1. Introduction
Spinal cord injury (SCI) in the pediatric population presents a unique and complex set of challenges for clinicians, researchers, and families. Every year, a considerable number of children sustain spinal cord injuries due to various causes, including trauma, congenital malformations, and acquired diseases. These injuries have profound implications on a child's overall health, functional abilities, and quality of life. Thus, understanding the intricacies of pediatric spinal cord injury and developing effective interventions are of utmost importance.
Pediatric spinal cord injury differs from its adult counterpart in several key aspects. Anatomically, the developing pediatric spinal cord exhibits distinct structural characteristics and different response mechanisms to injury. The pediatric spinal cord is more pliable and elastic, leading to different injury patterns and potential for recovery compared to adults. Moreover, children's growing bodies require unique considerations when planning treatment strategies to ensure optimal functional outcomes and minimize long-term complications.
Over the years, substantial progress has been made in advancing our knowledge and understanding of pediatric spinal cord injury. Medical advancements, improved diagnostic techniques, and multidisciplinary care approaches have contributed to enhanced outcomes and quality of life for children with spinal cord injuries. However, numerous challenges and unanswered questions still exist, necessitating further research and innovation.
This manuscript aims to comprehensively explore the current state of knowledge surrounding pediatric spinal cord injury, highlighting recent advancements, unresolved challenges, and promising interventions. By critically examining the existing literature, we aim to provide a comprehensive overview of the unique aspects of pediatric spinal cord injury, its clinical manifestations, underlying mechanisms, and potential therapeutic strategies.
The manuscript will delve into various aspects of pediatric spinal cord injury, including epidemiology, etiology, classification, and prognostic factors. We will explore the acute management of spinal cord injury in children, encompassing pre-hospital care, emergency stabilization, and surgical interventions. Additionally, we will review the comprehensive rehabilitation approaches, including physical therapy and occupational therapy.
2. Pediatric Spine Anatomy
The anatomy of the pediatric spine is distinct from adults due to ongoing skeletal maturation and central nervous system development. There are numerous differences that must be noted between the adult and pediatric spinal cord as they have important implications for the management of pediatric spinal cord injury (SCI). Briefly, this section of the review will go over the key radiographic features that differentiate the pediatric spine from adults, as well as the impact of skeletal maturation and CNS development on the spinal cord.
2.1. Contrast to adults
The emergency radiologic evaluation of pediatric spine cases can be taxing due to the wide range of normal anatomic variants and injuries that are unique in the pediatric population. Thus, it is important to understand the key differences that occur during normal embryonic development to avoid misinterpretations, as well as improving accuracy in image interpretation. To begin, the pediatric spine is made of 33 vertebrae, including 7 cervical, 12 thoracic, 5 lumbar, and 4 coccygeal vertebrae. This contrasts with the adult spine, where the number is reduced to 26 vertebrae, as some vertebrae become fused during normal growth and development. The anatomy of the spinal cord starts to resemble the adult spine by the age of 8-10 years. Importantly, in children, the center of rotation (COR) is shifted compared to in adults. Around the ages of 8-10 years old, the COR of the cervical spine is located at the C5-6 level, however prior to this age, the COR is located at C2-3. This shift is partially responsible for the increase in upper cervical spine injury in children compared to adults [
1].
Another key difference is that children have larger volumes of total and spinal CSF, with 50% in children and 33% in adults [
2]. In addition, spinal ligaments are less densely packed together, thus there is increased spine flexibility. The pediatric spine is considered hypermobile compared to that of adults due to many differences, including the shallow and angled facet joints, increased ligamentous laxity and underdeveloped spinous processes. Furthermore, younger children may also have incomplete ossification of the odontoid process extending from the C2 level, and along with a relatively large head and weaker neck muscles, increases the risk of spinal instability compared to adults.
2.2. Skeletal maturation
Skeletal maturation can significantly influence the anatomy of the pediatric spine, and thus can influence the degree of spinal cord injury depending on the level of maturation that has occurred. For instance, in children whose cervical spine has not yet undergone ossification, spinal cord injuries are more likely to occur at lower levels than in adults. The formation of the axial skeleton begins during early embryonic development. Around 5 to 6 weeks of gestation, the vertebral bodies of the spinal cord undergo chondrification, however growth, remodeling, and ossification persist for decades after birth before the complete development of the adult skeleton [
3].
Many studies have investigated the timing and pattern of skeletal maturation in the pediatric spine. Ossification centers first appear in the developing spine for the neural arches of the cervical and upper thoracic vertebrae. This process begins during the embryonic period and continues throughout fetal development. Using MRI to assess vertebral ossification in children, it is generally concluded that ossification begins in fetuses at around 10-11 weeks [
4] By the 10th week of gestation, ossification centers for vertebral centra are present in the lower seven thoracic and first lumbar vertebrae. By the end of the 11th week, ossification centers for vertebral centra are present in the lower four cervical, all thoracic, all lumbar, and four sacral vertebrae. The pattern of ossification for neural arches proceeds in a craniocaudal direction, while in vertebral centra, it progresses from the lower thoracic vertebrae in both directions. Between the ages of 3-6 years, the neural processes fuse with the centrum, and during puberty, 5 secondary ossification centers are formed at the top of the spinous processes on both surfaces of the vertebral body, responsible for the superior-inferior growth of the vertebrae. This process of ossification continues to occur until adulthood and ends around the age of 18-25.
2.3. CNS development
The development of the spinal cord in children is ongoing and affects the anatomy of the pediatric spine. In children, the spinal cord is more vulnerable to injury due to its greater blood supply, which can lead to more extensive secondary injury following SCI. Additionally, the immature CNS of children may not be able to respond as effectively to injury as in adults, which may result in worse functional outcomes [
5].
The development of the CNS of the pediatric spine involves multiple complex stages, including neural tube formation, neurogenesis, axon guidance and synaptogenesis, and myelination. The first stage of CNS development in the pediatric spine is the formation of the neural tube, which occurs during the first four weeks of gestation. The neural tube gives rise to the brain and spinal cord and is formed by the folding of the neural plate. The neural tube is closed in a rostral-to-caudal direction, and defects in this process can result in neural tube defects such as spina bifida, where the spinal column is not completely closed [
6]. Once the neural tube is formed, neurogenesis begins. This process involves the proliferation and differentiation of neural stem cells into neurons and glial cells. In the spinal cord, neurogenesis occurs primarily in the ventricular zone, which is located near the central canal. The timing and extent of neurogenesis are tightly regulated and play a critical role in the formation of the proper number and types of neurons and glia in the developing spinal cord [
7].
The next stage involves the formation of synapses and axon guidance. As neurons begin to differentiate, they extend axons to their targets and form synapses. Axon guidance and synaptogenesis are highly regulated processes that involve the interaction between growth cones on the tips of axons and guidance cues in the environment. These extracellular guidance cues include secreted factors such as netrins and semaphorins as well as cell adhesion molecules (CAMs), such as cadherins and ephrins [
8]. In the developing spinal cord, axon guidance and synaptogenesis are critical for the formation of functional circuits that allow for sensory and motor function. Lastly, the final stage of CNS development in the pediatric spine is myelination, which begins in the third trimester of gestation and continues into early adulthood. Myelination involves the formation of myelin sheaths around axons by oligodendrocytes. This process is critical for proper nerve conduction and allows for the efficient transmission of signals in the spinal cord.
3. Pediatric SCI Epidemiology
This section will focus on the epidemiology of SCI in pediatric populations, including a review of its incidence, etiology, and pathophysiology.
3.1. Incidence
Depending on the population studied and the definition of SCI used, the incidence of pediatric SCI will vary. According to the National Spinal Cord Injury Statistical Center, spinal cord injury in children is relatively rare and represents less than 4% of the overall SCI incidence annually [
9]. In the United States in particular, the annual incidence of SCI is approximately 54 cases per million, with a higher incidence in males than in females [
9]. The incidence of SCI in pediatric populations has also drastically changed over time. In adolescents, the incidence of SCI dramatically decreased from 13 per million people to 8 per million from 1997 to 2012.
With age, the incidence increases rapidly, where injuries at ages 17-23 represent more than 30% of injuries, and 16-30 represents 53% of injuries [
10]. Importantly, the rate of recovery after injury is significantly faster in the pediatric population compared to adults. Depending on age, the level of SCI also varies, with pre-teen groups prevailing in C2 lesions, teen groups with mostly C4 lesions, and adults with largely C4-5 lesions [
11].
Ultimately, neonatal population can also be affected by spinal cord injuries in an extremely rare incidence, estimated 1 case per 29,000 [
12,
13]. Studies revealed birth related SCI in 10% of perinatal neonatal deaths or stillbirths at autopsy [
12].
3.2. Etiology
The etiology of pediatric SCI differs from that of adult SCI. In adults, the most common cause of SCI is due to traumatic injuries, while in children, non-traumatic causes are more common [
14]. The most common non-traumatic causes of pediatric SCI include congenital anomalies, spinal cord tumors, infections, and vascular malformations [
15]. While traumatic causes of pediatric SCI include falls, sports-related injuries, motor vehicle accidents, and child abuse [
16]. Overall, there are generally 2 main causes of SCI in young children and adolescents. A retrospective study of consecutive spinal cord injuries at a single pediatric trauma center reported 52% of injuries to be caused by motor vehicle accidents (MVA) and 27% caused by sports injuries [
17]. Furthermore, most studies reporting spinal cord injury in pediatric populations indicate a greater prevalence of SCI in males than females [
10].
It is important to highlight that neonatal etiologies differ from the rest of the pediatric population and occurs mainly during delivery secondary to excessive extraction, rotation, or hyperextension of the neck [
18].
3.3. Pathophysiology
Spinal cord injury can be broken down into traumatic and non-traumatic forms of insult. Traumatic injuries result from a direct and abrupt mechanical insult to the spinal cord, due to the disruption of the vertebral column [
19]. These kinds of injuries are often accompanied by permanent motor deficits, ranging from mild limb numbness and weakness to complete paralysis, as well as sensory and autonomic impairments. Alternatively, non-traumatic SCI refers to injury to the spinal cord that is non-mechanical, and rather a result of a gradual damage to the spinal cord overtime. Despite the differences in etiology, both traumatic and non-traumatic SCI share various pathophysiological features, including apoptosis, inflammation, axonal degeneration, and changes to vascular permeability according to animal models of both diseases [
20].
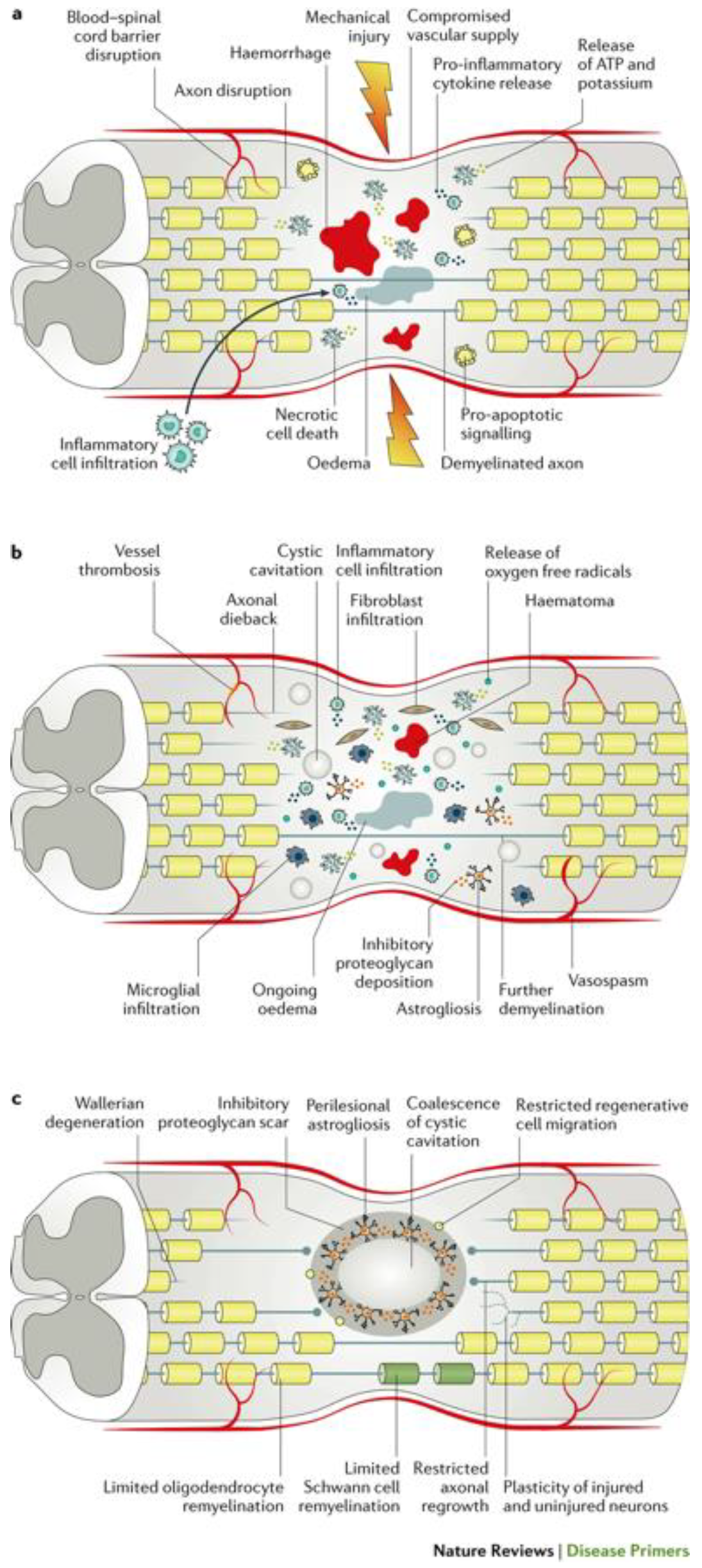
Figure 1.
The pathophysiology of traumatic spinal cord injury. a. Acute phase of traumatic SCI (0-48 hours post-injury) b. Subacute phase (2-4 days post-injury) c. Intermediate-to-chronic phase (2 weeks to 6 months post-injury).Figure from Ahuja et al. (2017) Traumatic spinal cord injury. Nat. Rev. Dis. Primers doi:10.1038/nrdp.2017.18.
Figure 1.
The pathophysiology of traumatic spinal cord injury. a. Acute phase of traumatic SCI (0-48 hours post-injury) b. Subacute phase (2-4 days post-injury) c. Intermediate-to-chronic phase (2 weeks to 6 months post-injury).Figure from Ahuja et al. (2017) Traumatic spinal cord injury. Nat. Rev. Dis. Primers doi:10.1038/nrdp.2017.18.
3.3.1. Traumatic SCI
3.3.1.1. Primary injury
Traumatic spinal cord injury can be further broken down into primary and secondary phases of injury. The primary phase refers to the initial mechanical damage that directly impacts the spinal cord. This involves the physical disturbance and structural damage to the spine, including bone fracturing and tearing of the spinal ligaments [
21]. The primary injury itself can be categorized into two main types: contusion and compression injuries. Contusion injuries are characterized by the direct impact or compression of the spinal cord, leading to localized hemorrhaging/bruising near the injury site. On the other hand, compression injuries occur when the spinal cord is indirectly compressed, for example by a bone structure or blood from a nearby hematoma. Thus, compression injuries often result in tissue deformation, vascular damage, and the disruption of axons.
3.3.1.2. Secondary injury
The secondary phase of injury is triggered by the primary phase and involves a series of molecular and cellular events that further exacerbate the chemical and mechanical damage to the spinal tissue. These events begin from hours to days after the initial injury, and ultimately leads to neuronal death and cellular dysfunction. Temporally, secondary injury can be subdivided into acute (0-48 hours post-injury), sub-acute (2-4 days post injury), and intermediate/chronic (2 weeks to 6 months post-injury) phases [
20].
The acute to sub-acute period occurs immediately after the initial injury and continues to last up to 4 days post-injury. Based on evidence from animal models, the primary insult triggers a significant inflammatory response, characterized by the activation of immune cells and release of pro-inflammatory cytokines and chemokines. This proinflammatory state is necessary for the phagocytosis of myelin debris, however, can contribute to additional tissue damage due to the production of free radicals as by-products of cytokine release [
20]. Reactive oxygen species (ROS) ultimately leads to oxidation of lipids, proteins, and DNA within the spinal cord tissue, causing further necrosis and apoptosis, worsening the state of the injury microenvironment [
22]. Furthermore, glutamate ecotoxicity occurs, which is characterized by the release of high levels of glutamate from apoptotic neurons and glia leading to the overactivation of glutamate receptors, leading to an influx of calcium ions, and excitotoxic neuronal death. Ultimately, these cellular processes of excitotoxic cell death and inflammation promote one another, perpetuating the secondary damage [
23]. In addition to this, traumatic SCI inevitably causes damage to the spinal cord vasculature, leading to ischemia that can persist up to weeks after the initial insult. Long-term ischemia plays a role in additional neuronal and glial cell death and spread of the injury. Importantly, the acute phase sets the foundation for subsequent phases, and provides a critical time window for interventions to potentially limit further secondary damage.
As the sub-acute secondary injury persists, it leads to the chronic secondary phase of SCI, characterized by the formation of cystic cavities, maturation of the glial scar, and axonal dieback [
21]. Beginning in the acute phase of injury, the formation of cystic cavities occurs, where extensive cell death leads to the formation of fluid-filled cysts containing macrophages and connective tissue. Eventually, these individual cystic cavities merge, forming a barrier that inhibits axonal regrowth and cell migration [
24]. Surrounding the cystic cavities a perilesional zone exists forms, where reactive astrocytes proliferate and interweave with one another, forming an inhibitory structure, known as the glial scar. Asides form astrocytes, the scar is formed by a combination of various ECM proteins, for example chondroitin sulfate proteoglycans (CSPGs) and NG2 proteoglycan. Along with astrocytes, these proteins form the glial scar, which inhibits axon regeneration by impeding neurite outgrowth [
25]. Importantly, the glial scar is believed to play a dual role, as it is beneficial in isolating the injury site to help limit the spread of cytotoxic molecules and inflammatory cells, although the role of the glial scar is still being investigated [
26].
3.3.2. Non-traumatic SCI
Alternatively, pediatric SCI may result from not only mechanical causes such as the ones mentioned above, but also non-mechanical mechanisms. For example, congenital anomalies can lead to the abnormal development of the spinal cord, which can result in SCI without any mechanical injury. The most common non-traumatic causes of SCI include neoplasms, or spinal cord tumors that can be either benign or malignant and lead to compression of the spinal cord that leads to SCI without direct trauma. Asides from tumours, transverse myelitis is another common cause of non-traumatic SCI in children, involving the inflammation of both sides of the spinal cord often leading to damage to myelin resulting in impairments in nerve signals [
27]. Compared to adults, neurological recovery following SCI is reported to be better in pediatric populations. However, the evidence is very weak for this as no studies have compared adults and children directly in terms of recovery following SCI.
3.3.3. Developmental variants and anomalies
Os odontoideum – this is an anomaly characterized by a well-corticated ossific density along the superior margin of a relatively hypoplastic or foreshortened base of the dens. There is debate on its etiology whether congenital or traumatic and it happens earlier in the development of the spine [
28].
Persistent ossiculum terminale – this refers to a failure of fusion of the secondary ossification center along the superior margin of the dens. The ossiculum terminale is supposed to ossify during the mid to latter half of the first decade of life and fuses during the second decade of life [
29,
30]
Odontoid synchondrotic slip: as known, there are 5 ossification centers for the axis present at birth and two for the odontoid. In between these centers there is a junction shown to have synchondrosis characteristics. It lies at a level below the C1-2 articulation and is weaker than the ligaments or bone, making it the least able to resist force [
31,
32]. With trauma, this structure can be avulsed or slip, even if there are no bony fractures [
33].
4. Pediatric SCI Diagnosis
4.1. X-ray, CT(A), MRI
Although identification of spine injuries is of huge importance it is always important to remember that imaging in the pediatric population has some particularities and concerns due to the increased risk of radiation in these patients [
1].
There is little evidence in thoracolumbar injury imaging, but for cervical there is a lot of literature [
35]. Injury mechanism would guide the decision making regarding which image should be considered, but more recently the Delphi study has been arguing against its role [
36]. It is important to remember, though, that specific mechanisms of injury and the presence of symptoms can guide the path to follow diving injuries, seatbelt type injuries, non-accidental trauma (NAT), presence of back or neck pain amongst others. It is also important to mention that the identification of an SCI at any level indicates the need to perform a full spinal column evaluation, in terms of imaging, as there are evidence to support the possibility of noncontiguous injuries up to 6% and contiguous in 32% [
37,
38].
The National Emergency X-Radiography Utilization Study (NEXUS) is one of the most used method to clear cervical pediatric spinal cord since its validation in a study perfume by Viccelio and colleagues [
39]. NEXUS utilizes 5 criteria: presence of neurologic deficits, midline spinal tenderness, altered level of consciousness, intoxication, and distracting injuries to define which is the best imaging option [
40]. Its is recommended to perform anteroposterior and lateral cervical spine x-rays or high-resolution CT scanning in children younger than 9 years with history of trauma, who are not alert or nonconversant, have a neurological deficit, neck pain or a painful distracting injury, are intoxicated, or have unexplained hypotension [
41]. In the population age 9 years and older, it is also recommended to add the open-mouth cervical spine x-ray (1a). Additionally, evidence supports cervical spine screening in children after a SCI who are not communicant due to age (< 3 years old) and were involved in motor vehicle collision, fall from height > 10 feet, or suspected NAT mechanisms, or GCS < 14 [
35].
Dynamic studies such as flexion and extension fluoroscopy or radiographs should be considered when the child’s clinic or static x-rays suggest cervical spinal instability [
42,
43]. Usage of CT studies is not definite to clear every cervical spine in the great majority of this populations and should be employed with caution due to the amount of the radiation in this test, unless there is risk of a potential AOD [
44,
45]. MRI is an important information provider about ligamentous injury that may influence surgical management, although it requests cooperation or sedation in cases where the child cannot tolerate it. This latter imaging study may also provide prognostic information regarding existing neurological deficits [
35,
46]. In MRIs T1-weighted image helps in evaluation with cord morphology and anatomy and T2 low signal shows acute hematomas [
47]. Subacute hematoma appears hyperintense on both T1 and T2 weighted imaging. Last, chronic hematomas appear hypointense on T2- weighted imaging. Edema appears hypertense in comparison with healthy normal nervous tissue in T2. This is important since these patients with hemorrhage tend to have worst poor outcome, while single level edema have a better outcome [
47,
48].
AANS/CNS Joint Guidelines Committee recommendations based on level 1 evidence suggests the use of CT in suspected atlanto-occipital dislocation to determine the condyle-C1 interval [
35,
40]. It is also relevant to highlight that CT cervical may be the chosen modality in patients who are obtunded, have polytrauma, or had a high-risk mechanism of injury [
49].Level 2 evidence supports the recommendation against performing imaging in children > 3 years of age without the criteria proposed above [
35,
40] with motor vehicle accident trauma mechanisms, fall from height greater than 10 feet and nonaccidental trauma [
50]. In cases that does not meet these criteria, cervical spine X rays or CT scans must be performed.
4.2. Contrast to adults
4.2.1. Upper cervical spine injuries
4.2.1.1. Craniocervical junction injuries
Due to anatomical differences mentioned priorly, atlanto-occipital distraction injuries are the most common cervical spine injuries in the pediatric population [
51].On radiographs, the most sensitive measurement for these injuries are the basion-dens interval and C1/2:C2/3 interspinous ratio [
52]. It is common that on those injuries the disruptions are incomplete causing minimal to no displacement in the craniocervical junction. In these cases, the measurements mentioned may not be sensitive, although soft tissue findings such as retroclival hematoma, fat stranding between the basion and dens, extrathecal collections, and hemorrhage can indicate an underlying distraction injury [
49]. Last, as occipital condyle fractures can be more common in children than in adults [
53], it is important to identify avulsion fractures on this structure as it can be associated with atlanto-occipital dissociation [
54].
4.2.1.2. Atlas injuries
Rarely seen in young children, these type of injuries are more frequent in adolescents [
49,
55]. C1 fractures can be missed on radiographs, but the displacement of the lateral aspect of the lateral mass of C1 relative to the lateral borders of C2 on an odontoid view can be an indication of the lesion. It is also important to highlight that unfused C1 synchondrosis can be mistaken with a fracture. In these cases, asymmetric widening of the synchondrosis or adjacent soft tissue edema suggests an injury in the region, which is better identified on the MRI.
Lastly, rare avulsion of the C1 anterior arch seen almost exclusively in the pediatric population is one lesion to be aware in cases where hyperextension was part of the injury mechanism [
55].
4.2.1.3. Atlanto-axial injuries
Less often then the craniocervical distractions injuries, atlanto-axial injuries happen usually as partial rather than complete lesions [
53]. Patients with underlying disorders that create ligament laxity such as Down, Morquio, and Marfan syndromes are at more risk of presenting these [
56]. Suggestive radiological findings in these case are widened atlantodental interval and disruption of the spinolaminar line and in cases that the subluxation of the atlas relative to the axis happen, spinal canal narrowing can be seen [
53]. It is important to highlight the possibility of occurrence of Grisel syndrome in the pediatric population [
57]. It consist in a non-traumatic subluxation of the atlanto-axial joint associated with retropharyngeal abscess, otitis media, and upper respiratory tract infection [
57].
Furthermore, young children can present with instability between C1 and C2 causing an atlanto-axial rotatory subluxation due to ligamentous and capsular hypermobility [
58].There are four types of these injuries: type 1 happens when there is an abnormal rotation to the atlas relative to the axis without subluxation across C1-C2 level. Type 2 are rotational deformities where there is mild anterior subluxation of C1-C2 corresponding to a widening of the atlantodental interval to 3-5mm. In type 3 rotational injuries, the interval is widened to more than 5mm. Finally, type 4 rotational injuries there are deformities with retrolisthesis at C1-C2 resulting in displacement of the C1 anterior arch to the dens. Rotational component of these injuries is demonstrated as medial offset of the C1 lateral mass, which is rotated anteriorly, and lateral offset of the opposite lateral mass on open-mouth view [
59].
4.2.1.4. Axis injuries
In the pediatric population, odontoid fractures (type 1 and type 3 dens fractures) occur frequently [
53]. Type 1 and type 3 occur through the apicodental and subdental synchondroses, respectively. Type 2 odontoid fractures become more prevalent in adolescents after the fusion of the syncondroses, similarly to injuries seen in adults [
53].
It is important to acknowledge Os odontoideum, a rare anatomical variant where the odontoid ossification center fails to fuse with the C2 vertebral body, and that can be distinguished from a dens fracture by the presence of smooth, corticated margins between the ossicle and C2 vertebral body. While Os odontoideum is not a fracture, it is important to identify and report it since it can lead to instability, pain, myelopathy, and vascular issues [
60].
The C2 vertebra is also susceptible to traumatic spondylosis, often called a “hangman’s fracture”, which refers to a fracture between the pedicle and inferior articular facet. Traumatic spondylolysis is rare in children, especially in those under the age of 9 years. Pseudosubluxation of the cervical spine is more common in the pediatric population, particularly those under 8 years of age [
61]. It is characterized by an apparent misalignment of the cervical spine, but it is actually a normal finding in this age group, with an offset of greater than 3 mm in the anterior and/or posterior longitudinal line while mantaining normal alignment of the spinolaminar line at the C2-C3 level [
55].
4.2.2. Subaxial cervical spine injuries
Injuries below the C2 level can be classified using the AOSpine subaxial CSI classification system [
62], which considers four criteria: injury morphology, facet injury, neurologic status, and case-specific modifiers. Morphology is divided into three categories: type A (compression fractures), type B (disruption of tension band without translation or dislocation), and type C (displacement or translation of vertebral bodies) [
62]. AOSpine A injuries are more common in patients older than 8 years, while AOSpine B and C injuries occur more frequently in children [
53]. Soft tissue changes may be the only evidence of subaxial injury, and close inspection of cervical soft tissues is crucial. Physiological vertebral body wedging and unfused epiphyseal ring ossifications are common mimics of fractures in the subaxial cervical spine [
63],[
64].
It is important to note that most vertebral body wedging in patients under 8 years is physiologic and not indicative of fractures. As the epiphyseal ring cartilage begins to ossify, it can resemble a small avulsion fracture from hyperextension injury, but this injury is rare in pediatric patients. Small calcifications along the corners of the vertebral body are likely normal physiologic mineralization in the absence of malalignment or soft tissue findings [
63].
4.3. SCIWORA
Spinal cord injury without radiographic abnormality remains an entity of concerning in the pediatric patients. Recent finding shows that the more widespread use of MRI improved the understanding regarding this particular pattern of injury in pediatric population [
65].
White matter integrity can be assessed by Diffusion-weighted MRI (DWI) as shown by Shen et al. [
66] where even with T1 and T2 weighted normal images, the DWI in SCI patients exhibited abnormal signal [
66]. For structural damage evaluation, the diffusion tensor imaging (DTI) is also another tool since it can examine and separate white matter from gray matter. Mulcahey et al. found a better association between DTI and the examination using the International Standards for Neurological Classification of SCI (ISNCSCI), although it is validated only for adults [
66].
MRI findings in children with SCIWORA range from normal to complete cord disruption along with injury of ligamentous and disk [
67,
68,
69]. Therefore, level III evidence [
70] suggests magnetic resonance should be performed in children with clinical spinal symptoms or suspected injury in addition to radiographic screening of the entire spinal column. In these patients it is recommended flexion-extension radiographs in acute setting and in late follow-up, even if MRI shows no extra neural injury. There are no recommendations regarding angiography or myelography in children population with SCIWORA [
70].
Lastly there are some recommendations in performing a somatosensory evoked potential (SSEP) to screen this group of patients especially in cases where there is subtle posterior column dysfunction and clinical findings are not conclusive [
69]. SSEPs also helps distinguishing intracranial, spinal, and peripheral nerve injuries in head injured, comatose or pharmacologically paralyzed patients [
69].
5. Pediatric SCI Classification
As discussed above pediatric SCI has unique characteristics that differentiate it from SCI in adults, including differences in etiology, spinal cord anatomy, and physiological responses to injury. As such, classification systems for pediatric SCI have been developed to aid in diagnosis, prognosis, and treatment planning. This section will review the current classification systems for pediatric SCI.
The most widely used classification system for SCI is the American Spinal Injury Association (ASIA) impairment scale, which classifies SCI based the neurological level of injury (NLI) and the completeness of injury [
71]. These set of standards is known as the International Standards for Neurological Classification of SCI [
71]. The NLI is determined by the lowest spinal segment with normal sensory and motor function, while the completeness of injury will either be classified as complete or incomplete. Complete injuries characterize injuries where there is an absence of motor and sensory function below the NLI, while incomplete injuries involve some motor and/or sensory function that is maintained below the NLI.
Currently, the ASIA classification system for SCI has been validated in adults, however it has not yet been validated in pediatric populations. Importantly, the ISNCSCI is not considered appropriate for use in children younger than 6 years old [
72]. To better apply the scale to pediatric populations, modifications must be made to account for differences in spinal cord anatomy and development. In its current state, the ISNCSCI may not be able to accurately measure the neurological consequence of SCI in young children, and only an estimated neurological level can be provided.
Developing tools for assessing neurological impairment in infants is important for improved diagnosis. For example, infant-specific motor scales may be beneficial to assess motor impairment more accurately in infants through monitoring physiological variables including heart rate and blood pressure during sensory testing. Alternatively, establishing correlations between imaging studies in children, such as CAT scans, MRI or DTI and the ISNCSCI motor and sensory scores may help establish a standardized assessment approach for young children that lack the cognitive ability to participate in the assessments [
73].
In addition to impairment-based classification systems, functional classification systems have been developed to assess the impact of SCI on a patient's daily life. One example of this is the Spinal Cord Independence Measure (SCIM-III), which assesses a child's ability to perform activities of daily living such as grooming, dressing, and bladder and bowel management. The SCIM-III is especially relevant for the pediatric SCI population as pediatric rehabilitation primarily focusses on restoring daily function and independent skills [
73]. The assessment also includes a self-report version, which provides a substitute to the performance-based version primarily in community-based settings or for long-term monitoring.
Importantly, classification systems for SCI should consider the etiology and the level and extent of injury, which is important in the prognosis and treatment of these patients. The etiology, for example traumatic versus nontraumatic causes of SCI can have serious implications on interventions required for treatment. Further, the level of injury and region of spinal cord tissue affected is important for understanding effects on motor and sensory function, and in guiding treatment planning. Ultimately, consideration of function, etiology, and the type of injury is important in classification systems for pediatric SCI. Current classification systems are continually evolving, and further research is needed to improve their accuracy and utility in the diagnosis, prognosis, and treatment of pediatric SCI.
6. Pediatric SCI Management
Fortunately, pediatric spinal cord injury remains a rare entity. However, given the rarity of occurrence, evidence surrounding management is also limited and much of the management is drawn from the adult population. Initial management follows that of ATLS guidelines which include management of airway, breathing and circulation. There are marked differences in the physiology of pediatric and adult populations which must be taken into consideration in the assessment of pediatric trauma. The acute and ICU management of children defers from adults due to the increased importance of airway control, given that the pediatric population is less tolerant of apnea prior to cardiac arrest [
74]. Additionally, it is prudent to maintain euthermia in pediatric populations; SCI can result in hypothermia, this is of greater consequence in pediatric patients given the increased metabolism and resultant O2 consumption in this population which has downstream implications of coagulopathy [
74,
75]. Therefore, due to the metabolic differences between adults and pediatric patients, euthermia is essential in preventing further complications of SCI.
The Congress of Neurological Surgeons published guidelines on the management of pediatric spinal cord injuries in which suggestions include closed reduction and halo immobilization of injuries to the C2 synchondrosis in children. Immobilization is imperative in preventing further SCI and resultant neurologic deficits.
Often implementing conservative management strategies are preferable in the pediatric population, given that pediatric patients have anticipated growth and anatomical changes which can be impeded by surgical fusion. It is suggested that in hyperextension injury conservative management be opted for unless there is ongoing compression, edema, progressive neurologic deterioration or increased intramedullary pressure in which case urgent decompression is suggested. Conservative treatment is the main treatment for children with pediatric acute hyperextension spinal cord injury (PAHSCI).
Surgical fusion is recommended for both spinal instability (irreducible AARF, ligamentous injuries and irreducible fractures resulting in deformity) and spinal cord injury resulting from canal compression. For SCIWORA the recommendation is for external immobilization for 12 weeks however, this was not seen to make a difference as demonstrated by Bosch et al in 2002. Most often, pediatric spinal cord injury is evaluated for surgical treatment on a case-by-case basis since consideration must be given to the implications on future growth after surgery.
In neonatal population, surgery is particularly challenging due to their unique anatomy, the difficulty achieving adequate internal fixation given bone immaturity, and the potential on growth. Cervical spine injuries in neonates is yet to be better studied and algorithms, as well as guidelines, are not standardized, but there is a recent report on the literature pointing the possibility of use of custom orthoses to treat these patients in a conservative approach [
18].
6.1. Steroidal therapy
The role of steroidal therapy in children is less clear given that children under the age of 13 were excluded from the NASCIS trials [
76] and additional studies have been limited in their sample size to draw conclusions. However, Zeng et al suggest that for pediatric acute hyperextension spinal cord injury steroids can be considered within 8 hours of injury. On the other hand, Pettiford et al explored the insufficient evidence of steroids, calling for increased randomized controlled trials in children to reach a solution; their study alluded to the increased risk of infection without neurologic improvement. It has also been advised that high dose steroids be reserved for the perioperative period given the risks of wound infection, PE and death [
77,
78]. In adults, it is suggested a 24-hour infusion of high dose methylprednisolone sodium succinate (MPSS) as a treatment option within 8 hours of the acute event [
1]. One can extrapolate this knowledge to older children given the paucity of specific pediatric literature.
Regarding high-dose methylprednisolone pulse Zeng et al.[
80] considered this treatment as an experimental option for PAHSCI in adults, considering children have a better potential for neurological recovery. Furthermore, there is no reliable pulse therapy for children available, at present, so it may be used according to the experience. A small pediatric study was published in 2011 showing total recovery in 13 out of 25 children between 8 and 16 year within 24 hour after administration of methylprednisolone, nevertheless the injuries of these patients were limited to spinal cord contusions and none of them needed surgery [
81].
6.2. Cardiovascular management
Patients with spinal cord injury can experience varying degrees of shock and it is a matter of utmost importance to differentiate between neurogenic shock (NS) and hemorrhagic shock to provide appropriate management [
80,
82].
As known, neurogenic shock is distributive in nature, causing hypotension without an accompanying increase in heart rate and for that patients may respond to intravenous fluid administration, but will often require vasoactive support[
83]. In such cases, pharmacological support involves the use of
agonists to address hypotension and
agonists to manage bradycardia[
80,
82].
It is suggested that a MAP >85 mmHg be maintained for 7 days post injury to prevent further ischemia in SCI for adults. We perceive this knowledge as potentially applicable to the older pediatric population, i.e., adolescents[
80,
84]. To date, a paucity of data exists to substantiate the establishment of cutoff points for blood pressure in the pediatric population so far and we suggest maintaining age appropriate blood pressure.
Finally, Parent et al through a systematic review, conclude that there is no evidence to support neuroprotective strategies such as hypothermia in the pediatric population [
10]. Overall, the timing of surgery, ICU and pharmacologic management of pediatric spinal cord injury presents a paucity in the literature [
85]. However, there is an abundance of literature surrounding post-injury complication management, care, and rehabilitation.
6.3. Surgical timing
Although literature is still limited to the pediatric population, a recent update in the guidelines for the adults was compiled by experts and is now in press. Previous 2017 guideline formulated a weakly suggestion on early surgery, based on a systematic review, using 24 hours as the threshold between early and late decompression [
79]
After this first guideline, several studies assessed this recommendation and new evidence emerged changing the strength of the recommendation to moderate. Early surgery (
24 hours after injury) should be offered as the primary approach for adults with acute SCI regardless of the level of injury, based on that recovery was more likely to happen in
2 grade ASIA impairment Score (AIS) within 6 and 12 months [
86,
87,
88,
89] when they were decompressed within 24 hours compared to after 24 hours.
At this point, it is not possible to determine the effectiveness of early surgery in different subpopulations, especially in the pediatric group, but these recommendations can guide surgical decisions. It is also important to point out that further studies on ultra-early surgery need to be done to figure out the impact on patient’s neurological recovery. Also, there is still lack of definition of what constitutes effective spinal cord decompression to individualize care, especially in the pediatric population, but most injuries don’t result in compression of neural elements.
7. Complication management
Although SCI complications in children are similar to the ones found in adult patients, pediatric population have a few particularities t
o be taken into consideration [
1] such as expressing difficulty which can be led to a delay in complications diagnosis. It is very important to active monitor for spinal deformity, hip dislocation, hypercalcemia, pain, venous thromboembolism and autonomic dysreflexia. Bowel and bladder are also usually involved in SCI patients and must be managed careful.
7.1. VTE prophylaxis
Venous Thromboembolism (VTE) is a well-known entity within hospitalized patients with limited mobility, that happens, especially, in the adult population. Under the age of 15, VTE is an occasional and rare event, with fewer than 5 cases per 100,000 [
46,
90] and this number tends to raise in an exponential rate after that [
90]. This risk can be elevated in polytraumatized patients and in cases with traumatic bran injury association [
91,
92]. In these cases, use of central venous catheters can increase the risk for VTE as well [
91,
92]. In pediatric traumatic spine injuries, the incidence of VTE also occurs in a low percentage of patients, and the risk is raised according to the severity of the trauma [
93] and concomitant injuries such as spinal cord injury, cranial hematoma, and lower extremities injuries [
92].
Consensus for VTE chemoprophylaxis is limited for the post-puberty children, as in the adult population, for 8 weeks [
94]. In pre-pubescent, there is few data for the recommendations, and the treatment is usually restricted to mechanical prophylaxis, such as the use of pneumatic compression devices and graduated compression stockings [
94] if proper sizing can be achieved. If commercially available ones are not possible in smaller children, for example, custom-made lower extremity stockings should be taken into consideration. Elastic wraps are discouraged in these patients due to the risk of venous obstruction, compartment syndrome amongst others [
95].
In cases where the children meet the criteria for the chemoprophylaxis it should be started soon after the injury [
94] if there is no active bleeding or the risk of it happening is not high. Enoxaparin dosage in patient younger than 2 months is 0.75mg/kg every twelve hours and 0.5mg/kg twice a day or 1mg/kg once a day in the older [
96].
7.2. Autonomic Dysreflexia (AD)
Development of autonomic dysreflexia usually happens in T6 or higher injuries as reported by Shcottler et al. [
97] in approximately 51% of children and it can be life threatening [
98]. Such as in the adult population, children with SCI show lower baseline blood pressure which might be measured, consistently [
47]. The diagnose for autonomic dysreflexia is made if baseline is exceeded by more than 20mmHg [
94]. Other symptoms described are sudden onset, severe headache with flushing and sweating above injury level and cool skin with piloerection below [
99].
It is also important to consider this diagnose in younger children that are unable to communicate or are too young to express themselves if they are presenting with unusual drowsiness or with a new onset irritability [
100].
Treatment involves conservative measures by removing all potential irritants. The popular pneumonic to remember is “6 Bs”: bladder (urinary retention or infection, nephrolithiasis, blocked catheters), bowel (impaction, constipation), back passage (hemorrhoids and fissures), boils (skin damage), bones (fractures) and babies (pregnancy, sexual intercourse) [
101].
Pharmacologic measures include fast acting anti-hypertensives such as nifedipine and for the recurrent cases prazosin or terazosin [
48]. For blood pressure control, it is also indispensable to position the patient upright to promote orthostatic drop and loosen any constrictors such as tight clothing or dressing [
94].
Correct management is extremely important since AD can lead to seizures, stroke, and intracerebral hemorrhage [
102].
7.3. Pain and Spasticity
Pain management in SCI can be a challenge matter and it is always better to have a experienced pain management team in these cases [
103]. Although it is always better to start with medications that do not impair respiratory drive and to avoid the ones that can cause cardiac and behavioral complications, many patients will need medications targeting neuropathic pain [
104]. Gabapentinoids, selective serotonin reuptake inhibitors, tricyclic antidepressants, and lidocaine patches should be considered if the patient’s condition allows their use.
Spasticity is another important feature of SCI patients that will only appear after the spinal cord shock period that is very variable and can be delayed to up to 2 months after the injury [
105]. Managing spasticity must be individualized since it some degree of it can be tolerable and even desirable in some patients. First step is to exclude potential nociceptive factors that can be exacerbating it and physical therapy must be introduced before considering oral medications [
105,
106,
107]. Afterwards, if the patient is still presenting with generalized spasticity an oral medication should be started. The most common used drugs include baclofen, diazepam, dantrolene sodium, tizanidine, and clonidine [
105] and the choice of which one to start is based on clinical experience, commencing at low dose and titration to optimal dose [
108]. Usually, baclofen is the drug of choice, with diazepam often added as an adjunct initially at night [
109]. Clonidine and tizanidine may also be added to diazepam, with one single nighttime dose at first [
109]. In supraspinal injuries, dantrolene may be preferred to avoid sedation from the cited drugs [
108]. Focal spasticity on the other hand is better managed with intramuscular botulinum toxin (BTX) injections and phenol/alcohol neurolysis. Electrical stimulation or ultrasonographic guidance may be used for better muscle localization [
105].
Patients with significant functional managements that are refractory to the medications should be treated with surgical management. Intrathecal baclofen (ITB) is highly effective and is delivered in the intrathecal space via a catheter connected to an implanted pump in the abdomen. This method allows a minimized nervous system depressive effect, although there is always the risk of potential complications such as infections, cerebrospinal fluid leaks and problems with the catheter [
110].
7.4. Bowel and bladder
Although it may not be presented right after the SCI, children can have bowel and bladder dysfunction. Depending on the level of the injury, patients will have either upper or lower motor neuron bowel patterns. The first one results in hyperreflexic bowels and spastic anal sphincter, and the patient will present with constipation and retention [
103]. The second pattern will present with an areflexic bowel and atonic external anal sphincter which will lead to a mix of constipation and incontinence [
103].
The goal for these patients is to achieve continence, but most important than that, patient should be started on a bowel regimen for regular timed bowel movements. In the upper motor neuron presentation, the program should include digital stimulation triggering retrocolic reflux along with oral and rectal medications, if needed [
103,
105]. It can also be helpful to have gravity-aided postures and suprapubic pressure (Credé maneuver) [
105,
111]. Retrograde irrigation systems such as enema and inflatable rectal catheters can be interesting additional strategies, as well [
112]. In Lower Motor Neuron programs, manual evacuation may be necessary [
113]. Ultimately, for children with significant difficulty in achieving continence it may be beneficial to perform the anterograde continence enema procedure [
113].
Neurogenic bladder is another important concern in these patients to manage. Primary goals are to preserve renal function and to promote continence [
111,
114]. Standard of care recommended by the Consortium of Spinal Cord Medicine [
94] is intermittent urinary self-catheterization. The pediatric patient is not able to perform it without help in young ages, but independence should be stimulated as soon as possible.
Patients presenting with a spastic detrusor muscle may benefit from anticholinergic drugs or detrusor BTX injections. If conservative methods aren’t able to help with satisfactory continence, surgical intervention may be considered [
115].
7.5. Spinal deformity and hip dislocation
One other important feature in children with SCI is spinal and hip deformities. Spinal deformities are common and scoliosis can develop in up to 97% of these patients before growth spurt [
105]. This is the cut point for the pediatric population and children injured before the growth spurt should be monitored closely. Usually, it can be treated with bracings, but if it fails and Cobb angle is > 40° surgical correction is indicated. Surgery can also be indicated in patients age greater than 10 years with a rapid deformity progression and functional problems or pain.
Hip dislocations and subluxation are also common in children under the age of 10 years old, occurring in > 90% according to McCarthy et al. [
116]. Positioning and surveillance are very important in these patients as surgical intervention may be needed [
105].
7.6. Hypercalcemia
Around 23% of children, especially adolescent males, can be affected by hypercalcemia [
117]. Classic symptoms are abdominal pain, polyuria, vomiting, generalized malaise, and psychosis. Acute abdomen is one misdiagnosis that can be made in these patients leading to unnecessary surgeries.
Hypercalcemia can also increase the risk of nephrocalcinosis, urolithiasis, and ultimately renal failure [
117]. Treatment consists generally in aggressive hydration with furosemide diuresis and bisphosphonates in some cases [
105,
117].
8. Pediatric SCI recovery
8.1. Rehabilitation
Although pediatric SCI is a devastating condition that can cause a lifelong neurological sequelae impacting the individual and their family researches on rehabilitation on this population is still scarce, focusing mainly on the adult group [
118]. Recently, a project named The Spinal Cord Injury Rehabilitation Evidence (SCIRE) start a combined effort to produce chapters on relevant topics related to SCI in the pediatric population [
118], but there is still an important lack of research on this area to fill the gaps needed.
Acknowledgment that rehabilitation of these patients is complex is important and involves mental, physical, and social health. Although protocols aren’t yet written for this population, addressing it and recognizing their potential damage to the pediatric population is vital.
Mental illness is often common in individuals with SCI, especially depression. It is estimated that 22% of the adult population present with depression (51), but in the pediatric population the prevalence seems to be lower [
118,
119] with a certain predominance in older adolescents (12-18 years). Anxiety is more common in older female adolescents and clinical factors such as short duration of injury are more associated with mental illness development [
120].
Physically these patients are impaired in many ways and proper management is required as stated previously regarding pain, venous thromboembolism, genitourinary issues, and deformities. Nevertheless, it is also important to focus on cardiovascular care and skin health. Although evidence in cardiovascular care is very limited in pediatric medicine [
118], relevant studies targeted in general outcomes including body composition (i.e., total lean mass, fat mass, % body fat, and bone mineral content/density), anthropometric measures (i.e., weight, height, and body mass index), metabolic efficiency (e.g., fasting lipids/glucose, resting metabolism rate), cardio-respiratory function (e.g., heart rate), and functional performance (e.g., muscle strength, power input) [
118]. Overall, the pediatric population with SCI exhibit body composition changes such as higher fat mass, lower total lean mass and body mass index amongst other changes that can predispose a poor cardiovascular health [
121,
122,
123,
124,
125,
126]. SCI can also predispose to pressure skin injury due to the impaired autonomic regulation of subcutaneous blood flow and skin moisture levels, reduced skin temperature reactivity, reduced immune response and changes in connective tissue composition [
127]. Aside from pressure injuries, it is also possible that other skin comorbidities arise in these patients, such as self-inflicted wounds [
128,
129,
130] and latex allergy [
131].
In patients with a mobility impairment leading to inability to sit upright, stand, sit-to-stand, and/or walk, a historical compensatory orthopedic approach is the solution to achieve alternative ways to function. It includes braces (e.g. parapodium, knee-ankle-foot orthoses) [
132,
133], assistive devices [
134] or electrical stimulation [
135,
136,
137,
138,
139,
140] alone or combined with braces.
Ultimately, the major life areas should be on the scope of the care in these patients. Returning to school is a primary rehabilitation goal and physical accessibility and social participation must be reassured. It is important to involve the physical and occupational therapist in this process, as well as use assistive technology and include the child’s perspective of this process [
118,
141,
142]. Community integration is also important, and it may mitigate some of long-term consequences of SCI. Individuals with pediatric SCI onset are more likely to report greater functional independence, less pain and fewer comorbidities requiring medical intervention compared to the individuals with adult-onset [
118].
9. Experimental research and future treatments
Spinal cord injury (SCI) is a devastating condition that results in partial or complete loss of motor, sensory, and autonomic functions. Traditional treatment approaches have focused on rehabilitation and symptom management, offering limited prospects for neural recovery. However, in recent years, neuroregenerative therapies have emerged as a promising avenue for promoting neural repair and functional restoration.
9.1. Cell-Based Therapies:
One of the most exciting areas of research in neuroregenerative therapies for SCI involves cell-based interventions [
143]. Stem cells, such as embryonic stem cells, induced pluripotent stem cells, and adult stem cells, have shown remarkable potential for regenerating damaged neural tissue. These cells can differentiate into various neural cell types and provide structural support, release growth factors, and modulate the inflammatory response [
144].
9.1.1. Neural Stem Cells (NSCs):
Neural stem cells are self-renewing, multipotent cells that have the ability to differentiate into various cell types found in the nervous system including oligodendrocytes and astrocytes [
145,
146]. Transplantation of NSCs into the injured spinal cord has demonstrated the potential to promote axonal regeneration, remyelination, and the formation of functional neural connections in large animal models [
144,
147,
148]. NSCs can also modulate the inflammatory response and provide trophic support to the damaged tissue.
9.1.2. Mesenchymal Stem Cells (MSCs):
Mesenchymal stem cells are multipotent cells that can be obtained from various sources, such as bone marrow, adipose tissue, or umbilical cord tissue [
149,
150]. MSCs have immunomodulatory properties and can secrete a range of factors that promote tissue repair and reduce inflammation through immunomodulation, neurotrophic factor secretion and pro-angiogenic signaling [
151,
152,
153]. Transplanted MSCs have shown the ability to enhance tissue preservation, stimulate endogenous regeneration mechanisms, and improve functional outcomes in SCI models.
9.1.3. Olfactory Ensheathing Cells (OECs):
Olfactory ensheathing cells are a specialized type of glial cells that are found in the olfactory system and are known for their high phagocytic abilities [
151,
154]. They also possess unique regenerative properties, including the ability to promote axonal growth and remyelination [
155,
156,
157]. OEC transplantation into the injured spinal cord has shown promise in promoting neural regeneration, improving locomotor function, and facilitating neuronal connectivity across the lesion site.
9.1.4. Schwann Cells:
Schwann cells are peripheral nervous system glial cells that play a crucial role in nerve regeneration. Transplantation of Schwann cells into the injured spinal cord has shown positive effects on axonal regrowth, myelination, and functional recovery. These cells can create a growth-promoting environment for regenerating axons and provide structural support to the injured tissue [
158,
159,
160,
161].
It is worth noting that cell-based therapies face challenges such as cell survival, integration into the host tissue, and guiding their differentiation into the desired cell types. Further research and clinical trials are required to optimize the cell types, delivery methods, and timing of cell transplantation to maximize their therapeutic potential in treating spinal cord injury [
145,
162,
163,
164].
9.2. Biomaterial Scaffolds:
Biomaterial scaffolds provide a supportive environment for axonal regrowth and neural tissue regeneration. These scaffolds can be made from natural or synthetic materials and are designed to mimic the extracellular matrix of the spinal cord. They provide physical support, guidance cues, and controlled release of therapeutic agents. Biomaterial scaffolds have shown promise in facilitating axonal regeneration, bridging the lesion site, and promoting tissue remodeling. However, challenges remain in achieving optimal integration with the host tissue and promoting functional connectivity. Amongst the therapies involved in this group, we can cite:
9.2.1. QL6
This is a water-soluble biomaterial that self assembles into a nanometer scale lattice-like conformation. It can be delivered prior to or in addition to NSCs[
162,
163] and it works on enhancing graft survival, reducing inflammation and glial scarring, and improving motor function in animal models [
156,
165,
166].
9.2.2. Hyaluronan/Methylcellulose (HAMC)
It is a biodegradable polymer blend [
167] that works on the support of NSCs in spinal cord injury and can be modified to carry and deliver growth factors [
162,
163] (e.g. PDGF-AA – platelet derived growth factor AA), scar-degrading drugs [
168] (e.g., chondroitinase ABC) and peptide ligands (e.g. RGD).
9.2.3. Granulocyte Colony-stimulating factor (G-CSF)
Animal models hypothesize that G-SCF suppresses neuronal apoptosis [
77]. Recent phase III clinical trial conducted to evaluate efficacy of intravenous G-CSF 400 given daily for 5 days didn’t show significant improvement in the American Spinal Injury Association (AIS) motor score [
78].
Research are actively investigating various modifications to biomaterial scaffolds, such as incorporating growth factors, controlling scaffold degradation rates, and combining multiple scaffold types to optimize their effectiveness in SCI treatment. Continued advancements in biomaterial design and fabrication techniques hold promise for developing scaffolds that can better mimic the spinal cord’s complex architecture and facilitate neural repair and functional recovery.
9.3. Trials
Riluzole – this is a sodium glutamate antagonist commonly used in patients with amyotrophic lateral sclerosis (ALS) and acts as a glutamatergic modulator. It has shown favourable results in preclinical trials promoting recovery in models of traumatic spinal cord injury (tSCI), and in early phase clinical trials [
169,
170,
171,
172]. Through a systematic review 3 studies evaluating its potential use in humans were found. Through a systematic review 3 studies evaluating its potential use in humans were found. They showed significant motor improvement with treatment but there was associated risks, including elevated liver enzymes. A more recent phase III trial (ClinicalTrials.gov: NCT01597518) was undertaken [
85,
169] and although the predetermined endpoint efficacy for riluzole was not achieved likely to insufficient power, it showed significant changes in all subgroups of cervical SCI (AIS A, B and C).
Granulocyte Colony-stimulating factor (G-CSF) – animal models hypothesize that G-SCF suppresses neuronal apoptosis [
173]. Recent phase III clinical trial conducted to evaluate efficacy of intravenous G-CSF 400 given daily for 5 days didn’t show significant improvement in the American Spinal Injury Association (AIS) motor score [
174].
SPRING – VX-210 – VX-210 is a recombinant engineered variant of C3 transferase and through a receptor-independent mechanism can cross the dura to permeate across the cell membrane. C3 transferase has shown to be important in promoting axonal outgrowth on inhibitory substrates in vitro and in vivo [
175].
ES135/rhFGF1 – this is a fibrin glue that contains fibroblast growth factor (aFGF) that is being studied as a possible repair strategy for SCI. It has been shown in animal studies to support axonal regeneration and formation of white matter to gray matter connections [
176,
177].
NOGO-A inhibition/antibody – Nogo-A is a protein present in the CNS myelin responsible for the inhibition of neurite growth [
178,
179]. Phase II trial (ClinicalTrials.gov:
NCT0393353210) investigating changes in upper extremity motor scores (UEMS) according to the International Standards for the Neurological Classification of Spinal Injury after the administration of the antibody therapy was completed earlier this year is now been analyzed for formal results.
RGMa inhibition with human monoclonal antibodies – repulsive guidance molecule A (RGMa) is found to be upregulated after a spinal cord injury and it is known for playing a role in neuronal apoptosis inducing, as well as axonal growth inhibition and remyelination [
180,
181,
182]. To neutralize this effect monoclonal antibodies are being administered by investigators to neutralize the inhibitory action of RGMa. Studies are showing improvement on the recovery of motor function and gait after its administration reassuring neuronal survival and plasticity on descending serotonergic pathways and corticospinal tract axons [
180,
181,
182]. There are two phase 2 trials investigating Elezanumab (ClinicalTrials.gov: NCT04295538) and MT-3921 (ClinicalTrials.gov: NCT04683848) as the inhibitor for the RGMa.
9.4. Functional Electrical Stimulation
Functional Electrical Stimulation (FES) holds a great potential as a non-invasive or implantable intervention for individuals with SCI. It is a therapeutic approach that utilizes electrical currents to activate nerves and muscles to restore or improve functional movements. It can be apply to various target areas, such as muscles, peripheral nerves, or the spinal cord itself [
183,
184].
The electrical stimuli apply to these structures will work on depolarizing excitable tissues, generating muscle contractions or activating sensory pathways. FES can be apple to improve upper and lower limb functions, as well as to increase bladder and bowel control, and can potentially be explored to improve respiratory function when applied directly to the respiratory muscles or even assisting with cough, deep breathing, and clearing secretions.
In conclusion, while significant strides have been made in the field of spinal cord injury (SCI) research, it is still notable that the pediatric population is yet to benefit from substantial clinical trials targeting this challenging condition. Research and therapeutic interventions have predominantly focused on adult populations and the unique anatomical, physiological, and developmental characteristics of children’s spines necessitate targeted investigations to address their distinct needs.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
All authors contributed equally to the conception and design of the study, analysis, and interpretation of the data, and drafting and critical revision of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We state that the manuscript was written in compliance with the Publication Ethics Guidelines.
Acknowledgments
The authors would like to extend their sincere appreciation to all individuals and institutions who have contributed to the completion of this review manuscript. We are grateful to the researchers, scholars, and experts whose valuable insights and groundbreaking work have shaped the field and provided the foundation for our study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dowdell, J.; Mikhail, C.; Robinson, J.; Allen, A. Anatomy of the pediatric spine and spine injuries in young athletes. Annals of Joint 2018, 3. [Google Scholar] [CrossRef]
- Gupta, A.; Saha, U. Spinal anesthesia in children: A review. J Anaesthesiol Clin Pharmacol 2014, 30, 10–18. [Google Scholar] [CrossRef]
- Sanders, J.O. Normal growth of the spine and skeletal maturation. Seminars in Spine Surgery 2015, 27, 16–20. [Google Scholar] [CrossRef]
- Skórzewska, A.; Grzymisławska, M.; Bruska, M.; Lupicka, J.; Woźniak, W. Ossification of the vertebral column in human foetuses: histological and computed tomography studies. Folia Morphol (Warsz) 2013, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wu, C.; Wu, Y.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review). Mol Med Rep 2021, 23, 417. [Google Scholar] [CrossRef] [PubMed]
- Copp, A.J.; Greene, N.D.E. Genetics and development of neural tube defects. J Pathol 2010, 220, 217–230. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef]
- Mandai, K.; Rikitake, Y.; Mori, M.; Takai, Y. Nectins and Nectin-Like Molecules in Development and Disease. Current topics in developmental biology 2015, 112, 197–231. [Google Scholar] [CrossRef]
- National Institute on Disability and Rehabilitation Research, O. of S.E. and R.Services.U.S.D. of Education. 2004 Annual Statistical Report for the Model Spinal Cord Injury Care System. 2004. Available online: https://www.nscisc.uab.edu/PublicDocuments/reports/pdf/2004StatReport.pdf.
- Parent, S.; Mac-Thiong, J.-M.; Roy-Beaudry, M.; Sosa, J.F.; Labelle, H. Spinal cord injury in the pediatric population: a systematic review of the literature. J Neurotrauma 2011, 28, 1515–1524. [Google Scholar] [CrossRef]
- Apple, D.F.; Anson, C.A.; Hunter, J.D.; Bell, R.B. Spinal cord injury in youth. Clin Pediatr (Phila) 1995, 34, 90–95. [Google Scholar] [CrossRef]
- Caird, M.S.; Reddy, S.; Ganley, T.J.; Drummond, D.S. Cervical spine fracture-dislocation birth injury: prevention, recognition, and implications for the orthopaedic surgeon. J Pediatr Orthop 2005, 25, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Chou, I.-J.; Chang, Y.-J.; Chiang, M.-C. Unusual Presentations of Birth Related Cervical Spinal Cord Injury. Front Pediatr 2020, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993-2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Klimo, P.; Rao, G.; Brockmeyer, D. Congenital anomalies of the cervical spine. Neurosurg Clin N Am 2007, 18, 463–478. [Google Scholar] [CrossRef]
- Spinal Cord Injury. National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/disorders/spinal-cord-injury (accessed on Jun 11, 2023).
- Brown, R.L.; Brunn, M.A.; Garcia, V.F. Cervical spine injuries in children: a review of 103 patients treated consecutively at a level 1 pediatric trauma center. J Pediatr Surg 2001, 36, 1107–1114. [Google Scholar] [CrossRef]
- Karthikeyan, V.; Breitbart, S.C.; Malhotra, A.K.; Fung, A.; Short, E.; Schmitz, A.; Lebel, D.E.; Ibrahim, G.M. Management of perinatal cervical spine injury using custom-fabricated external orthoses: design considerations, narrative literature review, and experience from the Hospital for Sick Children. Illustrative cases. Journal of Neurosurgery: Case Lessons 2023, 5. [Google Scholar] [CrossRef]
- David, G.; Mohammadi, S.; Martin, A.R.; Cohen-Adad, J.; Weiskopf, N.; Thompson, A.; Freund, P. Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat Rev Neurol 2019, 15, 718–731. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat Rev Dis Primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci 2020, 21, 7533. [Google Scholar] [CrossRef]
- Hausmann, O.N. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003, 41, 369–378. [Google Scholar] [CrossRef]
- Schwab, J.M.; Zhang, Y.; Kopp, M.A.; Brommer, B.; Popovich, P.G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol 2014, 258, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 1991, 75, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat Rev Neurosci 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.; Reyes, M.R.; Massagli, T.L. Pediatric Spinal Cord Injury. Curr Phys Med Rehabil Rep 2014, 2, 158–168. [Google Scholar] [CrossRef]
- O’Brien, W.T.; Shen, P.; Lee, P. The Dens: Normal Development, Developmental Variants and Anomalies, and Traumatic Injuries. J Clin Imaging Sci 2015, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Karwacki, G.M.; Schneider, J.F. Normal ossification patterns of atlas and axis: a CT study. AJNR Am J Neuroradiol. 2012, 33, 1882–1887. [Google Scholar] [CrossRef]
- Piatt, J.H.; Grissom, L.E. Developmental anatomy of the atlas and axis in childhood by computed tomography. J Neurosurg Pediatr 2011, 8, 235–243. [Google Scholar] [CrossRef]
- Sherk, H.H.; Nicholson, J.T.; Chung, S.M. Fractures of the odontoid process in young children. J Bone Joint Surg Am 1978, 60, 921–924. [Google Scholar] [CrossRef]
- McGrory, B.J.; Klassen, R.A.; Chao, E.Y.; Staeheli, J.W.; Weaver, A.L. Acute fractures and dislocations of the cervical spine in children and adolescents. J Bone Joint Surg Am 1993, 75, 988–995. [Google Scholar] [CrossRef]
- Connolly, B.; Emery, D.; Armstrong, D. The odontoid synchondrotic slip: an injury unique to young children. Pediatr Radiol 1995, 25 Suppl 1, S129–133. [Google Scholar] [CrossRef]
- Brenner, D.; Elliston, C.; Hall, E.; Berdon, W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001, 176, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Rozzelle, C.J.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Hurlbert, R.J.; Ryken, T.C.; Theodore, N.; Walters, B.C.; Hadley, M.N. Management of pediatric cervical spine and spinal cord injuries. Neurosurgery 2013, 72 Suppl 2, 205–226. [Google Scholar] [CrossRef]
- Herman, M.J.; Brown, K.O.; Sponseller, P.D.; Phillips, J.H.; Petrucelli, P.M.; Parikh, D.J.; Mody, K.S.; Leonard, J.C.; Moront, M.; Brockmeyer, D.L.; et al. Pediatric Cervical Spine Clearance: A Consensus Statement and Algorithm from the Pediatric Cervical Spine Clearance Working Group. J Bone Joint Surg Am 2019, 101, e1. [Google Scholar] [CrossRef] [PubMed]
- Hadley, M.N.; Zabramski, J.M.; Browner, C.M.; Rekate, H.; Sonntag, V.K. Pediatric spinal trauma. Review of 122 cases of spinal cord and vertebral column injuries. J Neurosurg 1988, 68, 18–24. [Google Scholar] [CrossRef]
- Mahan, S.T.; Mooney, D.P.; Karlin, L.I.; Hresko, M.T. Multiple level injuries in pediatric spinal trauma. J Trauma 2009, 67, 537–542. [Google Scholar] [CrossRef]
- Viccellio, P.; Simon, H.; Pressman, B.D.; Shah, M.N.; Mower, W.R.; Hoffman, J.R. ; NEXUS Group A prospective multicenter study of cervical spine injury in children. Pediatrics 2001, 108, E20. [Google Scholar] [CrossRef] [PubMed]
- Alexiades, N.G.; Parisi, F.; Anderson, R.C.E. Pediatric Spine Trauma: A Brief Review. Neurosurgery 2020, 87, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Hadley, M.N.; Walters, B.C.; Grabb, P.A.; Oyesiku, N.M.; Przybylski, G.J.; Resnick, D.K.; Ryken, T.C. Management of pediatric cervical spine and spinal cord injuries. Neurosurgery 2002, 50, S85–S99. [Google Scholar] [CrossRef]
- Ralston, M.E.; Chung, K.; Barnes, P.D.; Emans, J.B.; Schutzman, S.A. Role of flexion-extension radiographs in blunt pediatric cervical spine injury. Acad Emerg Med 2001, 8, 237–245. [Google Scholar] [CrossRef]
- Lui, T.N.; Lee, S.T.; Wong, C.W.; Yeh, Y.S.; Tzaan, W.C.; Chen, T.Y.; Hung, S.Y. C1-C2 fracture-dislocations in children and adolescents. J Trauma 1996, 40, 408–411. [Google Scholar] [CrossRef]
- Pang, D.; Nemzek, W.R.; Zovickian, J. Atlanto-occipital dislocation: part 1--normal occipital condyle-C1 interval in 89 children. Neurosurgery 2007, 61, 514–521, discussion 521. [Google Scholar] [CrossRef] [PubMed]
- Berne, J.D.; Velmahos, G.C.; El-Tawil, Q.; Demetriades, D.; Asensio, J.A.; Murray, J.A.; Cornwell, E.E.; Belzberg, H.; Berne, T.V. Value of complete cervical helical computed tomographic scanning in identifying cervical spine injury in the unevaluable blunt trauma patient with multiple injuries: a prospective study. J Trauma 1999, 47, 896–902, discussion 902-903. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.A.; Wheeler, H.B.; Goldberg, R.J.; Hosmer, D.W.; Patwardhan, N.A.; Jovanovic, B.; Forcier, A.; Dalen, J.E. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991, 151, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Achildi, O.; Betz, R.R.; Grewal, H. Lapbelt Injuries and the Seatbelt Syndrome in Pediatric Spinal Cord Injury. J Spinal Cord Med 2007, 30, S21–S24. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Yang, M.; Meng, M.; Li, Z.-H. Clinical characteristics and treatment of spinal cord injury in children and adolescents. Chin J Traumatol 2023, 26, 8–13. [Google Scholar] [CrossRef]
- McAllister, A.S.; Nagaraj, U.; Radhakrishnan, R. Emergent Imaging of Pediatric Cervical Spine Trauma | RadioGraphics 2019.
- Nypaver, M.; Treloar, D. Neutral cervical spine positioning in children. Ann Emerg Med 1994, 23, 208–211. [Google Scholar] [CrossRef]
- Leonard, J.R.; Jaffe, D.M.; Kuppermann, N.; Olsen, C.S.; Leonard, J.C. Cervical Spine Injury Patterns in Children | Pediatrics | American Academy of Pediatrics. Pediatrics 2014, 133, e1179–e1188. [Google Scholar] [CrossRef]
- du Plessis, J.-P.; Dix-Peek, S.; Hoffman, E.B.; Wieselthaler, N.; Dunn, R.N. Pediatric atlanto-occipital dissociation: radiographic findings and clinical outcome. Evid Based Spine Care J 2012, 03, 019–026. [Google Scholar] [CrossRef]
- Beckmann, N.M.; Chinapuvvula, N.R.; Zhang, X.; West, O.C. Epidemiology and Imaging Classification of Pediatric Cervical Spine Injuries: 12-Year Experience at a Level 1 Trauma Center. American Journal of Roentgenology 2020, 214, 1359–1368. [Google Scholar] [CrossRef]
- Neeman, Z.; Bloom, A.I. Occipital Condyle Fractures in the Pediatric Population. RadioGraphics 2003, 23, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- Junewick, J.J. Pediatric Craniocervical Junction Injuries | AJR. Pediatric Imaging 2011, 196. [Google Scholar]
- Riascos, R.; Bonfante, E.; Cotes, C.; Guirguis, M.; Hakimelahi, R.; West, C. Imaging of Atlanto-Occipital and Atlantoaxial Traumatic Injuries: What the Radiologist Needs to Know. RadioGraphics 2015, 35, 2121–2134. [Google Scholar] [CrossRef]
- Karkos, P.D.; Benton, J.; Leong, S.C.; Mushi, E.; Sivaji, N.; Assimakopoulos, D.A. Grisel’s syndrome in otolaryngology: A systematic review. International Journal of Pediatric Otorhinolaryngology 2007, 71, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Fielding, J.W.; Hawkins, R.J. Atlanto-axial rotatory fixation. (Fixed rotatory subluxation of the atlanto-axial joint). J Bone Joint Surg Am 1977, 59, 37–44. [Google Scholar] [CrossRef]
- Ghanem, I.; El Hage, S.; Rachkidi, R.; Kharrat, K.; Dagher, F.; Kreichati, G. Pediatric cervical spine instability. Journal of Children’s Orthopaedics 2008, 2, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Arvin, B.; Fournier-Gosselin, M.-P.; Fehlings, M.G. Os Odontoideum: Etiology and Surgical Management. Neurosurgery 2010, 66, A22. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Burnett, H.; Wilson, A.; Chan, O. Pseudosubluxation of C2 on C3 in Polytraumatized Children — Prevalence and Significance. Clinical Radiology 1999, 54, 377–380. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Koerner, J.D.; Radcliff, K.E.; Oner, F.C.; Reinhold, M.; Schnake, K.J.; Kandziora, F.; Fehlings, M.G.; Dvorak, M.F.; Aarabi, B.; et al. AOSpine subaxial cervical spine injury classification system | SpringerLink. European Spine Journal 2015, 25, 2173–2184. [Google Scholar] [CrossRef]
- Swischuk, L.E.; Swischuk, P.N.; John, S.D. Wedging of C-3 in infants and children: usually a normal finding and not a fracture. Radiology 1993, 188, 523–526. [Google Scholar] [CrossRef]
- Wang, M.X.; Beckmann, N.M. Imaging of pediatric cervical spine trauma. Emerg Radiol 2021, 28, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.A.; Hannon, M.; Lee, L.K. Pediatric spinal cord injury without radiographic abnormality in the era of advanced imaging. Curr Opin Pediatr 2017, 29, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tang, Y.; Huang, L.; Yang, R.; Wu, Y.; Wang, P.; Shi, Y.; He, X.; Liu, H.; Ye, J. Applications of diffusion-weighted MRI in thoracic spinal cord injury without radiographic abnormality. Int Orthop 2007, 31, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Pittman, T.; Bucholz, R.; Williams, D. Efficacy of barbiturates in the treatment of resistant intracranial hypertension in severely head-injured children. Pediatr Neurosci 1989, 15, 13–17. [Google Scholar] [CrossRef]
- Dickman, C.A.; Zabramski, J.M.; Hadley, M.N.; Rekate, H.L.; Sonntag, V.K. Pediatric spinal cord injury without radiographic abnormalities: report of 26 cases and review of the literature. J Spinal Disord 1991, 4, 296–305. [Google Scholar] [CrossRef]
- Pang, D. Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurgery 2004, 55, 1325–1342, discussion 1342-1343. [Google Scholar] [CrossRef]
- Rozzelle, C.J.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Hurlbert, R.J.; Ryken, T.C.; Theodore, N.; Walters, B.C.; Hadley, M.N. Spinal cord injury without radiographic abnormality (SCIWORA). Neurosurgery 2013, 72 Suppl 2, 227–233. [Google Scholar] [CrossRef]
- International Standards for Neurological Classification of SCI (ISNCSCI) Worksheet. American Spinal Injury Association. Available online: https://asia-spinalinjury.org/international-standards-neurological-classification-sci-isncsci-worksheet/%20 (accessed on 11 June 2023).
- Mulcahey, M.J.; Gaughan, J.P.; Chafetz, R.S.; Vogel, L.C.; Samdani, A.F.; Betz, R.R. Interrater reliability of the international standards for neurological classification of spinal cord injury in youths with chronic spinal cord injury. Arch Phys Med Rehabil 2011, 92, 1264–1269. [Google Scholar] [CrossRef]
- Mulcahey, M.J.; Calhoun, C.L.; Sinko, R.; Kelly, E.H.; Vogel, L.C. The spinal cord independence measure (SCIM)-III self report for youth. Spinal Cord 2016, 54, 204–212. [Google Scholar] [CrossRef]
- Benmelouka, A.; Shamseldin, L.S.; Nourelden, A.Z.; Negida, A. A Review on the Etiology and Management of Pediatric Traumatic Spinal Cord Injuries. Adv J Emerg Med 2019, 4, e28. [Google Scholar] [CrossRef]
- Seid, T.; Ramaiah, R.; Grabinsky, A. Pre–hospital care of pediatric patients with trauma. Int J Crit Illn Inj Sci 2012, 2, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Hoh, D.J.; Leary, S.P.; Griffith, P.; McComb, J.G. High rates of neurological improvement following severe traumatic pediatric spinal cord injury. Spine (Phila Pa 1976) 2004, 29, 1493–1497, discussion E266. [Google Scholar] [CrossRef] [PubMed]
- Spinal Cord Injury in Children - Injuries; Poisoning Available online:. Available online: https://www.merckmanuals.com/en-ca/professional/injuries-poisoning/spinal-trauma/spinal-cord-injury-in-children (accessed on Jun 12, 2023).
- Caruso, M.C.; Daugherty, M.C.; Moody, S.M.; Falcone, R.A.; Bierbrauer, K.S.; Geis, G.L. Lessons learned from administration of high-dose methylprednisolone sodium succinate for acute pediatric spinal cord injuries. Journal of Neurosurgery: Pediatrics 2017, 20, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.S.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Global Spine J 2017, 7, 84S–94S. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.-L.; Shen, X.-T.; Zhang, Z.-C.; Huang, G.-X.; Alshorman, J.; Serebour, T.B.; Tator, C.H.; Sun, T.-S.; Zhang, Y.-Z.; et al. Guidelines for management of pediatric acute hyperextension spinal cord injury. Chin J Traumatol 2023, 26, 2–7. [Google Scholar] [CrossRef]
- Arora, B.; Suresh, S. Spinal cord injuries in older children: is there a role for high-dose methylprednisolone? Pediatr Emerg Care. 2011, 27, 1192–1194. [Google Scholar] [CrossRef]
- Lemley, K.; Bauer, P. Pediatric Spinal Cord Injury: Recognition of Injury and Initial Resuscitation, in Hospital Management, and Coordination of Care. J Pediatr Intensive Care 2015, 4, 27–34. [Google Scholar] [CrossRef]
- Gerardi, M.J.; Sacchetti, A.D.; Cantor, R.M.; Santamaria, J.P.; Gausche, M.; Lucid, W.; Foltin, G.L. Rapid-sequence intubation of the pediatric patient. Pediatric Emergency Medicine Committee of the American College of Emergency Physicians. Ann Emerg Med 1996, 28, 55–74. [Google Scholar] [CrossRef]
- Squair, J.W.; Bélanger, L.M.; Tsang, A.; Ritchie, L.; Mac-Thiong, J.-M.; Parent, S.; Christie, S.; Bailey, C.; Dhall, S.; Street, J.; et al. Spinal cord perfusion pressure predicts neurologic recovery in acute spinal cord injury. Neurology 2017, 89, 1660–1667. [Google Scholar] [CrossRef]
- Walters, B.C.; Hadley, M.N.; Hurlbert, R.J.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Harrigan, M.R.; Rozelle, C.J.; Ryken, T.C.; Theodore, N.; et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013, 60, 82–91. [Google Scholar] [CrossRef]
- Aarabi, B.; Akhtar-Danesh, N.; Chryssikos, T.; Shanmuganathan, K.; Schwartzbauer, G.T.; Simard, J.M.; Olexa, J.; Sansur, C.A.; Crandall, K.M.; Mushlin, H.; et al. Efficacy of Ultra-Early (< 12 h), Early (12-24 h), and Late (>24-138.5 h) Surgery with Magnetic Resonance Imaging-Confirmed Decompression in American Spinal Injury Association Impairment Scale Grades A, B, and C Cervical Spinal Cord Injury. J Neurotrauma 2020, 37, 448–457. [Google Scholar] [CrossRef]
- Du, J.P.; Fan, Y.; Liu, J.J.; Zhang, J.N.; Meng, Y.B.; Mu, C.C.; Hao, D.J. Decompression for Traumatic Thoracic/Thoracolumbar Incomplete Spinal Cord Injury: Application of AO Spine Injury Classification System to Identify the Timing of Operation. World Neurosurg 2018, 116, e867–e873. [Google Scholar] [CrossRef]
- Haghnegahdar, A.; Behjat, R.; Saadat, S.; Badhiwala, J.; Farrokhi, M.R.; Niakan, A.; Eghbal, K.; Barzideh, E.; Shahlaee, A.; Ghaffarpasand, F.; et al. A Randomized Controlled Trial of Early versus Late Surgical Decompression for Thoracic and Thoracolumbar Spinal Cord Injury in 73 Patients. Neurotrauma Rep 2020, 1, 78–87. [Google Scholar] [CrossRef]
- Qadir, I.; Riew, K.D.; Alam, S.R.; Akram, R.; Waqas, M.; Aziz, A. Timing of Surgery in Thoracolumbar Spine Injury: Impact on Neurological Outcome. Global Spine J 2020, 10, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998, 158, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; McSwain, S.D.; Webb, S.A.; Stroud, M.A.; Streck, C.J. Venous thromboembolism prophylaxis in the pediatric trauma population. J Pediatr Surg 2013, 48, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Ugalde, V.; Franks, P.; Zhou, H.; White, R.H. Venous thromboembolism after spinal cord injury: incidence, time course, and associated risk factors in 16,240 adults and children. Arch Phys Med Rehabil 2005, 86, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Hauser, B.M.; Hoffman, S.E.; Gupta, S.; Zaki, M.M.; Xu, E.; Chua, M.; Bernstock, J.D.; Khawaja, A.; Smith, T.R.; Proctor, M.R.; et al. Association of venous thromboembolism following pediatric traumatic spinal injuries with injury severity and longer hospital stays. J Neurosurg Spine 2022, 36, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Consortium for Spinal Cord Medicine Prevention of Venous Thromboembolism in Individuals with Spinal Cord Injury: Clinical Practice Guidelines for Health Care Providers, 3rd ed. : Consortium for Spinal Cord Medicine. Top Spinal Cord Inj Rehabil 2016, 22, 209–240. [Google Scholar] [CrossRef]
- Vogel, L.C.; Lubicky, J.P. Lower extremity compartment syndrome in an adolescent with spinal cord injury. J Spinal Cord Med 2001, 24, 278–283. [Google Scholar] [CrossRef]
- Monagle, P.; Chan, A.K.C.; Goldenberg, N.A.; Ichord, R.N.; Journeycake, J.M.; Nowak-Göttl, U.; Vesely, S.K. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e737S–e801S. [Google Scholar] [CrossRef]
- Schottler, J.; Vogel, L.C.; Sturm, P. Spinal cord injuries in young children: a review of children injured at 5 years of age and younger. Dev Med Child Neurol 2012, 54, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Lakra, C.; Swayne, O.; Christofi, G.; Desai, M. Autonomic dysreflexia in spinal cord injury. Pract Neurol 2021, 21, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Eldahan, K.C.; Rabchevsky, A.G. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton Neurosci 2018, 209, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hickey, K.J.; Vogel, L.C.; Willis, K.M.; Anderson, C.J. Prevalence and etiology of autonomic dysreflexia in children with spinal cord injuries. J Spinal Cord Med 2004, 27 Suppl 1, S54–60. [Google Scholar] [CrossRef]
- Allen, K.J.; Leslie, S.W. Autonomic Dysreflexia. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Wan, D.; Krassioukov, A.V. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J Spinal Cord Med 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Thomas, A.X.; Riviello, J.J.; Davila-Williams, D.; Thomas, S.P.; Erklauer, J.C.; Bauer, D.F.; Cokley, J.A. Pharmacologic and Acute Management of Spinal Cord Injury in Adults and Children. Curr Treat Options Neurol 2022, 24, 285–304. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat Rev Dis Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- Powell, A.; Davidson, L. Pediatric spinal cord injury: a review by organ system. Phys Med Rehabil Clin N Am 2015, 26, 109–132. [Google Scholar] [CrossRef]
- Merritt, J.L. Management of spasticity in spinal cord injury. Mayo Clin Proc 1981, 56, 614–622. [Google Scholar]
- Hsieh, J.; Wolfe, D.; Connoly, S.; Townson, A. Spasticity After Spinal Cord Injury: An Evidence-Based Review of Current Interventions | Request PDF. Available online: https://www.researchgate.net/publication/285717446_Spasticity_After_Spinal_Cord_Injury_An_Evidence-Based_Review_of_Current_Interventions%20 (accessed on 30 March 2023).
- Kopec, K. Cerebral palsy: pharmacologic treatment of spasticity. US Pharm 2008, 33, 22–26. [Google Scholar]
- Mathew, A.; Mathew, M.C. Bedtime diazepam enhances well-being in children with spastic cerebral palsy. Pediatr Rehabil 2005, 8, 63–66. [Google Scholar] [CrossRef]
- Albright, A.L. Intrathecal baclofen for childhood hypertonia. Childs Nerv Syst 2007, 23, 971–979. [Google Scholar] [CrossRef]
- Goetz, L.L.; Hurvitz, E.A.; Nelson, V.S.; Waring, W. Bowel management in children and adolescents with spinal cord injury. J Spinal Cord Med 1998, 21, 335–341. [Google Scholar] [CrossRef]
- Del Popolo, G.; Mosiello, G.; Pilati, C.; Lamartina, M.; Battaglino, F.; Buffa, P.; Redaelli, T.; Lamberti, G.; Menarini, M.; Di Benedetto, P.; et al. Treatment of neurogenic bowel dysfunction using transanal irrigation: a multicenter Italian study. Spinal Cord 2008, 46, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Middleton, J.W.; Malcolm, A. Bowel Dysfunction in Spinal Cord Injury. Curr Gastroenterol Rep 2018, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Merenda, L.; Brown, J.P. Bladder and bowel management for the child with spinal cord dysfunction. J Spinal Cord Med 2004, 27 Suppl 1, S16–23. [Google Scholar] [CrossRef]
- Akbar, M.; Abel, R.; Seyler, T.M.; Bedke, J.; Haferkamp, A.; Gerner, H.J.; Möhring, K. Repeated botulinum-A toxin injections in the treatment of myelodysplastic children and patients with spinal cord injuries with neurogenic bladder dysfunction. BJU Int 2007, 100, 639–645. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.J.; Chafetz, R.S.; Betz, R.R.; Gaughan, J. Incidence and degree of hip subluxation/dislocation in children with spinal cord injury. J Spinal Cord Med 2004, 27 Suppl 1, S80–83. [Google Scholar] [CrossRef]
- Tori, J.A.; Hill, L.L. Hypercalcemia in children with spinal cord injury. Arch Phys Med Rehabil 1978, 59, 443–446. [Google Scholar]
- McIntyre, A.; Sadowsky, C.; Behrman, A.; Martin, R.; Augutis, M.; Cassidy, C.; Betz, R.; Ertzgaard, P.; Mulcahey, M. A Systematic Review of the Scientific Literature for Rehabilitation/Habilitation Among Individuals With Pediatric-Onset Spinal Cord Injury. Top Spinal Cord Inj Rehabil 2022, 28, 13–90. [Google Scholar] [CrossRef]
- Williams, R.; Murray, A. Prevalence of depression after spinal cord injury: a meta-analysis. Arch Phys Med Rehabil 2015, 96, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.E.; Kan, P.; Vanaman, M.; Rubsam, J.; Hansen, K.W.; Scaife, E.R.; Brockmeyer, D.L. Utility of a cervical spine clearance protocol after trauma in children between 0 and 3 years of age. J Neurosurg Pediatr 2010, 5, 292–296. [Google Scholar] [CrossRef]
- Johnston, T.E.; Smith, B.T.; Mulcahey, M.J.; Betz, R.R.; Lauer, R.T. A randomized controlled trial on the effects of cycling with and without electrical stimulation on cardiorespiratory and vascular health in children with spinal cord injury. Arch Phys Med Rehabil 2009, 90, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.E.; Smith, B.T.; Betz, R.R.; Lauer, R.T. Exercise testing using upper extremity ergometry in pediatric spinal cord injury. Pediatr Phys Ther 2008, 20, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.E.; Smith, B.T.; Oladeji, O.; Betz, R.R.; Lauer, R.T. Outcomes of a home cycling program using functional electrical stimulation or passive motion for children with spinal cord injury: a case series. J Spinal Cord Med 2008, 31, 215–221. [Google Scholar] [CrossRef]
- Widman, L.M.; Abresch, R.T.; Styne, D.M.; McDonald, C.M. Aerobic fitness and upper extremity strength in patients aged 11 to 21 years with spinal cord dysfunction as compared to ideal weight and overweight controls. J Spinal Cord Med 2007, 30 Suppl 1, S88–96. [Google Scholar] [CrossRef]
- Liusuwan, R.A.; Widman, L.M.; Abresch, R.T.; Styne, D.M.; McDonald, C.M. Body Composition and Resting Energy Expenditure in Patients Aged 11 to 21 Years With Spinal Cord Dysfunction Compared to Controls: Comparisons and Relationships Among the Groups. J Spinal Cord Med 2007, 30, S105–S111. [Google Scholar] [CrossRef]
- Zebracki, K.; Hwang, M.; Patt, P.L.; Vogel, L.C. Autonomic cardiovascular dysfunction and vitamin D deficiency in pediatric spinal cord injury. J Pediatr Rehabil Med 2013, 6, 45–52. [Google Scholar] [CrossRef]
- Houghton, P.; Campbell, K. CPG Panel Canadian best practice guidelines for the prevention and management of pressure ulcers in people with spinal cord injury A resource handbook for clinicians.
- Almeida, D.; da Costa, K.N.N.; de Castro, R.A.L.; Almeida, M.L.P.W.; Vianna, R.; Antonio, A.G. Self-inflicted oral injury in an infant with transverse myelitis. Spec Care Dentist 2009, 29, 254–258. [Google Scholar] [CrossRef]
- Vogel, L.C.; Anderson, C.J. Self-injurious behavior in children and adolescents with spinal cord injuries. Spinal Cord 2002, 40, 666–668. [Google Scholar] [CrossRef]
- Colville, G.A.; Mok, Q. Psychological management of two cases of self injury on the paediatric intensive care unit. Arch Dis Child 2003, 88, 335–336. [Google Scholar] [CrossRef]
- Vogel, L.C.; Krajci, K.A.; Anderson, C.J. Adults with pediatric-onset spinal cord injury: part 1: prevalence of medical complications. J Spinal Cord Med 2002, 25, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Choksi, A.; Townsend, E.L.; Dumas, H.M.; Haley, S.M. Functional recovery in children and adolescents with spinal cord injury. Pediatr Phys Ther 2010, 22, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Vogel, L.C.; Lubicky, J.P. Ambulation with parapodia and reciprocating gait orthoses in pediatric spinal cord injury. Dev Med Child Neurol 1995, 37, 957–964. [Google Scholar] [CrossRef]
- Altizer, W.; Noritz, G.; Paleg, G. Use of a dynamic gait trainer for a child with thoracic level spinal cord injury. BMJ Case Rep 2017, 2017, bcr2017220756. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, M.; Mullin, C.; Cohn, J.; Burns, C.A.; Halden, E.E.; Triolo, R.J.; Betz, R.R. Home use of a functional electrical stimulation system for standing and mobility in adolescents with spinal cord injury. Arch Phys Med Rehabil 1996, 77, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- McCain, K.J.; Farrar, M.; Smith, P.S. Gait recovery in a girl with ischemic spinal cord stroke. Pediatr Phys Ther 2015, 27, 190–199. [Google Scholar] [CrossRef]
- Murillo, N.; Kumru, H.; Opisso, E.; Padullés, J.M.; Medina, J.; Vidal, J.; Kofler, M. Recovery of assisted overground stepping in a patient with chronic motor complete spinal cord injury: a case report. NeuroRehabilitation 2012, 31, 401–407. [Google Scholar] [CrossRef]
- Johnston, T.E.; Betz, R.R.; Smith, B.T.; Benda, B.J.; Mulcahey, M.J.; Davis, R.; Houdayer, T.P.; Pontari, M.A.; Barriskill, A.; Creasey, G.H. Implantable FES system for upright mobility and bladder and bowel function for individuals with spinal cord injury. Spinal Cord 2005, 43, 713–723. [Google Scholar] [CrossRef]
- Betz, R.R.; Johnston, T.E.; Smith, B.T.; Mulcahey, M.J.; McCarthy, J.J. Three-year follow-up of an implanted functional electrical stimulation system for upright mobility in a child with a thoracic level spinal cord injury. J Spinal Cord Med 2002, 25, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Bonaroti, D.; Akers, J.M.; Smith, B.T.; Mulcahey, M.J.; Betz, R.R. Comparison of functional electrical stimulation to long leg braces for upright mobility for children with complete thoracic level spinal injuries. Arch Phys Med Rehabil 1999, 80, 1047–1053. [Google Scholar] [CrossRef]
- Massagli, T.L. Medical and rehabilitation issues in the care of children with spinal cord injury. Phys Med Rehabil Clin N Am 2000, 11, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.; Weingarden, S.; Murphy, P. School reintegration: a rehabilitation goal for spinal cord injured adolescents. Rehabil Nurs 1991, 16, 122–127. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Ahuja, C.S.; Fehlings, M.G. Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine 2018, 30, 1–18. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat Neurosci 2017, 20, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Ahuja, C.S.; Siddiqui, A.M.; Fehlings, M.G. Transplantation of human-induced pluripotent stem cell-derived neural precursor cells for treatment of spinal cord injury. In Gene Therapy in Neurological Disorders; Elsevier, 2018; pp. 299–325. ISBN 978-0-12-809821-9.
- Khazaei, M.; Ahuja, C.S.; Fehlings, M.G. Induced Pluripotent Stem Cells for Traumatic Spinal Cord Injury. Front Cell Dev Biol 2017, 4, 152. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 2006, 26, 3377–3389. [Google Scholar] [CrossRef]
- Parr, A.M.; Kulbatski, I.; Zahir, T.; Wang, X.; Yue, C.; Keating, A.; Tator, C.H. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 2008, 155, 760–770. [Google Scholar] [CrossRef]
- Fellows, C.R.; Matta, C.; Zakany, R.; Khan, I.M.; Mobasheri, A. Adipose, Bone Marrow and Synovial Joint-Derived Mesenchymal Stem Cells for Cartilage Repair. Front Genet 2016, 7, 213. [Google Scholar] [CrossRef]
- Law, S.; Chaudhuri, S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am J Stem Cells 2013, 2, 22–38. [Google Scholar]
- Ahuja, C.S.; Schroeder, G.D.; Vaccaro, A.R.; Fehlings, M.G. Spinal Cord Injury-What Are the Controversies? J Orthop Trauma 2017, 31 Suppl 4, S7–S13. [Google Scholar] [CrossRef]
- Hachem, L.D.; Ahuja, C.S.; Fehlings, M.G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J Spinal Cord Med 2017, 40, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.C.; Noonan, V.; Cadotte, D.W.; Fehlings, M.G. Timing of Decompressive Surgery of Spinal Cord after Traumatic Spinal Cord Injury: An Evidence-Based Examination of Pre-Clinical and Clinical Studies. J Neurotrauma 2011, 28, 1371–1399. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Cooke, M.J.; Tam, R.Y.; Sousa, N.; Salgado, A.J.; Reis, R.L.; Shoichet, M.S. The effects of peptide modified gellan gum and olfactory ensheathing glia cells on neural stem/progenitor cell fate. Biomaterials 2012, 33, 6345–6354. [Google Scholar] [CrossRef]
- Ramer, L.M.; Au, E.; Richter, M.W.; Liu, J.; Tetzlaff, W.; Roskams, A.J. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol 2004, 473, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, P.; Wang, Q.; Chen, Y.; Yu, H.; Ma, J.; Guo, M.; Piao, M.; Ren, W.; Xiang, L. Meta analysis of olfactory ensheathing cell transplantation promoting functional recovery of motor nerves in rats with complete spinal cord transection. Neural Regen Res 2014, 9, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Watzlawick, R.; Rind, J.; Sena, E.S.; Brommer, B.; Zhang, T.; Kopp, M.A.; Dirnagl, U.; Macleod, M.R.; Howells, D.W.; Schwab, J.M. Olfactory Ensheathing Cell Transplantation in Experimental Spinal Cord Injury: Effect size and Reporting Bias of 62 Experimental Treatments: A Systematic Review and Meta-Analysis. PLoS Biol 2016, 14, e1002468. [Google Scholar] [CrossRef]
- Biernaskie, J.; Sparling, J.S.; Liu, J.; Shannon, C.P.; Plemel, J.R.; Xie, Y.; Miller, F.D.; Tetzlaff, W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci 2007, 27, 9545–9559. [Google Scholar] [CrossRef]
- Honmou, O.; Felts, P.A.; Waxman, S.G.; Kocsis, J.D. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci 1996, 16, 3199–3208. [Google Scholar] [CrossRef]
- Kanno, H.; Pearse, D.D.; Ozawa, H.; Itoi, E.; Bunge, M.B. Schwann cell transplantation for spinal cord injury repair: its significant therapeutic potential and prospectus. Rev Neurosci 2015, 26, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Sparling, J.S.; Bretzner, F.; Biernaskie, J.; Assinck, P.; Jiang, Y.; Arisato, H.; Plunet, W.T.; Borisoff, J.; Liu, J.; Miller, F.D.; et al. Schwann Cells Generated from Neonatal Skin-Derived Precursors or Neonatal Peripheral Nerve Improve Functional Recovery after Acute Transplantation into the Partially Injured Cervical Spinal Cord of the Rat. J Neurosci 2015, 35, 6714–6730. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Tam, R.Y.; Zahir, T.; Tator, C.H.; Shoichet, M.S. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials 2013, 34, 3775–3783. [Google Scholar] [CrossRef]
- Mothe, A.J.; Zahir, T.; Santaguida, C.; Cook, D.; Tator, C.H. Neural stem/progenitor cells from the adult human spinal cord are multipotent and self-renewing and differentiate after transplantation. PLoS One 2011, 6, e27079. [Google Scholar] [CrossRef] [PubMed]
- Neuhuber, B.; Timothy Himes, B.; Shumsky, J.S.; Gallo, G.; Fischer, I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res 2005, 1035, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Wilcox, J.T.; Nishimura, Y.; Zweckberger, K.; Suzuki, H.; Wang, J.; Liu, Y.; Karadimas, S.K.; Fehlings, M.G. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials 2014, 35, 2617–2629. [Google Scholar] [CrossRef]
- Zweckberger, K.; Ahuja, C.S.; Liu, Y.; Wang, J.; Fehlings, M.G. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater 2016, 42, 77–89. [Google Scholar] [CrossRef]
- Gupta, D.; Tator, C.H.; Shoichet, M.S. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 2006, 27, 2370–2379. [Google Scholar] [CrossRef]
- Pakulska, M.; Tator, C.; Shoichet, M. Local delivery of chondroitinase ABC with or without stromal cell-derived factor 1α promotes functional repair in the injured rat spinal cord. Biomaterials 2017, 134. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Moghaddamjou, A.; Harrop, J.S.; Stanford, R.; Ball, J.R.; Aarabi, B.; Freeman, B.J.; Arnold, P.; Guest, J.; Kurpad, S.N.; et al. Safety and Efficacy of Riluzole in Acute Spinal Cord Injury (RISCIS): A Multi-Center, Randomized, Placebo-Controlled, Double-Blinded Trial. J Neurotrauma 2023. [Google Scholar] [CrossRef]
- Nagoshi, N.; Nakashima, H.; Fehlings, M.G. Riluzole as a neuroprotective drug for spinal cord injury: from bench to bedside. Molecules 2015, 20, 7775–7789. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, A.; Salehpour, F.; Aghazadeh, J.; Mirzaei, F.; Naseri Alavi, S.A. Riluzole Can Improve Sensory and Motor Function in Patients with Acute Spinal Cord Injury. Asian J Neurosurg 2018, 13, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.H.; Coleman, W.P.; Grieco, G.; Poonian, D. ; Sygen Study Group The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976) 2001, 26, S87–S98. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.L.; Jones, N.C.; Prior, M.J.W.; Bath, P.M.W.; Murphy, S.P. G-CSF suppresses edema formation and reduces interleukin-1beta expression after cerebral ischemia in mice. J Neuropathol Exp Neurol 2005, 64, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Wali, A.R.; Pham, M.H. Efficacy of riluzole in the treatment of spinal cord injury: a systematic review of the literature. Neurosurg Focus 2019, 46, E6. [Google Scholar] [CrossRef]
- Lord-Fontaine, S.; Yang, F.; Diep, Q.; Dergham, P.; Munzer, S.; Tremblay, P.; McKerracher, L. Local inhibition of Rho signaling by cell-permeable recombinant protein BA-210 prevents secondary damage and promotes functional recovery following acute spinal cord injury. J Neurotrauma 2008, 25, 1309–1322. [Google Scholar] [CrossRef]
- Cheng, H.; Cao, Y.; Olson, L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 1996, 273, 510–513. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Hsiao, I.; Lin, V.W. Peripheral nerve grafts and aFGF restore partial hindlimb function in adult paraplegic rats. J Neurotrauma 2002, 19, 1203–1216. [Google Scholar] [CrossRef]
- Zörner, B.; Schwab, M.E. Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci 2010, 1198 Suppl 1, E22–34. [Google Scholar] [CrossRef]
- Freund, P.; Wannier, T.; Schmidlin, E.; Bloch, J.; Mir, A.; Schwab, M.E.; Rouiller, E.M. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J Comp Neurol 2007, 502, 644–659. [Google Scholar] [CrossRef]
- Jacobson, P.B.; Goody, R.; Lawrence, M.; Mueller, B.K.; Zhang, X.; Hooker, B.A.; Pfleeger, K.; Ziemann, A.; Locke, C.; Barraud, Q.; et al. Elezanumab, a human anti-RGMa monoclonal antibody, promotes neuroprotection, neuroplasticity, and neurorecovery following a thoracic hemicompression spinal cord injury in non-human primates. Neurobiol Dis 2021, 155, 105385. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Coelho, M.; Huang, L.; Monnier, P.P.; Cui, Y.-F.; Mueller, B.K.; Jacobson, P.B.; Tator, C.H. Delayed administration of the human anti-RGMa monoclonal antibody elezanumab promotes functional recovery including spontaneous voiding after spinal cord injury in rats. Neurobiol Dis 2020, 143, 104995. [Google Scholar] [CrossRef]
- Mothe, A.J.; Jacobson, P.B.; Caprelli, M.; Ulndreaj, A.; Rahemipour, R.; Huang, L.; Monnier, P.P.; Fehlings, M.G.; Tator, C.H. Delayed administration of elezanumab, a human anti-RGMa neutralizing monoclonal antibody, promotes recovery following cervical spinal cord injury. Neurobiol Dis 2022, 172, 105812. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, N.; Masani, K.; Catharine Craven, B.; Giangregorio, L.M.; Hitzig, S.L.; Richards, K.; Popovic, M.R. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J Spinal Cord Med 2014, 37, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Thrasher, T.A.; Flett, H.M.; Popovic, M.R. Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord 2006, 44, 357–361. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).