1. Introduction
The frequency of
Candida species that cause drug resistance is increasing, and for this purpose, the design and synthesis of black seed nanoliposome has been carried out. Recently, in hospitalized patients with
Candida infections who are constantly taking antifungal drugs, drug resistance with different species of
Candida has been reported.
C.parapsilosis is the most prevalent fungal pathogen that causes numerous infections and has a high adaptability which enables it to transit from commensal to pathogen due to many virulence factors, especially by biofilm formation [
1]. In the meantime,
Candida parapsilosis has been established as a common fungal pathogen with many pathogenic factors such as biofilm formation in order to create internal and potential drug resistance [
2]. C.
parapsilosis is intrinsically resistant to antifungal drugs and has little susceptibility to common treatments is due to biofilm cell wall regulators [
3]. BCR1and EFG1 are mainly transcription factors in
C. albicans and
C. parapsilosis also BCR1 is the main essential transcription factor in the early stage for adhesion and biofilm formation in
C. albicans and
C. parapsilosis [
4,
5]. EFG1 is the other transcription factor which is required for hyphal growth in biofilm formation of
C. parapsilosis [
6]. Among countless herbal medicines, black seed (Nigella sativa) is a miraculous plant with a rich history and religious background, so many inventors call it a plant with a wide range and potential medicinal power [
7]. Liposomes have been used to improve drug absorption, reduce metabolism and toxicity, prolong biological half-life Moreover, they prevent to produce pyrogenic or antigenic reactions [
8,
9].
2. Methods
2.1. Standard fungal strains
In our current study, 15 isolates of C. parapsilosis were obtained from Hospitalized patients at Iran (Mashhad Medical university of science).
2.2. Preparation and Characterization techniques of Nano liposomes
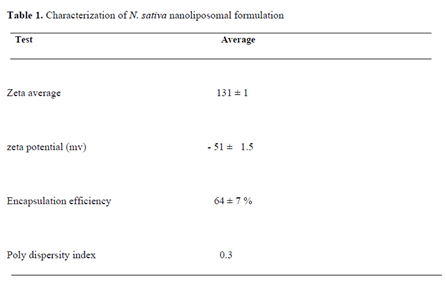
Various liposomal formulations were prepared using lipid film hydration (passive loading) method. Finally, the best formulations were selected by considering the total characteristics of the lipid structure, size, zeta potential, homogeneity, retention percentage and the final concentration of the encapsulated drug. The information related to the preparation of selected liposomal formulations is explained in the results section (
Table 1). Suitable and clean glass tubes, washed 3 times with normal methanol, 3 times with normal chloroform and 1 time with HPLC grade chloroform. Then, to prepare the 50 mM liposome formulation, the required amounts of each lipid along with the desired drug were added to the glass tube using a Hamilton syringe. In order to prepare the lipid film, certain molarities of the mentioned drugs and lipidd stocks were used. The organic solvent (chloroform) was removed by placing it in a rotary device for 2 hours. Then it was placed in a freeze dryer for 2 hours to completely remove the remaining solvent. Then, to the obtained lipid film, we added one milliliter of PBS buffer with pH = 7.4, which was pre-heated at 65 °C, and it was quickly sealed in argon-filled container. Hydration was done in Bain-Marie at a temperature of 65 degrees and vertexing was done several times for 60 minutes so that all lipids were dispersed and completely dissolved in the aqueous phase and liposomal vesicles were formed. Then we placed the prepared liposomes under bath sonication at a temperature of 65°C until the desired drug nanoliposome became clear. The entrapment efficiency of
N. Sativa oil in liposomal formulation was estimated to be ∼87% (
Figure 1). Microscopic evaluation using transmission electron microscope is as follows: first, a certain concentration of nano drug carrying liposomal compounds is added with 9% uranium acetate, then a few drops of the prepared solution are added to the carbon surfaces, and finally, it is used with a digital camera in different sizes. It is taken with a picture (
Figure 5).
We speculate the size of nano lipids with sterile sugar serum in such a way that 20 microliters of the sample are mixed with one cc of sterile sugar serum and PDI, Zeta Potential and Zeta Potential can be measured with 4.7 maps buffer. In this investigation, the size is measured three times for a more detailed investigation and the zeta potential is measured once. All the above items are measured with the desired materials and buffer by DLS (thermal light scattering) device, and the analysis and size of the particles are also checked. Also, the charge on the surface of the particles is determined by measuring the zeta potential of the suspension using a Zetasizer.
Table 1 and
Figure 2 and
Figure 3. In this study, a buffer in which the desired drugs are well formulated liposomal with 2 types of pH including: 7.4 and 2: (neutral environment of blood and this in vitro to check drug sensitivity to check drug release and exposure to fungal cells) and an acidic environment with pH 2, such as normal stomach acid, which includes the addition of the desired buffer in the vicinity of hydrochloric acid 0.2 normal + NACL 0.2 normal + DW has been used. First, 1000 microliters of nanoliposome with the desired drug was poured into the dialysis bag, then 100ml of buffer with the desired pH was poured into the bottle at zero time point, first 2ml of the contents of the buffer and nanoliposome were inserted into the microtube to be seen with a spectrophotometer. then add 2 cc of buffer to the bottle at the same moment and place it in a 37 degree incubator with a shaker, then after 15 minutes, take 2 cc of the contents of the bottle and read its absorption, then immediately place it at 37 degrees and Again, we add 2 cc of buffer with the desired pH, and after 30 minutes, we take 2 ml of buffer and insert it into the microtube, and by mentioning the characteristics of the drug and the buffer with the desired pH, we read its absorption at the desired wavelength. In this way, in 0.5-1-2-4-6-8-24 hours, the amount of the collected sample was absorbed in the desired pH with a spectrophotometer and compared to the standard absorption of the desired drug in the Excel chart. we give (
Figure 3(.
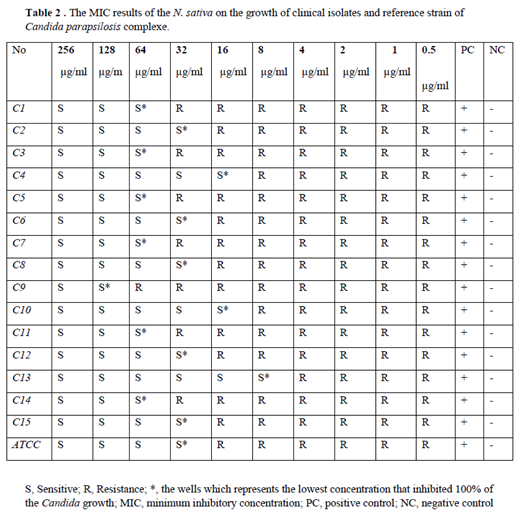
2.3. Minimum inhibitory concentration evaluation
Adjusting by CLSI M27 A3 protocol
First, from 15 clinical isolates of
Candida parapsilosis samples stored in the fungal bank of Ghaem Hospital, subculture was performed to obtain fresh yeast colonies and kept in an incubator for 48 hours. With an autoclave, RPMI culture medium is prepared along with Mapes buffer powder according to the protocol and the amount of the required sample, and in order to stabilize the pH, a few drops of 1/1 normal sodium chloride are used, and after that, the desired sample is filtered with a 0. 22-syringe filter and the sample is finally stored in sterile bottles The steps of yeast suspension: First, the spectrophotometer device was placed in the transmittance solution at a wavelength of 530 nm, and under sterile conditions, next to the flame, freshly cultured yeast samples were dipped with a sterile tip and placed in sterile distilled water in a vial containing a specific volume of about 1.5 After absorption with the desired wavelength and 73-75 percent transmittance (equal to half McFarland's formula), the samples are prepared for the next steps. First of all, 100% of RPMI medium was poured into each of the wells from the second to the tenth well, and well 11 corresponds to the positive control (RPMI medium and fungal suspension) and well 12 is the negative control (nanoparticles and drug with RPMI). 200 microliters of the drug sample were poured into the first well and dilution was performed, and the tenth sample was also extracted. Finally, 100 microliters of the fungal suspension isolated from the patients was poured into each of the wells up to the tenth well and the microplates were read for 48 hours. We put it in the incubator (
Table 2 and
Table 3).
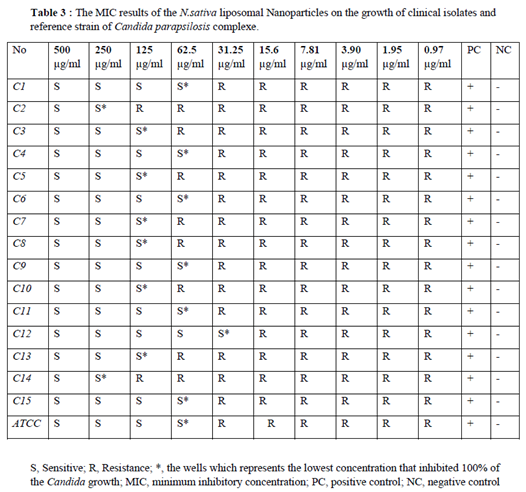
Minimum inhibitory concentration was conducted on
N. Sativa-Lip-NPs according to CLSI M27 A3/S4 protocol. For this purpose, 2.5×10 3 cells of the standard strain of
C. parapsilosis (ATCC 22019) were counted by slide hemocytometer and prepared in RPMI 1640 culture medium. Then 100 μL of this suspension was kept and seeded into a 96-well microplate [
10]. Two-fold serial diluted concentrations of
N. Sativa-Lip-NPs (0.97 to 500 µg/mL), and free
N. sativa (0.5 to 256 µg/mL) was adjusted in RPMI 1640. Then 100 μL of each of them was added to the wells. The microplate was incubated at 37°C for 24 hours, and the lowest concentration which inhibited the growth of fungal cells was defined as the MIC. Fungal cell suspension without treatment was used as a positive control, and the culture medium (RPMI)was used as a negative control (
Table 2 and
Table 3).
2.4. Evaluation of PBMCs viability to liposomal formulation (MTT assay procedure)
First, we count first 5×10 3 cells and pour 100 μL of these cells into microplate wells. After 24 hours of incubation at 37 degrees, add 100 μL of drugs with C02 and then again incubated at 37°C for 24 hours with 5% CO2 and then for every 10 ml of culture medium, 1 ml of MTT (5 mg in one ml of RPMI) which is needed in the calculation of about 10 ml per well, then we kept the plate for 4 hours in the dark room and then remove the supernatant on sterile gas and the contents include purple cells and crystals. Then DMSO is added to the amount of 100 microliters as a solvent for these crystals and for about 15 to 20 minutes in the dark, and finally we read the absorbance of each well at 545 and 630 nm with an ELISA reader, and then the final average is calculated for the calculation of the viable percentage. We calculate persistence and toxicity (
Figure 4)
Cell viability (%) = Absorbance of the test / Absorbance of the control ×.
2.5. Statistical analysis
The results analyzed and obtained as the average of the measurements by using Mann-Whitney Test, with procedure of SPSS Version 23 and finally, p-value: < 0. 5 was adjusted.
3. Results
3.1. Characterization techniques of NPs
The synthesized
N. Sativa-Lip-NP was approved by using the TEM, particle size, zeta potential, and UV-Vis. Size measurements were carried out in triplicate and the average of recordings was reported (
Table 1).
3.2. Evaluation of particle size (ZAve) and zeta potential
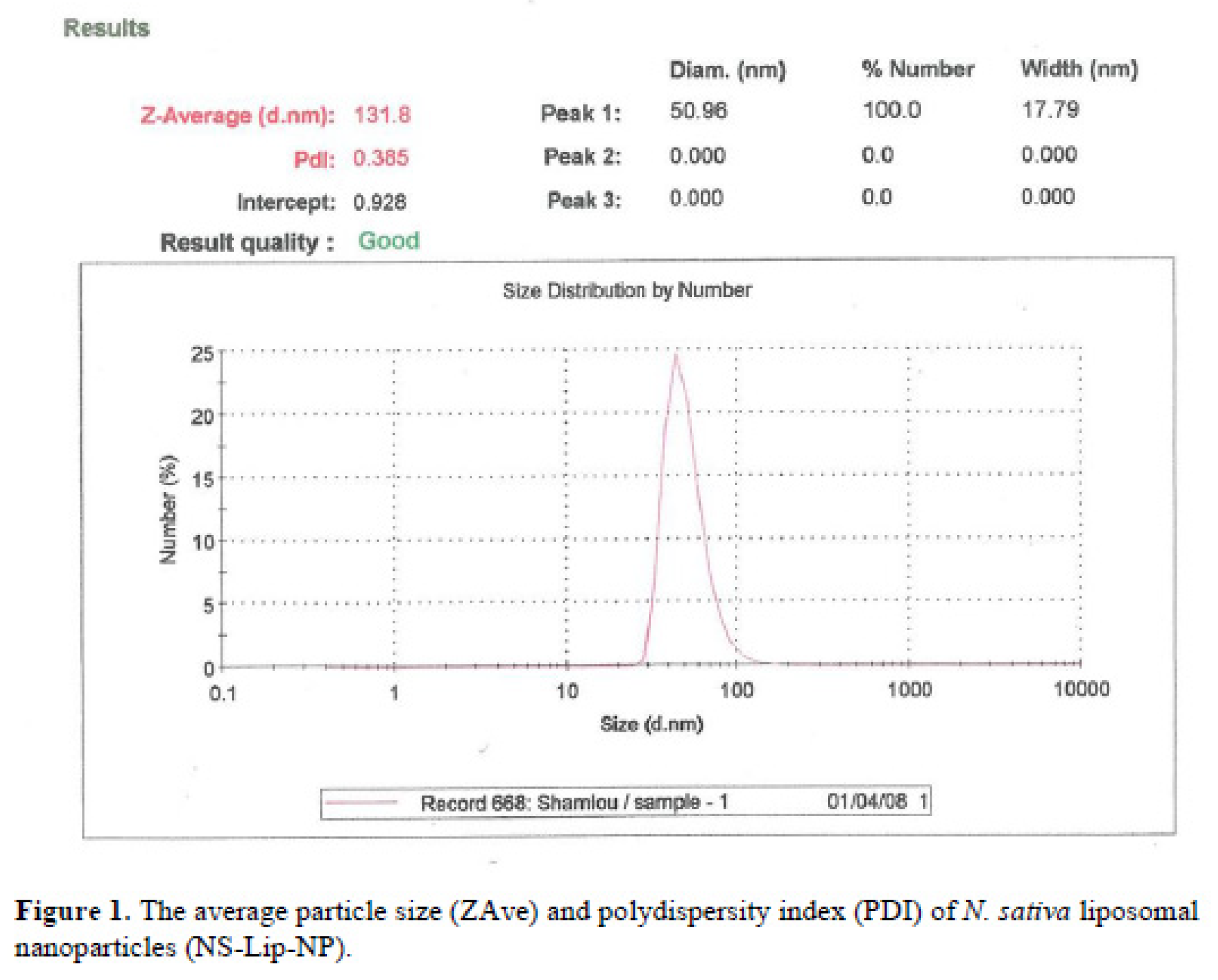
The average particle size (ZAve) results of
N. Sativa-Lip-NP and polydispersity index (PDI) range showed a good mean average size and polydispersity in liposomal formulation (
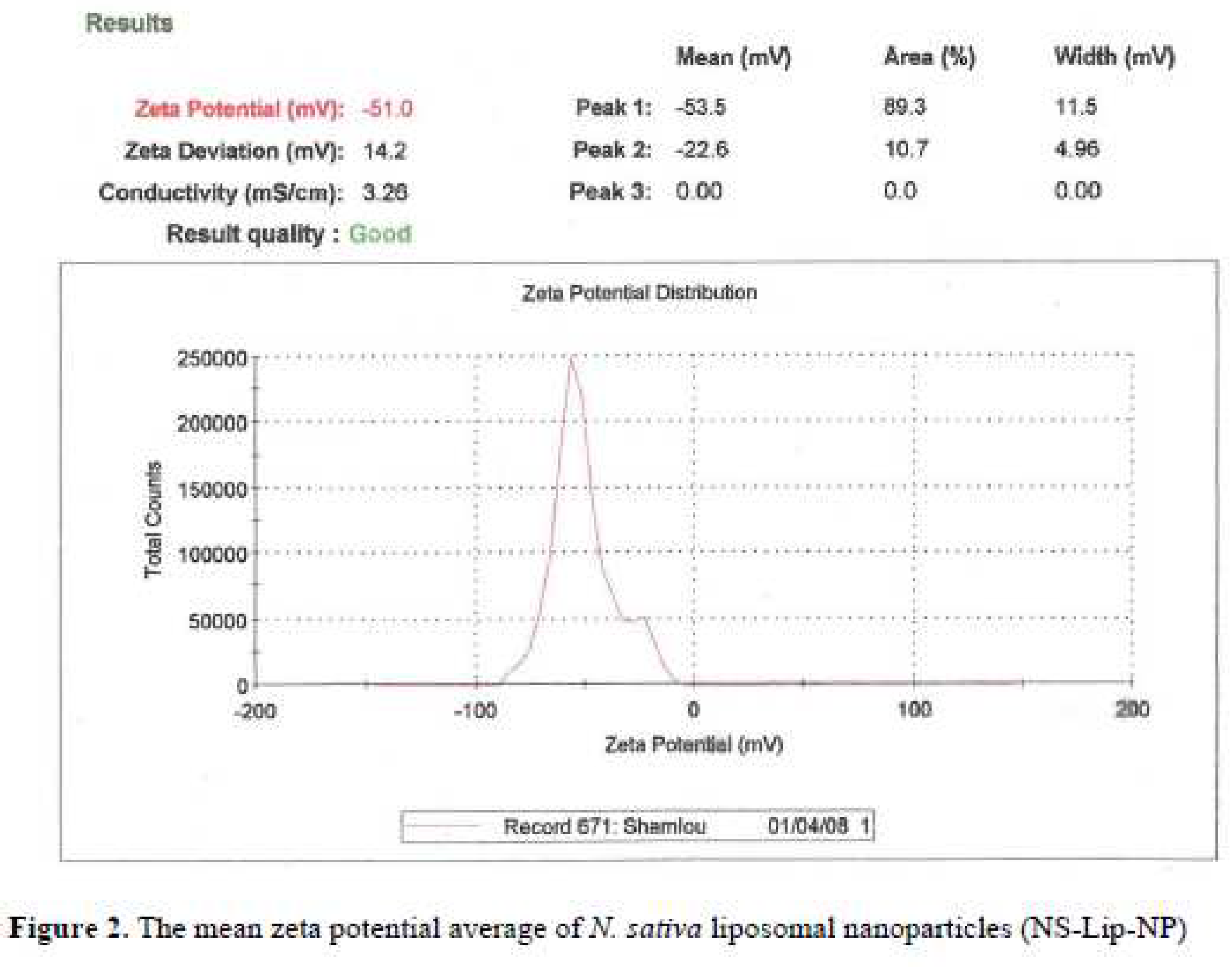
Figure 1). The mean average of zeta potential with -51 (mv), showed a good negative voltage charge with no agglutination and aggregation of nanoliposomes (
Figure 2).
3.3. The Encapsulation efficiency (EE%)
The Encapsulation efficiency (EE%) of
N. Sativa-Lip-NP was 64 ± 7 % (
Table 1).
3.4. Release profile
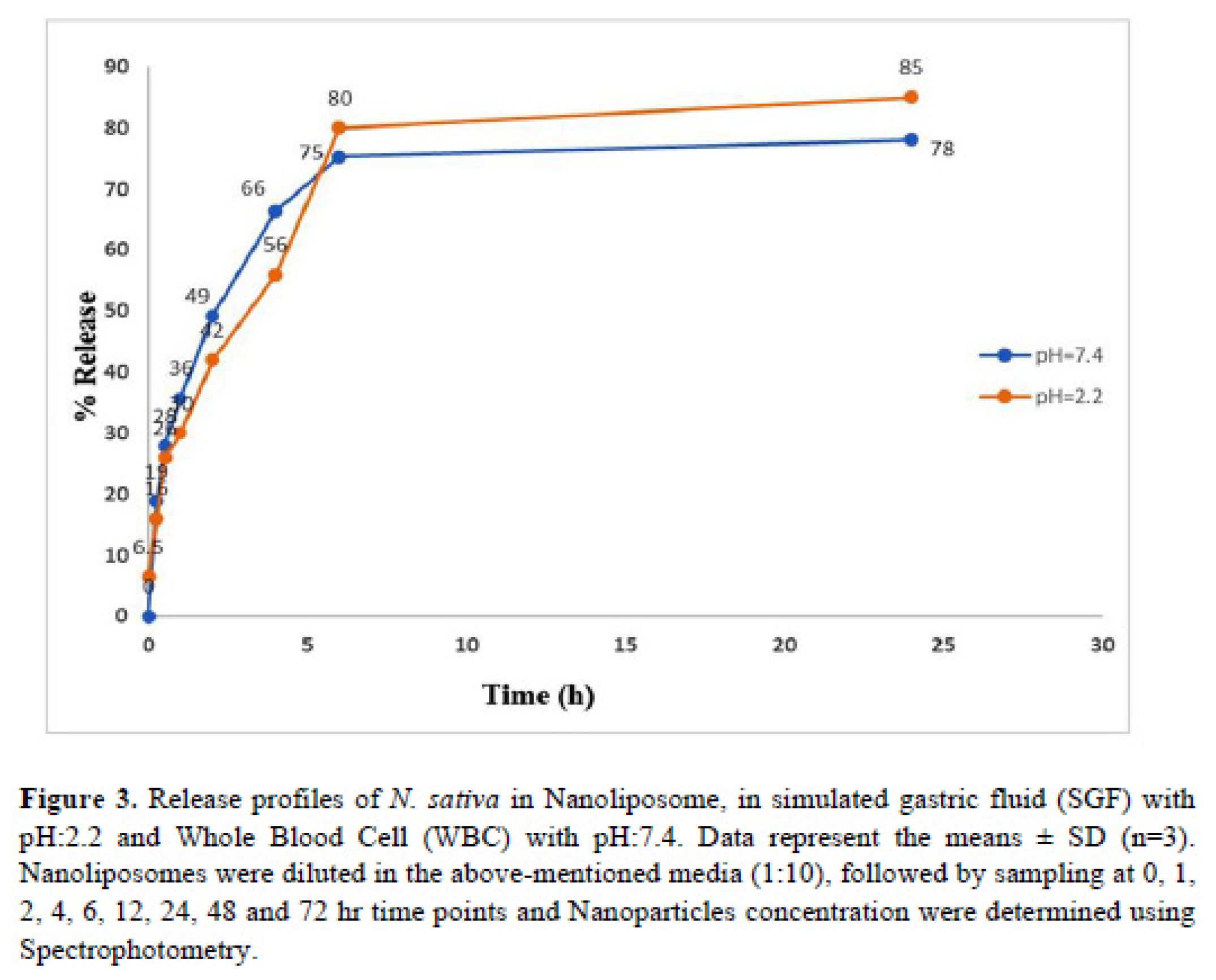
Release efficiency of
N. Sativa-Lip-NP in acidity media like gastric fluid with 85 % is higher than bufferic media (PV< 0.5) (
Figure 3).
3.5. The viability percentages of cells treated with N. Sativa-Lip-NPs, and free N. sativa
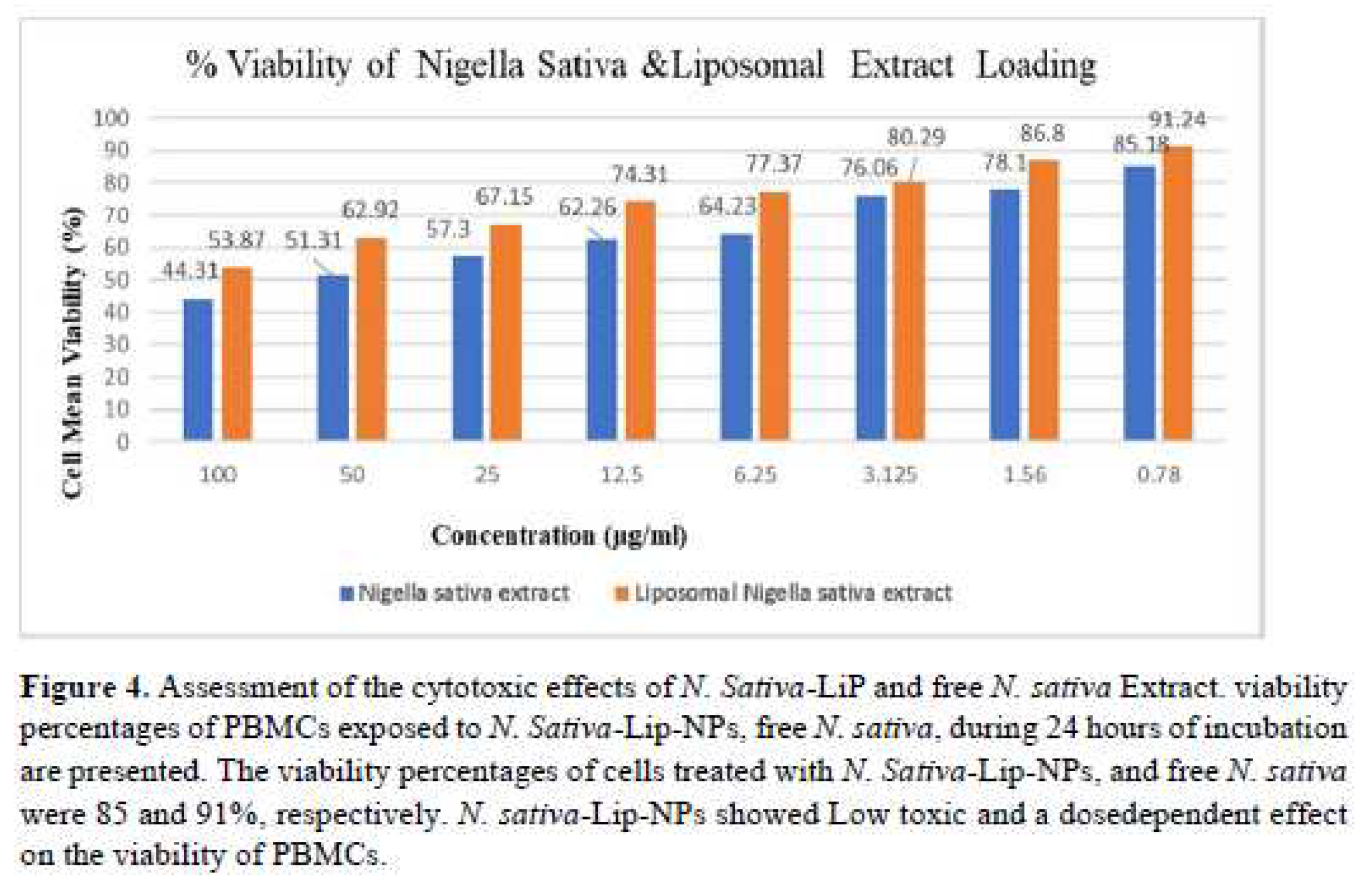
The viability percentage results of cells treated with liposomal formulation, and free
N. sativa oil were respectively 91 % and 85% therefore liposomal formulation showed low toxic effect and a dosedependent range on the viability of PBMCs (
Figure 4).
3.6. The MIC range of free N. sativa and liposomal formulation
N. sativa oil and liposomal formulation with inhibitory effects on
candida isolates was between 128 to 8 and 250 to 31.25 µg/mL, in current study MIC50 and MIC90 values were also 125,187 and 32,96 µg/mL, respectively showed little inhibitory effect on fungal cell growth. MIC values of liposomal formulation of
N. sativa oil were significantly much more those of free liposomes, in current study,6 Isolates showed high significant resistance to
N. sativa oil and 8 Isolates Showed more Siginificant resistance to liposomal formulation of
N. sativa oil than the others (p-value < 0.5) (
Table 2 and
Table 3).
4. Discussion
Currently, a large number of mucosal and systemic anti-fungal drugs such as fluconazole, itraconazole, and echinocandin are available for the treatment of candida infections, but due to the toxicity and side effects of long-term use in persistent systemic infections, as well as resistance and the high cost of the drugs, treatment Combinations of plant extracts and compounds derived from plants are used effectively.
Black seed oil and its important compounds, especially thymoquinone, have inhibitory properties against Candida yeast infections and filamentous fungal agents which produces aflatoxins [
10]. The aqueous extract of
N. sativa seed exhibited inhibitory effect against candidiasis in the groups of animals post-treated with the plant extract and finally the results were confirmed by Histopathological examination of examined organs [
11]. The curmments in Animal was basically confirmed by effect on neutrophils and production of nitric oxide (NO)-synthetase [
12]. The fact is that black seed plant extracts and their components directly stimulate granulocytes and monocytes in order to produce nitric oxide in order to create antifungal properties and among other black seed compounds that have antifungal properties against candida agent’s, Beta-sitosterol and oleic acid, which are two oils and the main composition of black seeds and long-chain fatty acid have antifungal effect against a few strains of
C. parapsilosis [
13,
14]. Thymoquinone with a concentration of 9 µg/mL makes 42% peripheral blood macrophage cells viable, while in our study, a concentration of 12 µg/mL of
N. sativa oil makes 64% of peripheral blood cells viable [
15]. In another study, adding polyethylene glycol carrier to thymoquinone increases the viability of macrophage cells by 80%, while in the present study, the percentage of viable cells reaches 90% by adding nanoliposomal carriers. Thymoquinone is one of the main components of
N. sativa oil, which causes the death of fungal cells due to the production of oxidants and their agents [
16]. TQ (an active component of
N. sativa) has no inhibitory effect of
Candida isolates from systemic infections because its activity is diminished in serum proteins [
17].
Nowadays, the emergence of drug-resistant species has led to the development of new drugs to solve this problematic issue, and the use of essential oils like TQ
as the major components of N. sativa improves the effectiveness of antifungal agents and reduce the antifungal resistance [
18].
5. Conclusion
N. sativa oil release simply and slowly in the body with bioactive phytochemical effects and numerous pharmacological therapies with no side effects. Considering the prevalence of invasive candida infections in recent decades, especially patients hospitalized in intensive care units and people with immunosuppression such as patients exposed to chemotherapy and the presence of persistent infections caused by biofilms created in catheters, as well as the need to diagnose and treat the involved candidate species, since the Candida parapsilosis complex is the second fungal species after albicans in creating an invasive form and biofilm, which has been widely reported in the world and is created due to biofilm resistance. By this type, against common antifungal drugs such as itraconazole, the use of herbal agents as a medicinal aid in the treatment of this type of infections has been proposed, and due to the active role of liposomal compounds in helping chemical and physical properties. The intended drug was used and considering that such research has not been done in Iran so far, if the research is fruitful, it will be proposed as an effective antifungal agent in the field of candidiasis treatment.
Author Contributions
MRJ; adjusted all the experiments. AGS, HZ, MHY; designed the study and tools for analysis. AGS; performed the tests, wrote and edited the manuscript and All authors have read, confirmed and agreed to the final manuscript to be published.
Funding
The project was financially supported by the Tarbiat Modares university, faculty of medical medicene (Date :2022-05-02. No :89166).
Institutional Review Board Statement
All the experiments were carried out according to the regulations and guidelines by Biomedical Research Ethics Committee of Tarbiat Modares university, faculty of medical medicene, Iran, Tehran (IR.MODARES.REC.1401.025).
Informed Consent Statement
All the sample isolation of the patients and data was acquired during the last years and many of patients was dead by the way informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data from this study are not allowed to be shared with anyonedue to national legal regulations. And the datasets used and/or Analysed during the current study available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to express gratitude and appreciation to the management and respected expert of the Department of Medical Mycology and Nanomedicine of Mashhad University of Medical Sciences.
Conflicts of Interest
The authors declare no conflict of interest exist.
References
- Cecilia, M.; Riccardo, T.; Theun, G.; Elena Grazia, A.M.; Giulia, D.A.; Posteraro, B.; et al. Prevalence and Clonal Distribution of Azole- Resistant Candida parapsilosis Isolates Causing Bloodstream Infections in a Large Italian Hospital. Front. Cell Infect Microbiol. 2020, 10, 232. [Google Scholar] [CrossRef]
- Modiri, M.; Hashemi S j Daie ghazvini, R.; Khodavaisy, S.; Ahmadi, A.; Ghaffari, M.; et al. Antifungal susceptibility pattern and biofilm-related genes expression in planktonic and biofilm cells of Candida parapsilosis species complex. Current Medical Mycology. 2019, 5(4), 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Martínez, J.P.; López-Ribot, J.L. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006, 6(7), 979–86. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.M.; Schröder, M.S.; Turner, S.A.; Taff, H.; Andes, D.; Grózer, Z.; et al. Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Patho. 2014, 10(9). [CrossRef]
- Ding C, Vidanes GM, Maguire SL, Guida A, Synnott JM, Andes DR, et al. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PloS One 2011, 6(12), e28151. [CrossRef]
- Connolly, L.A.; Riccombeni, A.; Grózer, Z.; Holland, L.M.; Lynch, D.B.; Andes, D.R.; et al. The APSES transcription factor Efg 1 is a global regulator that controls morphogenesis and biofilm formation in Candida parapsilosis. Mol Microbiol. 2013, 90(1), 36–53. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; et al. review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013, 3, 337–352 [. [Google Scholar] [CrossRef] [PubMed]
- Çagdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery, Application of Nanotechnology 2014. [CrossRef]
- Mizushima, Y.; Hamano, T.; Yokohama, K. Use of a Lipid Emulsion as a Novel Carrier for Corticosteriods. J Pharm Pharmacol. 1982 Jan;34(1):49-50. [CrossRef]
- Gupta, S.; Satishkumar, M.N.; Duraiswamy, B.; Das, S.; Chhajed, M. Potential herbs and its phytoconstituents against fungal infection: A systematic review. World Journal of Pharmaceutical Research (WJPR); Res Volume 1, Issue 2012; 1, 01-20. Available online: http//ajp.mums.ac.irarticle_6190.
- Khan, M.A.; Ashfaq, M.K.; Zuberi, H.S.; Zuberi, A.H. The in vivo anti-fungal activity of the aqueous extract from Nigella sativa seed. Phytother Res. 2003; 17:183–186. [CrossRef]
- Fierro, M.; Fidalgo, C.B. The involvement of nitrous oxide in the anti-Candida albicans activity of rat neutrophils. Immunology.1996;89(2):295-300. [CrossRef]
- Asdadi, A.; Harhar, H.; Gharby, S.; Bouzoubaâ, Z.; Yadini, A.E.; Moutaj, R.; et al. Chemical composition and antifungal activity of Nigella Sativa L. oil seed cultivated in Morocco. Int J Pharma Sci Invent. 2014; 3:9–15. Available online: https://www.ncbi.nlm.nih.gov›articles›PMC4884215.
- Bita, A.; Rosu, A.F.; Calina, D.; Rosu, L.; Zlatian, O.; Dindere, C.; et al. An alternative treatment for Candida infections with Nigella sativa extracts. Eur J Hosp Pharm. 2012; 19:162. [CrossRef]
- Bhattacharya, S.; Ahir, M.; Patra, P.; Mukherjee, S.; Ghosh, S.; Mazumdar, M.; et al. PEGylated thymoquinone-nanoparticle mediated retardation of breast cancer cell migration by deregulation of cytoskeletal actin polymerization through miR34a. Biomaterials. 2015; 51:91-107. [CrossRef]
- İşcan, G.; İşcan, A.; Demirci, F. Anticandidal effects of thymoquinone: Mode of action determined by transmission electron microscopy (TEM). Nat Prod Commun 2016 Jul;11(7):977-978.
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; et al. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis.2010; 15(2):183-95. [CrossRef]
- Donadu, M.G.; Peralta-Ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.B.; Rizzo, D.; et al. Colombian essential oil of Ruta graveolens against nosocomial antifungal resistant Candida strains. Journal of Fungi 2021, 7, 383. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).