso HP, Drucker JA, Rovira EZ, Fabre-Teste B, Sow A, et al. Clinical efficacy of a new, enhanced-potency, inactivated poliovirus vaccine. Lancet. 1988;1:897–9. [CrossRef]
- Estívariz CF, Pallansch MA, Anand A, Wassilak SGF, Sutter RW, Wenger JD, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr Opin Virol. 2013; 3:309–15. [CrossRef]
- Shulman LM, Gavrilin E, Jorba J, Martin J, Burns CC, Manor Y, et al.; Genotype - Phenotype Identification (GPI) group. Molecular epidemiology of silent introduction and sustained transmission of wild poliovirus type 1, Israel, 2013. Euro Surveill. 2014; 19:20709. [CrossRef]
- Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8:e1002599. [CrossRef]
- John J, Giri S, Karthikeyan AS, Iturriza-Gomara M, Muliyil J, Abraham A, et al. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open-label, randomised controlled trial. Lancet. 2014; 384:1505 12. [CrossRef]
- Jafari H, Deshpande JM, Sutter RW, Bahl S, Verma H, Ahmad M, et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science. 2014; 345:922–5. [CrossRef]
- Habib MA, Soofi S, Cousens S, Anwar S, ul Haque N, Ahmed I, Ali N, Tahir R, Bhutta ZA. Community engagement and integrated health and polio immunisation campaigns in conflict-affected areas of Pakistan: a cluster randomised controlled trial. The Lancet Global Health. 2017 Jun 1;5(6): e593-603.
- WHO Department of Immunization, Vaccines and Biologicals. Polio laboratory manual. Geneva: World Health Organization; 2004 (WHO/IVB/04.10.).
- World Health Organization, Guidelines for environmental surveillance of poliovirus circulation. http://polioeradication.org/wp-content/uploads/2016/07/WHO_V-B_03.03_eng.pdf.
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, November 2012—conclusions and recommendations. Wkly Epidemiol Rec. 2013; 88:1–16.
- Asturias EJ, Bandyopadhyay AS, Self S, Rivera L, Saez-Llorens X, Lopez E, Melgar M, Gaensbauer JT, Weldon WC, Oberste MS, Borate BR. Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open-label randomised controlled trial. The Lancet. 2016 Jul 9;388(10040):158-69. [CrossRef]
- O’Ryan M, Bandyopadhyay AS, Villena R, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis 2015; 15: 1273–82. [CrossRef]
- Sutter RW, Suleiman AJ, Malankar P, Al-Khusaiby S, Mehta F, Clements GB, Pallansch MA, Robertson SE. Trial of a supplemental dose of four poliovirus vaccines. New England Journal of Medicine. 2000 Sep 14;343(11):767-73. [CrossRef]
- Patriarca PA, Wright PF, John TJ. Factors aff ecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis 1991; 13: 926–39. [CrossRef]
- Sutter RW, Bahl S, Deshpande JM, et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial. Lancet 2015;386: 2413–21. [CrossRef]
- Estivariz CF, Anand A, Gary HE Jr, et al. Immunogenicity of three doses of bivalent, trivalent, or type 1 monovalent oral poliovirus vaccines with a 2 week interval between doses in Bangladesh: an open-label, non-inferiority, randomised, controlled trial.Lancet Infect Dis 2015; 15: 898–904. [CrossRef]
- Gamage D, Mach O, Palihawadana P, Zhang Y, Weldon WC, Oberste MS, Gunasena S, Sutter RW. Boosting of mucosal immunity after fractional-dose inactivated poliovirus vaccine. The Journal of infectious diseases. 2018 Nov 5;218(12):1876-82. [CrossRef]
- Saleem AF, Mach O, Quadri F, Khan A, Bhatti Z, ur Rehman N, Zaidi S, Weldon WC, Oberste SM, Salama M, Sutter RW. Immunogenicity of poliovirus vaccines in chronically malnourished infants: a randomized controlled trial in Pakistan. Vaccine. 2015 Jun 4;33(24):2757-63. [CrossRef]
- Habib MA, Soofi S, Mach O, Samejo T, Alam D, Bhatti Z, Weldon WC, Oberste SM, Sutter R, Bhutta ZA. Effect of booster doses of poliovirus vaccine in previously vaccinated children, clinical trial results 2013. Vaccine. 2016 Jul 19;34(33):3803-9. [CrossRef]
- Parker EP, Grassly NC. Unravelling mucosal immunity to poliovirus. Lancet Infect Dis 2016; 16:1310–1.
- Onorato IM, Modlin JF, McBean AM, Thoms ML, Losonsky GA, Bernier R. Mucosal immunity induced by enhanced potency IPV and OPV. J Infect Dis 1991; 163:1–6.
- Nishio O, Ishihara Y, Sakae K, et al. The trend of acquired immunity with live poliovirus vaccine and the effect of revaccination: follow-up of vaccinees for ten years. J Biol Stand 1984; 12:1–10. [CrossRef]
- Grassly NC, Jafari H, Bahl S, et al. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J Infect Dis 2012; 205:1554–61. [CrossRef]
- John J, Giri S, Karthikeyan AS, et al. The duration of intestinal immunity after an inactivated poliovirus vaccine booster dose in children immunized with oral vaccine: a randomized controlled trial. J Infect Dis 2017; 215:529–36. [CrossRef]
- Bandyopadhyay AS, Asturias EJ, O'Ryan M, Oberste MS, Weldon W, Clemens R, Rüttimann R, Modlin JF, Gast C. Exploring the relationship between polio type 2 serum neutralizing antibodies and intestinal immunity using data from two randomized controlled trials of new bOPV-IPV immunization schedules. Vaccine. 2017 Dec 19;35(52):7283-91. [CrossRef]
- Herremans TM, Reimerink JH, Buisman AM, Kimman TG, Koopmans MP. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol 1999; 162:5011–8. [CrossRef]
- Wright PF, Connor RI, Wieland-Alter WF, et al. Vaccineinduced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants. Lancet Infect Dis 2016; 16:1377–84. [CrossRef]
- Saez-Llorens X, Clemens R, Leroux-Roels G, et al. Immunogenicity and safety of a novel monovalent highdose inactivated poliovirus type 2 vaccine in infants: a comparative, observer-blind, randomised, controlled trial. Lancet Infect Dis 2016; 16:321–30. [CrossRef]
- Ogra PL, Karzon DT, Righthand F, MacGillivray M. Immunoglobulin response in serum and secretions after immunization with live and inactivated poliovaccine and natural infection. N Engl J Med 1968; 279:893–900. [CrossRef]
- Cuba IPV Study Collaborative Group. Randomized, placebo- controlled trial of inactivated poliovirus vaccine in Cuba. N Engl J Med 2007; 356:1536–44. [CrossRef]
- Brickley EB, Strauch CB, Wieland-Alter WF, Connor RI, Lin S, Weiner JA, Ackerman ME, Arita M, Oberste MS, Weldon WC, Sáez-Llorens X. Intestinal immune responses to type 2 oral polio vaccine (OPV) challenge in infants previously immunized with bivalent OPV and either high-dose or standard inactivated polio vaccine. The Journal of infectious diseases. 2018 Jan 17;217(3):371-80. [CrossRef]
- Parker EP, Molodecky NA, Pons-Salort M, O’Reilly KM, Grassly NC. Impact of inactivated poliovirus vaccine on mucosal immunity: implications for the polio eradication endgame. Expert Rev Vaccines 2015; 14: 1113–23. [CrossRef]
- Sutter, RW. Unraveling the mucosal immunity of inactivated poliovirus vaccine. J Infect Dis 2018; 217: 344–46. [CrossRef]
- Macklin GR, Grassly NC, Sutter RW, Mach O, Bandyopadhyay AS, Edmunds WJ, O'Reilly KM. Vaccine schedules and the effect on humoral and intestinal immunity against poliovirus: a systematic review and network meta-analysis. The Lancet Infectious Diseases. 2019 Oct 1;19(10):1121-8. [CrossRef]
- Behrend MR, Hu H, Nigmatulina KR, Eckhoff P. A quantitative survey of the literature on poliovirus infection and immunity. Int J Infect Dis 2014; 18:4–13. [CrossRef]
- Anand A, Zaman K, Estívariz CF, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine 2015; 33:6816–22. [CrossRef]
- Anis E, Kopel E, Singer SR, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveill 2013; 18:pii: 20586.
- Crothers JW, Colgate ER, Cowan KJ, Dickson DM, Walsh M, Carmolli M, Wright PF, Norton EB, Kirkpatrick BD. Intradermal fractional-dose inactivated polio vaccine (fIPV) adjuvanted with double mutant Enterotoxigenic Escherichia coli heat labile toxin (dmLT) is well-tolerated and augments a systemic immune response to all three poliovirus serotypes in a randomized placebo-controlled trial. Vaccine. 2022 Apr 26;40(19):2705-13.
- Estivariz CF, Kovacs SD, Mach O. Review of use of inactivated poliovirus vaccine in campaigns to control type 2 circulating vaccine derived poliovirus (cVDPV) outbreaks. Vaccine. 2022 Mar 29.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).