1. Introduction
The introduction should briefly place the study in a broad context and highlight why it is important. It should define the purpose of the work and its significance. The current state of the research field should be carefully reviewed and key publications cited. Please highlight controversial and diverging hypotheses when necessary. Finally, briefly mention the main aim of the work and highlight the principal conclusions. As far as possible, please keep the introduction comprehensible to scientists outside your particular field of research.
Infertility affects 15% reproductive-aged couples globally based on World Health Organization reports. In recent decades, the fertility outcome of In-vitro fertilization (IVF) treatment has been greatly improved by the introduction of new methods of assisted reproductive techniques, such as assisted hatching [
1], intracytoplasmic sperm injection (ICSI) [
2], assisted oocyte activation (AOA) [
3], spindle imaging [also known as spindle view (SV) or polarization microscopy] [
4], and so on. However, according to previous studies, the fertilization rate and the clinical pregnancy rate of ICSI treatment had stabilized around 70% to 80% and 40% to 45% [
5,
6], and the total fertilization failure remained between 1% to 5% [
7,
8].
ICSI was primarily indicated for male factor infertility, but the fertility outcome of ICSI treatment, such as fertilization rate and clinical pregnancy rate, seems to remain constant [
5]. Polarization microscopy has been proved to improve fertilization rate of ICSI treatment, but lowered cleavage rate and top-quality embryo formation were also found [
9]. The cause of fertilization failure has been attributable to oocyte activation failure [
10], which might be caused by impaired sperm parameter and lower oocyte quality [
11]. Fertilization rate could be greatly elevated by means of AOA in patients with clear indication of oocyte activation deficiency [
12]. AOA will increase intracellular calcium ions in oocyte to trigger its activation [
13], through physiological stimulation of calcium ion oscillation by sperm-specific phospholipase C zeta (PLCz) proteins [
14]. Mutation in PLCz protein leads to a failure to induce calcium oscillation interfering further pathways of oocytes activation [
15]. Three mechanisms of AOA were used: mechanical (through microinjection), electrical (through electric field application), and chemical (through calcium ionophore) [
8], and chemical AOA seems to be widely used so far.
Both SV and AOA had been proved to improve fertility outcome of ICSI treatment [
9,
12], but no study had ever focused on the combination of these two techniques. Hence, this study aims to find out if there any synergic effect among AOA (
Figure 1), SV (Figure 2), and ICSI in specific patient groups, such as previous fertilization rate was lower less than 25% [
16]. We compare the fertility outcome, such as fertilization rate, blastocyst formation rate, and clinical pregnancy rate, between intervention group (AOA-SV-ICSI) and control group (SV-ICSI).
2. Materials and Methods
The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. Please note that the publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
Study design:
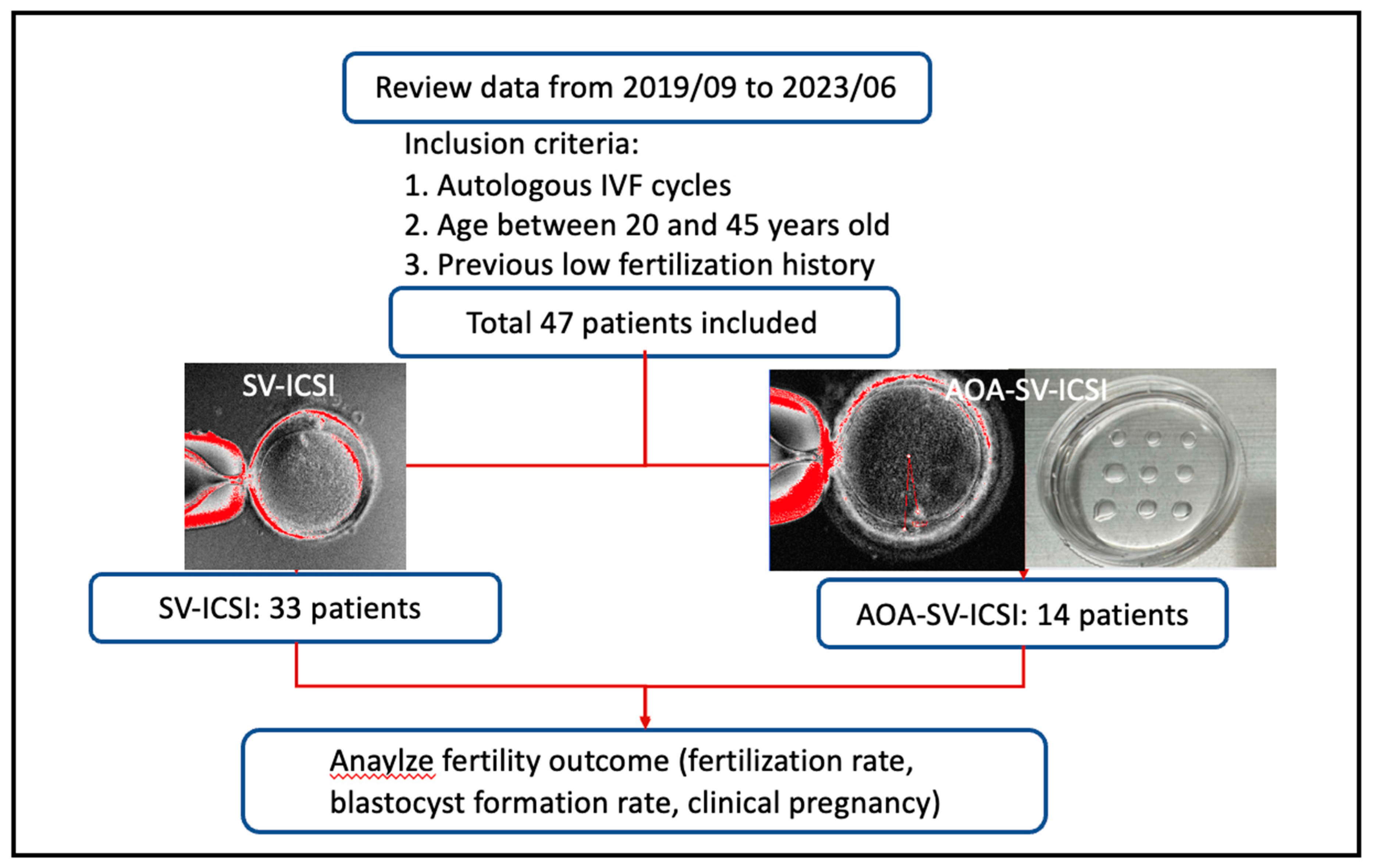
This retrospective study was held in single tertiary medical center, Chang-Gung Memorial hospital, Linkou branch, Taiwan, from 2019/09~2023/06, and was approved by the ethical committee of Chang-Gung Medical Foundation Institution Review Board (230300506B0, 2023/04/17).
The inclusion criteria were 1) autologous IVF cycles, 2) aged between 20 and 45, 3) indications of previous low fertilization after ICSI, which defined as fertilization rate less than 25%. After exclusion, total 47 patients were included in this study and separated into two groups: intervention group (n=14), which SV-ICSI combined with AOA (AOA-SV-ICSI) was performed; control group (n=33), which SV-assisted ICSI (SV-ICSI) was performed. The study flow chart was showed in
Figure 1 and the instrument and methods used were revealed in the
Appendix A.
Treatment protocol:
Either gonadotropin antagonist protocol (Cetrotide™, Merck-Serono) or progestin-primed ovarian stimulation protocol (PPOS, Duphaston®, Abbott) were used for controlled ovarian stimulation based on clinical condition, so did ovarian stimulation dosage (Gonal-f™, Merck-Serono; Menopur™, Ferring Pharmaceuticals; Pergoveris®, Merck-Serono). The stimulation protocols started from menstrual cycle day 2–3 and ended on trigger day for the final oocyte maturation. Serum levels of estradiol, progesterone, LH and the number and size of follicles were monitored every 3 to 5 days. Adjusted by the patient’s age, baseline FSH, anti-mullerian hormone, antral follicle count and body mass index, the starting dose of gonadotropin ranged from 150 to 300 IU. In antagonist protocol, GnRH antagonist was administered when the leading follicle reached 12–14 mm in diameter. Dual trigger (Ovidrel™, 2500 to 5000IU, Merck-Serono; Decapeptyl® 0.1 to 0.2 mg, Ferring Co.) was administrated when two or more follicles reached 18 mm in diameter. Oocytes were retrieved by transvaginal ultrasound 34–36 hours after dual trigger application. The collected mature oocytes were cultivated till blastocyst stage as possible.
The primary (laboratory) outcome evaluated fertilization rate, which defined as the numbers of 2 pronuclei divided by the numbers of metaphase II oocytes retrieval, and blastocyst formation rate, which defined as the number of embryos reaching blastocyst stage at day 5 divided by the numbers of 2 pronuclei. The secondary (clinical) outcome assessed clinical pregnancy, which defined pregnancy diagnosed by ultrasound with visualization of one or more intrauterine gestational sacs with fetal heartbeat.
Statistical analyses:
All data were analyzed using SPSS 25 (IBM SPSS, Armonk, New York, USA). The data were presented as median (IQR) for continuous variables and percentages for categorical variables. The Mann-Whitney U test on continuous variables and Fisher’s exact test on categorical variables. The p-value descriptive data was generated for all variables and a two-sided p-value <0.05 was considered as an index of statistical significance.
3. Results
In this study (
Table 1), all the variables between groups were analyzed and it appeared that both female and male participants were significantly older in the AOA-SV-ICSI group. It makes sense because all the patients in intervention groups had experienced previous low fertilization, possibly more than once, so they were older than the control groups. Also, the blastocyst numbers in AOA-SV-ICSI group were significantly higher than in SV-ICSI group. It suggested that AOA did help increase usable embryos than SV-ICSI only, but the mechanism was not well-understood. Despite higher numbers of MII and 2PN numbers were observed in AOA-SV-ICSI group, they did not reach statistical significance. None of other variables, such as female BMI, AMH level, number of stimulation days, estradiol level at trigger day, number of retrieved oocytes and endometrial thickness at trigger days, had showed statistical significance.
The fertility outcome of this study was presented in
Table 2. The blastocyst formation rate was significantly better in the AOA-SV-ICSI group than in SV-ICSI group (p=0.02), which proves our initial assumption that there is a possible synergic effect between AOA and SV-ICSI to improve fertility outcome. The more blastocyst forms, the more usable embryos could be used in following embryo transfer. Though the fertilization rate did increase in AOA-SV-ICSI group, but no statistical significance was achieved. In addition, the clinical pregnancy seemed better in SV-ICSI group than in AOA-SV-ICSI group. The older age means poor prognostic outcome and those who suffered from previous low fertilization history might prefer embryo collection before proceeding to embryo transfer. Hence, less participants in AOA-SV-ICSI group, compared to SV-ICSI group, had embryo transfer immediately after oocyte retrieval. The less the embryo transfer, the less clinical pregnancy happen.
4. Discussion
Our study manifested that AOA combined with SV-assisted ICSI could improve the blastocyst formation rate in these patients with previous low fertilization history. Even though non-significant, the fertilization rate seems to be mildly improved in intervention group. To our knowledge, this is the first research to explore the possible synergic effect of AOA and SV in intracytoplasmic sperm injection with patients of low fertilization history. So far, the exact mechanism of this possible synergic effect is still unknown.
As we mentioned before, three different methods of AOAs had been studied for years and the well-accepted indication of AOA were previous total fertilization failures or male factor infertility. In Meerschaut’s study [
17], ICSI-AOA group (mechanical AOA) had a mean fertilization rate of 74.2% compared to 43.5% in conventional ICSI group for couples with suspected oocyte-activation problem, so did cumulative pregnancy rate and live birth rate per cycle. A 17-year-duration retrospective study [
12] used mechanical AOA after ICSI and had highlighted a significant clinical improvement in patient with clear oocyte-activation deficiency.
In Mansour’s prospective study [
18]. The ICSI-AOA group (electrical AOA), compared to sibling control groups, had significantly improved the fertilization rate in severe oligoasthenoteratospermia and nonobstructive azoospermia. Besides, the oocyte degeneration rate was not different between two groups. Chemical AOA has been widely used and studied. In a laboratory study [
19], ionomycin-treated oocytes had been observed with significant elevated mean fertilization rates and cleavage rates 72 hours after ICSI in patients with severe teratospermia. The meta-analysis later proved that calcium ionophore treatment after ICSI improved significantly fertilization, cleavage, blastulation, implantation, overall pregnancy, and live birth rates [
20]. Two other studies [
21,
22] had also concluded that electrical and chemical AOA made oocytes produce better-quality and healthy embryos in infertile couples with previous TFF history.
How to select adequate patient to maximize the possibility of successful fertilization benefited from AOA? A prospective study collected patients from previous low fertilization rate ICSI cycles [
23] and categorize different groups according to previous fertilization rate. The result showed that AOA favors those who had previous fertilization rate lower than 30% after ICSI. Through the introduce of both SV and AOA into ICSI cycle, we would like to observe a synergic effect among these three and hopefully benefit those who suffered from previous low fertilization.
Though two meta-analyses [
8,
24] claimed that there is insufficient evidence to judge the efficacy and safety of ICSI-AOA. The difficulty of declaring these issues were the heterogenous study design, patient inclusion criteria, non-standardized outcome assessment, restricted sample size and animal model limitation. In our study, the study patients were strictly selected for only those who with previous low fertilization and outcomes were clearly defined as international guideline do. Besides, our sample size is growing gradually to allow us to strengthen gradually the study findings.
Our result showed the addition of AOA into SV-ICSI improved significantly blastocyst formation rate in patients with previous low fertilization history. The exact mechanism of this possible synergic effect is still unknown for now, but the disadvantages of polarization microscopy, such as lower cleavage stage and less top-quality embryos [
9] seem to be recovered by the introduce of AOA. Though both MII and 2PN numbers in our study did not show statistical significance in AOA-SV-ICSI group, but the increased trend was observed. Also, more blastocyst formation meaned more usable embryos for future ET. However, the clinical pregnancy rate in AOA-SV-ICSI group is 0%, which might be explained by fewer people in this group proceed to ET right after ovulation induction. For example, some patients of the intervention group decided to collect more usable embryos before further ET, which lowered the total numbers of ET in AOA-SV-ICSI group. In addition, the older age distribution in AOA-SV-ICSI group further worsened the expected result of clinical pregnancy, even though more blastocysts were collected than ever.
The limitation of our study was retrospective nature and small sample size. However, the sample size would enlarge as time grows and provide more prominent evidence for the combination of AOA, SV, and ICSI. The strength of our study was strict inclusion criteria and international guidelines for fertility outcome assessment was utilized. All patients of this study had been suffered from multiple IVF failure due to low fertilization rate. We focus only on how to produce more available embryos for future IVF purpose and do find positive findings. A large, randomized control study must be held in the future to evaluate this possible synergic effect of AOA-SV-ICSI.
5. Conclusions
There was a significant improvement of blastocyst formation rate in assisted oocyte activation combined with spindle view-assisted intracytoplasmic sperm injection in patients with previous low fertilization rate history. To explore the possible synergic effect, larger RCTs should be executed in the future.
Author Contributions
Investigation, Chia-Jung Li; Resources, Chiung-Hui Hou; Data curation, Chieh-Yu Lin and Lin-Hsuan Dai; Writing—original draft, Wei-Che Lo; Writing—review & editing, Hsien-Ming Wu.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank Yu-Ching Wang for the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3N0011) at Chang Gung Memorial Hospital for study design and monitor, data analysis and interpretation.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Figure A1 was the spindle view platform performed in this study (the Oosight Imaging system, Hamilton Thorne, Beverly, USA). And
Figure A2 was the assisted oocyte activation procedure, followed by manufactural manual (GM 508 CultiActive, GYNEMED GmbH & Co. KG, Germany).
Figure A1.
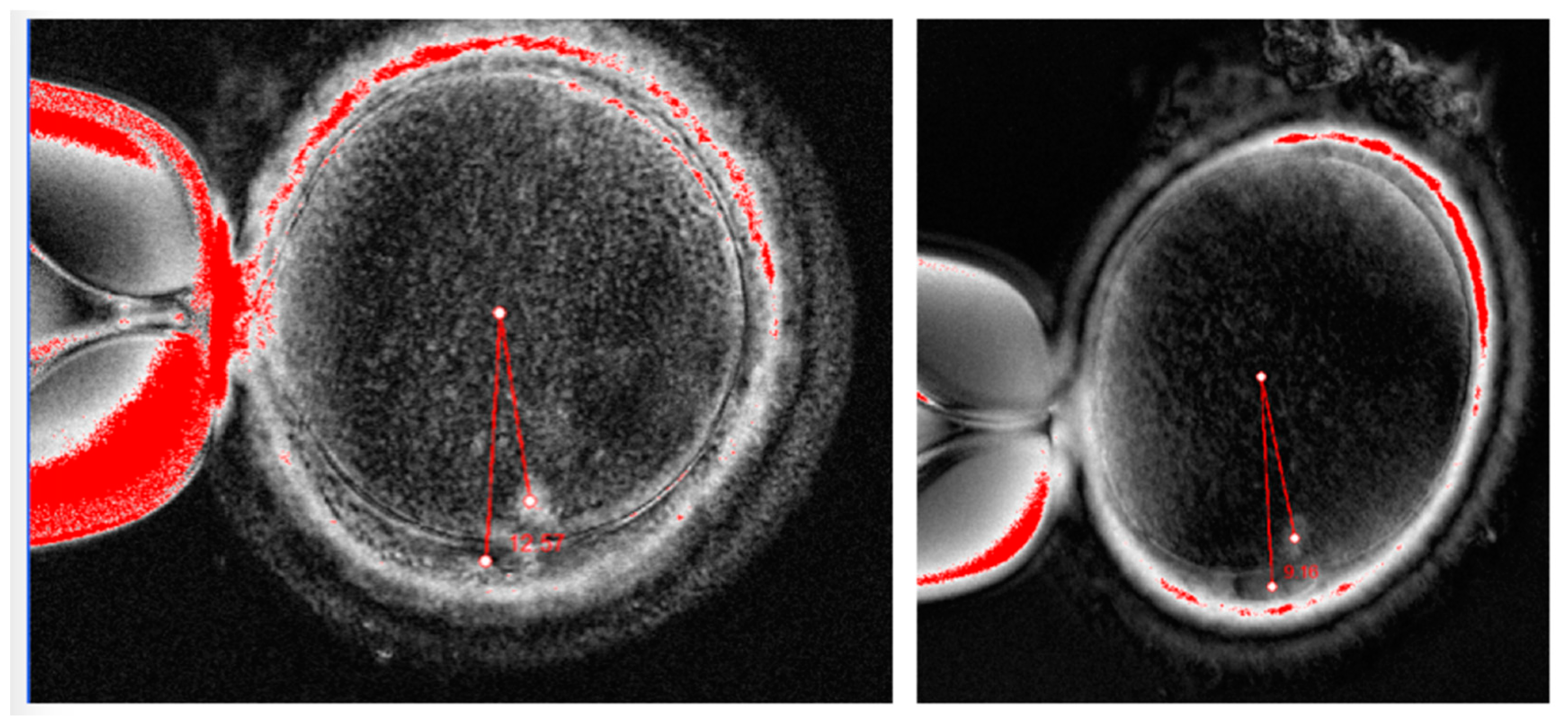
Examples of spindle views from our patients (Oosight Imaging system, Hamilton Throne, Beverly, USA).
Figure A1.
Examples of spindle views from our patients (Oosight Imaging system, Hamilton Throne, Beverly, USA).
Figure A2.
AOA procedure, followed by manufactural manual (GM508 CultActive, GYNEMED Gmbh & Co. KG, Germany).
Figure A2.
AOA procedure, followed by manufactural manual (GM508 CultActive, GYNEMED Gmbh & Co. KG, Germany).
References
- Cohen, J.; Malter, H.; Fehilly, C.; Wright, G.; Elsner, C.; Kort, H.; et al. Implantation of embryos after partial opening of oocyte zona pellucida to facilitate sperm penetration. The Lancet 1988, 332, 162. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza, C.; Greco, E. The activity (calcium oscillator?) responsible for human oocyte activation after injection with round spermatids is associated with spermatid nuclei. Fertil Steril. 2000, 74, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Malcov, M.; Schwartz, T.; Mey-Raz, N.; Carmon, A.; Cohen, T.; et al. Spindle imaging: a new marker for optimal timing of ICSI? Human Reproduction 2004, 19, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.D.; Neri, Q.V.; Takeuchi, T.; Rosenwaks, Z. ICSI: where we have been and where we are going. Semin Reprod Med. 2009, 27, 191–201. [Google Scholar] [CrossRef]
- O’Neill, C.L.; Chow, S.; Rosenwaks, Z.; Palermo, G.D. Development of ICSI. Reproduction 2018, 156, F51–F58. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Javed, M.H.; Gotlieb, L.; Casper, R.F. Complete failed fertilization after intracytoplasmic sperm injection--analysis of 10 years’ data. Int J Fertil Womens Med. 2005, 50, 187–192. [Google Scholar]
- Kashir, J.; Ganesh, D.; Jones, C.; Coward, K. Oocyte activation deficiency and assisted oocyte activation: mechanisms, obstacles and prospects for clinical application. Hum Reprod Open 2022, 2022, hoac003. [Google Scholar] [CrossRef]
- Picinato, M.C.; Martins, W.P.; Giorgenon, R.C.; Santos, C.K.; Ferriani, R.A.; Navarro, P.A.; et al. The impact of examining the meiotic spindle by polarization microscopy on assisted reproduction outcomes. Fertil Steril. 2014, 101, 379–384. [Google Scholar] [CrossRef]
- Neri, Q.V.; Lee, B.; Rosenwaks, Z.; Machaca, K.; Palermo, G.D. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium. 2014, 55, 24–37. [Google Scholar] [CrossRef]
- Peultier, A.S.; Fréour, T.; Cazenave, N.; Barrière, P. Fertilization failure in IVF and ICSI. J Gynecol Obstet Biol Reprod 2015, 44, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Bonte, D.; Ferrer-Buitrago, M.; Dhaenens, L.; Popovic, M.; Thys, V.; De Croo, I.; et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril. 2019, 112, 266–274. [Google Scholar] [CrossRef]
- Ferrer-Buitrago, M.; Bonte, D.; De Sutter, P.; Leybaert, L.; Heindryckx, B. Single Ca2+ transients vs oscillatory Ca2+ signaling for assisted oocyte activation: limitations and benefits. Reproduction 2018, 155, R105–R109. [Google Scholar] [CrossRef] [PubMed]
- Hachem, A.; Godwin, J.; Ruas, M.; Lee, H.C.; Ferrer Buitrago, M.; Ardestani, G.; et al. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 2017, 144, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Jones, C.; Lee, H.C.; Rietdorf, K.; Nikiforaki, D.; Durrans, C.; et al. Loss of activity mutations in phospholipase C zeta (PLCζ) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum Reprod. 2011, 26, 3372–3387. [Google Scholar] [CrossRef] [PubMed]
- van der Westerlaken, L.; Helmerhorst, F.; Dieben, S.; Naaktgeboren, N. Intracytoplasmic sperm injection as a treatment for unexplained total fertilization failure or low fertilization after conventional in vitro fertilization. Fertil Steril. 2005, 83, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Vanden Meerschaut, F.; Nikiforaki, D.; De Gheselle, S.; Dullaerts, V.; Van den Abbeel, E.; Gerris, J.; et al. Assisted oocyte activation is not beneficial for all patients with a suspected oocyte-related activation deficiency. Hum Reprod. 2012, 27, 1977–1984. [Google Scholar] [CrossRef]
- Mansour, R.; Fahmy, I.; Tawab, N.A.; Kamal, A.; El-Demery, Y.; Aboulghar, M.; et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009, 91, 133–139. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.H.; Razavi, S.; Javdan, Z.; Tavalaee, M. Artificial oocyte activation in severe teratozoospermia undergoing intracytoplasmic sperm injection. Fertil Steril. 2008, 90, 2231–2237. [Google Scholar] [CrossRef]
- Murugesu, S.; Saso, S.; Jones, B.P.; Bracewell-Milnes, T.; Athanasiou, T.; Mania, A.; et al. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil Steril. 2017, 108, 468–482.e3. [Google Scholar] [CrossRef]
- Baltaci, V.; Ayvaz, O.U.; Unsal, E.; Aktaş, Y.; Baltaci, A.; Turhan, F.; et al. The effectiveness of intracytoplasmic sperm injection combined with piezoelectric stimulation in infertile couples with total fertilization failure. Fertil Steril. 2010, 94, 900–904. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Lian, Y.; Li, M.; Lin, S.; Zhuang, X.; et al. Artificial oocyte activation improves cycles with prospects of ICSI fertilization failure: a sibling oocyte control study. Reprod Biomed Online 2019, 39, 199–204. [Google Scholar] [CrossRef]
- Montag, M.; Köster, M.; van der Ven, K.; Bohlen, U.; van der Ven, H. The benefit of artificial oocyte activation is dependent on the fertilization rate in a previous treatment cycle. Reprod Biomed Online 2012, 24, 521–526. [Google Scholar] [CrossRef]
- Sfontouris, I.A.; Nastri, C.O.; Lima, M.L.; Tahmasbpourmarzouni, E.; Raine-Fenning, N.; Martins, W.P. Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs. Hum Reprod. 2015, 30, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).