Submitted:
28 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
3. Results and Discussion
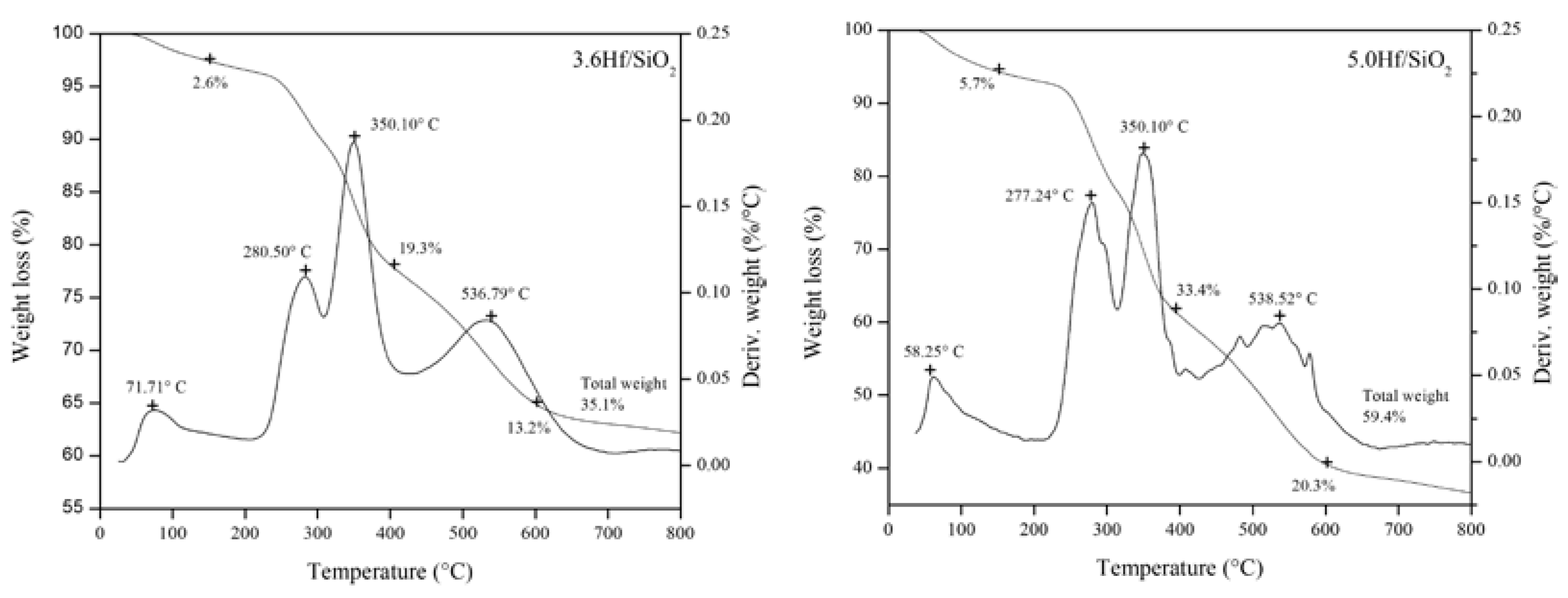
3.1. Thermal Analysis
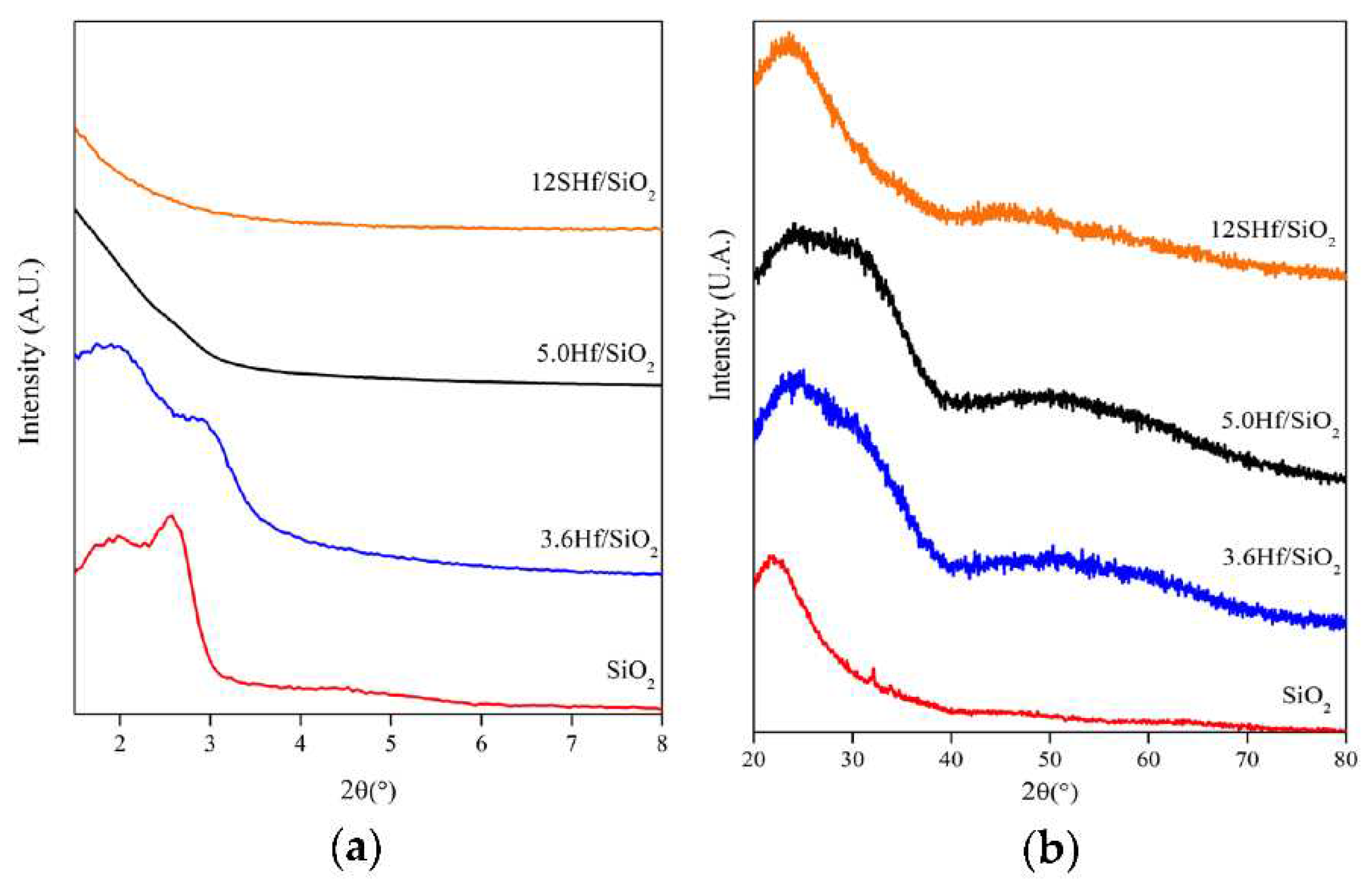
3.2. X-ray Diffraction
3.3. Textural Properties
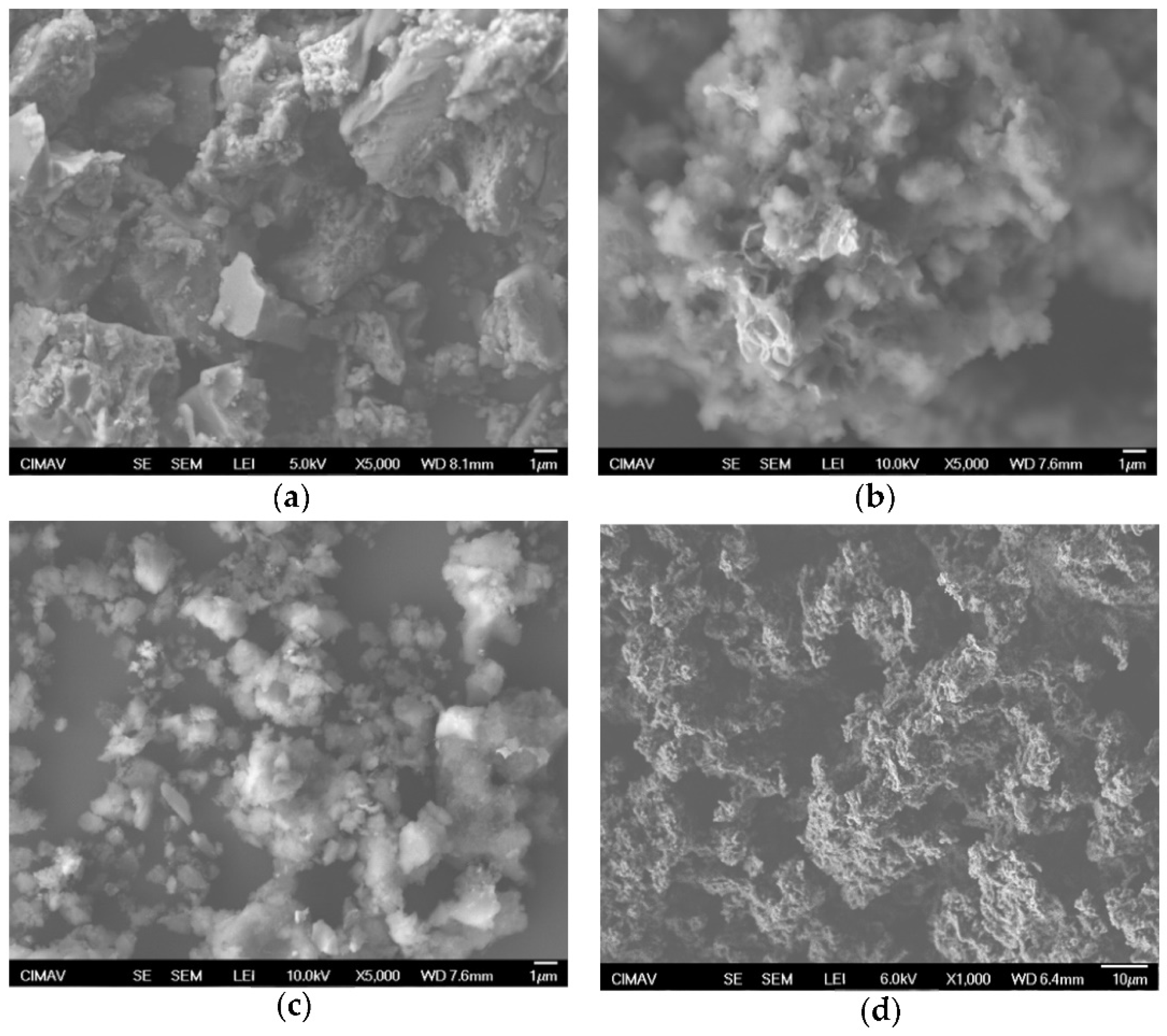
3.4. Scanning Electron Microscopy
3.5. Transmission Electron Microscopy
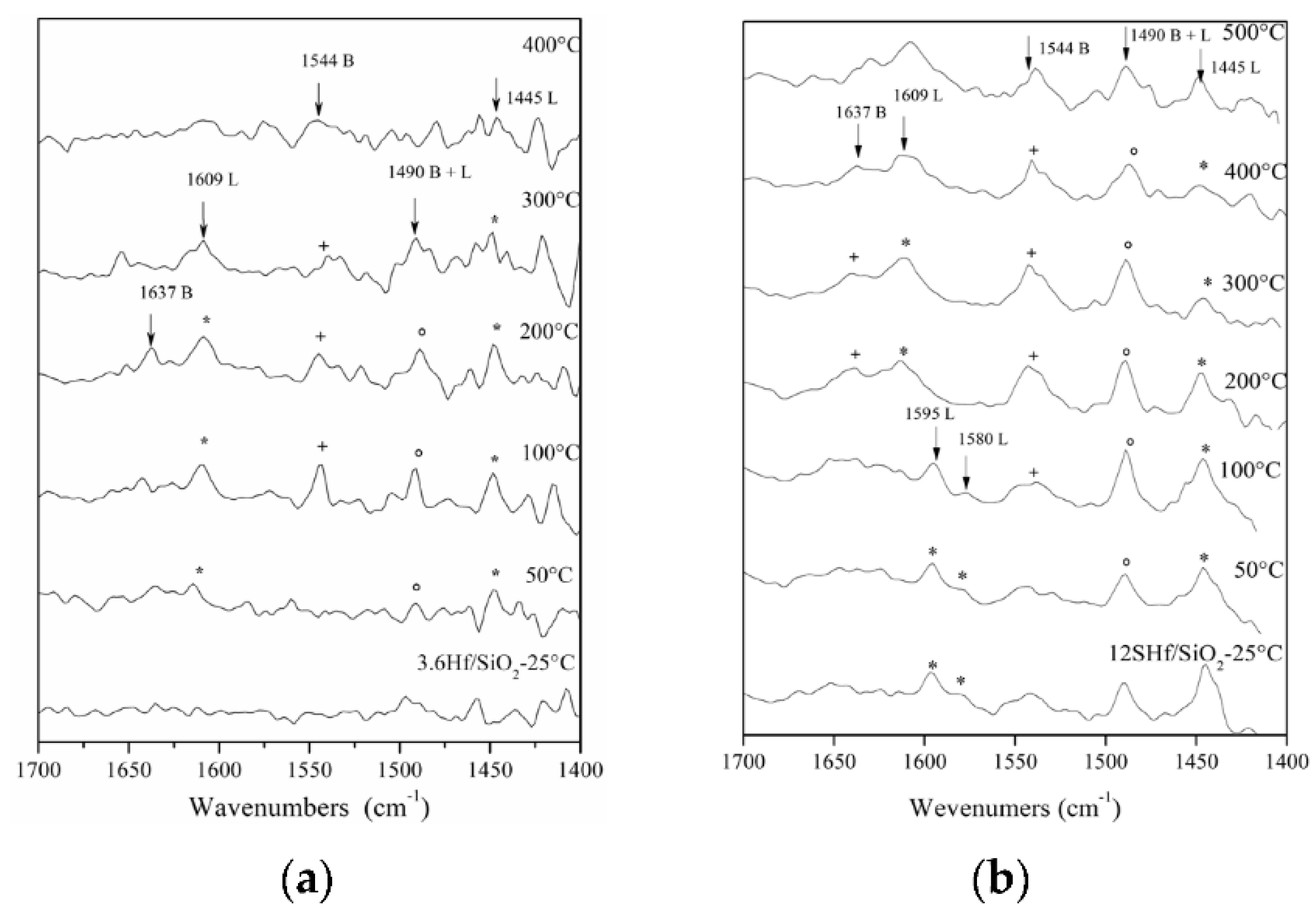
3.6. FTIR Spectroscopy
3.7. Potentiometric Titration with n-Butylamine
3.8. FTIR-Pyridine Spectroscopy
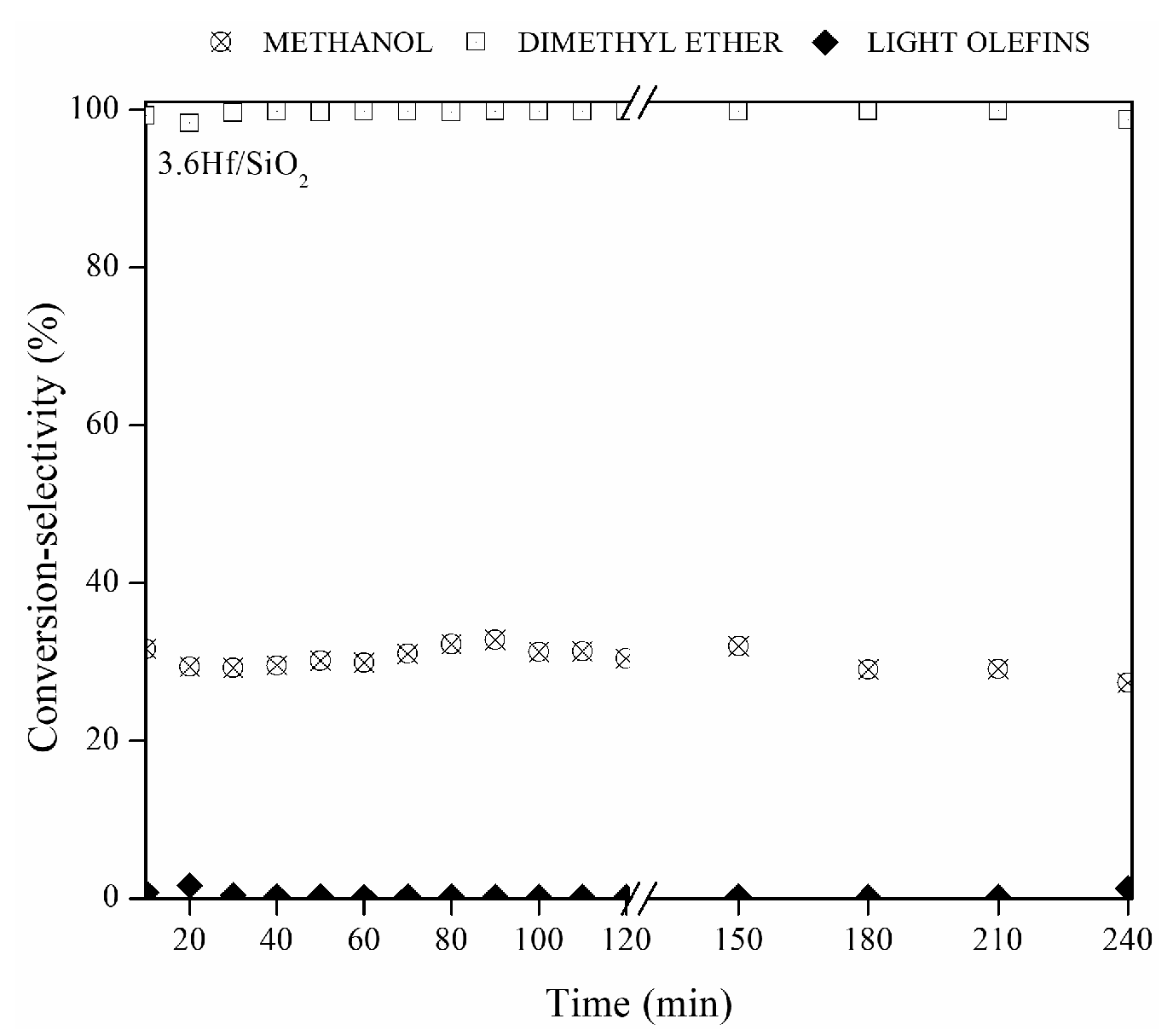
3.9. Catalytic Activity
3.9.1. Ethanol Dehydration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dhengale, S.D.; Bhosale, T.R.; Shinde, S.B.; Rode, C.V.; Kolekar, G.B.; Anbhule, P.V. An Efficient and Convenient Heterogeneous Cu/MCM-41 Catalyst for the Synthesis of 7,10,11,12-Tetrahydrobenzo[c]Acridin-8(9H)-One Derivatives. Res. Chem. Intermed. 2023, 49, 1581–1600. [Google Scholar] [CrossRef]
- Afanasiev, P.; Thiollier, A.; Breysse, M.; Dubois, J.L. Control of the Textural Properties of Zirconium Oxide. Top. Catal. 1999, 8, 147–160. [Google Scholar] [CrossRef]
- Du, Y.; Sun, Y.; Di, Y.; Zhao, L.; Liu, S.; Xiao, F.-S. Ordered Mesoporous Sulfated Silica-Zirconia Materials with High Zirconium Contents in the Structure. J. Porous Mater. 2006, 13, 163–171. [Google Scholar] [CrossRef]
- Claure Zeballos, M.; Pardo Tarifa, F.L.; Lopez N., L. G.; Cabrera M., S. Mesoporous Silicoaluminate Materials (MCM-41, SBA-15 And MCF) By Atrane Route For Cobalt Catalyst. Rev. Boliv. Quím. 2022, 39. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Zirconium Doped MCM-41 Supported WO3 Solid Acid Catalysts for the Esterification of Oleic Acid with Methanol. Appl. Catal. Gen. 2010, 379, 61–68. [Google Scholar] [CrossRef]
- Naik, S.P.; Bui, V.; Ryu, T.; Miller, J.D.; Zmierczak, W. Al-MCM-41 as Methanol Dehydration Catalyst. Appl. Catal. Gen. 2010, 381, 183–190. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Wang, G.; Liu, Y.; Wang, F. Synthesis, Characterization and Catalytic Activities of Bimetallic Modified MCM-41 for Epoxidation of Styrene. J. Sol-Gel Sci. Technol. 2011, 57, 185–192. [Google Scholar] [CrossRef]
- Xie, C.; Liu, F.; Yu, S.; Xie, F.; Li, L.; Zhang, S.; Yang, J. Catalytic Cracking of Polypropylene into Liquid Hydrocarbons over Zr and Mo Modified MCM-41 Mesoporous Molecular Sieve. Catal. Commun. 2008, 10, 79–82. [Google Scholar] [CrossRef]
- Broqvist, P.; Pasquarello, A. Amorphous Hafnium Silicates: Structural, Electronic and Dielectric Properties. Microelectron. Eng. 2007, 84, 2416–2419. [Google Scholar] [CrossRef]
- Kopani, M.; Mikula, M.; Pinčík, E.; Kobayashi, H.; Takahashi, M. FT IR Spectroscopy of Nitric Acid Oxidation of Silicon with Hafnium Oxide Very Thin Layer. Appl. Surf. Sci. 2014, 301, 24–27. [Google Scholar] [CrossRef]
- O’Dell, L.A.; Gunawidjaja, P.N.; Holland, M.A.; Mountjoy, G.; Pickup, D.M.; Newport, R.J.; Smith, M.E. Characterisation of Sol–Gel Prepared (HfO2)x(SiO2)1−x (X=0.1, 0.2 and 0.4) by 1H, 13C, 17O and 29Si MAS NMR, FTIR and TGA. Solid State Nucl. Magn. Reson. 2008, 33, 16–24. [Google Scholar] [CrossRef]
- Ahmed, M.A. Surface Characterization and Catalytic Activity of Sulfated-Hafnia Promoted Zirconia Catalysts for n-Butane Isomerization. Fuel Process. Technol. 2011, 92, 1121–1128. [Google Scholar] [CrossRef]
- Khatri, C.; Mishra, M.K.; Rani, A. Synthesis and Characterization of Fly Ash Supported Sulfated Zirconia Catalyst for Benzylation Reactions. Fuel Process. Technol. 2010, 91, 1288–1295. [Google Scholar] [CrossRef]
- Jakóbik-Kolon, A.; Bok-Badura, J. Stripping of Hafnium and Zirconium from Chelating Ion-Exchange Resin. Polyhedron 2022, 224, 116023. [Google Scholar] [CrossRef]
- Ahmed, A.I.; El-Hakam, S.A.; Samra, S.E.; EL-Khouly, A.A.; Khder, A.S. Structural Characterization of Sulfated Zirconia and Their Catalytic Activity in Dehydration of Ethanol. Colloids Surf. Physicochem. Eng. Asp. 2008, 317, 62–70. [Google Scholar] [CrossRef]
- Bharali, P.; Thrimurthulu, G.; Katta, L.; Reddy, B.M. Preparation of Highly Dispersed and Thermally Stable Nanosized Cerium–Hafnium Solid Solutions over Silica Surface: Structural and Catalytic Evaluation. J. Ind. Eng. Chem. 2012, 18, 1128–1135. [Google Scholar] [CrossRef]
- Moradi, G.R.; Yaripour, F.; Vale-Sheyda, P. Catalytic Dehydration of Methanol to Dimethyl Ether over Mordenite Catalysts. Fuel Process. Technol. 2010, 91, 461–468. [Google Scholar] [CrossRef]
- Phung, T.K.; Proietti Hernández, L.; Lagazzo, A.; Busca, G. Dehydration of Ethanol over Zeolites, Silica Alumina and Alumina: Lewis Acidity, Brønsted Acidity and Confinement Effects. Appl. Catal. Gen. 2015, 493, 77–89. [Google Scholar] [CrossRef]
- Sepehrian, H.; Khanchi, A.R.; Rofouei, M.K.; Waqif Husain, S. Non-Thermal Synthesis of Mesoporous Zirconium Silicate and Its Characterization. J. Iran. Chem. Soc. 2006, 3, 253–257. [Google Scholar] [CrossRef]
- De Souza, L.K.C.; Pardauil, J.J.R.; Zamian, J.R.; Da Rocha Filho, G.N.; Da Costa, C.E.F. Influence of the Incorporated Metal on Template Removal from MCM-41 Type Mesoporous Materials. J. Therm. Anal. Calorim. 2011, 106, 355–361. [Google Scholar] [CrossRef]
- Occelli, M.L.; Biz, S.; Auroux, A. Effects of Isomorphous Substitution of Si with Ti and Zr in Mesoporous Silicates with the MCM-41 Structure. Appl. Catal. Gen. 1999, 183, 231–239. [Google Scholar] [CrossRef]
- Chen, C.-L.; Li, T.; Cheng, S.; Lin, H.-P.; Bhongale, C.J.; Mou, C.-Y. Direct Impregnation Method for Preparing Sulfated Zirconia Supported on Mesoporous Silica. Microporous Mesoporous Mater. 2001, 50, 201–208. [Google Scholar] [CrossRef]
- Ghorbani, F.; Habibollah, Y.; Mehraban, Z.; Çelik, M.S.; Ghoreyshi, A.A.; Anbia, M. Preparation and Characterization of Highly Pure Silica from Sedge as Agricultural Waste and Its Utilization in the Synthesis of Mesoporous Silica MCM-41. J. Taiwan Inst. Chem. Eng. 2013, 44, 821–828. [Google Scholar] [CrossRef]
- Kim, J.; Yong, K. Characterization of Hafnium Silicate Thin Films Grown by MOCVD Using a New Combination of Precursors. J. Cryst. Growth 2004, 263, 442–446. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface Area and Pore Texture of Catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Hong, G.-B.; Ruan, R.-T.; Chang, C.-T. MCM-41 from Spent Glasses for Volatile Organic Compounds Treatment. Chem. Eng. J. 2013, 215–216, 472–478. [Google Scholar] [CrossRef]
- Mahendran, N.; Johnson Jeyakumar, S.; Jothibas, M.; Ponnar, M.; Muthuvel, A. Synthesis, Characterization of Undoped and Copper-Doped Hafnium Oxide Nanoparticles by Sol–Gel Method. J. Mater. Sci. Mater. Electron. 2022, 33, 10439–10449. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, T. Preparation and Characterization of Sodium Sulfate/Silica Composite as a Shape-Stabilized Phase Change Material by Sol-Gel Method. Chin. J. Chem. Eng. 2014, 22, 360–364. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Vázquez, P.G.; Cáceres, C.V.; Blanco, M.N. Supported Keggin Type Heteropolycompounds for Ecofriendly Reactions. Appl. Catal. Gen. 2003, 256, 125–139. [Google Scholar] [CrossRef]
- Jung, S.M.; Grange, P. TiO2–SiO2 Mixed Oxide Modified with H2SO4. Appl. Catal. Gen. 2002, 228, 65–73. [Google Scholar] [CrossRef]
- Alharbi, W.; Brown, E.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Dehydration of Ethanol over Heteropoly Acid Catalysts in the Gas Phase. J. Catal. 2014, 319, 174–181. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Yang, X.; Zhang, F. Comparison of Four Catalysts in the Catalytic Dehydration of Ethanol to Ethylene. Microporous Mesoporous Mater. 2008, 116, 210–215. [Google Scholar] [CrossRef]
- Sheng, Q.; Ling, K.; Li, Z.; Zhao, L. Effect of Steam Treatment on Catalytic Performance of HZSM-5 Catalyst for Ethanol Dehydration to Ethylene. Fuel Process. Technol. 2013, 110, 73–78. [Google Scholar] [CrossRef]
- Said, A.E.-A.A.; Abd El-Wahab, M.M.; El-Aal, M.A. The Catalytic Performance of Sulfated Zirconia in the Dehydration of Methanol to Dimethyl Ether. J. Mol. Catal. Chem. 2014, 394, 40–47. [Google Scholar] [CrossRef]
- Yaripour, F.; Baghaei, F.; Schmidt, I.; Perregaard, J. Catalytic Dehydration of Methanol to Dimethyl Ether (DME) over Solid-Acid Catalysts. Catal. Commun. 2005, 6, 147–152. [Google Scholar] [CrossRef]
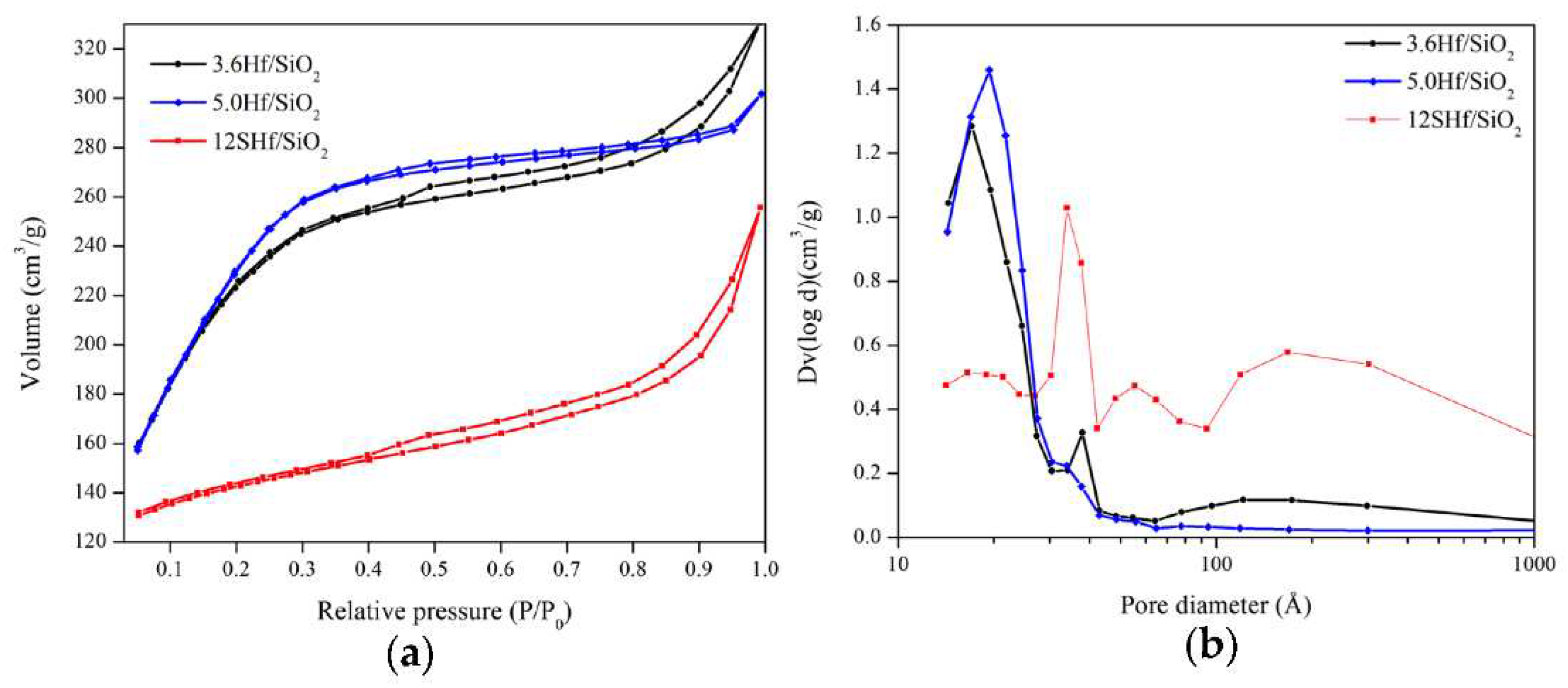
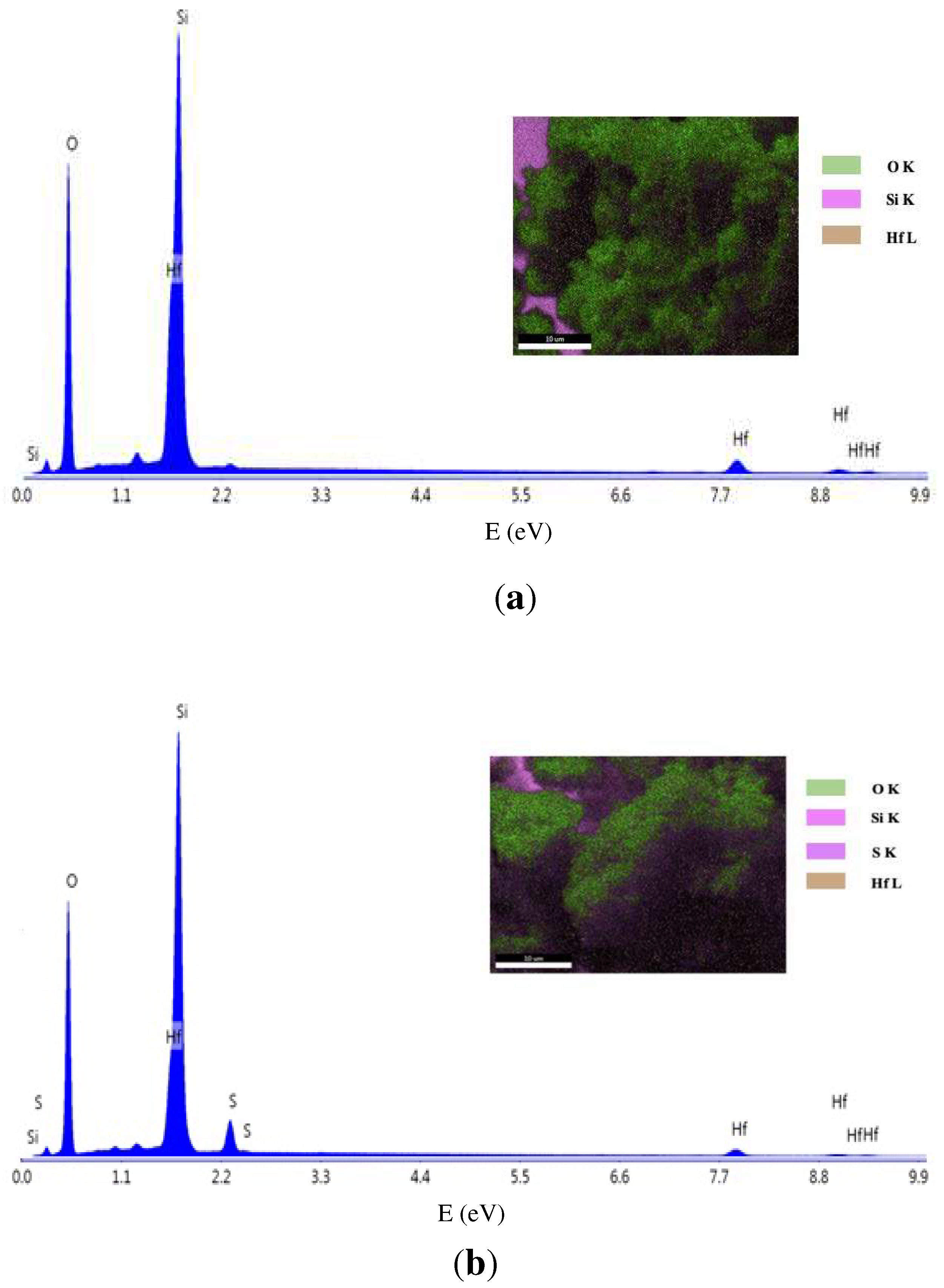
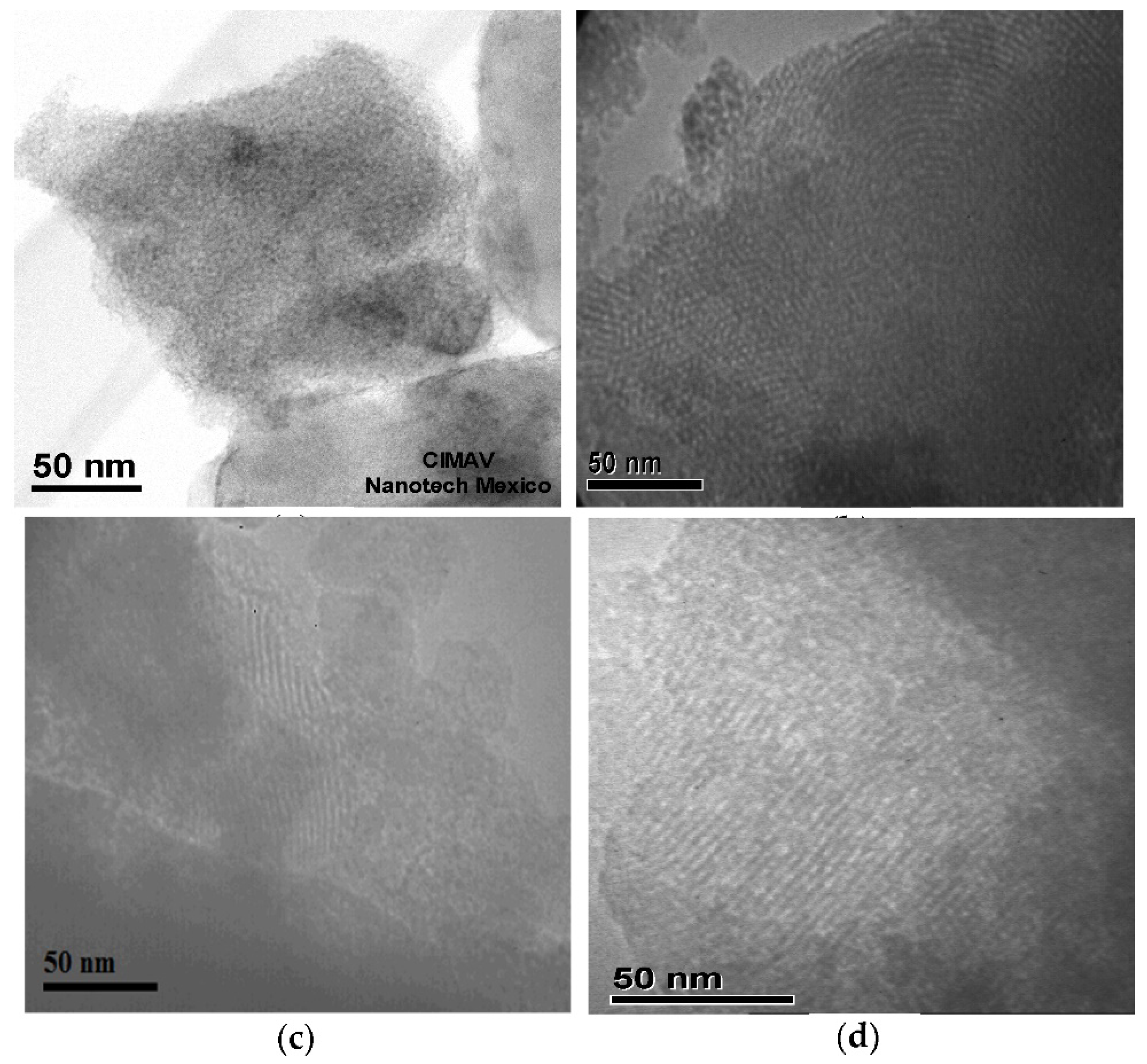
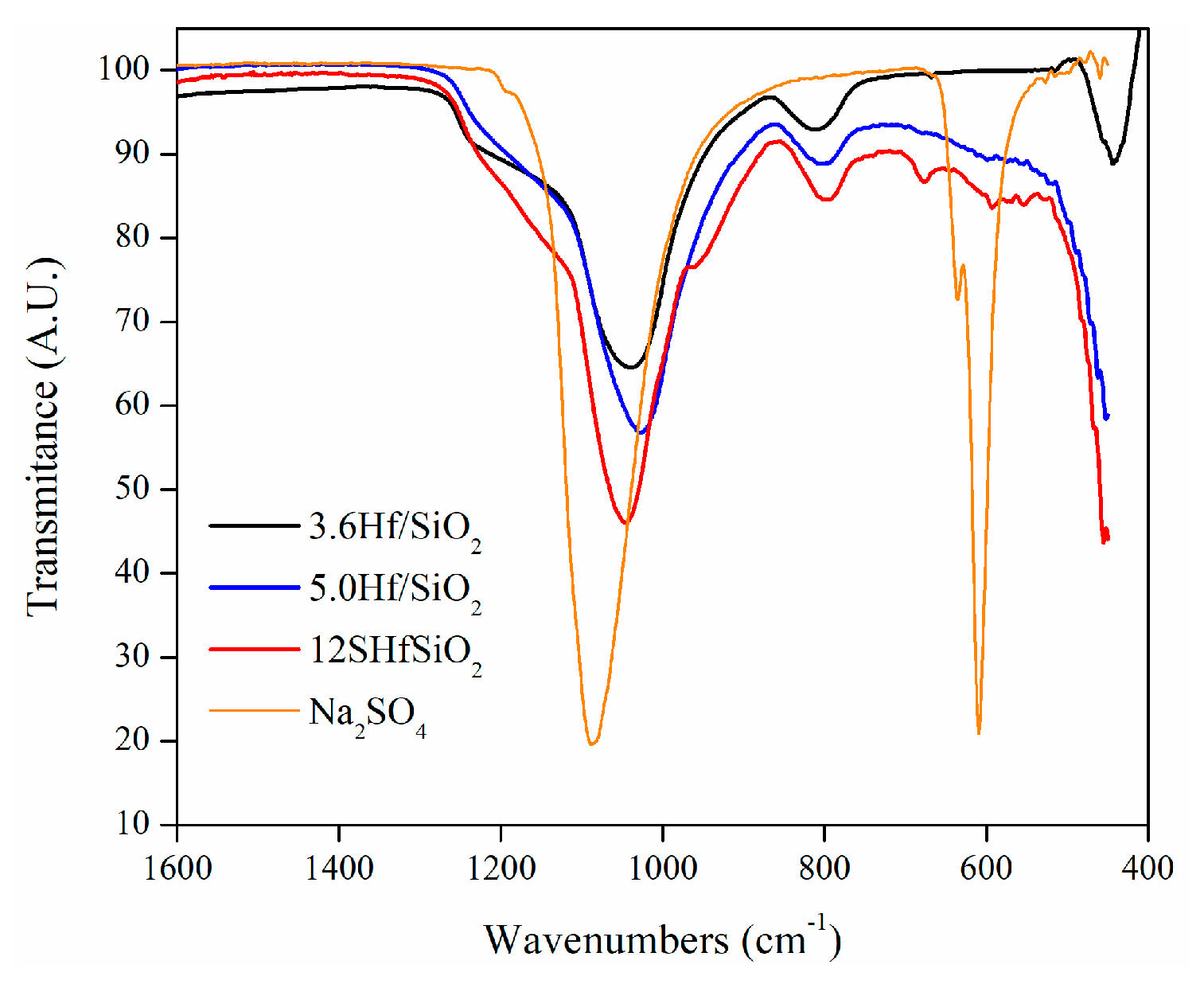
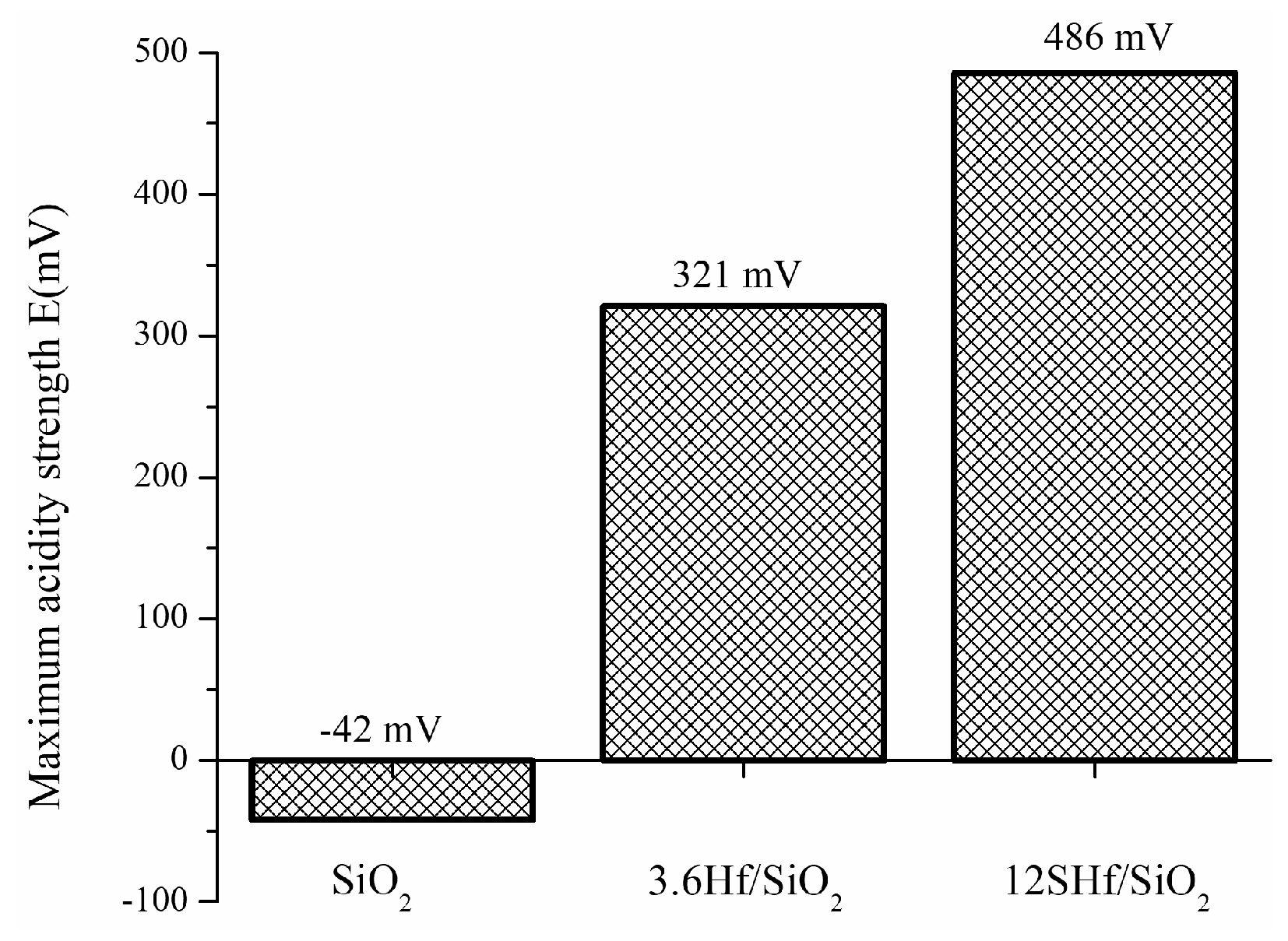
| Material | Area (m2g-1) |
Average Pore Diameter (Å) | Pore Volume (cm3/g) |
|---|---|---|---|
| 3.6Hf/SiO2 | 784 | 26 | 0.47 |
| 5.0Hf/SiO2 | 831 | 22 | 0.45 |
| 12SHf/SiO2 | 152 | 64 | 0.24 |
| 3.6Hf/SiO2 | 12Hf/SiO2 | ||
|---|---|---|---|
| Element | Atomic (%) | Element | Atomic (%) |
| O K | 65.44 | O K | 61.90 |
| Si K | 30.58 | Si K | 31.31 |
| Hf L | 3.98 | Hf L | 3.42 |
| S K | 3.37 | ||
| T = 300 °C | T = 325 °C | T = 350 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalyst | XA* (%) |
** (%) |
*** (%) |
XA (%) |
(%) |
(%) |
XA (%) |
(%) |
|
| SiO2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3.6Hf/SiO2 | 50 | 86 | 14 | 80 | 96 | 4 | 94 | 99 | 1 |
| 12SHf/SiO2 | 100 | 100 | 0 | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





