Submitted:
28 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Root and rhizospheric soil sampling of halophyte plants
2.2. Physical and chemical analysis of rhizospheric soil
2.3. Propagation of AMF and DSF consortia
2.4. Establishment of fungal trap cultures
2.5. Evaluation and selection of endorhizal fungi consortia
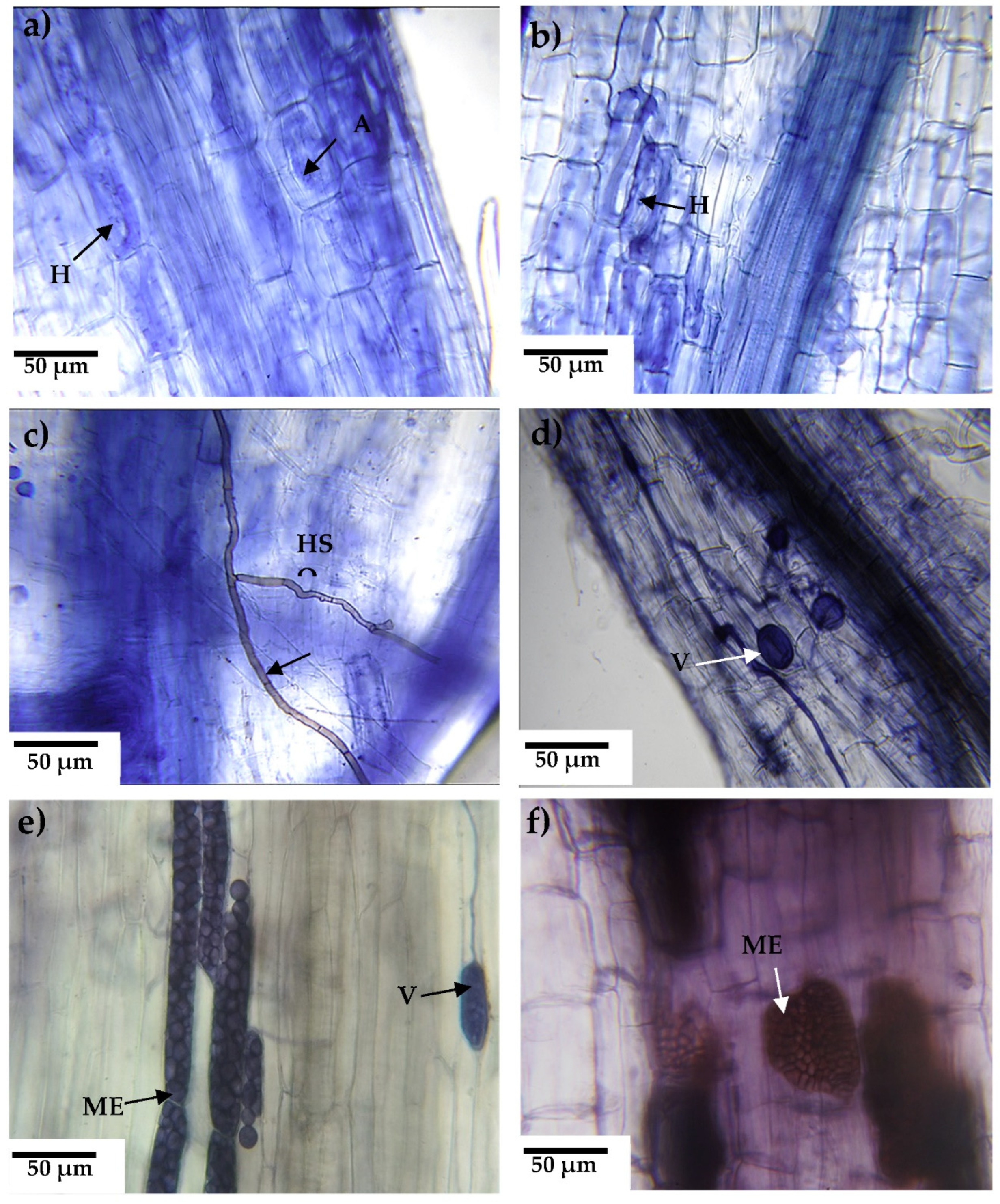
2.5.1. Fungal infectivity
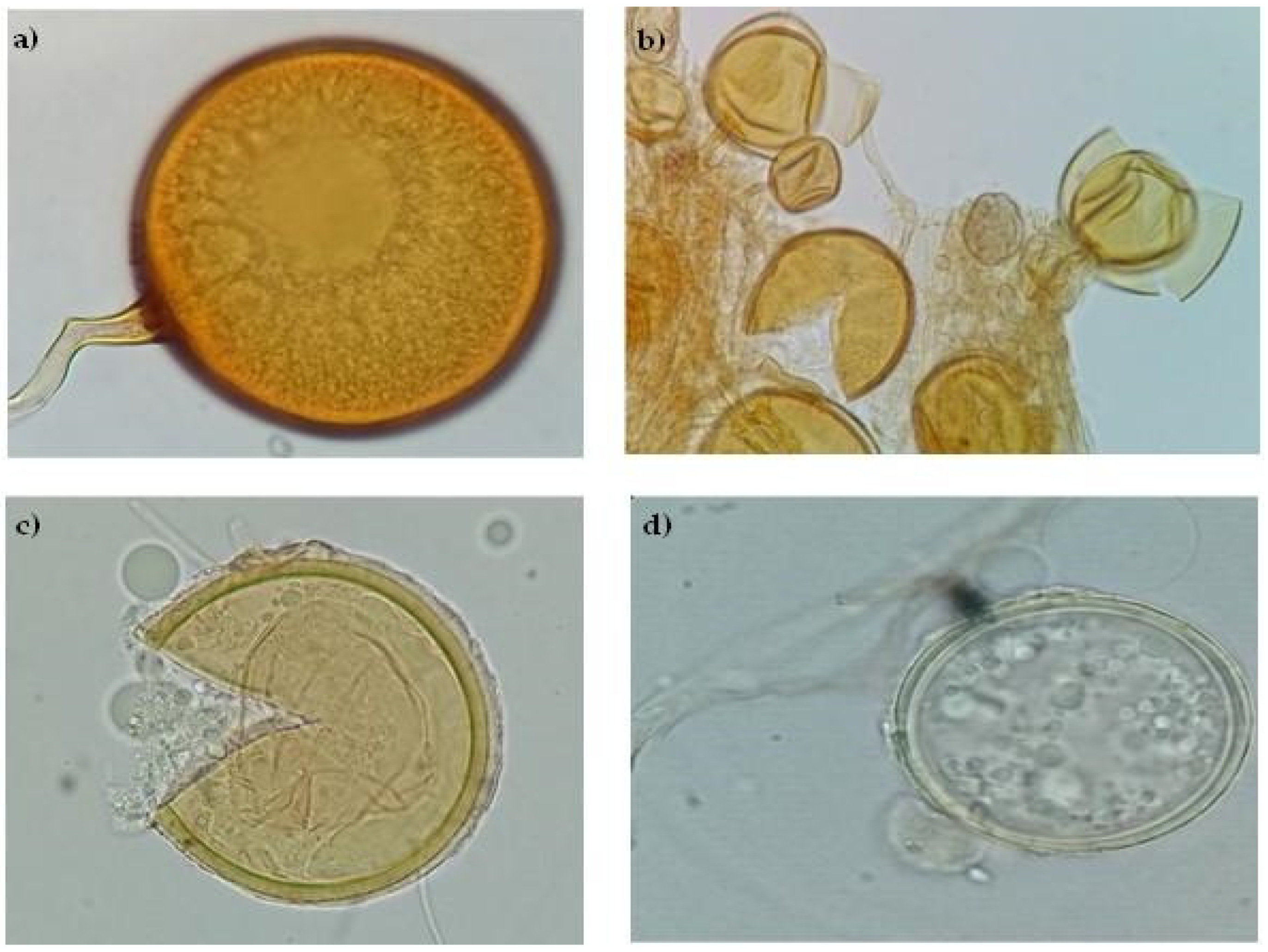
2.5.2. Taxonomical morphotypes identification of spores of AMF
2.5.3. Fungal effectiveness
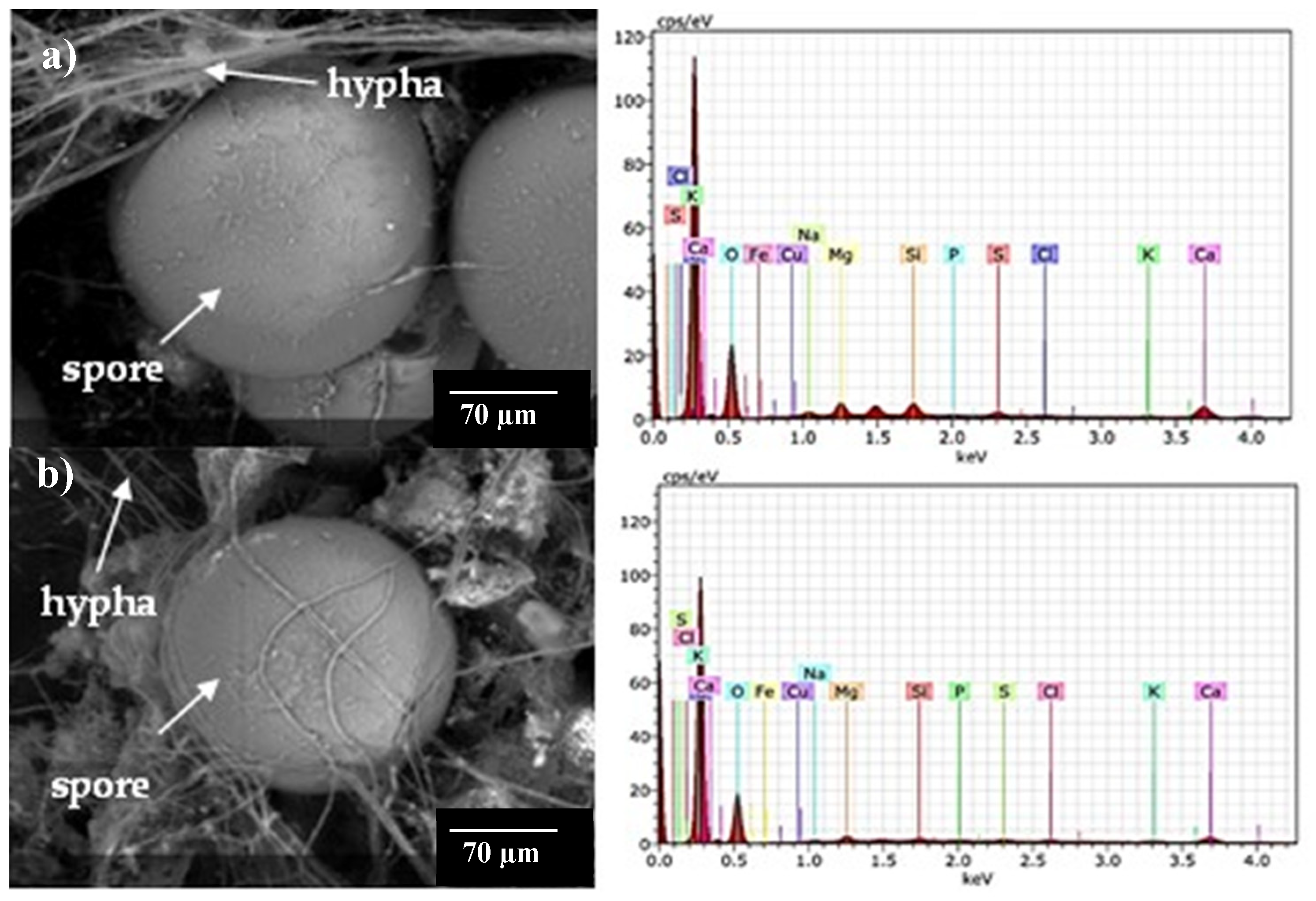
2.5.4. Elemental analysis in AMF spores and hyphae
2.6. Surface disinfection of roots, DNA extraction, and PCR amplification
2.7. Experimental design and statistical analysis
3. Results and discussion
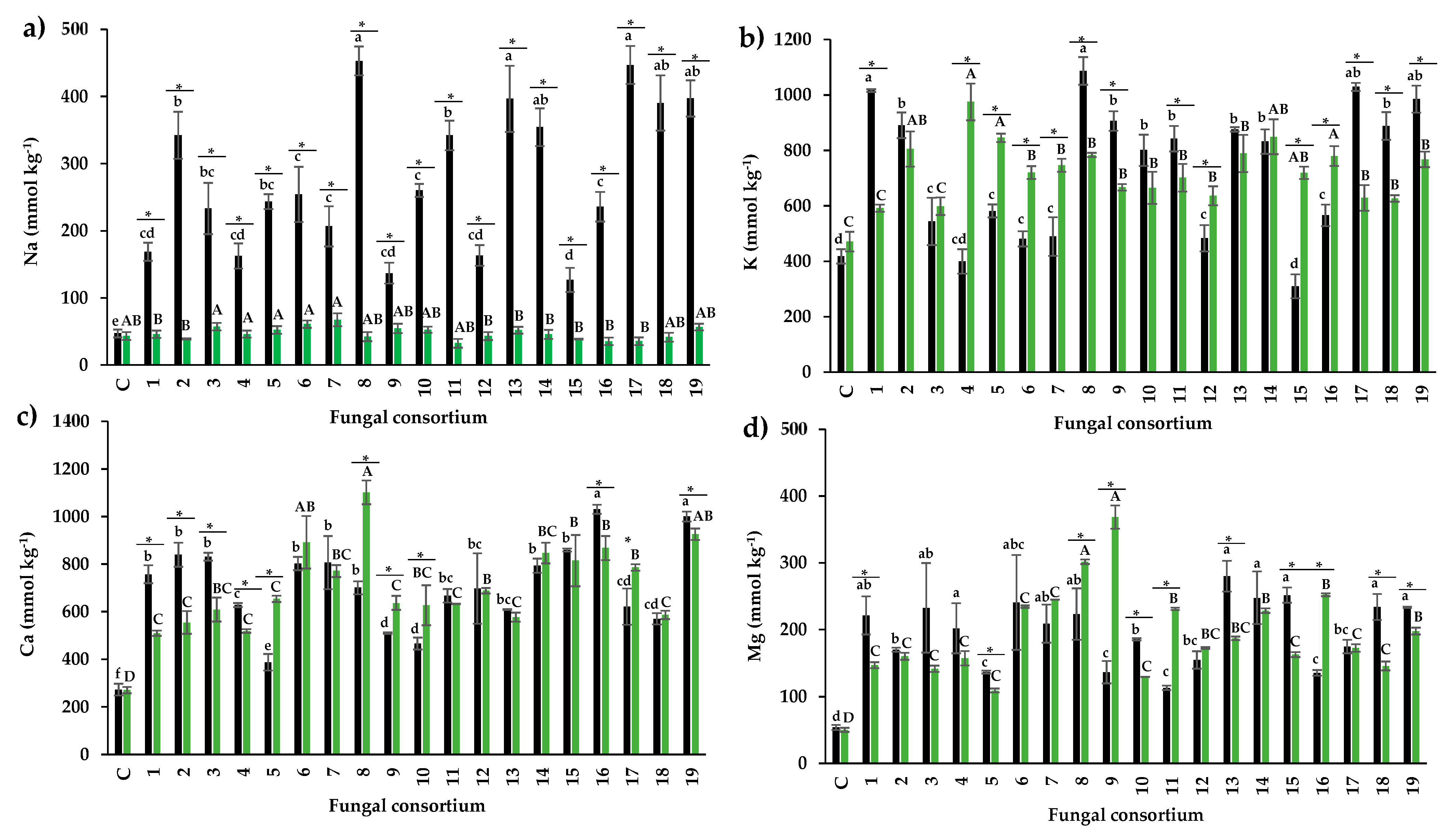
3.1. Physical and chemical analysis of the rhizospheric soil of each composite sample
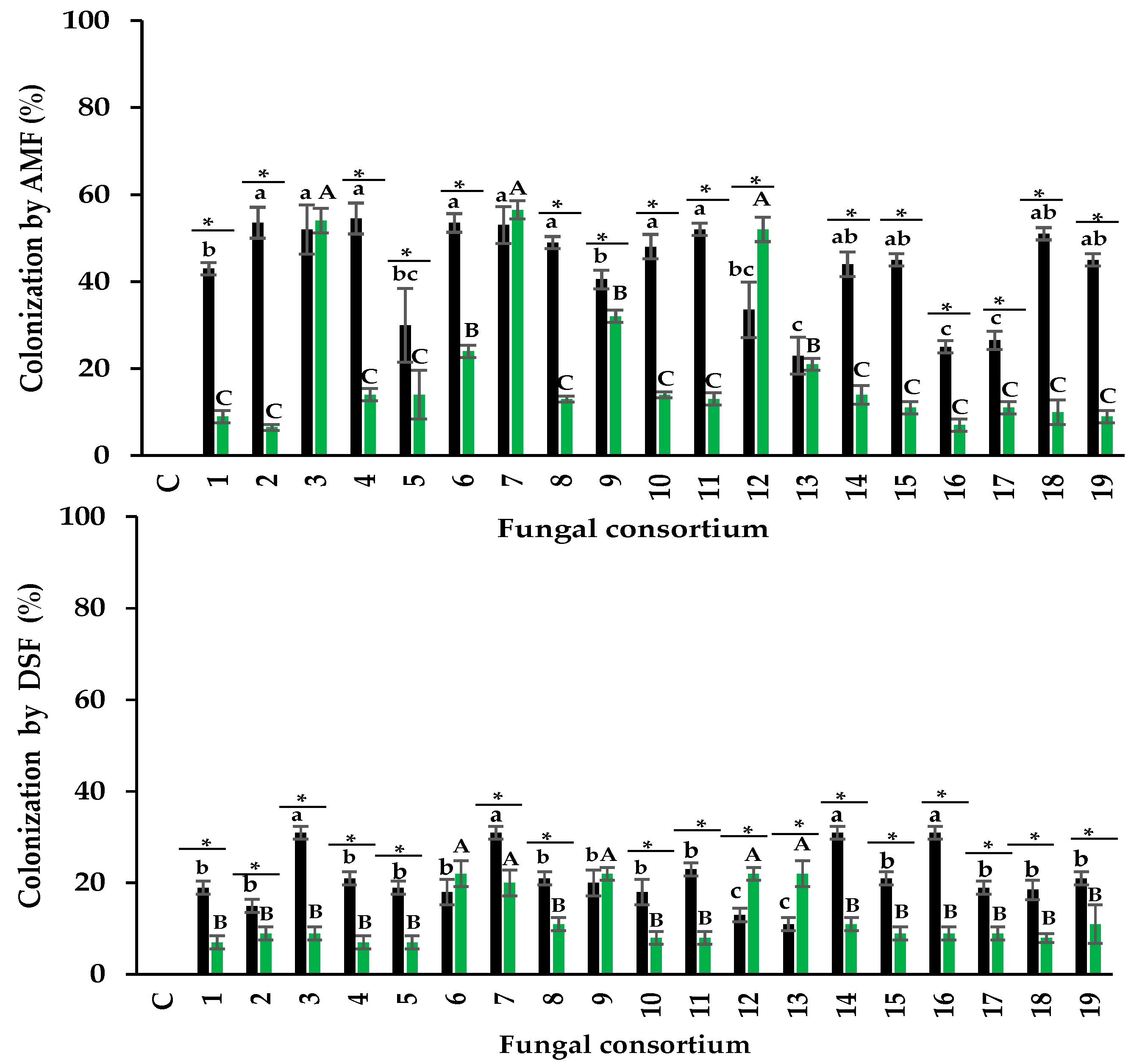
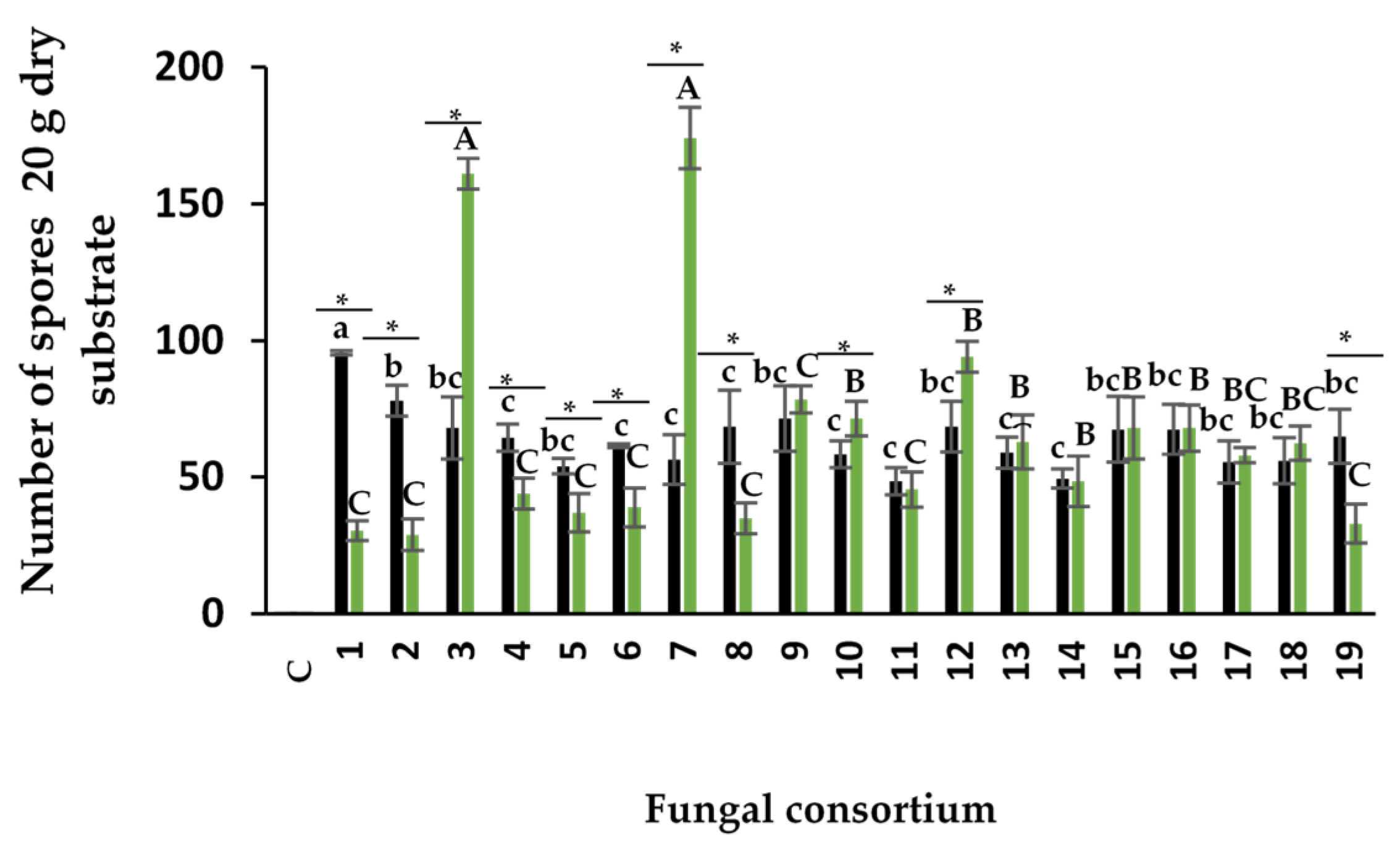
3.2. Infectivity of fungal consortia
3.2.1. Arbuscular mycorrhizal fungi
Mycorrhizal colonization
Number of spores
3.2.2. Dark septate fungi
3.2.2. Dark septate fungi
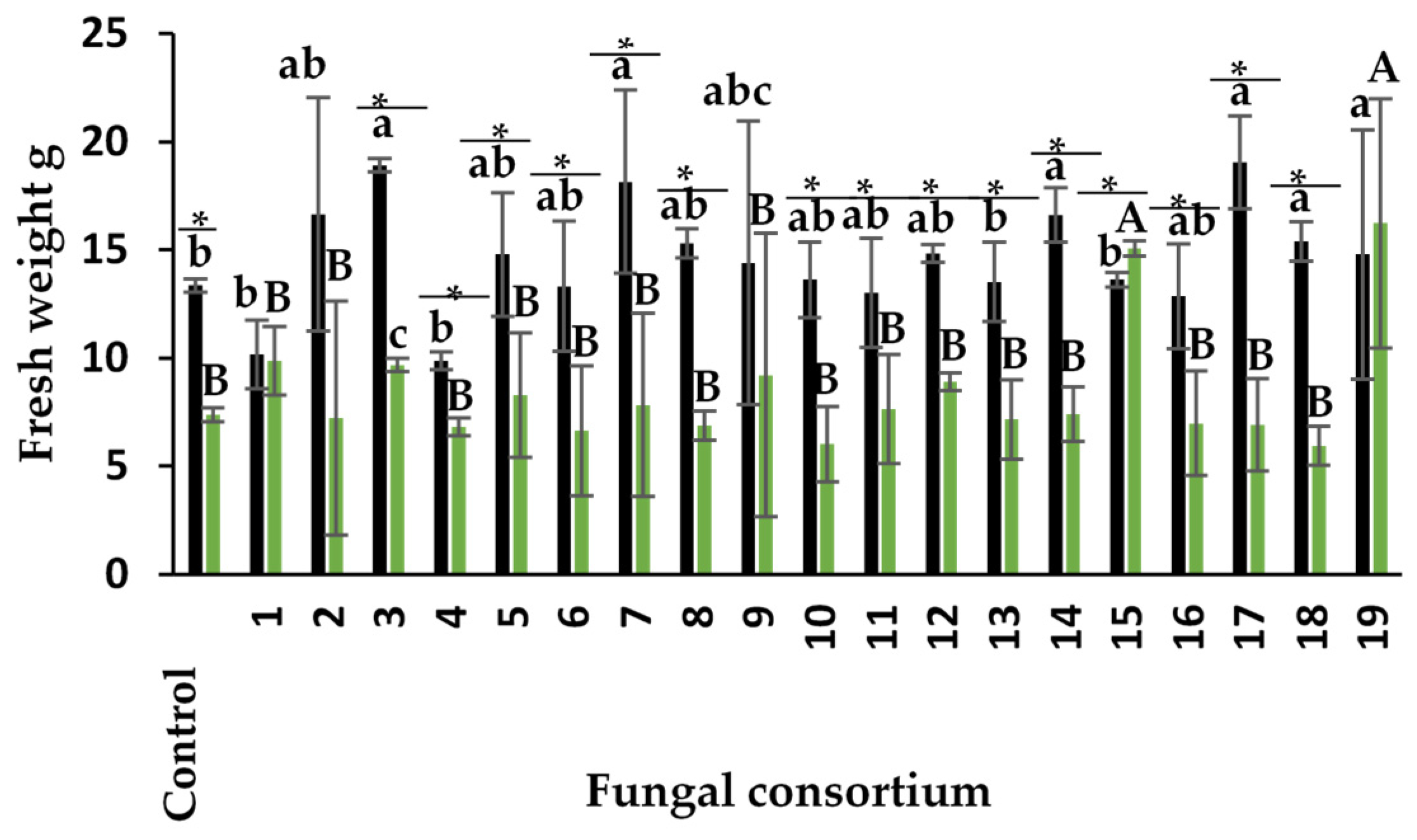
3.3. Mitigation of salt stress in alfalfa plants
3.3.1. Effectiveness of fungal consortia propagated on alfalfa
Fresh weight
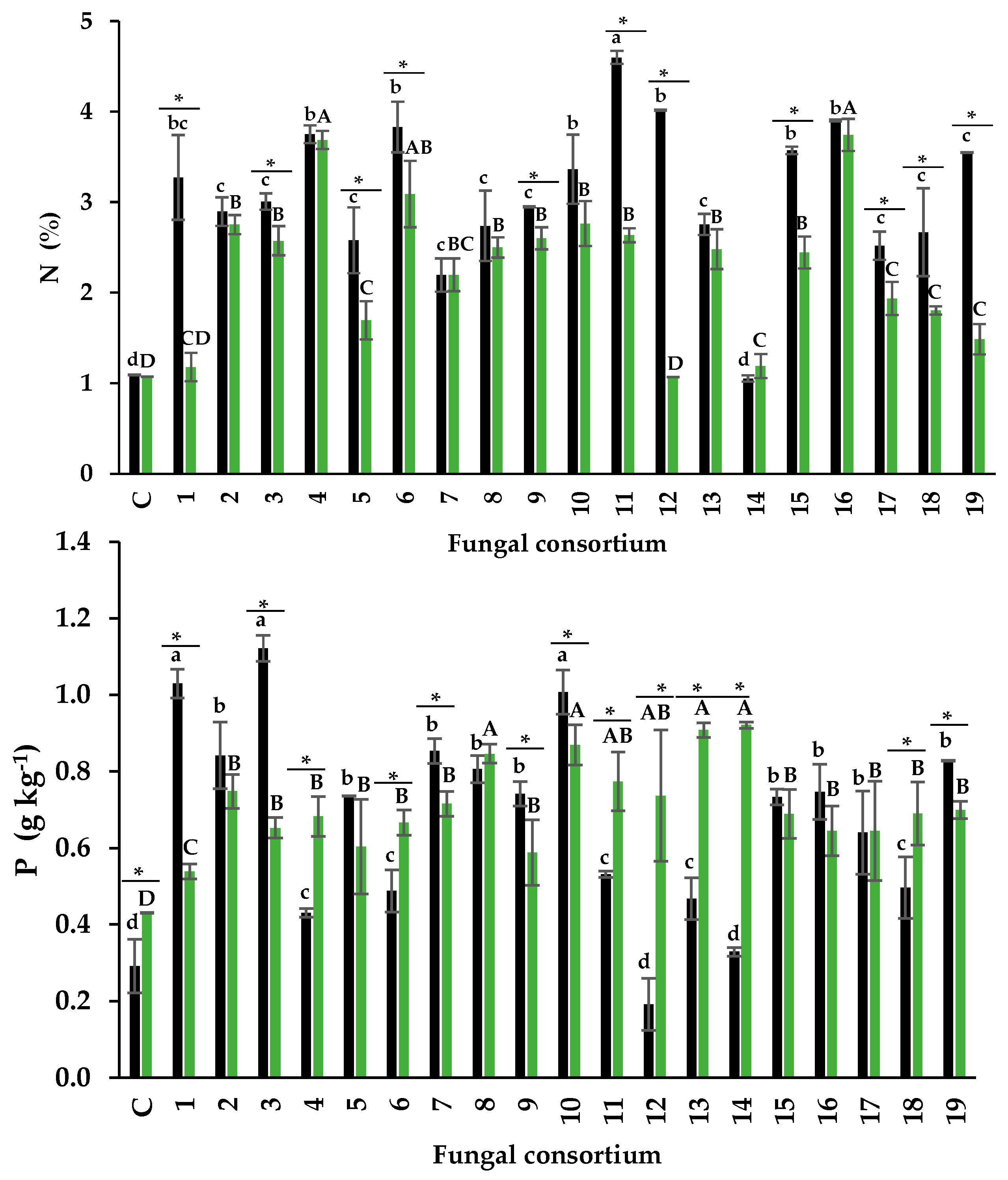
Foliar concentration of N and P
Foliar concentration of Na and protective osmolytes
3.4. Scanning electron microscopy (SEM) and elemental analysis in AMF fungal structures
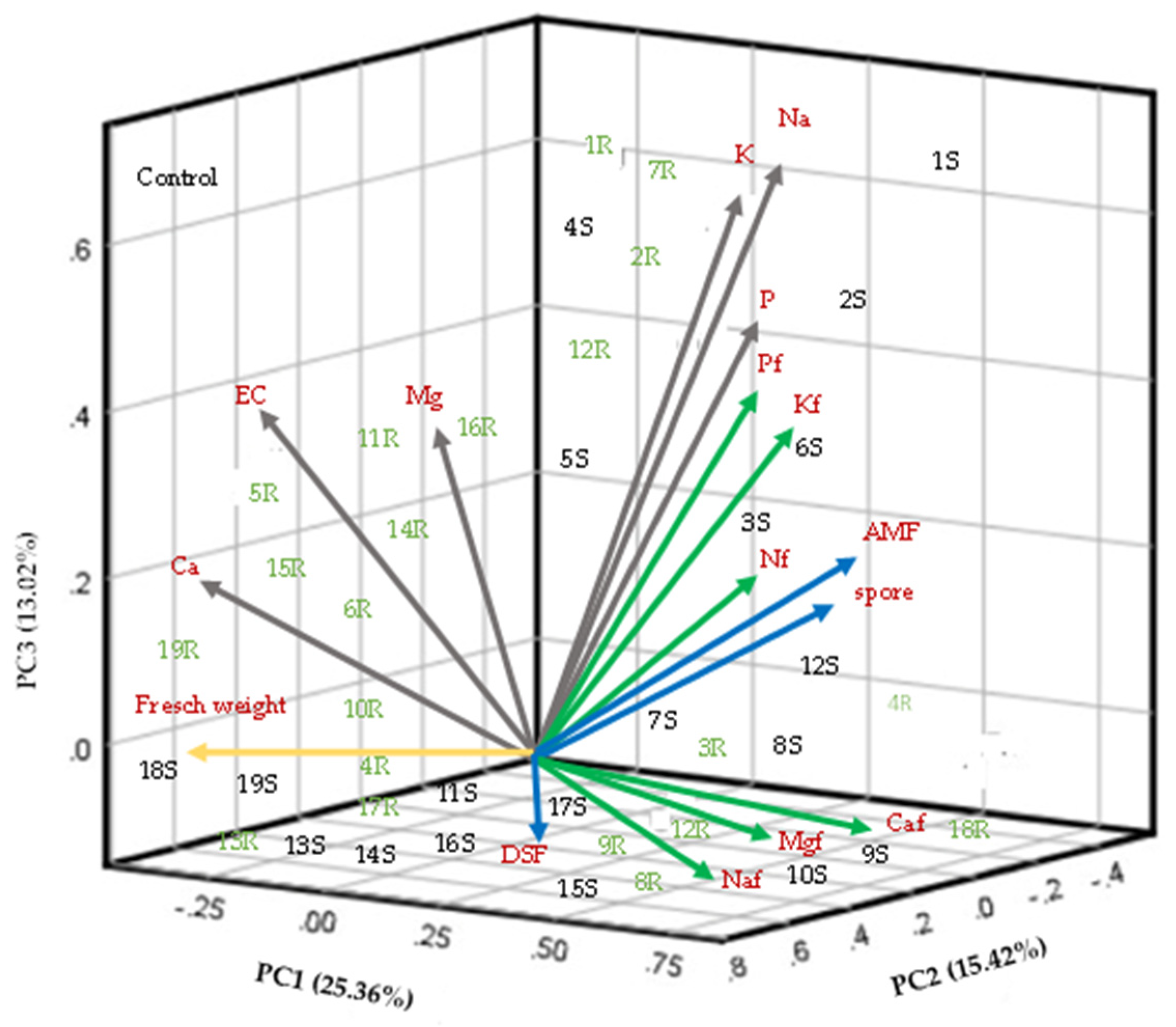
3.5. Principal component analysis (PCA)
3.6. Selection of fungal inoculants
3.7. Identification of AMF spore morphospecies in the selected consortium
3.8. Endophytic microbial community of the selected consortium
4. Conclusions
References
- Abbott, L. K., and Gazey, C. An ecological view of the formation of VA mycorrhizas. Plant Soil 1994, 159, 69–78. [CrossRef]
- Abbott, L. K., Robson, A. D., & Gazey, C. Selection of Inoculant Vesicular-arbuscular Mycorrhizal Fungi. In Methods in microbiology 1992, (Vol. 24, pp. 1-21). Academic Press. [CrossRef]
- Aliasgharzadeh, N., Rastin, S. N., Towfighi, H., & Alizadeh, A. Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 2001, 11, 119-122. [CrossRef]
- Anand, A., Baig, M. J., & Mandal, P. K. Response of alfalfa genotypes to saline water irrigation. Biologia Plantarum 2000, 43(3), 455-457. [CrossRef]
- Ashraf, M., McNeilly, T., & Bradshaw, A. D. The potential for evolution of salt (NaCl) tolerance in seven grass species. New Phytologist 1986, 103(2), 299-309. [CrossRef]
- Ban, Y., Tang, M., Chen, H., Xu, Z., Zhang, H., & Yang, Y. The response of dark septate endophytes (DSE) to heavy metals in pure culture. PLoS one, 2012, 7(10), e47968. [CrossRef]
- Barrow, J. R., Havstad, K. M., & McCaslin, B. D. Fungal root endophytes in fourwing saltbush, Atriplex canescens, on arid rangelands of southwestern USA. Arid Land Research and Management 1997, 11(2), 177-185. [CrossRef]
- Beltrán-Hernández, R. I., Luna-Guido, M. L., & Dendooven, L. (2007). Emission of carbon dioxide and dynamics of inorganic N in a gradient of alkaline saline soils of the former lake Texcoco. Applied soil ecology 2007, 35(2), 390-403. [CrossRef]
- Bencherif, K., Laruelle, F., Tisserant, B., Dalpé, Y., & Lounés-Hadj Sahraoui, A. Engineering approach for production of arbuscular mycorrhizal inoculum adapted to saline soil management. Stresses 2023, 3(2), 404-423. [CrossRef]
- Ben Laouane, R., Baslam, M., Ait-El-Mokhtar, M., Anli, M., Boutasknit, A., Ait-Rahou, Y., & Meddich, A. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms 2020, 8(11), 1695. [CrossRef]
- Berthelot, C., Chalot, M., Leyval, C., & Blaudez, D. From darkness to light: emergence of the mysterious dark septate endophytes in plant growth promotion and stress alleviation. Endophytes for a growing world 2019, 143-164. [CrossRef]
- Bertram, N. A., Alfonso, C., Grande, S., Chiacchiera, S., Ohanian, A., Bonvillani, J., & Angeletti, F. R. Efecto de la concentración salina y el régimen hídrico sobre la germinación, emergencia y establecimiento de alfalfa (Medicago sativa L.). RIA. Revista de investigaciones agropecuarias 2021, 47(2), 267-272.
- Biermann, B., & Linderman, R. G. Use of vesicular-arbuscular mycorrhizal roots, intra-radical vesicles and extra-radical vesicles as inoculum. New Phytologist 1983, 95(1), 97-105.
- Campanelli, A., Ruta, C., De Mastro, G., y Morone-Fortunato, I. The role of arbuscular mycorrhizal fungi in alleviating salt stress in Medicago sativa L. var. icon. Symbiosis 2013, 59(2), 65-76. [CrossRef]
- Castro Silva, C. Análisis del cambio en las comunidades microbianas en un suelo salino-alcalino del exlago de Texcoco en presencia de antraceno 2013, Tesis doctoral. Cinvestav, Unidad Zacatenco.
- Chandra, P., Singh, A., Prajapat, K., Rai, A. K., & Yadav, R. K. Native arbuscular mycorrhizal fungi improve growth, biomass yield, and phosphorus nutrition of sorghum in saline and sodic soils of the semi–arid region. Environmental and Experimental Botany 2022, 201, 104982. [CrossRef]
- Cofré, M. N., Becerra, A. G., Nouhra, E. R., & Soteras, M. F. (2012). Arbuscular mycorrhizae and dark-septate endophytes on Atriplex cordobensis in saline sites from Argentina. Journal of Agricultural Technology 2012, 8(7), 2201-2214.
- Corkidi, L., Allen, E. B., Merhaut, D., Allen, M. F., Downer, J., Bohn, J., & Evans, M. (2004). Assessing the infectivity of commercial mycorrhizal inoculants in plant nursery conditions. Journal of Environmental Horticulture 2004, 22(3), 149-154. [CrossRef]
- Currah, R.S., Tsuneda, A., and Murakami, S. Morphology and ecology of Phialocephala fortinii in roots of Rhododendron brachycarpum. Canadian Journal Botany 1993, 71: 1639–1644. [CrossRef]
- Daft, M. J., & Nicolson, T. H. (1972). Effect of Endogone mycorrhiza on plant growth. IV. Quantitative relationships between the growth of the host and the development of the endophyte in tomato and maize. New Phytologist 1972, 287-295.
- David, D. J. Determination of calcium in plant material by atomic-absorption spectrophotometry. Analyst 1959, 84(1002), 536-545. [CrossRef]
- Della Mónica, I. F., Saparrat, M. C., Godeas, A. M., & Scervino, J. M. The co-existence between DSE and AMF symbionts affects plant P pools through P mineralization and solubilization processes. Fungal Ecology 2015, 17, 10-17. [CrossRef]
- Dhiman, P., Rajora, N., Bhardwaj, S., Sudhakaran, S. S., Kumar, A., Raturi, G., ... & Deshmukh, R. Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiology and Biochemistry 2021, 162, 110-123. [CrossRef]
- Douds Jr, D. D., Nagahashi, G., Pfeffer, P. E., Kayser, W. M., & Reider, C. On-farm production and utilization of arbuscular mycorrhizal fungus inoculum. Canadian Journal of Plant Science 2005, 85(1), 15-21.
- Estrada, B., Aroca, R., Maathuis, F. J., Barea, J. M., & RUIZ-LOZANO, J. M. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant, cell & environment 2013, 36(10), 1771-1782. [CrossRef]
- Evelin, H., Giri, B., & Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenumgraecum. Mycorrhiza 2012, 22, 203-217. [CrossRef]
- Evelin, H., Kapoor, R., y Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Annals of botany 2009, 104(7), 1263-1280. [CrossRef]
- Farias, G. C., Nunes, K. G., Soares, M. A., de Siqueira, K. A., Lima, W. C., Neves, A. L. R., y de Lacerda, C. F. Dark septate endophytic fungi mitigate the effects of salt stress on cowpea plants. Brazilian Journal of Microbiology 2020, 51(1), 243-253. [CrossRef]
- Fuchs, B., & Haselwandter, K. Red list plants: colonization by arbuscular mycorrhizal fungi and dark septate endophytes. Mycorrhiza 2004, 14(4), 277-281. [CrossRef]
- Gaber, D. A., Berthelot, C., Camehl, I., Kovacs, G. M., Blaudez, D., y Franken, P. Salt stress tolerance of dark septate endophytes is independent of melanin accumulation. Frontiers in microbiology 2020, 3158. [CrossRef]
- Gerdemann, J. W., & Nicolson, T. H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological society 1963, 46(2), 235-244. [CrossRef]
- Ghabooli., M. Effect of Piriformospora indica inoculation on some physiological traits of barley (Hordeum vulgare) under salt stress. Chemistry of Natural Compounds 2014, 50:1082–1087. [CrossRef]
- Giri, B., & Mukerji, K. G. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 2004, 14, 307-312. [CrossRef]
- Gonzalez Mateu, M., Baldwin, A. H., Maul, J. E., & Yarwood, S. A. Dark septate endophyte improves salt tolerance of native and invasive lineages of Phragmites australis. The ISME journal 2020, 14(8), 1943-1954. [CrossRef]
- Grattan, S. R., & Grieve, C. M. Salinity mineral nutrient relations in horticultural crops. Scientia horticulturae 1998, 78(1-4), 127-157. [CrossRef]
- Hammer, E. C., Nasr, H., Pallon, J., Olsson, P. A., & Wallander, H. Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 2011, 21, 117-129. [CrossRef]
- Hartmond, U., Schaesberg, N. V., Graham, J. H., & Syvertsen, J. P. (1987). Salinity and flooding stress effects on mycorrhizal and non-mycorrhizal citrus rootstock seedlings. Plant and Soil 1987, 104, 37-43. [CrossRef]
- Havre, G. N. The flame photometric determination of sodium, potassium and calcium in plant extracts with special reference to interference effects. Analytica Chimica Acta 1961, 25(6), 557-566. [CrossRef]
- Hawksworth, D. L., & Rossman, A. Y. Where are all the undescribed fungi? Phytopathology 1997, 87(9), 888-891. [CrossRef]
- Hayman, D. S. Endogone spore numbers in soil and vesicular-arbuscular mycorrhiza in wheat as influenced by season and soil treatment. Transactions of the British mycological Society 1970, 54(1), 53-IN12. [CrossRef]
- Hirrel, M.C. The effect of sodium and chloride salts on the germination of Gigaspora margarita. Mycologia 1981, 73:610-617. [CrossRef]
- Hou, L., Yu, J., Zhao, L., & He, X. (2020). Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Frontiers in microbiology 2020, 10, 3061. [CrossRef]
- INVAM. Results of infectivity (MIP) assays of commercial Inoculants. Available at: http://invam.caf.wvu.edu/otherinfo/commercial/commercial_MIPs.pdf. Accessed 29 December 2011 2011.
- Jahromi, F., Aroca, R., Porcel, R., & Ruiz-Lozano, J. M. Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microbial Ecology 2008, 55, 45-53. [CrossRef]
- Jumpponen, A. R. I., & Trappe, J. M. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. The New Phytologist 1998, 140(2), 295-310. [CrossRef]
- Juniper, S. The effect of sodium chloride on some vesicular–arbuscular mycorrhizal fungi. Ph.D. thesis 1996, The University of Western Australia.
- Juniper, S., y Abbott, L. K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16(5), 371-379. [CrossRef]
- Karagiannidis, N., Bletsos, F., & Stavropoulos, N. Effect of Verticillium wilt (Verticillium dahliae Kleb.) and mycorrhiza (Glomus mosseae) on root colonization, growth and nutrient uptake in tomato and eggplant seedlings. Scientia horticulturae 2002, 94(1-2), 145-156. [CrossRef]
- Karley, A. J., & White, P. J. Moving cationic minerals to edible tissues: potassium, magnesium, calcium. Current opinion in plant biology 2009, 12(3), 291-298. [CrossRef]
- Kassambara, A., y Mundt, F. (2017). Package ‘factoextra’. Extract and visualize the results of multivariate data analyses 2017, 76(2).
- Kendall, C. Tracing nitrogen sources and cycling in catchments. In Isotope tracers in catchment hydrology 1998, (pp. 519-576). Elsevier. [CrossRef]
- Kim, C.K., & Weber D.J. Distribution of VA mycorrhiza on halophytes on inland salt playas. Plant Soil 1985, 83:207-214. [CrossRef]
- Kitson, R. E., & Mellon, M. G. Colorimetric determination of germanium as molybdigermanic acid. Industrial & Engineering Chemistry Analytical Edition 1944, 16(2), 128-130. [CrossRef]
- Knapp, D. G., Kovács, G. M., Zajta, E., Groenewald, J. Z., & Crous, P. W. (2015). Dark septate endophytic Pleosporalean genera from semiarid areas. Persoonia-Molecular Phylogeny and Evolution of Fungi 2015, 35(1), 87-100. [CrossRef]
- Koske, R. E., y Gemma, J. N. A modified procedure for staining roots to detect VA mycorrhizas. Mycological research 1989, 92(4), 486. [CrossRef]
- LaHaye, P., & Epstein, E. Salt toleration by plants: enhancement with calcium. Science 1969, 166(3903), 395-396. [CrossRef]
- Laouane, B., Meddich, A., Bechtaoui, N., Oufdou, K., & Wahbi, S. Effects of arbuscular mycorrhizal fungi and rhizobia symbiosis on the tolerance of Medicago sativa to salt stress. Gesunde Pflanzen 2019, 71(2), 135-146. [CrossRef]
- Latef, A. A. H. A., & Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Scientia Horticulturae 2011, 127(3), 228-233. [CrossRef]
- Levy, Y., Dodd, J., & Krikun, J. Effect of irrigation, water salinity and rootstock on the vertical distribution of vesicular-arbuscular mycorrhiza in citrus roots. New Phytologist 1983, 95(3), 397-403. [CrossRef]
- Maciá-Vicente, J. G., Ferraro, V., Burruano, S., & Lopez-Llorca, L. V. Fungal assemblages associated with roots of halophytic and non-halophytic plant species vary differentially along a salinity gradient. Microbial ecology 2012, 64, 668-679. [CrossRef]
- Maggio, A., Chiaranda, F. Q., Cefariello, R., y Fagnano, M. Responses to ozone pollution of alfalfa exposed to increasing salinity levels. Environmental Pollution 2009, 157(5), 1445-1452. [CrossRef]
- Mahmood, I. A., Arshad, A., Aslam, M., Shahzad, A., Sultan, T., & Hussain, F. Phosphorus availability in different salt-affected soils as influenced by crop residue incorporation. International Journal of Agriculture and Biology 2013, 15(3).
- Malhotra, H., Sharma, S., & Pandey, R. Phosphorus nutrition: plant growth in response to deficiency and excess. Plant nutrients and abiotic stress tolerance 2018, 171-190. [CrossRef]
- Mapperson, R. R., Kotiw, M., Davis, R. A., & Dearnaley, J. D. The diversity and antimicrobial activity of Preussia sp. endophytes isolated from Australian dry rainforests. Current microbiology 2014, 68, 30-37. [CrossRef]
- Marschner, H., & Cakmak, I. High light intensity enhances chlorosis and necrosis in leaves of zinc, potassium, and magnesium deficient bean (Phaseolus vulgaris) plants. Journal of Plant physiology 1989, 134(3), 308-315. [CrossRef]
- Martínez, C. M., Marcos, M. F., & Rodriguez, E. A. Factors influencing phosphorus adsorption in mine soils in Galicia, Spain. Science of the total environment 1996, 180(2), 137-145. [CrossRef]
- Martinez, V., & Läuchli, A. Salt-induced inhibition of phosphate uptake in plants of cotton (Gossypium hirsutum L.). New Phytologist 1994, 126(4), 609-614.
- Mattson Jr, W. J. Herbivory in relation to plant nitrogen content. Annual review of ecology and systematics 1980, 11(1), 119-161.
- McMillen, B. G., Juniper, S., & Abbott, L. K. Inhibition of hyphal growth of a vesicular-arbuscular mycorrhizal fungus in soil containing sodium chloride limits the spread of infection from spores. Soil Biology and Biochemistry 1998, 30(13), 1639-1646. [CrossRef]
- Millner, P. D., y Kitt, D. G. The Beltsville method for soilless production of vesicular-arbuscular mycorrhizal fungi. Mycorrhiza 1992, 2(1), 9-15. [CrossRef]
- Nagy, R., Drissner, D., Amrhein, N., Jakobsen, I., & Bucher, M. (2009). Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytologist 2009, 181(4), 950-959. [CrossRef]
- Olsen, S. R. Estimation of available phosphorus in soils by extraction with sodium bicarbonate (No. 939). US Department of Agriculture 1954.
- Olsson, P. A., Hammer, E. C., Pallon, J., Van Aarle, I. M., & Wallander, H. (2011). Elemental composition in vesicles of an arbuscular mycorrhizal fungus, as revealed by PIXE analysis. Fungal biology 2011, 115(7), 643-648. [CrossRef]
- Pan, X., Qin, Y., & Yuan, Z. Potential of a halophyte-associated endophytic fungus for sustaining Chinese white poplar growth under salinity. Symbiosis 2018, 76, 109-116. [CrossRef]
- Phillips, J. M., y Hayman, D. S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British mycological Society 1970, 55(1), 158-IN18. [CrossRef]
- Piercey, M. M., Graham, S. W., & Currah, R. S. Patterns of genetic variation in Phialocephala fortinii across a broad latitudinal transect in Canada. Mycological Research 2004, 108(8), 955-964. [CrossRef]
- Porcel, R., Aroca, R., Azcon, R., & Ruiz-Lozano, J. M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673-684. [CrossRef]
- Porras-Alfaro, A., & Bayman, P. Hidden fungi, emergent properties: endophytes and microbiomes. Annual review of phytopathology 2011, 49, 291-315. [CrossRef]
- Redman, R. S., Anderson, J. A., Biaggi, T. M., Malmberg, K. E., Rienstra, M. N., Weaver, J. L., & Rodriguez, R. J. Symbiotic modulation as a driver of niche expansion of coastal plants in the San Juan Archipelago of Washington State. Frontiers in Microbiology 2022, 13. [CrossRef]
- Rodrı́guez-Navarro, A. (2000). Potassium transport in fungi and plants. Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes 2000, 1469(1), 1-30. [CrossRef]
- Ruiz-Lozano, J. M., Porcel, R., Azcon, C., & Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. Journal of Experimental Botany 2012, 63(11), 4033-4044. [CrossRef]
- Santoyo de la Cruz, M. F., Flores-Magdaleno, H., Khalil-Gardezi, A., Mancilla-Villa, Ó. R., & Rubiños-Panta, J. E. Composición iónica y comparación de índices de salinidad de suelo agrícola de Texcoco, México. Nova scientia 2021, 13(27).
- Shi, Y. W., Lou, K., Li, C., Wang, L., Zhao, Z. Y., Zhao, S., & Tian, C. Y. Illumina-based analysis of bacterial diversity related to halophytes Salicornia europaea and Sueada aralocaspica. Journal of Microbiology 2015, 53, 678-685. [CrossRef]
- Shrivastava, P., y Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi journal of biological Sciences 2015, 22(2), 123-131. [CrossRef]
- Smith, S. E., y Read, D. J. Mycorrhizal symbiosis. 2008, (Academic Press Ltd: Cambridge, UK).
- Song, M., Chai, Q., Li, X., Yao, X., Li, C., Christensen, M.J, & Nan, Z. An asexual Epichloë endophyte modifies the nutrient stoichiometry of wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil 2015, 387:153–165. [CrossRef]
- Sonjak, S., Udovič, M., Wraber, T., Likar, M., & Regvar, M. Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Sečovlje salterns. Soil Biology and Biochemistry 2009, 41(9), 1847-1856. [CrossRef]
- Saqib, M., Zörb, C., & Schubert, S. Silicon-mediated improvement in the salt resistance of wheat (Triticum aestivum) results from increased sodium exclusion and resistance to oxidative stress. Functional Plant Biology 2008, 35(7), 633-639. [CrossRef]
- Tekaya, M., Mechri, B., Mbarki, N., Cheheb, H., Hammami, M., & Attia, F. Arbuscular mycorrhizal fungus Rhizophagus irregularis influences key physiological parameters of olive trees (Olea europaea L.) and mineral nutrient profile. Photosynthetica 2017, 55(2), 308-316. [CrossRef]
- Trejo-Aguilar, D., & Banuelos, J. Isolation and culture of arbuscular mycorrhizal fungi from field samples. Arbuscular Mycorrhizal Fungi: Methods and Protocols 2020, 1-18. [CrossRef]
- Valenzuela-Encinas, C., Neria-González, I., Alcántara-Hernández, R. J., Enríquez-Aragón, J. A., Estrada-Alvarado, I., Hernández-Rodríguez, C., & Marsch, R. Phylogenetic analysis of the archaeal community in an alkaline-saline soil of the former lake Texcoco (Mexico). Extremophiles 2008, 12, 247-254. [CrossRef]
- Vergara, C., Araujo, K. E. C., Sperandio, M. V. L., Santos, L. A., Urquiaga, S., y Zilli, J. É. Dark septate endophytic fungi increase the activity of proton pumps, efficiency of 15N recovery from ammonium sulphate, N content, and micronutrient levels in rice plants. Brazilian Journal of Microbiology 2019, 50(3), 825-838. [CrossRef]
- Vergara, C., Araujo, K. E., Urquiaga, S., Schultz, N., Balieiro, F. D. C., Medeiros, P. S., & Zilli, J. E. Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Frontiers in Microbiology 2017, 8, 2437. [CrossRef]
- Wang, F. Y., Liu, R. J., Lin, X. G., & Zhou, J. M. Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta. Mycorrhiza 2004, 14, 133-137. [CrossRef]
- Waqas, M., Khan, A.L., Kamran M, Hamayun, M., Kang S-M, Kim Y-H, Lee I-J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17:10754–10773. [CrossRef]
- Xie, W., Yang, J., Gao, S., Yao, R., & Wang, X. The effect and influence mechanism of soil salinity on phosphorus availability in coastal salt-affected soils. Water 2022, 14(18), 2804. [CrossRef]
- Yin, L., Ren, A., Wei, M., Wu, L., Zhou, Y., Li X., & Gao, Y. Neotyphodium coenophialum-infected tall fescue and its potential application in the phytoremediation of saline soils. International Journal Phytoremediation 2014, 16:235–246. [CrossRef]
- Yuan, Z., Druzhinina, I. S., Gibbons, J. G., Zhong, Z., Van de Peer, Y., Rodriguez, R. J., & Martin, F. M. Divergence of a genomic island leads to the evolution of melanization in a halophyte root fungus. The ISME journal 2021, 15(12), 3468-3479. [CrossRef]
- Yuan, Z., Druzhinina, I. S., Labbé, J., Redman, R., Qin, Y., Rodriguez, R., ... & Lin, F. Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Scientific reports 2016, 6(1), 32467. [CrossRef]
- Yun P, Xu L, Wang S-S, Shabala L, Shabala S, Zhang W. Y. Piriformospora indica improves salinity stress tolerance in Zea mays L. plants by regulating Na+ and K+ loading in root and allocating K+ in shoots. Plant Growth Regul 2018, 86:323–331. [CrossRef]
- Zare-Maivan, H., Khanpour-Ardestani, N., & Ghanati, F. Influence of mycorrhizal fungi on growth, chlorophyll content, and potassium and magnesium uptake in maize. Journal of plant Nutrition 2017, 40(14), 2026-2032. [CrossRef]
- Zhang, Q., Acuña, J. J., Inostroza, N. G., Mora, M. L., Radic, S., Sadowsky, M. J., & Jorquera, M. A. Endophytic bacterial communities associated with roots and leaves of plants growing in Chilean extreme environments. Scientific reports 2019, 9(1), 4950. [CrossRef]
- Zuccarini, P., & Okurowska, P. Effects of mycorrhizal colonization and fertilization on growth and photosynthesis of sweet basil under salt stress. Journal of Plant Nutrition 2008, 31(3), 497-513. [CrossRef]

| Consortium | # of individual soil sample † | Cations concentration (mM)†† |
EC (dS m-1) †† | pH†† | SAR (mM)†† | Soil type | PO4-2 Olsen (mg kg-1) †† |
Vegetal species | ||||
| Na | K | Ca | Mg | S | SS | |||||||
| 1 | 5 | 655.9 | 60.2 | 2.0 | 0.7 | 42.5 | 7.8 | 580.4 | + | 10.91 | Distichlis spicata | |
| 2 | 8, 10 | 585.3 | 70.8 | 5.1 | 1.4 | 36.3 | 8.1 | 325.1 | + | 15.46 | Distichlis spicata | |
| 3 | 19 | 322.9 | 39.3 | 3.3 | 0.8 | 20.3 | 8.1 | 225.1 | + | 12.19 | Kochia scoparia | |
| 4 | 29 | 272.5 | 15.2 | 3.0 | 0.5 | 17.5 | 9.2 | 206.4 | + | 15.12 | Eragrostis obtusiflora | |
| 5 | 13 | 222.0 | 18.3 | 3.3 | 0.8 | 15.4 | 8.3 | 154.0 | + | 13.86 | Distichlis spicata | |
| 6 | 2, 18 | 212.0 | 24.7 | 3.3 | 2.4 | 14.8 | 8.5 | 213.6 | + | 15.69 | Suaeda torreyana | |
| 7 | 6 | 94.9 | 12.6 | 2.3 | 1.5 | 8.5 | 8.1 | 69.2 | + | 13.28 | Distichlis spicata | |
| 8 | 9, 11 | 131.2 | 18.6 | 4.4 | 3.1 | 9.1 | 8.4 | 67.2 | + | 16.49 | Baccharis salicifolia | |
| 9 | 20, 23 | 52.4 | 16.8 | 8.2 | 2.1 | 8.7 | 8.6 | 23.0 | + | 12.91 | Distichlis spicata | |
| 10 | 7 | 80.8 | 9.1 | 3.9 | 1.6 | 6.0 | 9.4 | 49.0 | + | 17.38 | Kochia scoparia | |
| 11 | 12, 14, 16 | 181.6 | 12.3 | 5.7 | 3.2 | 6.4 | 9.6 | 86.5 | + | 15.00 | Eragrostis obtusiflora | |
| 12 | 32, 37 | 212.0 | 12.6 | 3.1 | 1.7 | 6.6 | 8.2 | 135.2 | + | 6.41 | Eragrostis obtusiflora | |
| 13 | 17 | 50.5 | 9.1 | 3.9 | 1.0 | 3.8 | 8.5 | 32.5 | + | 8.13 | Cynodon dactylon | |
| 14 | 4 | 52.5 | 8.1 | 4.9 | 1.4 | 3.1 | 8.7 | 29.6 | + | 9.96 | Baccharis salicifolia | |
| 15 | 3 | 131.1 | 8.1 | 6.0 | 1.4 | 2.6 | 8.7 | 68.3 | + | 16.15 | Kochia scoparia | |
| 16 | 21, 22 | 38.3 | 7.3 | 3.9 | 3.0 | 2.2 | 8.4 | 20.6 | + | 10.76 | Distichlis spicata | |
| 17 | 1, 27, 28, 30, 31, 33 | 141.3 | 3.4 | 3.1 | 1.2 | 3.8 | 7.9 | 96.8 | + | 7.18 | Eragrostis obtusiflora | |
| 18 | 15, 34, 35, 36, 38 | 31.3 | 6.3 | 6.3 | 3.3 | 2.0 | 8.0 | 14.3 | + | 8.79 | Baccharis salicifolia | |
| 19 | 24, 25 | 31.3 | 6.0 | 5.2 | 1.6 | 0.9 | 8.4 | 17.0 | + | 12.14 | Kochia scoparia | |
| Normal in the soil solution | 0.2††† | 20†††† | 0.1‡ | 8‡‡ | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





