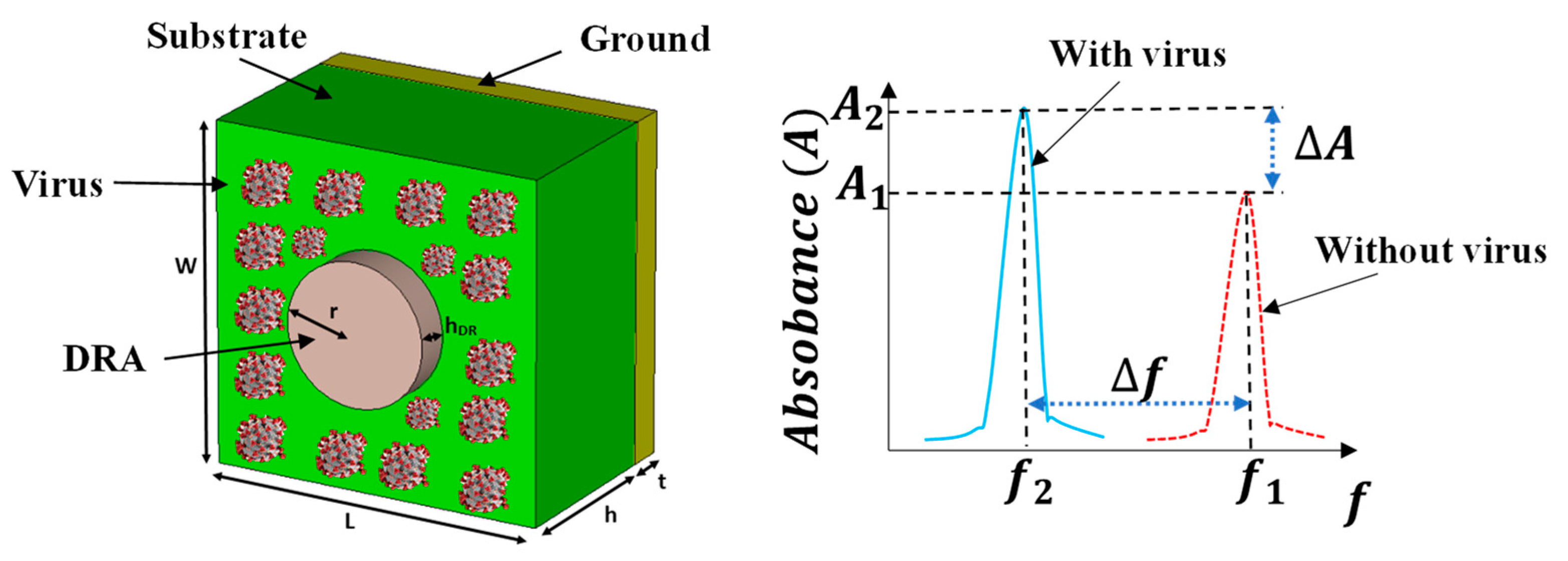
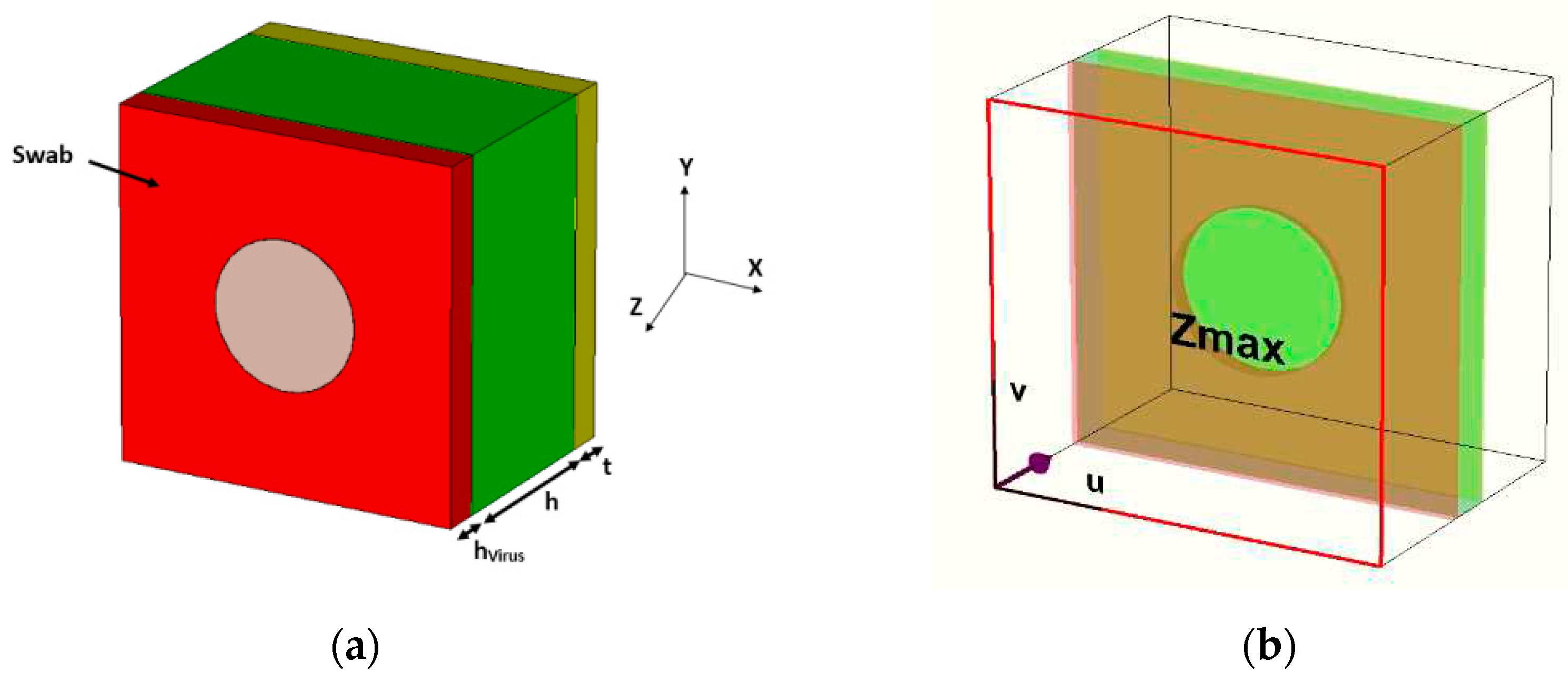
3.2. Variations of DR radius (r) with fixed DR thickness for different swab layers with the virus
Different layers of the H1N1 virus are placed on the quartz substrate around the DR. The DR thickness is kept the same
i.e., 4 µm as in
Section 3.1. Its radii are varied from 9 µm to 19 µm and its impact is analyzed on the absorbance of the biosensor with the different swab layers of the H1N1 virus.
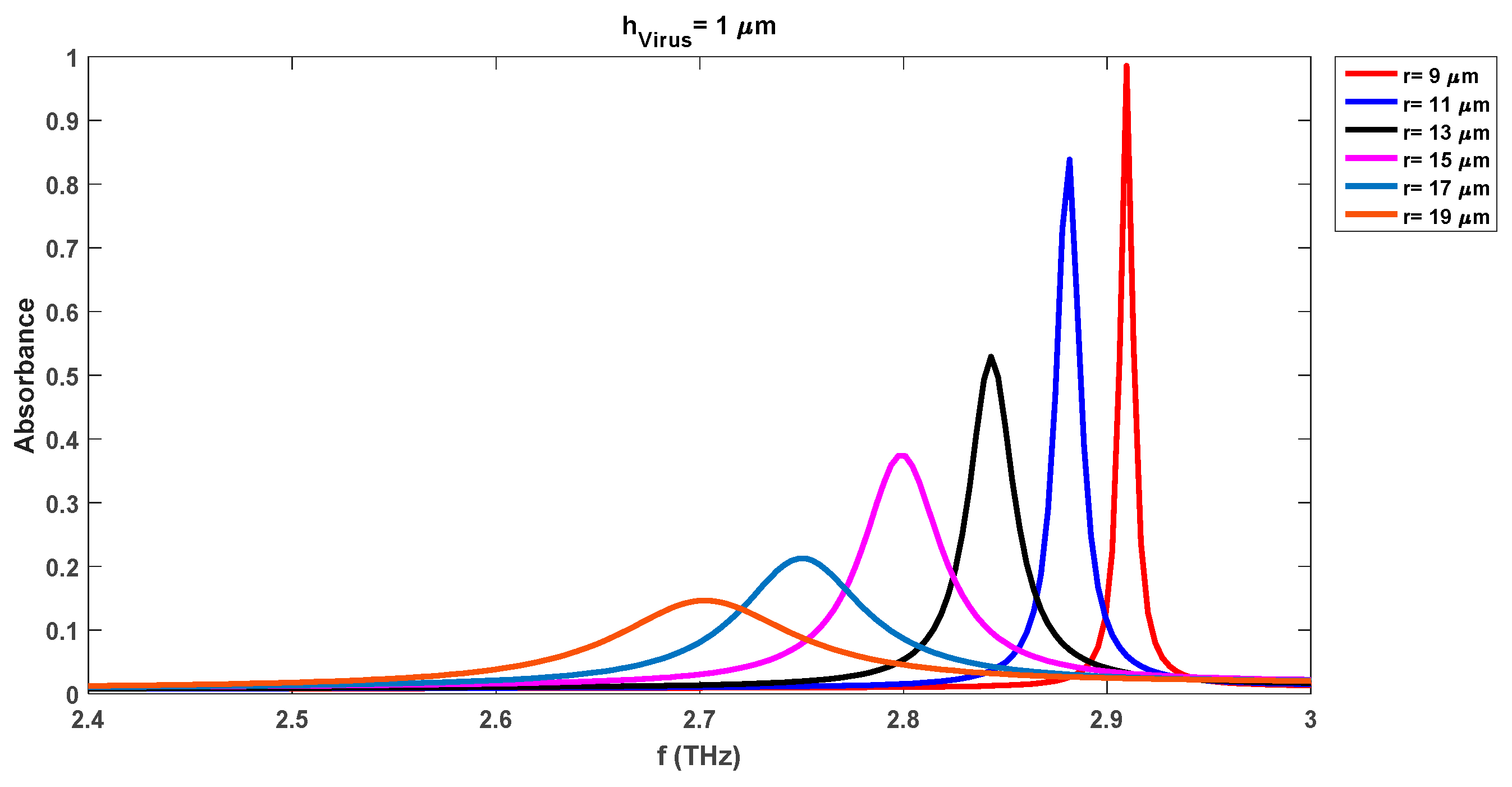
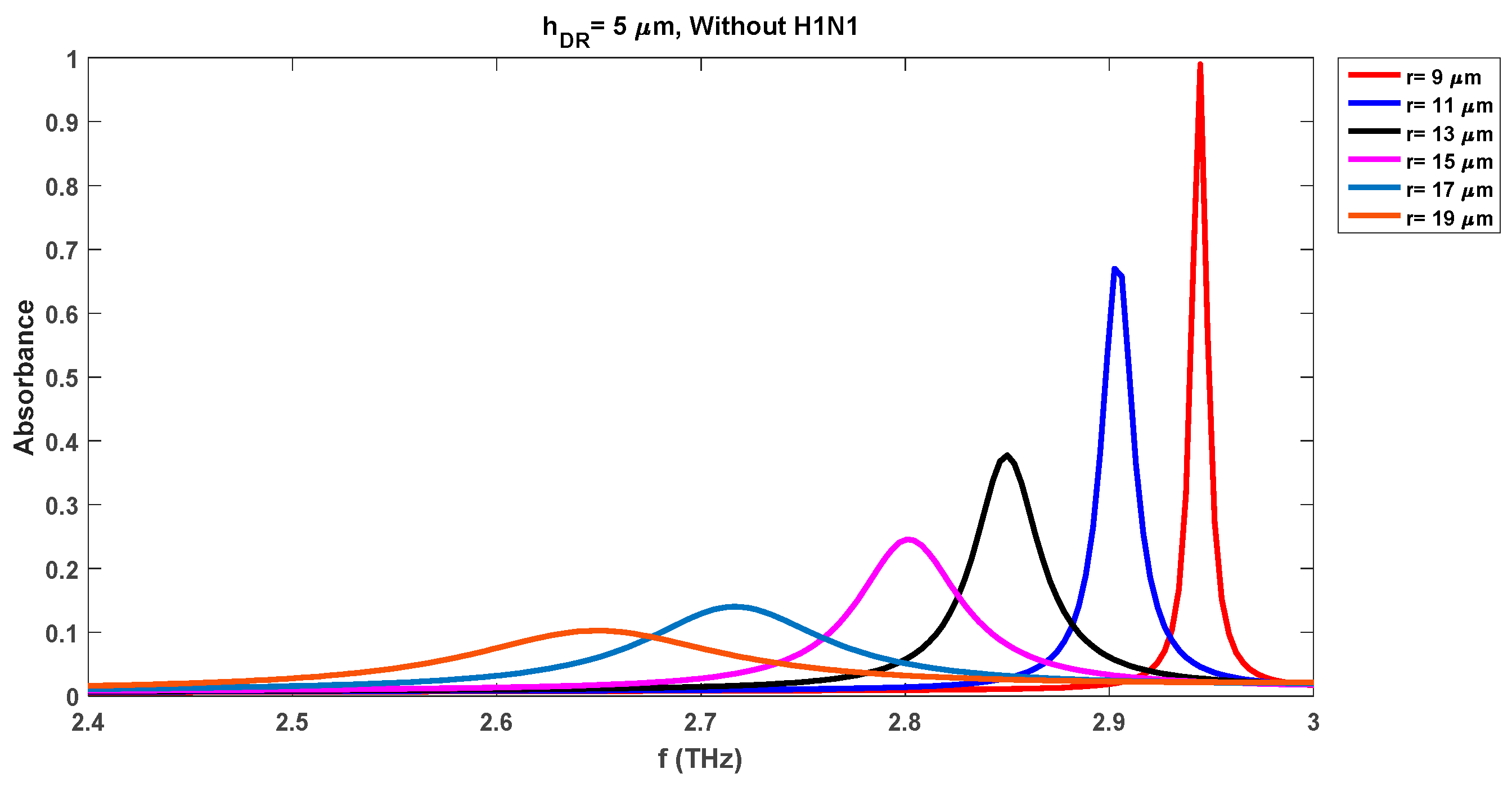
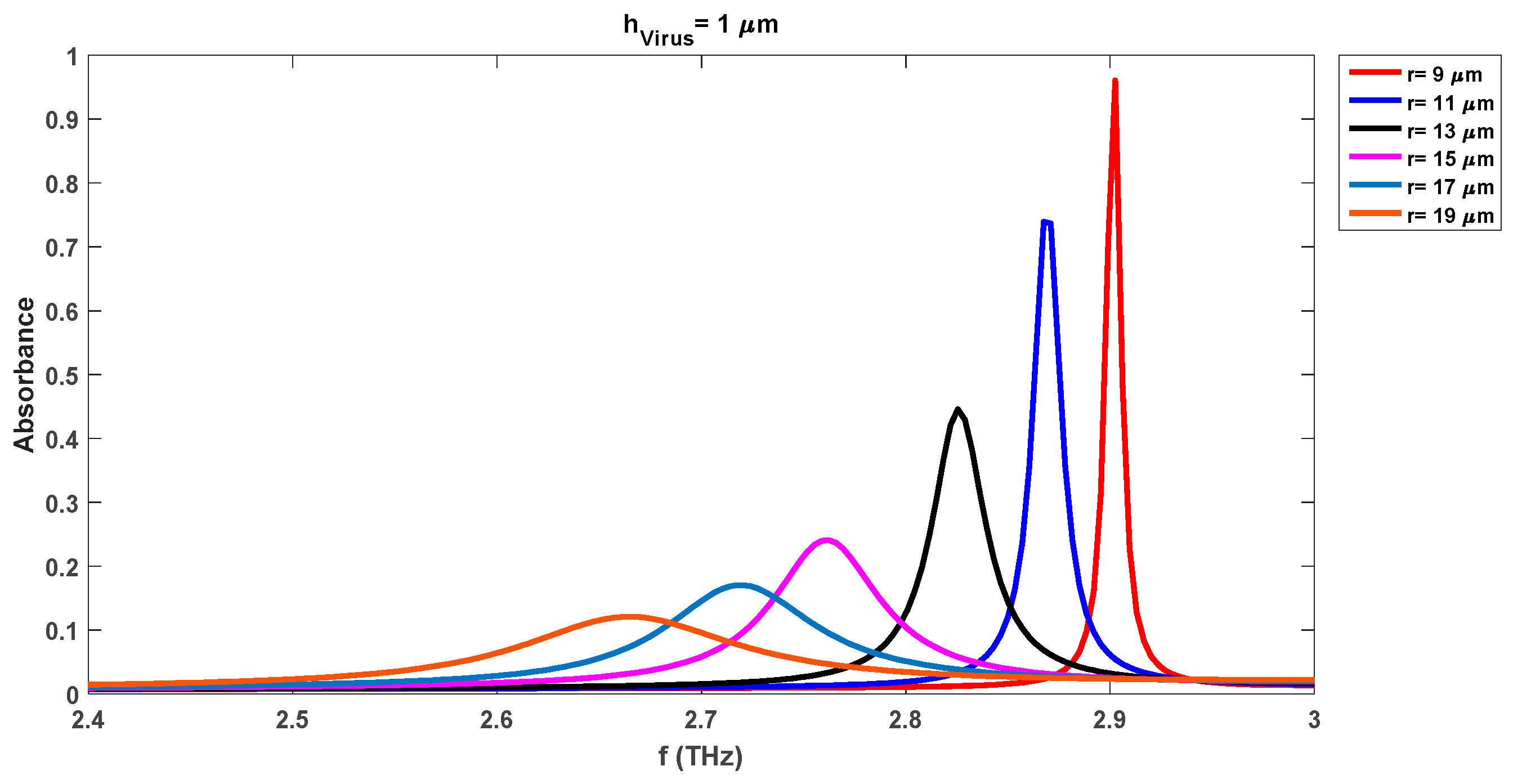
Figure 4 depicts the variations in the absorbance attributes of the sensor when DR is changed from 9 µm to 19 µm with a fixed swab layer of 1 µm. The resonance frequency of the waveform with a DR radius of 9 µm is 2.91 THz while the corresponding absorbance level is 0.98. We note that the absorbance level of the detection waveforms has been increased by 0.14 (0.98 from 0.84 for the radius of 9 µm) with the insertion of the virus swab layer on the substrate as compared to the same case without virus results of
Figure 3. The increase in the absorbance level is due to the presence of microorganisms of the virus. Also, the resonance frequency for this case has been shifted to the lower level of 2.91 THz as compared to the 2.95 THz frequency of the waveform with a DR radius of 9 µm in
Figure 3.
Overall comparison of
Figure 4 results with
Figure 3 reveals that the presence of the virus increases the absorbance level while reducing the resonance frequency for all cases of different DR radii.
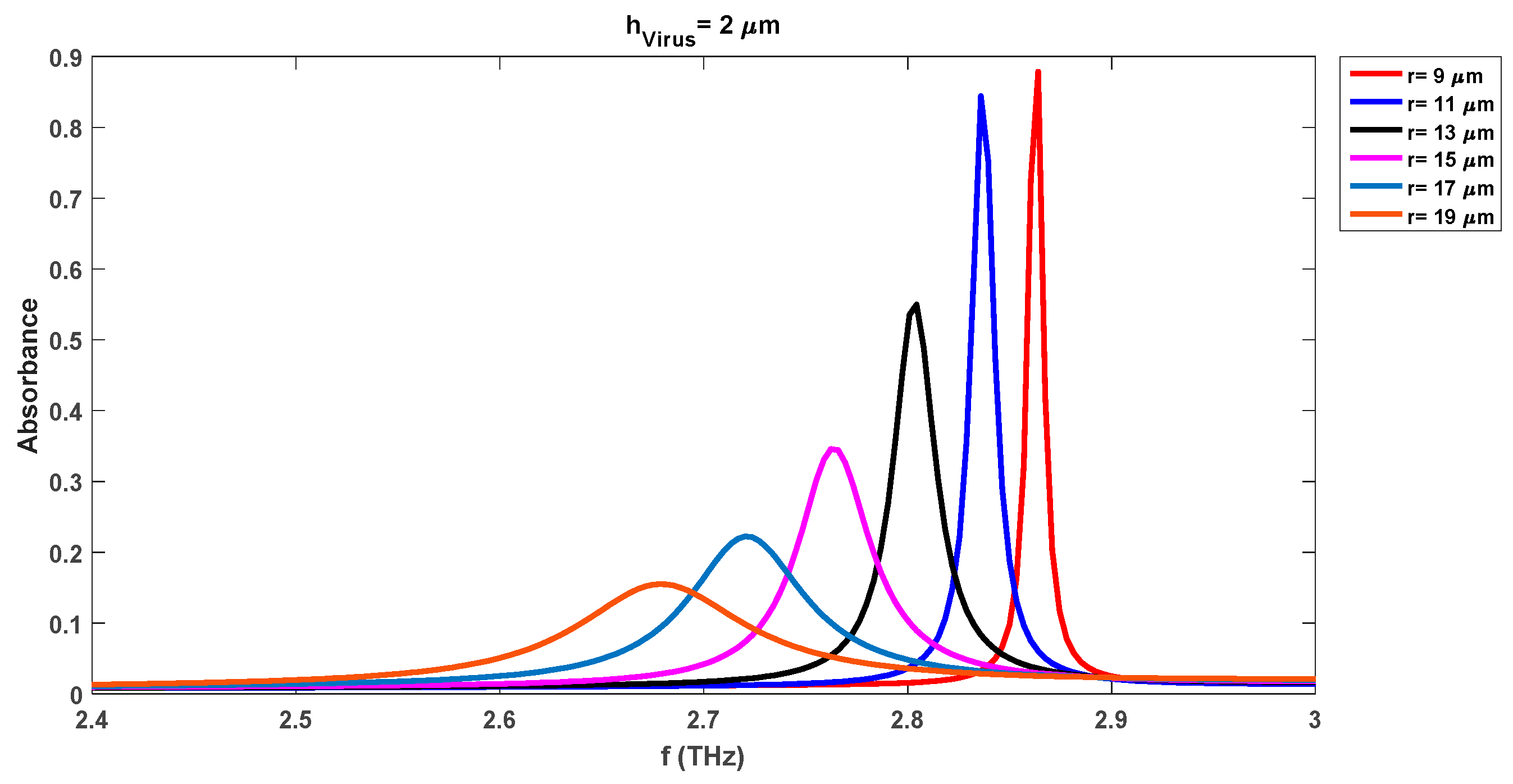
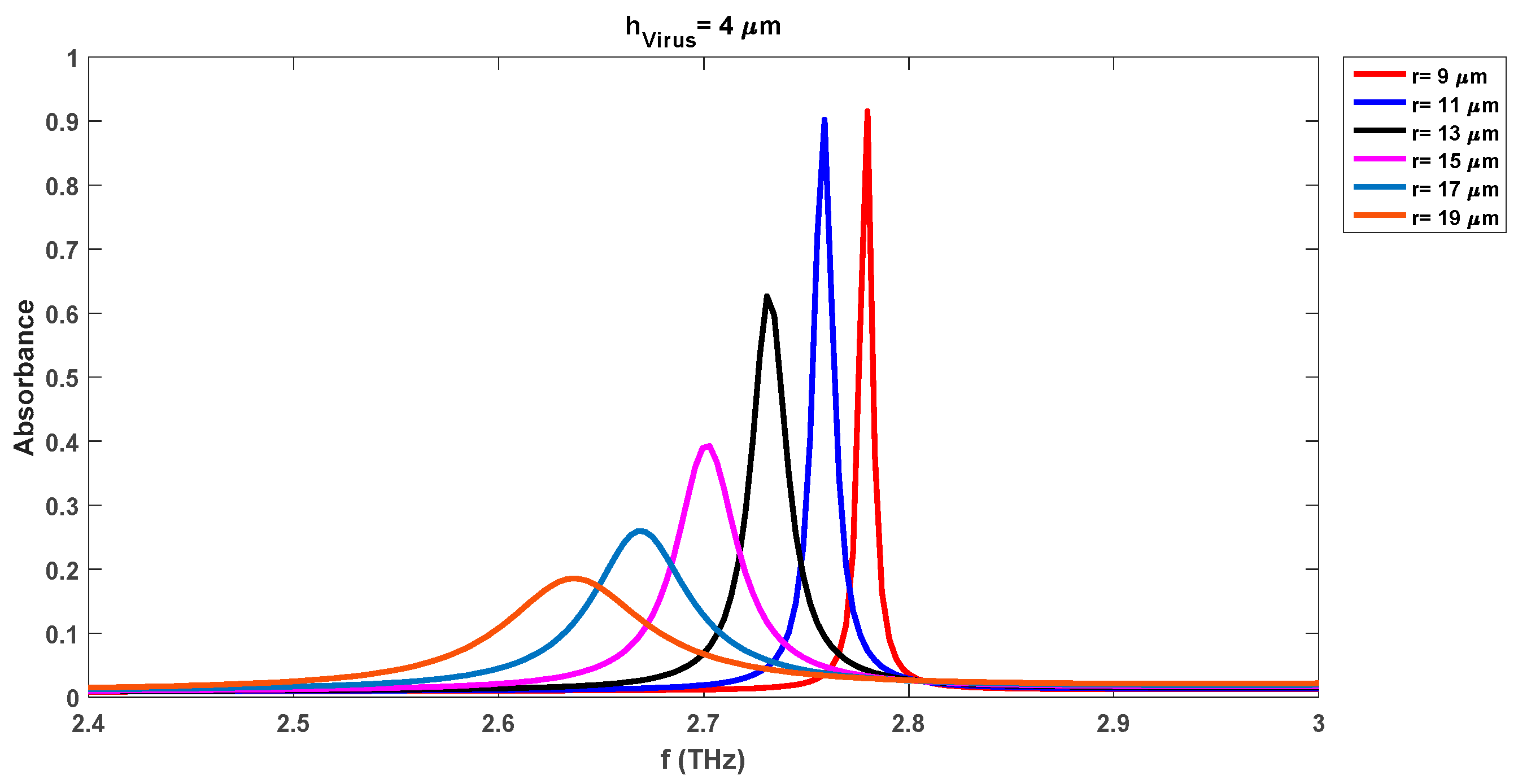
The absorption variations are also investigated by increasing the virus swab layer thickness (h
virus) to 2 µm, 4 µm, 6 µm, 8 µm, and 10 µm respectively. The characteristic waveforms for the cases of h
virus = 2 µm, h
virus = 4 µm, and h
virus = 10 µm are shown in
Figure 5,
Figure 6, and
Figure 7 respectively. The results for the other cases of h
virus (6 µm and 8 µm) are not reported here for brevity.
Table 3 lists the absorption levels and corresponding resonance frequencies of each waveform for all analyzed different thicknesses of the virus swab. As the thickness of the DR is fixed here
i.e., 4 µm, the virus swab layer completely covers the DR and quartz substrate when its thickness is increased beyond 4 µm. For a fixed DR radius (
r) of 9 µm, we can note from
Table 3 and
Figure 4,
Figure 5,
Figure 6 and
Figure 7 that as the thickness of the virus swab layer enhances, the pick of frequency levels continuously shifts to lower levels. We note from
Figure 7 that no absorbance waveform is obtained with a DR radius of 9 µm when the thickness of the virus level is increased to its maximum analyzed level of 10 µm. The absorption waveforms for h
virus = 10 µm appear as we move toward the higher DR radii. The change in the DR radius to 11 µm decreases both pick-up frequencies and absorbance levels. We note that the pick-up frequency decreases to 2.88 THz, 2.83 THz, and 2.75 THz for the virus swab layers of 1 µm, 2 µm, and 4 µm respectively as compared to the same case values with DR radius of 9 µm. Also, the absorbance level reduces to the values of 0.83, 0.844, and 0.90 for these frequencies while the level of 0.98, 0.87, and 0.91 was observed for the same frequencies when the DR radius was 9 µm. Contrary to the biosensor detection results with
r = 9 µm, DR with a radius of 11 µm can detect the virus swab layer of 10 µm with detection frequency and absorbance levels of 2.55 THz and 0.89 respectively. This shows that we notice that the absorbance level for this case (
r = 11 µm) is comparatively lower than the noted levels of the biosensor with a radius of 9 µm. However, still, the sensor is detecting the different virus swab layers with more than 83% absorbance levels which shows good performance of the realized sensor.
The further increase of the proposed biosensor DR radius to 13 µm brings the reduction in both pick-up resonance frequencies and absorbance levels. We observe the pick-up frequencies of 2.84 THz (hVirus = 1 µm), 2.80 THz (hVirus = 2 µm), 2.73 THz (hVirus = 4 µm), 2.65 THz (hVirus = 6 µm), 2.59 THz (hVirus = 8 µm), and 2.53 THz (hVirus = 10 µm) respectively. These observed frequencies have significant variations from the observed frequencies with virus layers which show the good sensitivity of the realized sensor with different swab layers. The minimum and maximum absorbance levels for this case are 52% and 79% respectively for the virus swab layers of 1 µm and 10 µm. These absorbance levels are in a very good range for the essay and rapid detection of various virus layers.
For a fixed layer of a virus,
e.g., 1 µm, the increase in the DR radius also reduces the resonance frequency and absorbance levels. We can notice in
Table 3 that the resonance frequency decreases to the levels of 2.88 THz, 2.84 THz, 2.79 THz, 2.74 THz, and 2.69 THz from 2.91 THz with the increase in the radius of DR for the virus thickness of 1 µm. The same pattern is observed for the reduction in the absorption properties where the absorption values reduce to 0.83 (
r = 11 µm), 0.52 (
r = 13 µm), 0.37 (
r = 15 µm), and 0.14 (
r = 19 µm) when compared with the value of 0.98 (
r = 9 µm) for the same 1 µm thickness of virus swab. Similar observations can be made for other thicknesses of the virus in
Table 3.
The increase of the DR radius to 15 µm, 17 µm, and 19 µm reduces the absorbance levels. The minimum and absorbance levels for 15 µm cases are 37 % (hVirus = 1 µm) and 61% (hVirus = 10 µm). These level changes to 21 % (hVirus = 1 µm) and 45 % (hVirus = 10 µm) for the DR radius of 17 µm. The lowest absorbance level is noted for the case of 19 µm DR radius case which is 14 % and 32 % for the virus layers of 1 µm and 10 µm respectively. This shows that although the absorbance level reduces to 32 % for the maximum DR radius of 19 µm, it is in an acceptable range for the rapid detection of the virus layer of 10 µm. The detection sensitivity of the realized sensor could be lower for the thinner virus swab layers with the increase in the DR radii. The DR covers more area on the substrate layer with the increase in its radius for the fixed virus thickness and thus results in lowering of the pick of frequency of absorption waveform.
The change in the resonance frequency (∆
f) and absorbance level (∆A) with and without the presence of a virus swab is computed using (2) and (3) respectively.
Table 4 presents the ∆
f and ∆A values for the analyzed cases of
Table 1 and
Table 2. The change in resonance frequencies and absorbance levels with a swab layer of 1 µm are 0.04 THz/0.14 (
r = 9 µm), 0.04 THz/0.01 (
r = 11 µm), 0.04 THz/0.02 (
r = 13 µm), 0.04 THz/0.08 (
r = 15 µm), and 0.04 THz/0.02 (
r = 17 µm) and 0.03 THz/0.01 (
r = 19 µm) respectively. The overall change in absorption levels (∆A) and resonance frequencies (∆
f) for the biosensor with a DR radius of 11 µm with and without virus swab layers are 0.04/0.01, 0.09/0.024, 0.17/0.08, 0.25/0.06, 0.32/0.09, and 0.37/0.07 respectively (see
Table 4). This change in the pick of frequencies and absorbance level could be used for the detection of the different concentrations of the virus with fixed DR radius or the same concertation of the virus with different DR radii.
While comparing the results of
Table 2 and
Table 3, we notice that the overall better absorbance levels are obtained with a DR radius of 11 µm for all concentrations of the virus swab. The observed absorption values for these cases are 0.83 (h
virus = 1 µm), 0.844 (h
virus = 2 µm), 0.90 (h
virus = 4 µm), 0.88 (h
virus = 6 µm), 0.91 (h
virus = 8 µm), and 0.89 (h
virus = 10 µm) respectively. The higher absorption values provide better sensitivity for the early, rapid, and accurate detection of the virus. These values are higher than all other cases of DR radii except for a couple of values for the 9 µm DR radius case. Although the absorbance levels are comparatively higher for certain concentrations of virus with a lower DR radius of 9 µm, it fails to detect the virus concentration of more than its radius value
i.e., 10 µm.
3.3. Impact of varying DR thickness (hDR) with different DR radii and virus swab layers
Section 3.1 and 3.2 have described the impact of the changing DR radii and concentrations of the virus swab on the absorption levels and resonance frequencies with a fixed DR thickness
i.e., h
DR = 4 µm. This section presents the analysis of the change in h
DR on the absorption properties of the realized biosensor.
The numerical analysis is performed by varying the DR thickness to 4.5 µm and 5 µm with all different levels of DR radii (
r) and h
virus as done in
Section 3.1 and 3.2.
Figure 8 and
Figure 9 illustrate the results of biosensors without viruses for the DR thicknesses of 4.5 µm and 5 µm respectively. We note that the absorbance (A) levels are 0.99 (h
DR = 4.5 µm) and 0.98 (h
DR = 5 µm) for the case of a DR radius of 9 µm. The observed absorption of the same case with a DR thickness of 4 µm was 0.84 without the virus. This reflects that the enhancement of the DR thickness increases the absorption level when no virus is placed on the biosensor. The impact of the placement of the virus swab with different concentration layers (h
virus) of 1 µm, 2 µm, 4 µm, 6 µm, 8 µm, and 10 µm is analyzed for the DR thickness of 4.5 µm and 5 µm respectively.
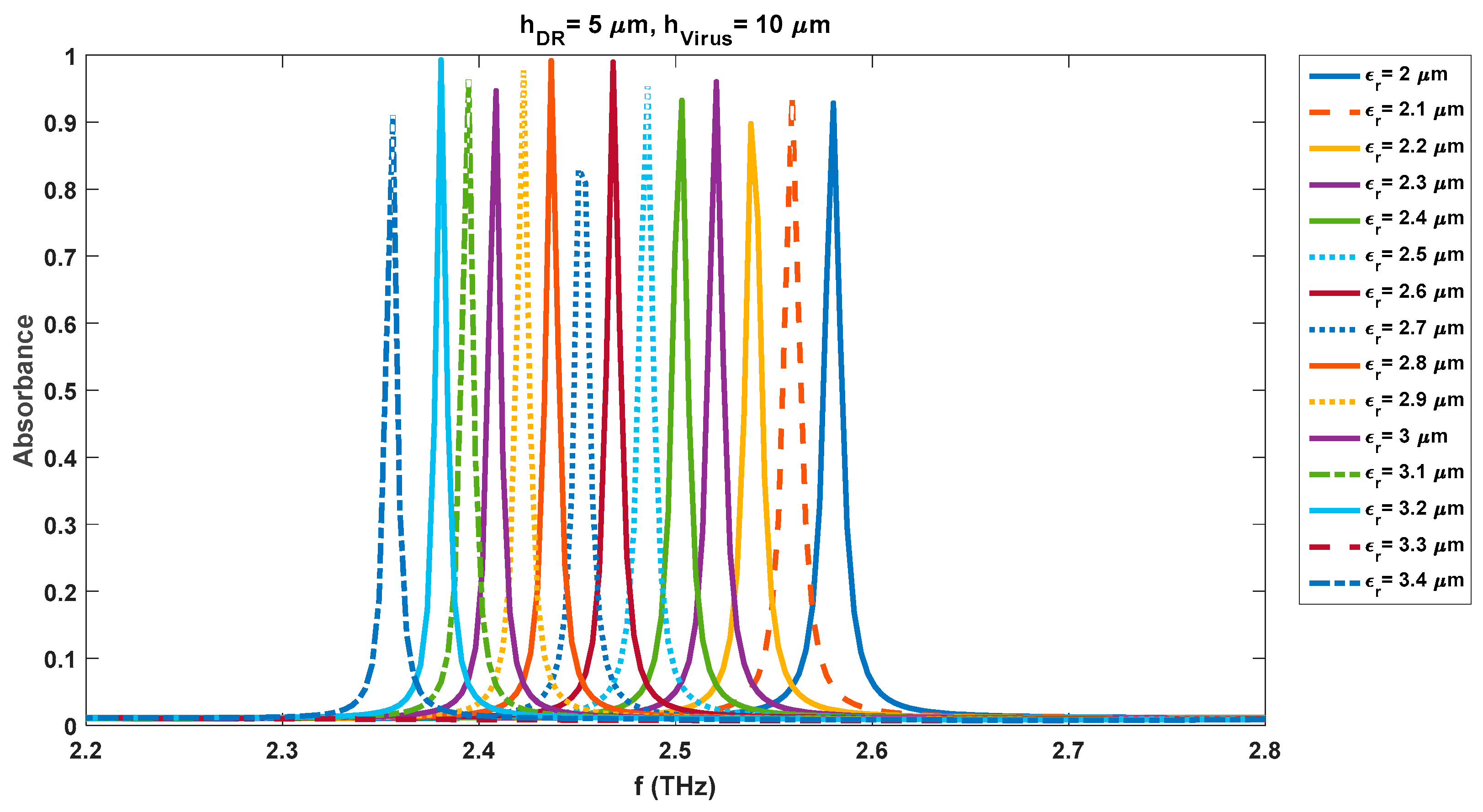
Figure 10 and
Figure 11 show the change in the waveform properties when the quartz substrate is covered with a virus swab layer of 1 µm. Similar results were obtained for the aforementioned concentrations of viruses but are not reported here for brevity. However, the tabular comparison of the variations in absorbance and resonance frequency levels is shown in
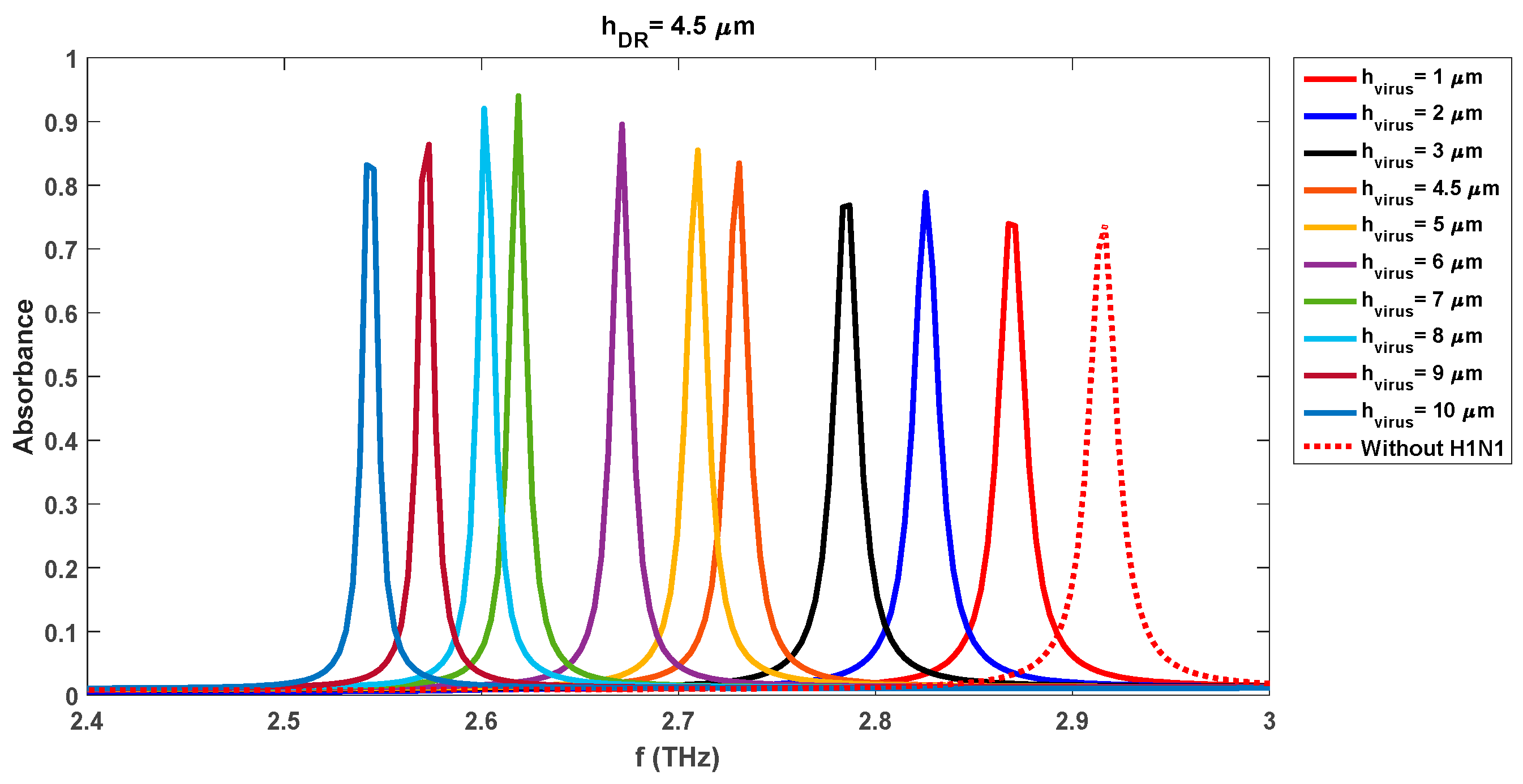
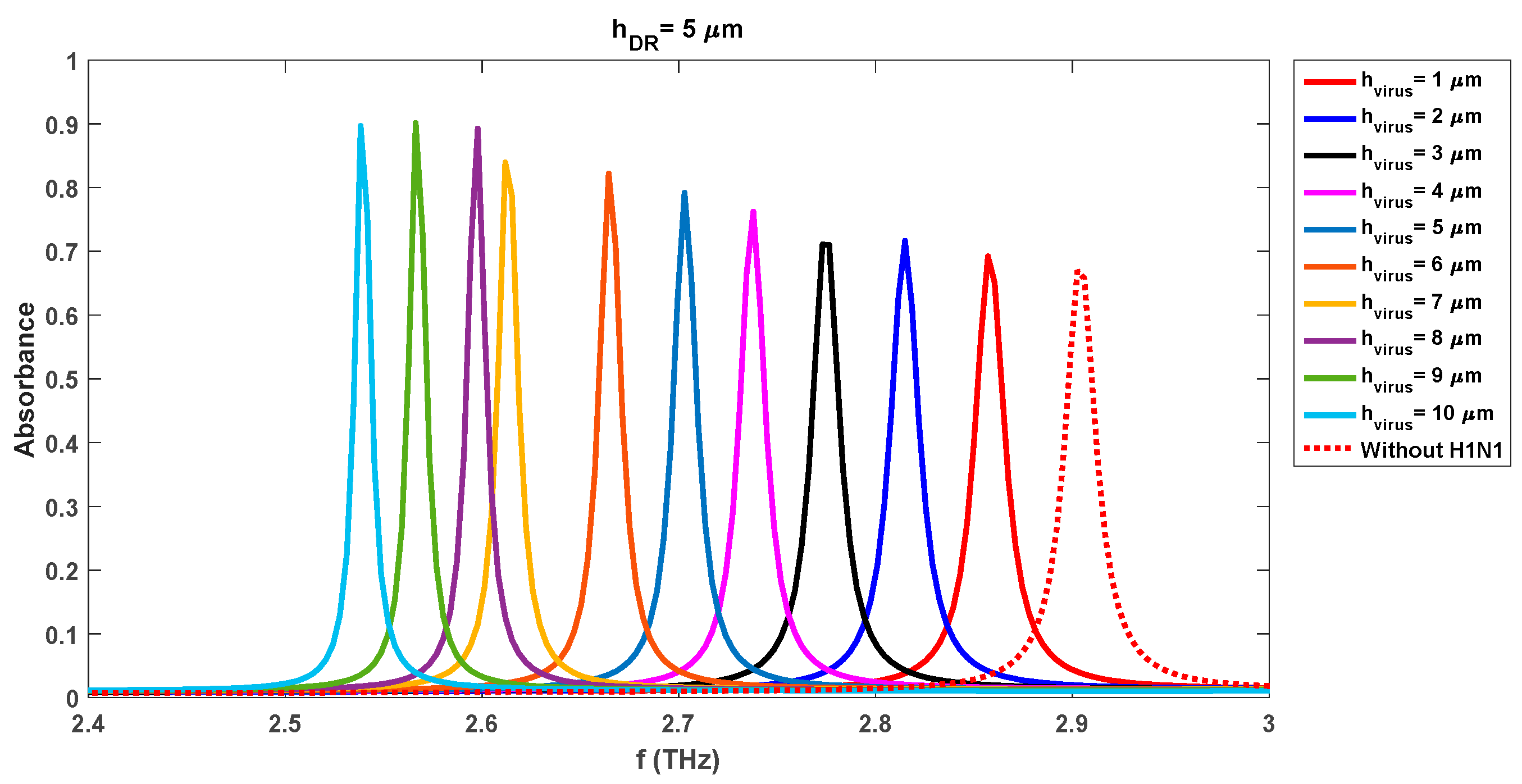
Tables 5, 6, 7, and 8 respectively. Similar analysis observations can be made for the individual cases of 4.5 µm and 5 µm DR thicknesses as was made for the 4 µm case. The sensing capabilities of the realized sensor with a DR thickness of 4.5 µm vary with the change in the virus swab layers too. The observed resonance frequencies and absorbance levels are 2.90 THz/0.96 (h
virus = 1 µm), 2.85 THz/0.84 (h
virus = 2 µm), 2.74 THz/0.94 (h
virus = 4 µm), 2.68/0.79 (h
virus = 6 µm), and 2.55 THz/0.73 (h
virus = 10 µm) respectively. These values are different than the observed values for the DR thickness of 4 µm for the same radius of 9 µm which depicts the sensitive nature of the realized sensor. As in the earlier case of DR thickness of 4 µm, the increase in the radii of DR decreases the absorbance levels. The maximum absorbance levels for radii of 11 µm, 13 µm, 15 µm, 17 µm, and 19 µm are 91 %, 82 %, 53 %, 36 %, and 26 % respectively which shows the good detection attributes of the proposed sensor even with the larger radii sensor.
The observed resonance frequencies and absorption levels for the DR thickness of 4.5 µm and 5 µm are 2.86 THz/0.73 (
r = 11 µm) and 2.85 THz/0.69 (
r = 11 µm) which are lower than the observed levels of 2.88 THz/0.83 (
r = 11 µm) for the same case of the sensor with DR thickness of 4 µm. The comparison of
Table 3,
Table 5 and
Table 7 shows that the increase of the h
DR decreases the resonance frequency and absorption attributes of the sensor for the fixed DR radius and virus swab layer. Also, we note from
Table 3,
Table 5 and
Table 7 that better results in terms of the sensitivity and selectivity of the biosensor are obtained for the DR radius of 11 µm for all three cases of different DR and virus thicknesses.