Submitted:
26 June 2023
Posted:
27 June 2023
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
METHODS
RESULTS
DISCUSSION
CONCLUSIONS
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Humboldt, A. Essai sur la géographie des plantes: accompagne d'un tableau physique des régions équinoxiales, fondé sur des mesures exécutées, depuis le dixième degré la latitude boréale juasqu'au dixième degré de latitude australe, pendant les années 1799, 1800, 1801, 1802 et 1803 par Al. de Humboldt et A. Bonpland; Schoell et Cie.: Chez Levrault, France, 1805. [Google Scholar]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catalina Mountains, Arizona: a gradient analysis of the south slope. Ecology 1965, 46, 429–452. [Google Scholar] [CrossRef]
- Clark, D.B.; Palmer, M.W.; Clark, D.A. Edaphic factors and the landscape-scale distributions of tropical rain forest trees. Ecology 1999, 80, 2662–2675. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. Journal of Ecology 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Zuleta, D.; Russo, S.E.; Barona, A.; Barreto-Silva, J.S.; Cardenas, D.; Castaño, N.; Davies, S.J.; Detto, M.; Sua, S.; Turner, B.L. Importance of topography for tree species habitat distributions in a terra firme forest in the Colombian Amazon. Plant and Soil 2020, 450, 133–149. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.-C.; Hernandez, C.; Romoleroux, K.; Losos, E.; Magard, E.; Balslev, H. Tree species distributions and local habitat variation in the Amazon: large forest plot in eastern Ecuador. J Ecology 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Salinas, N.; Malhi, Y.; Meir, P.; Silman, M.; Roman Cuesta, R.; Huaman, J.; Salinas, D.; Huaman, V.; Gibaja, A.; Mamani, M. The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New phytologist 2011, 189, 967–977. [Google Scholar] [CrossRef]
- Malhi, Y.; Girardin, C.A.; Goldsmith, G.R.; Doughty, C.E.; Salinas, N.; Metcalfe, D.B.; Huaraca Huasco, W.; Silva-Espejo, J.E.; del Aguilla-Pasquell, J.; Farfán Amézquita, F. The variation of productivity and its allocation along a tropical elevation gradient: a whole carbon budget perspective. New Phytologist 2017, 214, 1019–1032. [Google Scholar] [CrossRef]
- Feeley, K.J.; Silman, M.R.; Bush, M.B.; Farfan, W.; Cabrera, K.G.; Malhi, Y.; Meir, P.; Revilla, N.S.; Quisiyupanqui, M.N.R.; Saatchi, S. Upslope migration of Andean trees. Journal of Biogeography 2011, 38, 783–791. [Google Scholar] [CrossRef]
- Feeley, K.J.; Hurtado, J.; Saatchi, S.; Silman, M.R.; Clark, D.B. Compositional shifts in Costa Rican forests due to climate-driven species migrations. Global Change Biology 2013, 19, 3472–2480. [Google Scholar] [CrossRef]
- Duque, A.; Stevenson, P.; Feeley, K.J. Thermophilization of adult and juvenile tree communities in the northern tropical Andes. Proceedings of the National Academy of Sciences USA 2015, 112, 10744–10749. [Google Scholar] [CrossRef]
- Girardin, C.A.J.; Malhi, Y.; Aragao, L.; Mamani, M.; Huaraca Huasco, W.; Durand, L.; Feeley, K.; Rapp, J.; SILVA-ESPEJO, J.; Silman, M. Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Global Change Biology 2010, 16, 3176–3192. [Google Scholar] [CrossRef]
- Rapp, J.; Silman, M. Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Climate Research 2012, 55, 17–32. [Google Scholar] [CrossRef]
- Malizia, A.; Blundo, C.; Carilla, J.; Osinaga Acosta, O.; Cuesta, F.; Duque, A.; Aguirre, N.; Aguirre, Z.; Ataroff, M.; Baez, S.; et al. Elevation and latitude drives structure and tree species composition in Andean forests: Results from a large-scale plot network. PLOS ONE 2020, 15, e0231553. [Google Scholar] [CrossRef]
- Homeier, J.; Breckle, S.W.; Günter, S.; Rollenbeck, R.T.; Leuschner, C. Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich Ecuadorian montane rain forest. BIOTROPICA 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Báez, S.; Malizia, A.; Carilla, J.; Blundo, C.; Aguilar, M.; Aguirre, N.; Aquirre, Z.; Álvarez, E.; Cuesta, F.; Duque, Á.; et al. Large-scale patterns of turnover and basal area change in Andean forests. PLoS ONE 2015, 10, e0126594. [Google Scholar] [CrossRef]
- Quiroga, M.P.; Pacheco, S.; Malizia, L.R.; Premoli, A.C. Shrinking forests under warming: evidence of Podocarpus parlatorei (pino del cerro) from the subtropical Andes. Journal of Heredity 2012, 103, 682–691. [Google Scholar] [CrossRef]
- Krömer, T.; Kessler, M.; Gradstein, S.R. Vertical stratification of vascular epiphytes in submontane and montane forest of the Bolivian Andes: the importance of the understory. Plant Ecology 2007, 189, 261–278. [Google Scholar] [CrossRef]
- Fadrique, B.; Báez, S.; Duque, Á.; Malizia, A.; Blundo, C.; Carilla, J.; Osinaga-Acosta, O.; Malizia, L.; Silman, M.; Farfán-Ríos, W.; et al. Widespread but heterogeneous responses of Andean forests to climate change. Nature 2018, 564, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Feeley, K.J.; Silman, M.R. Land-use and climate change effects on population size and extinction risk of Andean plants. Global Change Biology 2010, 16, 3215–3222. [Google Scholar] [CrossRef]
- Feeley, K.J.; Davies, S.J.; Perez, R.; Hubbell, S.P.; Foster, R.B. Directional changes in the species composition of a tropical forest. Ecology 2011, 92, 871–882. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E. Compositional response of Amazon forests to climate change. Global change biology 2019, 25, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Feeley, K.J.; Bravo-Avila, C.; Fadrique, B.; Perez, T.M.; Zuleta, D. Climate-driven changes in the composition of New World plant communities. Nature Climate Change 2020. [CrossRef]
- Vuille, M.; Bradley, R.S.; Werner, M.; Keimig, F. 20th century climate change in the tropical Andes: observations and model results. Climate variability and change in high elevation regions: Past, present & future 2003, 75–99.
- Urrutia, R.; Vuille, M. Climate change projections for the tropical Andes using a regional climate model: Temperature and precipitation simulations for the end of the 21st century. Journal of Geophysical Research 2009, 114. [Google Scholar] [CrossRef]
- Bruijnzeel, L.; Mulligan, M.; Scatena, F.N. Hydrometeorology of tropical montane cloud forests: emerging patterns. Hydrological Processes 2011, 25, 465–498. [Google Scholar] [CrossRef]
- Martin, P.H.; Sherman, R.E.; Fahey, T.J. Tropical montane forest ecotones: climate gradients, natural disturbance, and vegetation zonation in the Cordillera Central, Dominican Republic. Journal of Biogeography 2007, 34, 1792–1806. [Google Scholar] [CrossRef]
- Santiago, L.S.; Schuur, E.A.; Silvera, K. Nutrient cycling and plant–soil feedbacks along a precipitation gradient in lowland Panama. Journal of Tropical Ecology 2005, 21, 461–470. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Whitaker, J.; Turner, B.L.; Salinas, N.; Zimmermann, M.; Malhi, Y.; Meir, P. Climate warming and soil carbon in tropical forests: insights from an elevation gradient in the Peruvian Andes. Bioscience 2015, 65, 906–921. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Dawson, T.E.; Draguljić, D. Variation in the resilience of cloud forest vascular epiphytes to severe drought. New Phytologist 2018, 219, 900–913. [Google Scholar] [CrossRef]
- Horwath, A.B.; Royles, J.; Tito, R.; Gudiño, J.A.; Salazar Allen, N.; Farfan-Rios, W.; Rapp, J.M.; Silman, M.R.; Malhi, Y.; Swamy, V. Bryophyte stable isotope composition, diversity and biomass define tropical montane cloud forest extent. Proceedings of the Royal Society B 2019, 286, 20182284. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meir, P.; Silman, M.; Fedders, A.; Gibbon, A.; Malhi, Y.; Urrego, D.; Bush, M.; Feeley, K.; Garcia, K.; et al. No differences in soil carbon stocks across the tree line in the Peruvian Andes. Ecosystems 2010, 13, 62–74. [Google Scholar] [CrossRef]
- Rehm, E.M.; Feeley, K.J. Forest patches and the upward migration of timberline in the southern Peruvian Andes. Forest Ecology and Management 2013, 305, 204–211. [Google Scholar] [CrossRef]
- Venturas, M.D.; Sperry, J.S.; Hacke, U.G. Plant xylem hydraulics: what we understand, current research, and future challenges. Journal of integrative plant biology 2017, 59, 356–389. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proceedings of the National Academy of Sciences 2016, 113, 13098–13103. [Google Scholar] [CrossRef] [PubMed]
- Kerstiens, G. Cuticular water permeability and its physiological significance. Journal of experimental botany 1996, 47, 1813–1832. [Google Scholar] [CrossRef]
- Slot, M.; Nardwattanawong, T.; Hernández, G.G.; Bueno, A.; Riederer, M.; Winter, K. Large differences in leaf cuticle conductance and its temperature response among 24 tropical tree species from across a rainfall gradient. New Phytologist 2021, 232, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New phytologist 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Volaire, F. A unified framework of plant adaptive strategies to drought: crossing scales and disciplines. Global change biology 2018, 24, 2929–2938. [Google Scholar] [CrossRef]
- Agarie, S.; Hanaoka, N.; Kubota, F.; Agata, W.; Kaufman, P.B. Measurement of cell membrane stability evaluated by electrolyte leakage as a drought and heat tolerance test in rice (Oryza sativa L.). 1995. [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat 1. Crop Science 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Shimada, T. The measurement of cell membrane stability using polyethylene glycol as a drought tolerance test in wheat. Japanese Journal of Crop Science 1987, 56, 92–98. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. The Journal of Agricultural Science 1990, 115, 63–66. [Google Scholar] [CrossRef]
- Fadrique, B.; Baraloto, C.; Bravo-Avila, C.H.; Feeley, K.J. Bamboo climatic tolerances are decoupled from leaf functional traits across an Andean elevation gradient. Oikos 2022, 2022, e09229. [Google Scholar] [CrossRef]
- Zuleta, D.; Muller-Landau, H.C.; Duque, A.; Caro, N.; Cardenas, D.; Castaño, N.; León-Peláez, J.D.; Feeley, K.J. Interspecific and intraspecific variation of tree branch, leaf and stomatal traits in relation to topography in an aseasonal Amazon forest. Functional Ecology 2022, 36, 2955–2968. [Google Scholar] [CrossRef]
- Santiago, L.S.; De Guzman, M.E.; Baraloto, C.; Vogenberg, J.E.; Brodie, M.; Hérault, B.; Fortunel, C.; Bonal, D. Coordination and trade-offs among hydraulic safety, efficiency and drought avoidance traits in Amazonian rainforest canopy tree species. New Phytologist 2018, 218, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Killeen, T.J.; Douglas, M.; Consiglio, T.; Jørgensen, P.M.; Mejia, J. Dry spots and wet spots in the Andean hotspot. Journal of Biogeography 2007, 34, 1357–1373. [Google Scholar] [CrossRef]
- Lenz, T.I.; Wright, I.J.; Westoby, M. Interrelations among pressure–volume curve traits across species and water availability gradients. Physiologia Plantarum 2006, 127, 423–433. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Schulze, E.-D.; Zimmermann, R. Measurement of transpiration and leaf conductance. Plant physiological ecology: field methods and instrumentation 2000, 137–160.
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regulation 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Whitlow, T.H.; Bassuk, N.L.; Ranney, T.G.; Reichert, D.L. An improved method for using electrolyte leakage to assess membrane competence in plant tissues. Plant Physiology 1992, 98, 198–205. [Google Scholar] [CrossRef]
- Premachandra, G.; Saneoka, H.; Ogata, S. Nutrio-physiological evaluation of the polyethylene glycol test of cell membrane stability in maize. Crop science 1989, 29, 1287–1292. [Google Scholar] [CrossRef]
- França, M.G.C.; Thi, A.T.P.; Pimentel, C.; Rossiello, R.O.P.; Zuily-Fodil, Y.; Laffray, D. Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environmental and Experimental Botany 2000, 43, 227–237. [Google Scholar] [CrossRef]
- Saneoka, H.; Moghaieb, R.E.; Premachandra, G.S.; Fujita, K. Nitrogen nutrition and water stress effects on cell membrane stability and leaf water relations in Agrostis palustris Huds. Environmental and Experimental Botany 2004, 52, 131–138. [Google Scholar] [CrossRef]
- Team, R.C. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Tobin, M.F.; Lopez, O.R.; Kursar, T.A. Responses of Tropical Understory Plants to a Severe Drought: Tolerance and Avoidance of Water Stress 1. BIOTROPICA 1999, 31, 570–578. [Google Scholar] [CrossRef]
- Jane, G.; Green, T. Patterns of stomatal conductance in six evergreen tree species from a New Zealand cloud forest. Botanical gazette 1985, 146, 413–420. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods in Ecology and Evolution 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Cavelier, J. Tissue water relations in elfin cloud forest tree species of Serrania de Macuira, Guajira, Colombia. Trees 1990, 4, 155–163. [Google Scholar] [CrossRef]
- Lenz, T.I.; Wright, I.J.; Westoby, M. Interrelations among pressure-volume curve traits across species and water availability gradients. Physiologia Plantarum 2006, 127, 423–433. [Google Scholar] [CrossRef]
- Goldsmith, G.R.; Matzke, N.J.; Dawson, T.E. The incidence and implications of clouds for cloud forest plant water relations. Ecology Letters 2013, 16, 307–314. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Fujita, K.; Ogata, S. Leaf water relations, osmotic adjustment, cell membrane stability, epicuticular wax load and growth as affected by increasing water deficits in sorghum. Journal of experimental botany 1992, 43, 1569–1576. [Google Scholar] [CrossRef]
- Yang, G.; Rhodes, D.; Joly, R. Effects of High Temperature on Membrane Stability and Chlorophyll Fluorescence in Glycinebetaine-Deficient and Glycinebetaine-Containing Maize Lines. Functional Plant Biology 1996, 23, 437–443. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Archives of Biochemistry and Biophysics 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Fadrique, B.; Baraloto, C.; Bravo-Avila, C.H.; Feeley, K.J. Bamboo climatic tolerances are decoupled from functional traits across an Andean elevation gradient. Oikos 2022. In Press. [Google Scholar] [CrossRef]
- Haylock, M.R.; Peterson, T.C.; Alves, L.M.; Ambrizzi, T.; Anunciação, Y.M.T.; Baez, J.; Barros, V.R.; Berlato, M.A.; Bidegain, M.; Coronel, G.; et al. Trends in Total and Extreme South American Rainfall in 1960–2000 and Links with Sea Surface Temperature. Journal of Climate 2006, 19, 1490–1512. [Google Scholar] [CrossRef]
- Halladay, K.; Malhi, Y.; New, M. Cloud frequency climatology at the Andes/Amazon transition: 2. Trends and variability. Journal of Geophysical Research: Atmospheres (1984–2012) 2012, 117.
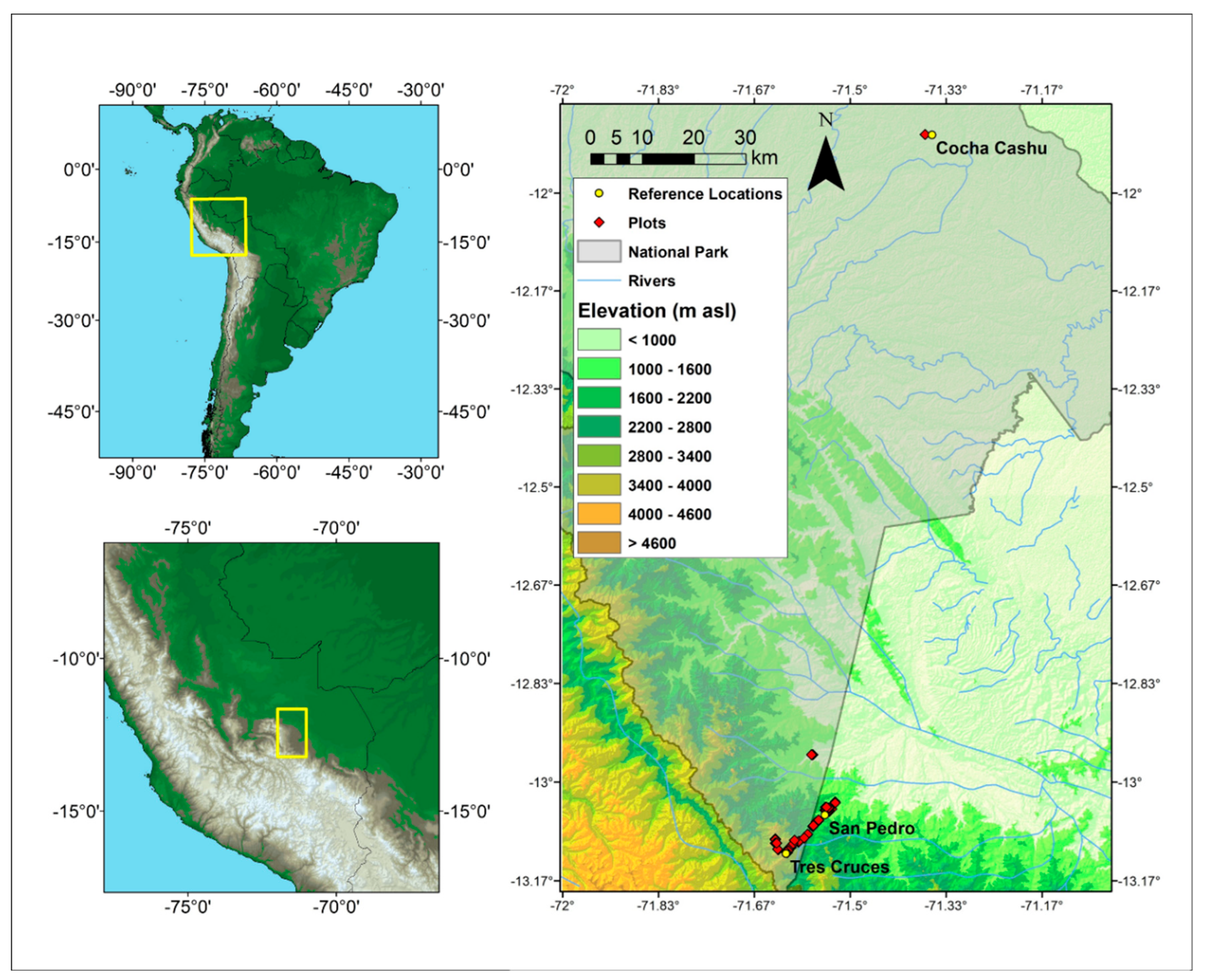
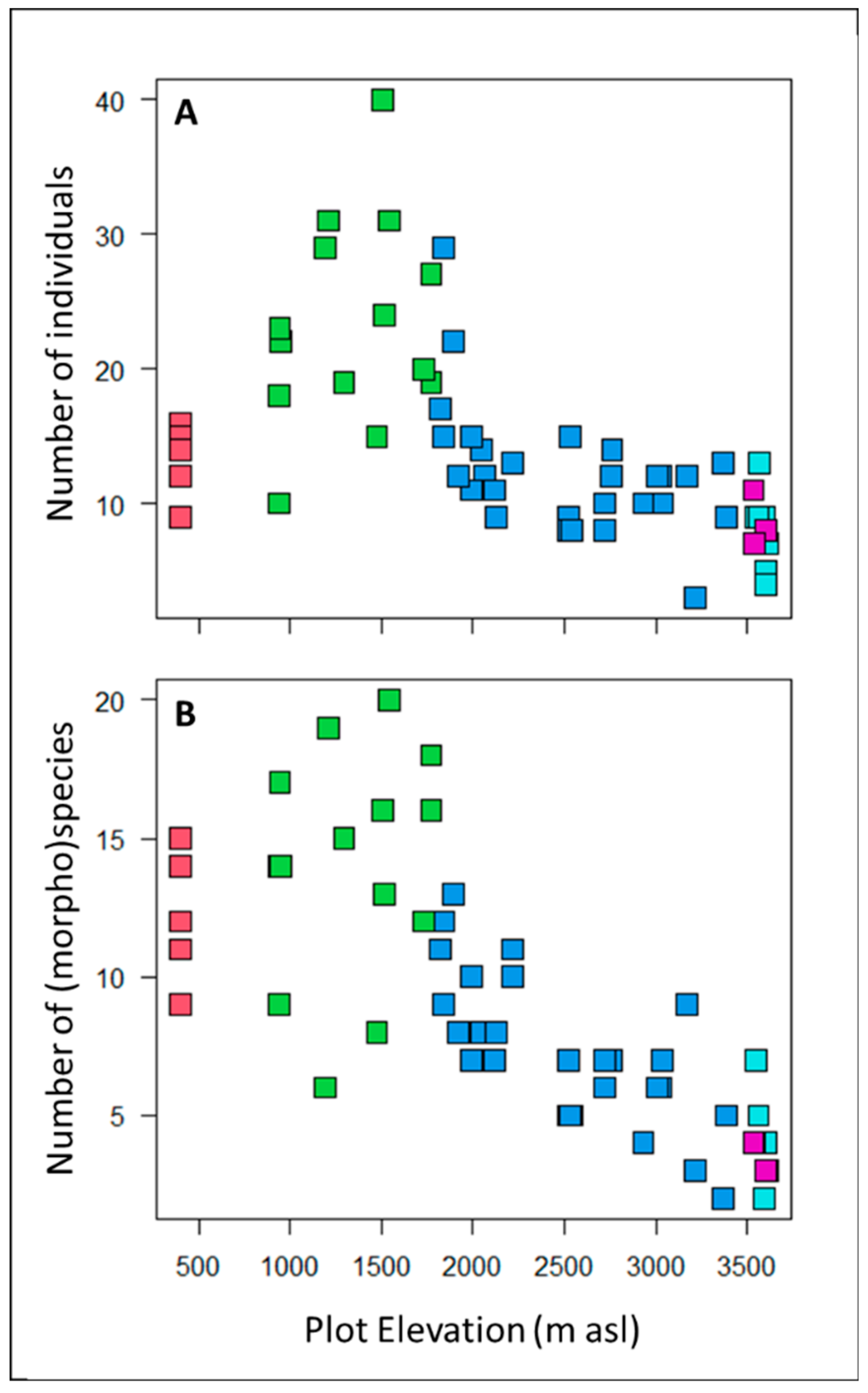
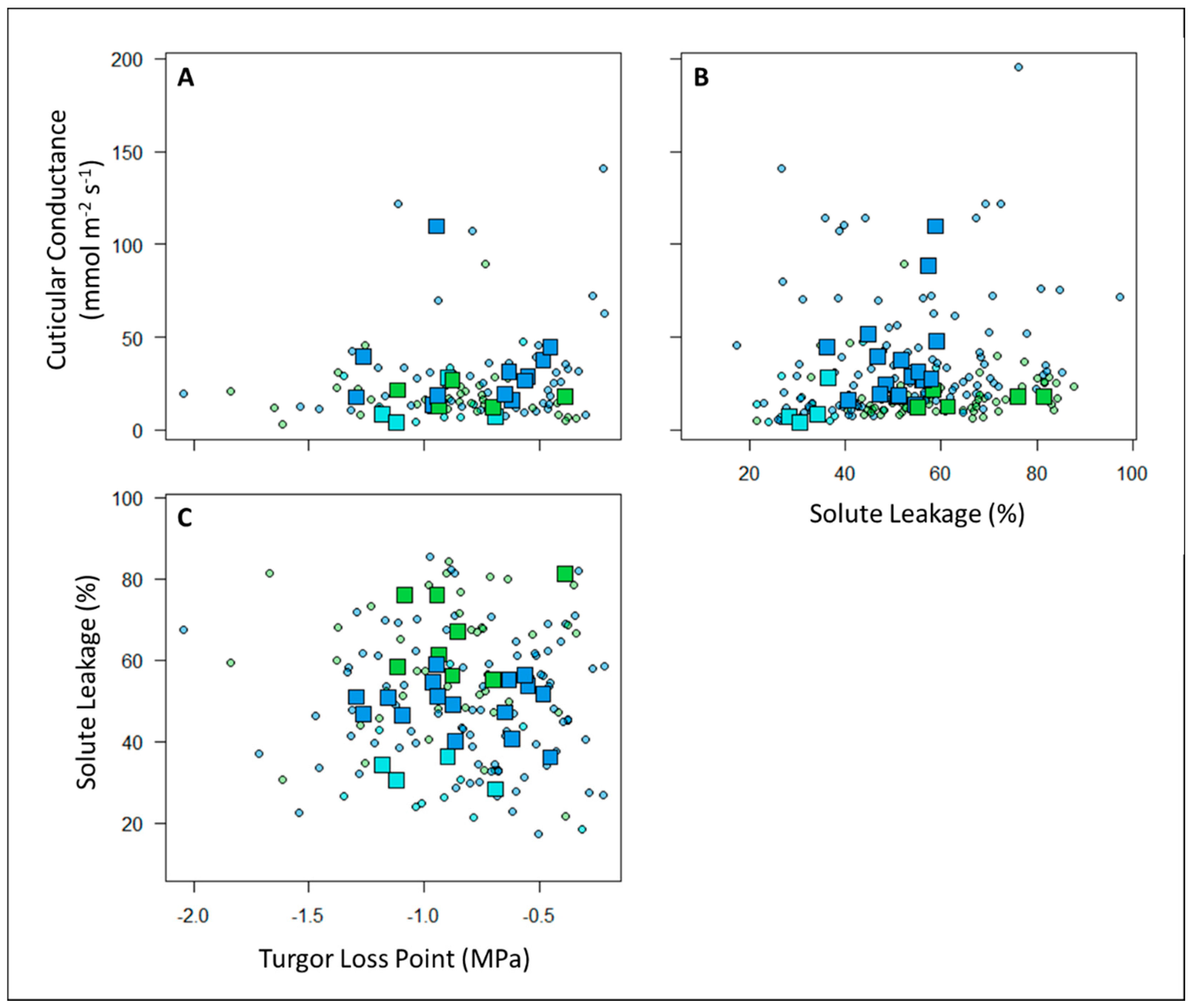
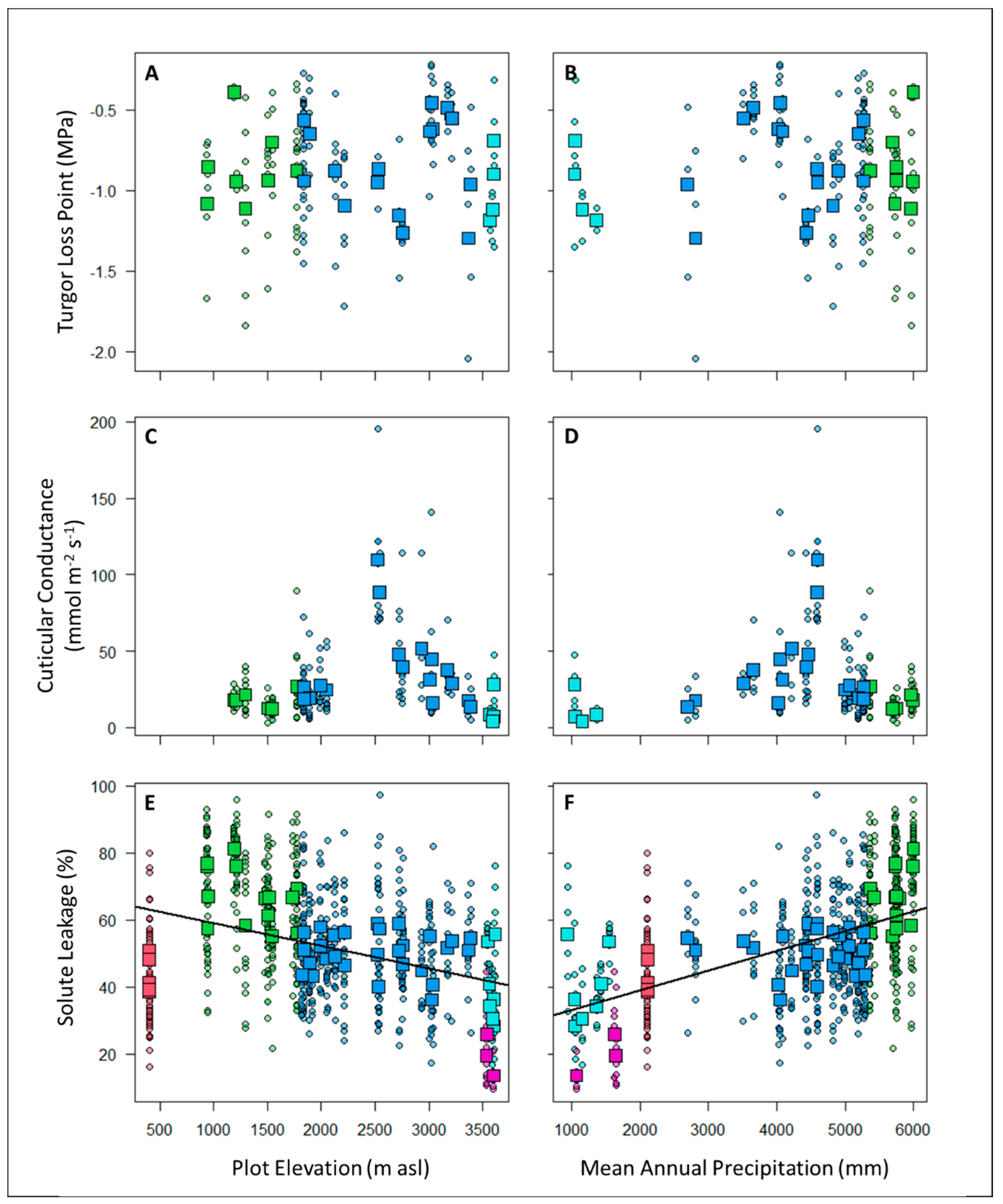
| Plot | Latitude (º) | Longitude (º) | Elevation (m asl) | MAT (º C) | MAP (mm/year) | Habitat | N. Individuals | N. Shrubs | N. Sapling | N. Families | N. Genera | N. (Morpho)Species |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | -13.096 | -71.630 | 3604 | 8.2 | 1617 | gallery forest | 5 | 3 | 2 | 3 | 4 | 4 |
| 2 | -13.097 | -71.630 | 3569 | 8.2 | 1437 | gallery forest | 13 | 5 | 8 | 3 | 3 | 4 |
| 3 | -13.103 | -71.630 | 3603 | 8.0 | 1054 | gallery forest | 4 | 3 | 1 | 2 | 2 | 4 |
| 4 | -13.103 | -71.629 | 3592 | 18.9 | 1157 | gallery forest | 9 | 0 | 9 | 1 | 2 | 2 |
| 5 | -13.114 | -71.625 | 3548 | 18.9 | 1549 | gallery forest | 9 | 5 | 4 | 2 | 1 | 7 |
| 6 | -13.113 | -71.626 | 3562 | 10.1 | 1515 | gallery forest | 9 | 3 | 6 | 3 | 2 | 5 |
| 7 | -13.103 | -71.628 | 3615 | 12.5 | 937 | gallery forest | 7 | 1 | 6 | 3 | 2 | 3 |
| 8 | -13.110 | -71.603 | 3172 | 18.7 | 3654 | cloud forest | 12 | 7 | 5 | 4 | 5 | 9 |
| 9 | -13.110 | -71.604 | 3214 | 18.7 | 3507 | cloud forest | 3 | 3 | 0 | 2 | 3 | 3 |
| 10 | -13.114 | -71.607 | 3366 | 17.7 | 2812 | cloud forest | 13 | 9 | 4 | 2 | 2 | 2 |
| 11 | -13.112 | -71.607 | 3387 | 17.7 | 2692 | cloud forest | 9 | 1 | 8 | 4 | 4 | 5 |
| 12 | -13.033 | -71.526 | 1189 | 10.1 | 5992 | sub-montane | 29 | 21 | 8 | 5 | 5 | 6 |
| 13 | -13.035 | -71.526 | 1212 | 17.7 | 5990 | sub-montane | 31 | 16 | 15 | 5 | 5 | 19 |
| 14 | -13.045 | -71.532 | 1296 | 20.2 | 5989 | sub-montane | 19 | 7 | 12 | 10 | 9 | 15 |
| 15 | -13.047 | -71.544 | 1771 | 19.7 | 5363 | sub-montane | 27 | 9 | 18 | 6 | 8 | 18 |
| 16 | -13.047 | -71.542 | 1837 | 17.7 | 5266 | cloud forest | 29 | 6 | 23 | 5 | 6 | 12 |
| 17 | -13.049 | -71.536 | 1503 | 20.1 | 5744 | sub-montane | 40 | 8 | 32 | 6 | 9 | 16 |
| 18 | -13.048 | -71.537 | 1545 | 8.0 | 5694 | sub-montane | 31 | 19 | 12 | 7 | 8 | 20 |
| 19 | -13.119 | -71.609 | 3601 | 10.1 | 1082 | puna shrubs | 8 | 8 | 0 | 0 | 0 | 3 |
| 20 | -13.116 | -71.608 | 3539 | 12.5 | 1082 | puna shrubs | 7 | 7 | 0 | 0 | 0 | 4 |
| 21 | -13.118 | -71.611 | 3536 | 10.1 | 1082 | puna shrubs | 11 | 11 | 0 | 0 | 0 | 4 |
| 22 | -13.101 | -71.590 | 2754 | 13.3 | 4428 | cloud forest | 12 | 5 | 7 | 4 | 3 | 7 |
| 23 | -13.101 | -71.590 | 2762 | 13.6 | 4421 | cloud forest | 14 | 5 | 9 | 7 | 6 | 7 |
| 24 | -13.100 | -71.589 | 2721 | 11.2 | 4457 | cloud forest | 10 | 5 | 5 | 5 | 5 | 6 |
| 25 | -13.100 | -71.589 | 2724 | 12.5 | 4455 | cloud forest | 8 | 4 | 4 | 5 | 4 | 7 |
| 26 | -13.089 | -71.575 | 2525 | 14.4 | 4595 | cloud forest | 9 | 6 | 3 | 4 | 4 | 7 |
| 27 | -13.089 | -71.575 | 2526 | 14.4 | 4594 | cloud forest | 8 | 4 | 4 | 2 | 1 | 5 |
| 28 | -13.088 | -71.574 | 2542 | 13.6 | 4584 | cloud forest | 8 | 1 | 7 | 4 | 1 | 5 |
| 29 | -13.094 | -71.580 | 2532 | 17.7 | 4590 | cloud forest | 15 | 12 | 3 | 3 | 4 | 5 |
| 30 | -13.104 | -71.599 | 3036 | 22.1 | 4021 | cloud forest | 10 | 7 | 3 | 3 | 2 | 7 |
| 31 | -13.104 | -71.599 | 3025 | 14.5 | 4045 | cloud forest | 12 | 8 | 4 | 3 | 4 | 6 |
| 32 | -13.104 | -71.599 | 3006 | 21.8 | 4084 | cloud forest | 12 | 1 | 11 | 3 | 3 | 6 |
| 33 | -13.098 | -71.597 | 2933 | 17.7 | 4213 | cloud forest | 10 | 4 | 6 | 3 | 3 | 4 |
| 34 | -13.070 | -71.560 | 2064 | 8.7 | 4973 | cloud forest | 12 | 3 | 9 | 6 | 7 | 8 |
| 35 | -13.070 | -71.560 | 2048 | 9.2 | 4991 | cloud forest | 14 | 4 | 10 | 7 | 7 | 8 |
| 36 | -13.068 | -71.559 | 1994 | 8.2 | 5056 | cloud forest | 11 | 6 | 5 | 6 | 6 | 7 |
| 37 | -13.067 | -71.559 | 1992 | 8.2 | 5059 | cloud forest | 15 | 3 | 12 | 6 | 8 | 10 |
| 38 | -13.065 | -71.556 | 1891 | 10.1 | 5191 | cloud forest | 22 | 8 | 14 | 8 | 9 | 13 |
| 39 | -13.066 | -71.556 | 1920 | 8.2 | 5152 | cloud forest | 12 | 7 | 5 | 6 | 6 | 8 |
| 40 | -13.065 | -71.555 | 1839 | 13.3 | 5265 | cloud forest | 15 | 1 | 14 | 7 | 9 | 9 |
| 41 | -13.064 | -71.555 | 1820 | 14.4 | 5292 | cloud forest | 17 | 8 | 9 | 4 | 6 | 11 |
| 42 | -13.074 | -71.565 | 2217 | 17.7 | 4789 | cloud forest | 13 | 6 | 7 | 7 | 6 | 10 |
| 43 | -13.075 | -71.565 | 2121 | 17.7 | 4789 | cloud forest | 11 | 1 | 10 | 5 | 5 | 7 |
| 44 | -13.074 | -71.565 | 2217 | 18.7 | 4789 | cloud forest | 13 | 10 | 3 | 4 | 5 | 11 |
| 45 | -13.073 | -71.564 | 2130 | 18.7 | 4789 | cloud forest | 9 | 3 | 6 | 7 | 7 | 8 |
| 46 | -13.044 | -71.536 | 1474 | 17.7 | 5754 | sub-montane | 15 | 2 | 13 | 5 | 6 | 8 |
| 47 | -13.043 | -71.537 | 1514 | 17.7 | 5754 | sub-montane | 24 | 8 | 16 | 10 | 10 | 13 |
| 48 | -13.042 | -71.543 | 1772 | 18.9 | 5395 | sub-montane | 19 | 11 | 8 | 13 | 12 | 16 |
| 49 | -13.042 | -71.541 | 1732 | 18.9 | 5395 | sub-montane | 20 | 2 | 18 | 7 | 11 | 12 |
| 50 | -12.954 | -71.565 | 937 | 17.7 | 5849 | sub-montane | 18 | 4 | 14 | 9 | 10 | 14 |
| 51 | -12.954 | -71.566 | 938 | 18.9 | 5849 | sub-montane | 10 | 6 | 4 | 6 | 6 | 9 |
| 52 | -12.954 | -71.567 | 946 | 22.1 | 5849 | sub-montane | 22 | 13 | 9 | 7 | 8 | 14 |
| 53 | -12.954 | -71.567 | 944 | 21.8 | 5849 | sub-montane | 23 | 13 | 10 | 8 | 11 | 17 |
| 54 | -11.900 | -71.370 | 400 | 25.0 | 1366 | lowland forest | 9 | 0 | 9 | 0 | 0 | 9 |
| 55 | -11.900 | -71.370 | 400 | 25.0 | 1366 | lowland forest | 16 | 0 | 16 | 11 | 11 | 11 |
| 56 | -11.900 | -71.370 | 400 | 25.0 | 1366 | lowland forest | 15 | 0 | 15 | 0 | 0 | 15 |
| 57 | -11.900 | -71.370 | 400 | 25.0 | 1366 | lowland forest | 14 | 0 | 14 | 0 | 0 | 14 |
| 58 | -11.900 | -71.370 | 400 | 25.0 | 1366 | lowland forest | 12 | 0 | 12 | 11 | 12 | 12 |
| Plot | Leakage (%) | L_se | TLP (MPa) | TLP_se | Gmin (mmol m-2 s-1) | Gmin_se |
|---|---|---|---|---|---|---|
| 1 | 36.40 | 6.74 | -0.95 | 0.15 | 26.43 | 5.00 |
| 2 | 34.26 | 2.30 | -1.20 | 0.02 | 8.87 | 2.04 |
| 3 | 28.37 | 4.07 | -0.69 | 0.12 | 7.06 | 0.22 |
| 4 | 30.47 | 3.77 | -1.04 | 0.04 | 4.18 | 0.00 |
| 5 | 53.59 | 1.90 | NA | NA | NA | NA |
| 6 | 41.06 | 1.28 | NA | NA | NA | NA |
| 7 | 55.77 | 6.78 | NA | NA | NA | NA |
| 8 | 51.76 | 4.23 | -0.44 | 0.04 | 33.88 | 6.53 |
| 9 | 53.79 | 20.95 | -0.55 | 0.10 | 28.86 | 3.21 |
| 10 | 50.96 | 2.07 | -1.42 | 0.31 | 21.11 | 4.92 |
| 11 | 54.59 | 4.64 | -0.96 | 0.22 | 12.73 | 1.65 |
| 12 | 81.36 | 1.17 | -0.36 | 0.01 | 23.20 | 2.61 |
| 13 | 76.05 | 2.06 | -0.94 | 0.03 | 22.48 | 1.99 |
| 14 | 58.32 | 4.62 | -0.96 | 0.15 | 26.02 | 3.02 |
| 15 | 56.19 | 3.21 | -0.93 | 0.09 | 20.46 | 4.26 |
| 16 | 56.33 | 2.65 | -0.51 | 0.03 | 17.98 | 3.37 |
| 17 | 61.42 | 2.02 | -0.89 | 0.06 | 11.60 | 0.84 |
| 18 | 55.12 | 2.16 | -0.65 | 0.07 | 11.97 | 1.10 |
| 19 | 13.62 | 1.24 | NA | NA | NA | NA |
| 20 | 26.02 | 4.38 | NA | NA | NA | NA |
| 21 | 19.50 | 2.62 | NA | NA | NA | NA |
| 22 | 46.82 | 2.68 | -1.28 | 0.02 | 35.85 | 7.35 |
| 23 | 52.47 | 3.55 | NA | NA | NA | NA |
| 24 | 50.92 | 5.19 | -1.16 | 0.07 | NA | NA |
| 25 | 59.04 | 5.72 | NA | NA | 47.17 | 8.20 |
| 26 | 58.89 | 6.56 | -0.96 | 0.04 | 99.11 | 14.28 |
| 27 | 49.76 | 6.76 | NA | NA | NA | NA |
| 28 | 57.37 | 8.18 | NA | NA | 96.76 | 8.34 |
| 29 | 40.11 | 2.95 | -0.87 | 0.00 | NA | NA |
| 30 | 40.69 | 5.38 | -0.62 | 0.06 | 15.63 | 2.25 |
| 31 | 36.21 | 3.47 | -0.37 | 0.07 | 74.28 | 22.50 |
| 32 | 55.23 | 3.52 | -0.60 | 0.06 | 31.21 | 0.00 |
| 33 | 44.89 | 2.93 | NA | NA | 57.21 | 19.77 |
| 34 | 50.80 | 4.98 | NA | NA | NA | NA |
| 35 | 48.40 | 2.65 | NA | NA | 21.30 | 5.44 |
| 36 | 52.36 | 3.51 | NA | NA | NA | NA |
| 37 | 57.97 | 3.21 | NA | NA | 23.95 | 3.87 |
| 38 | 47.25 | 2.53 | -0.70 | 0.06 | 17.71 | 3.38 |
| 39 | 43.25 | 2.94 | NA | NA | NA | NA |
| 40 | 51.18 | 4.49 | -0.96 | 0.09 | 17.76 | 2.39 |
| 41 | 43.68 | 2.71 | NA | NA | NA | NA |
| 42 | 46.59 | 4.25 | -1.02 | 0.09 | NA | NA |
| 43 | 55.56 | 3.60 | NA | NA | NA | NA |
| 44 | 56.27 | 4.06 | NA | NA | NA | NA |
| 45 | 49.21 | 4.06 | -0.81 | 0.11 | NA | NA |
| 46 | 66.56 | 3.82 | NA | NA | NA | NA |
| 47 | 66.67 | 3.39 | NA | NA | NA | NA |
| 48 | 69.45 | 3.56 | NA | NA | NA | NA |
| 49 | 66.79 | 4.22 | NA | NA | NA | NA |
| 50 | 75.95 | 3.47 | -1.01 | 0.11 | NA | NA |
| 51 | 76.98 | 3.78 | NA | NA | NA | NA |
| 52 | 66.99 | 4.14 | -0.84 | 0.03 | NA | NA |
| 53 | 57.47 | 3.80 | NA | NA | NA | NA |
| 54 | 46.46 | 5.40 | NA | NA | NA | NA |
| 55 | 38.61 | 3.47 | NA | NA | NA | NA |
| 56 | 48.30 | 3.35 | NA | NA | NA | NA |
| 57 | 41.27 | 3.77 | NA | NA | NA | NA |
| 58 | 39.19 | 3.52 | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





