Submitted:
27 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
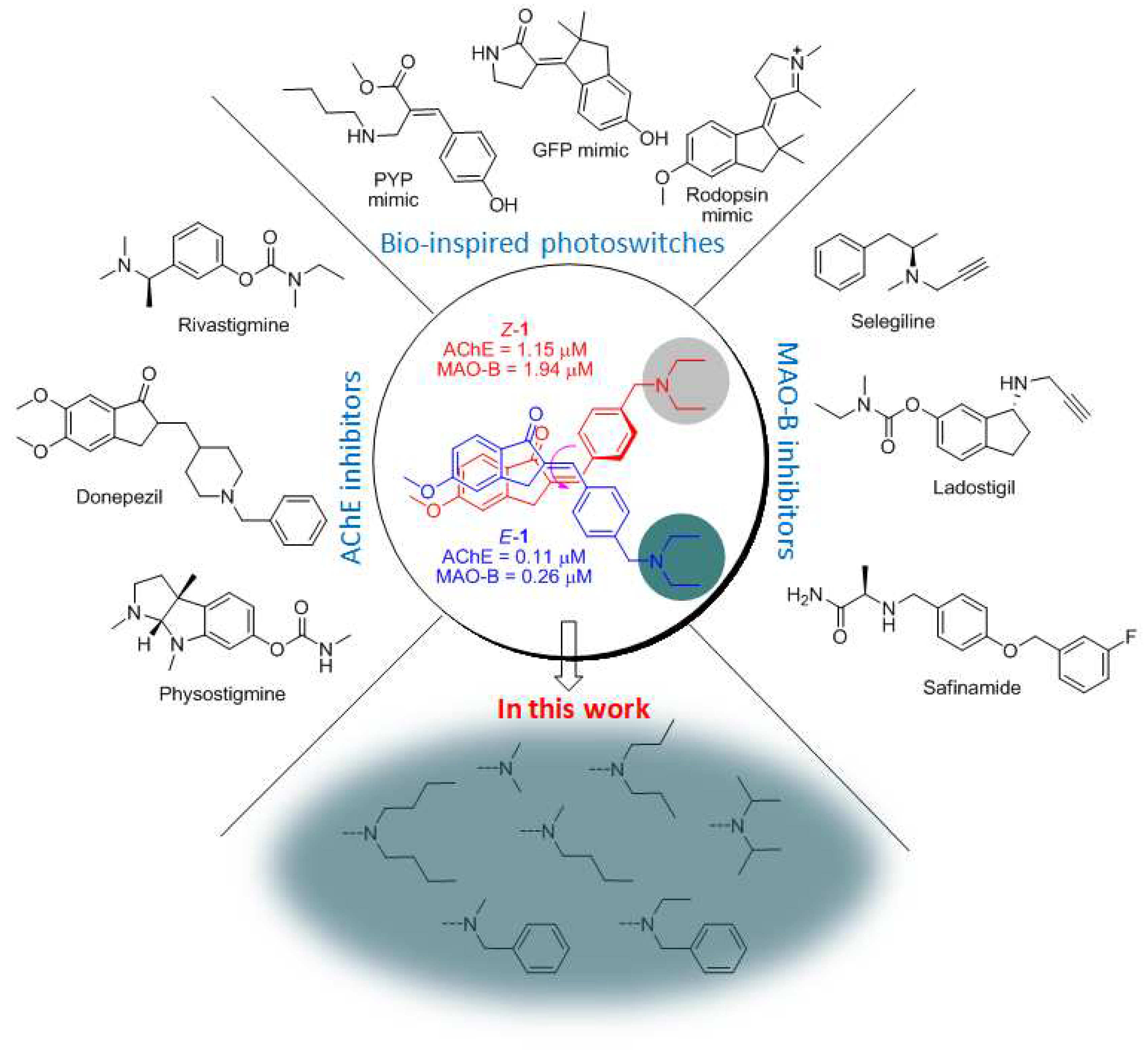
1. Introduction
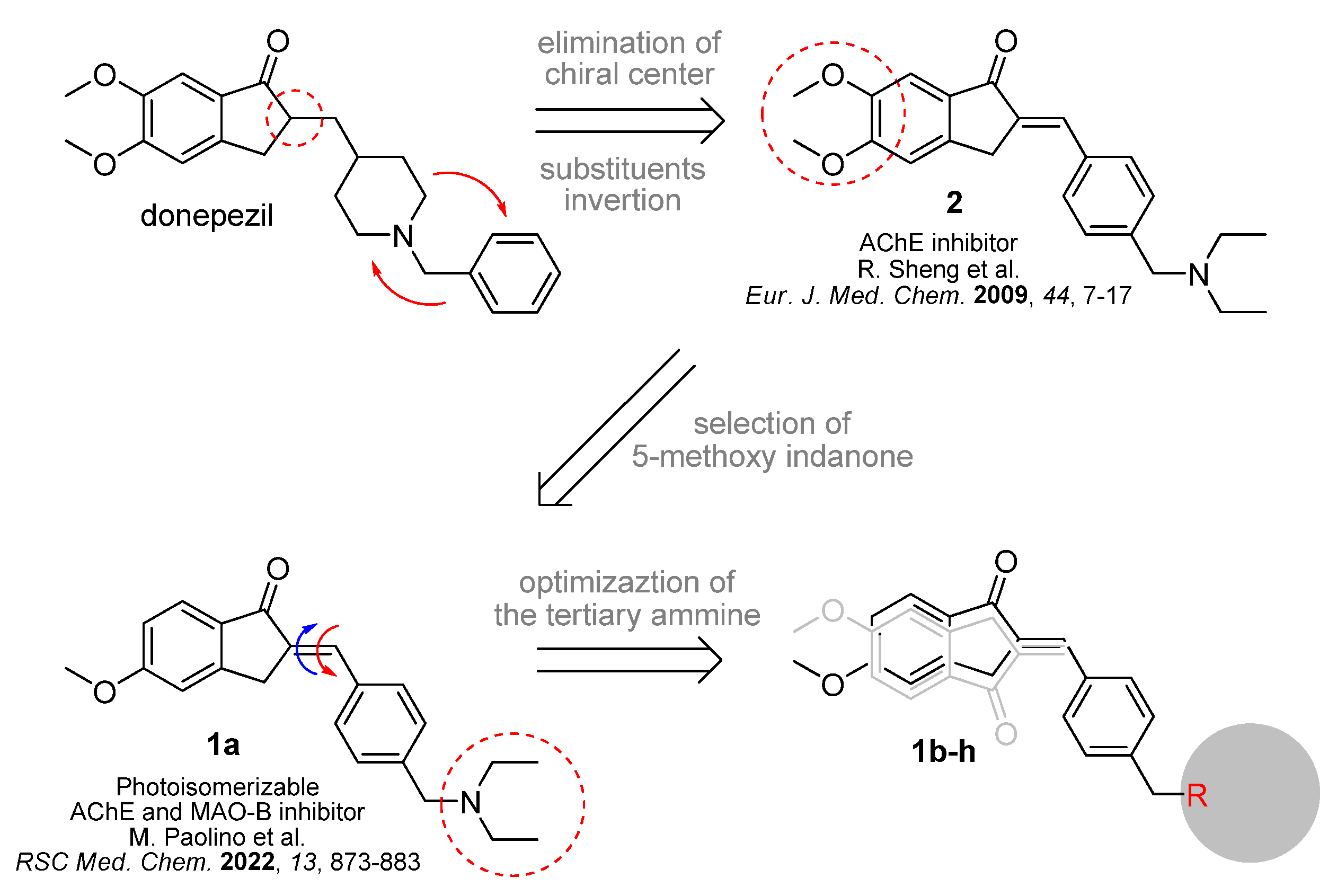
2. Design and Synthesis
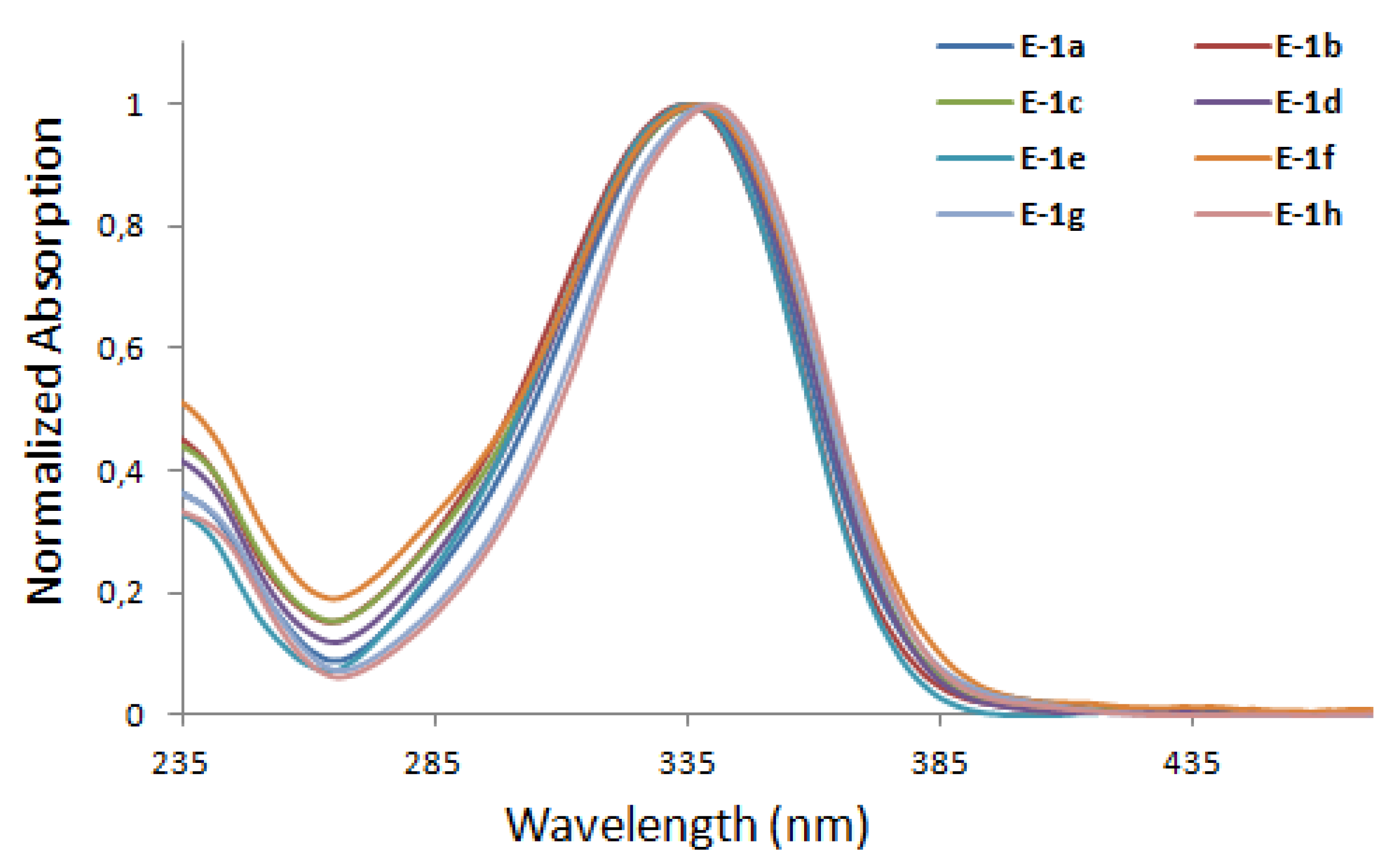
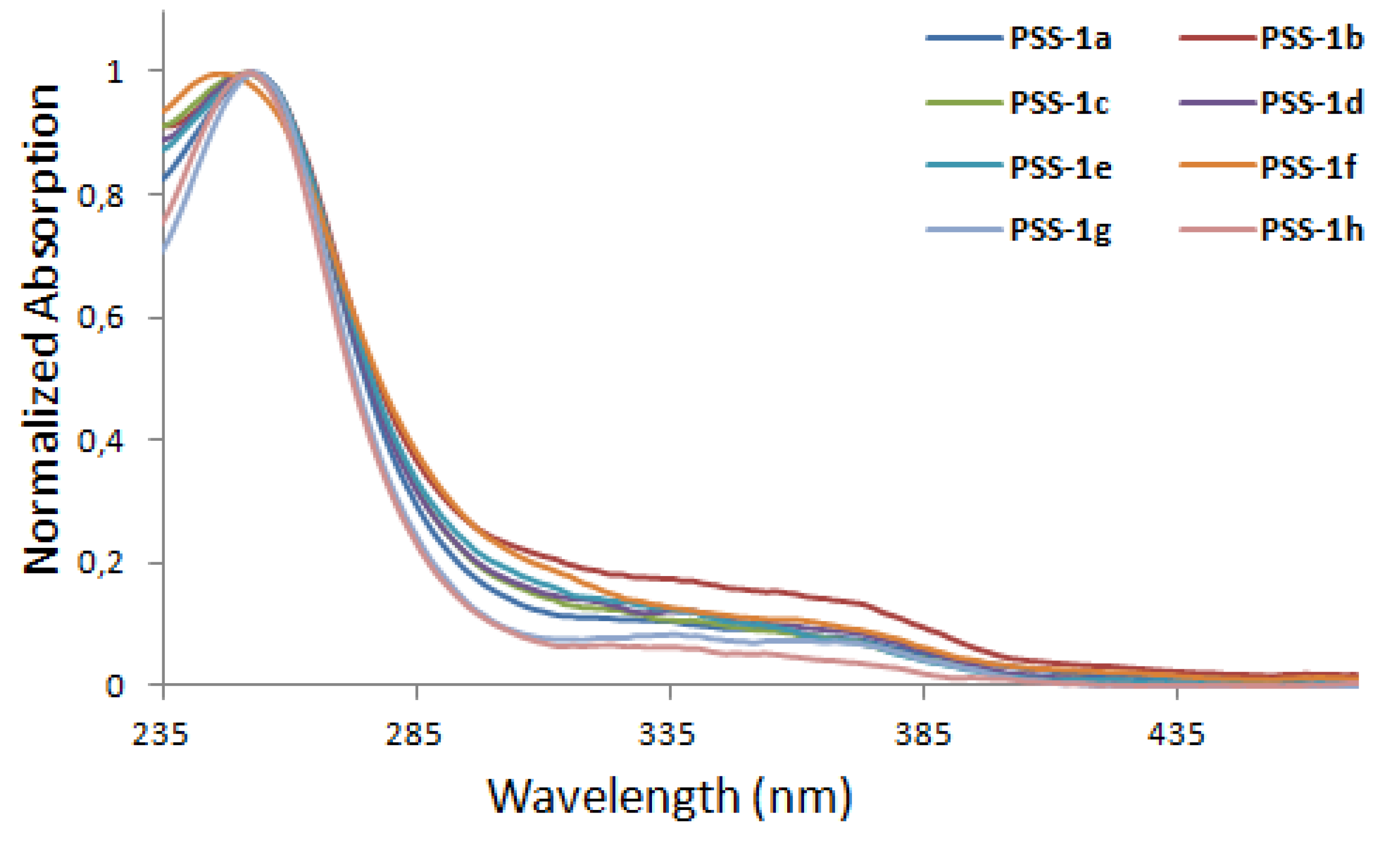
3. Photophysical and Photochemical Characterization
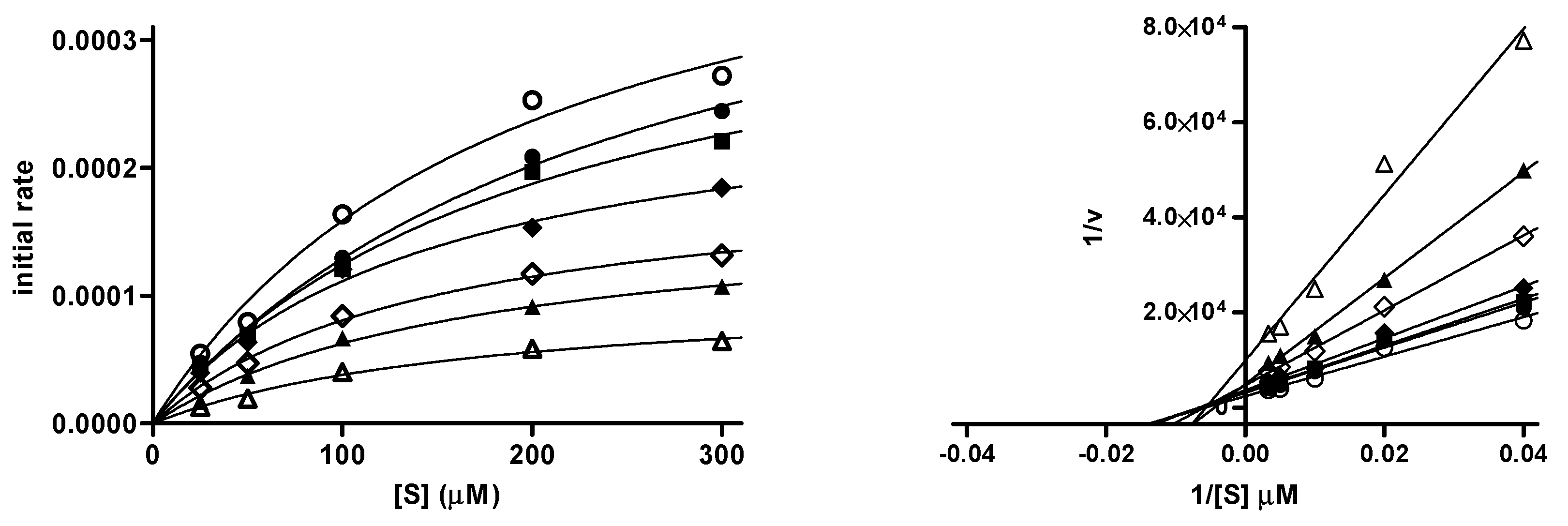
4. Biological Evaluation
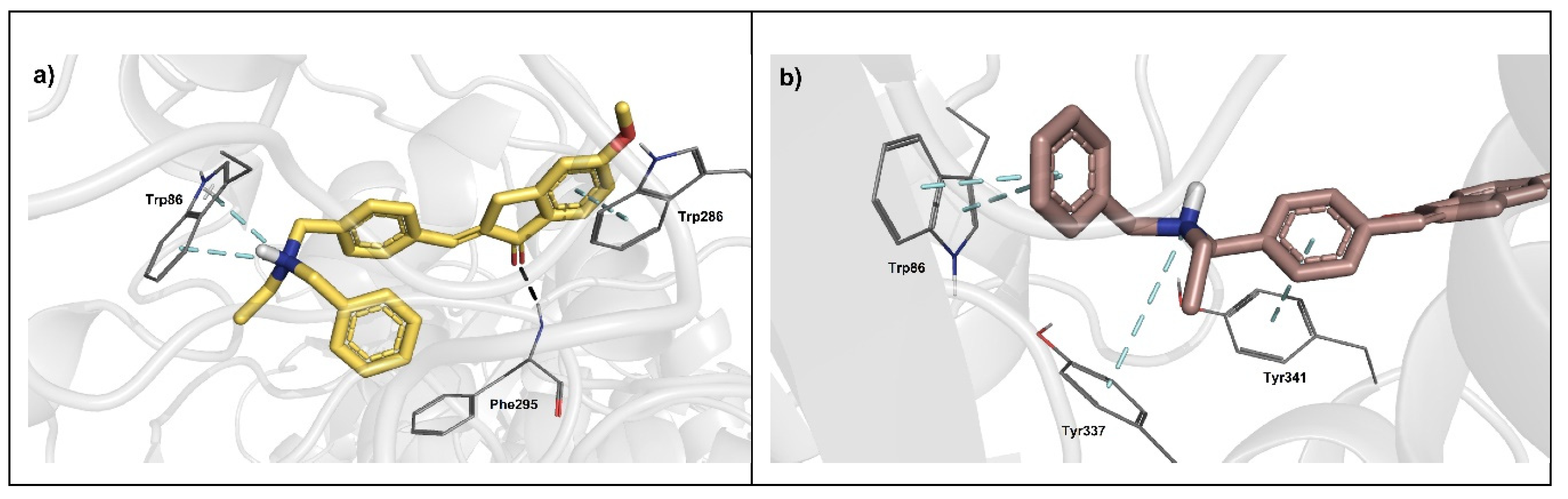
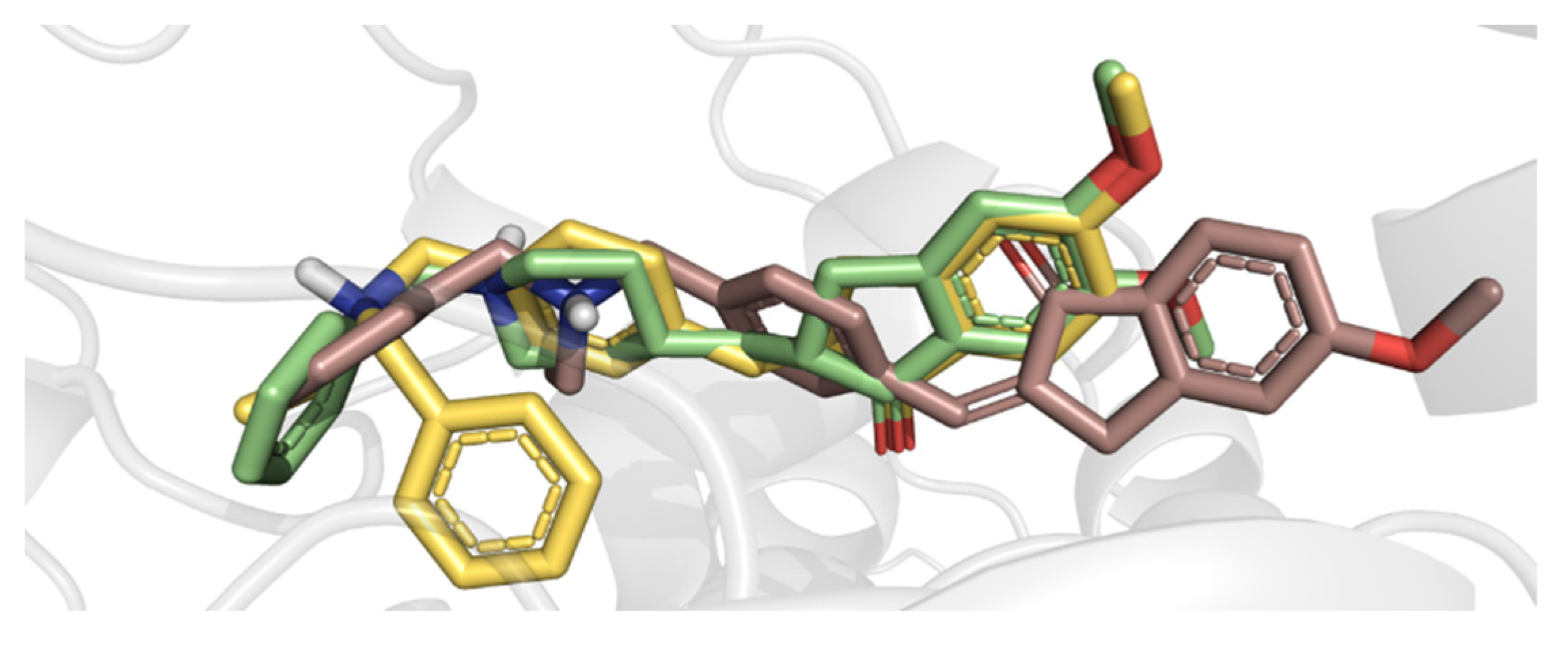
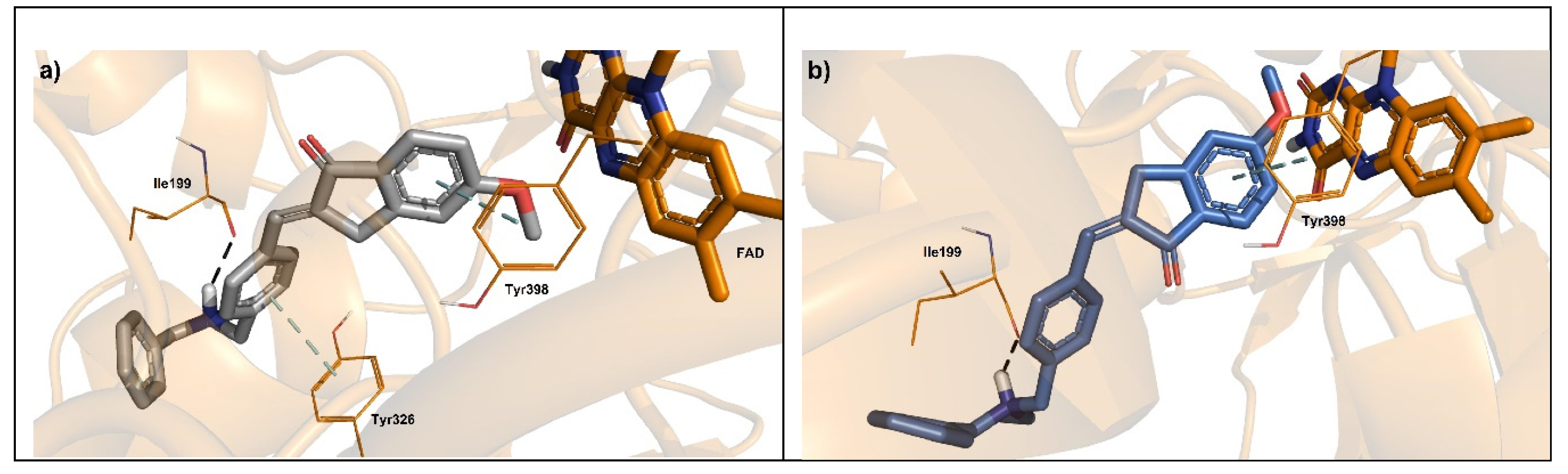
5. Molecular Docking Analysis
6. Materials and Methods
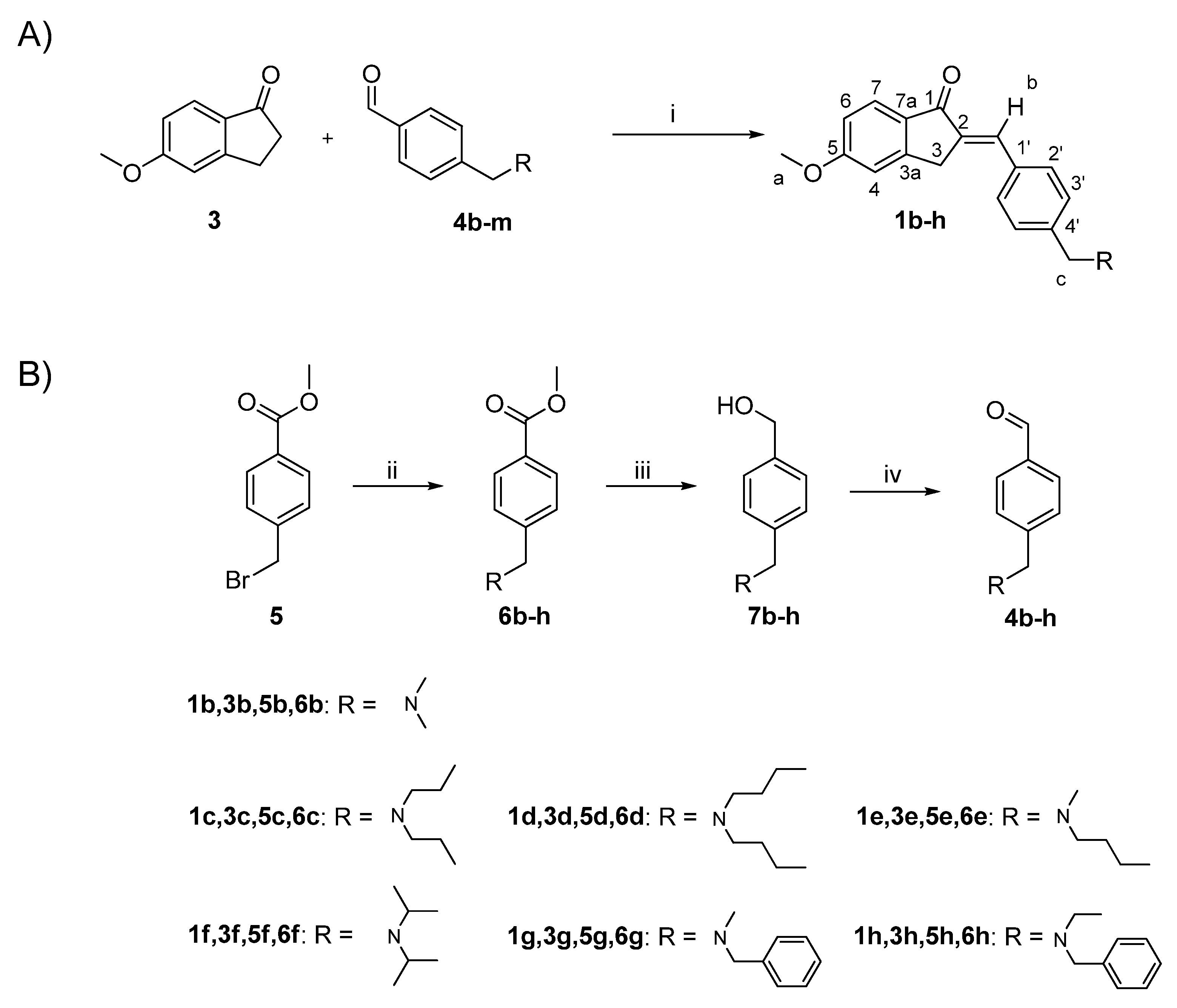
6.1. Chemistry
6.1.1. General Procedure for the Synthesis of Compounds 1b-h.
6.1.2. General Procedure for the Synthesis of Compounds 6b-h.
6.1.3. General Procedure for the Synthesis of the Alcohol Derivatives 7b-h.
6.1.4. General Procedure for the Synthesis of Aldehyde Derivatives 4b-h.
4-((Dipropylamino)methyl)benzaldehyde (4c)
4-((Dibutylamino)methyl)benzaldehyde (4d)
4-((Butyl(methyl)amino)methyl)benzaldehyde (4e)
4-((Diisopropylamino)methyl)benzaldehyde (4f)55
4-((Benzyl(methyl)amino)methyl)benzaldehyde (4g)56
4-((Benzyl(ethyl)amino)methyl)benzaldehyde (4h)56
6.2. Inhibition of Cholinesterases and Monoamine Oxidases
6.3. Molecular Docking Calculations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Available online: https://www.alzint.org/resource/world-alzheimer-report-2022/.
- Gerrow, K.; Triller, A. Synaptic stability and plasticity in a floating world. Curr. Opin. Neurobiol. 2010, 20, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.M.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z. Monoamine oxidase inhibitors: Promising therapeutic agents for Alzheimer’s disease (Review). Mol. Med. Rep. 2014, 9, 1533–1541. [Google Scholar] [CrossRef]
- Carradori, S.; Silvestri, R. New Frontiers in Selective Human MAO-B Inhibitors. J. Med. Chem. 2015, 58, 6717–6732. [Google Scholar] [CrossRef]
- Kumar, B.; Sheetal; Mantha, A. K.; Kumar, V. Recent developments on the structure–activity relationship studies of MAO inhibitors and their role in different neurological disorders. RSC Adv. 2016, 6, 42660–42683. [Google Scholar] [CrossRef]
- Cereda, E.; Cilia, R.; Canesi, M.; Tesei, S.; Mariani, C.B.; Zecchinelli, A.L.; Pezzoli, G. Efficacy of rasagiline and selegiline in Parkinson’s disease: a head-to-head 3-year retrospective case–control study. J. Neurol. 2017, 264, 1254–1263. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Spencer, C.M.; Noble, S. Rivastigmine. Drugs Aging 1998, 13, 391–411. [Google Scholar] [CrossRef]
- Dooley, M.; Lamb, H.M. Donepezil. Drugs Aging 2000, 16, 199–226. [Google Scholar] [CrossRef]
- Maramai, S.; Benchekroun, M.; Gabr, M.T.; Yahiaoui, S. Multitarget Therapeutic Strategies for Alzheimer’s Disease: Review on Emerging Target Combinations. Biomed Res. Int. 2020, 2020, 5120230. [Google Scholar] [CrossRef] [PubMed]
- Rullo, M.; Cipolloni, M.; Catto, M.; Colliva, C.; Miniero, D.V.; Latronico, T.; de Candia, M.; Benicchi, T.; Linusson, A.; Giacchè, N.; Altomare, C.D.; Pisani, L. Probing Fluorinated Motifs onto Dual AChE-MAO-B Inhibitors: Rational Design, Synthesis, Biological Evaluation, and Early-ADME Studies. J. Med. Chem. 2022, 65, 3962–3977. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Oh, J.M.; Baty, R.S.; Batiha, G.E.-S.; Parambi, D.G.T.; Gambacorta, N.; Nicolotti, O.; Kim, H. Piperazine-substituted chalcones: a new class of MAO-B, AChE, and BACE-1 inhibitors for the treatment of neurological disorders. Environ. Sci. Pollut. Res. 2021, 28, 38855–38866. [Google Scholar] [CrossRef] [PubMed]
- Guieu, B.; Lecoutey, C.; Legay, R.; Davis, A.; Sopkova de Oliveira Santos, J.; Altomare, C.D.; Catto, M.; Rochais, C.; Dallemagne, P. First Synthesis of Racemic Trans Propargylamino-Donepezil, a Pleiotrope Agent Able to Both Inhibit AChE and MAO-B, with Potential Interest against Alzheimer's Disease. Molecules 2020, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef]
- Duran-Corbera, A.; Catena, J.; Otero-Viñas, M.; Llebaria, A.; Rovira, X. Photoswitchable Antagonists for a Precise Spatiotemporal Control of β2-Adrenoceptors. J. Med. Chem. 2020, 63, 8458–8470. [Google Scholar] [CrossRef]
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist's Perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef]
- Rodríguez-Soacha, D.A.; Fender, J.; Ramírez, Y.A.; Collado, J.A.; Muñoz, E.; Maitra, R.; Sotriffer, C.; Lorenz, K.; Decker, M. "Photo-Rimonabant": Synthesis and Biological Evaluation of Novel Photoswitchable Molecules Derived from Rimonabant Lead to a Highly Selective and Nanomolar "Cis-On" CB1R Antagonist. ACS Chem. Neurosci. 2021, 12, 1632–1647. [Google Scholar] [CrossRef]
- Qiao, Z.; Fu, W.; Zhang, Y.; Chen, R.; Xu, Z.; Li, Z.; Shao, X. Azobenzene-Semicarbazone Enables Optical Control of Insect Sodium Channels and Behavior. J. Agric. Food Chem. 2021, 69, 15554–15561. [Google Scholar] [CrossRef]
- Yue, L.; Pawlowski, M.; Dellal, S.S.; Xie, A.; Feng, F.; Otis, T.S.; Bruzik, K.S.; Qian, H.; Pepperberg, D.R. Robust photoregulation of GABAA receptors by allosteric modulation with a propofol analogue. Nat. Commun. 2012, 3, 1095. [Google Scholar] [CrossRef] [PubMed]
- Mourot, A.; Kienzler, M.A.; Banghart, M.R.; Fehrentz, T.; Huber, F.M.E.; Stein, M.; Kramer, R.H.; Trauner, D. Tuning Photochromic Ion Channel Blockers. ACS Chem. Neurosci. 2011, 2, 536–543. [Google Scholar] [CrossRef]
- Szymanski, W.; Ourailidou, M.E.; Velema, W.A.; Dekker, F.J.; Feringa, B.L. Light-Controlled Histone Deacetylase (HDAC) Inhibitors: Towards Photopharmacological Chemotherapy. Chem. – A Eur. J. 2015, 21, 16517–16524. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, B.; Kuzmanovic, N.; Löffler, P.; Merkl, R.; König, B.; Sterner, R. Exploiting Protein Symmetry To Design Light-Controllable Enzyme Inhibitors. Angew. Chem. Int. Ed. 2014, 53, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Rovira, X.; Trapero, A.; Pittolo, S.; Zussy, C.; Faucherre, A.; Jopling, C.; Giraldo, J.; Pin, J.-P.; Gorostiza, P.; Goudet, C.; et al. OptoGluNAM4.1, a Photoswitchable Allosteric Antagonist for Real-Time Control of mGlu4 Receptor Activity. Cell Chem. Biol. 2016, 23, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wehle, S.; Kuzmanovic, N.; Merget, B.; Holzgrabe, U.; König, B.; Sotriffer, C.A.; Decker, M. Acetylcholinesterase Inhibitors with Photoswitchable Inhibition of β-Amyloid Aggregation. ACS Chem. Neurosci. 2014, 5, 377–389. [Google Scholar] [CrossRef]
- Broichhagen, J.; Jurastow, I.; Iwan, K.; Kummer, W.; Trauner, D. Optical Control of Acetylcholinesterase with a Tacrine Switch. Angew. Chem. Int. Ed. 2014, 53, 7657–7660. [Google Scholar] [CrossRef]
- Paolino, M.; Gueye, M.; Pieri, E.; Manathunga, M.; Fusi, S.; Cappelli, A.; Latterini, L.; Pannacci, D.; Filatov, M.; Léonard, J.; et al. Design, Synthesis, and Dynamics of a Green Fluorescent Protein Fluorophore Mimic with an Ultrafast Switching Function. J. Am. Chem. Soc. 2016, 138, 9807–9825. [Google Scholar] [CrossRef]
- Paolino, M.; Giovannini, T.; Manathunga, M.; Latterini, L.; Zampini, G.; Pierron, R.; Léonard, J.; Fusi, S.; Giorgi, G.; Giuliani, G.; et al. On the Transition from a Biomimetic Molecular Switch to a Rotary Molecular Motor. J. Phys. Chem. Lett. 2021, 12, 3875–3884. [Google Scholar] [CrossRef]
- Tassone, G.; Paolino, M.; Pozzi, C.; Reale, A.; Salvini, L.; Giorgi, G.; Orlandini, M.; Galvagni, F.; Mangani, S.; Yang, X.; et al. Xanthopsin-Like Systems via Site-Specific Click-Functionalization of a Retinoic Acid Binding Protein. ChemBioChem 2022, 23, e202100449. [Google Scholar] [CrossRef]
- Gueye, M.; Manathunga, M.; Agathangelou, D.; Orozco, Y.; Paolino, M.; Fusi, S.; Haacke, S.; Olivucci, M.; Léonard, J. Engineering the vibrational coherence of vision into a synthetic molecular device. Nature Communications 2018, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Pagano, K.; Paolino, M.; Fusi, S.; Zanirato, V.; Trapella, C.; Giuliani, G.; Cappelli, A.; Zanzoni, S.; Molinari, H.; Ragona, L.; Olivucci, M. Bile acid binding protein functionalization leads to a fully synthetic rhodopsin mimic. J. Phys. Chem. Lett. 2019, 10, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Filatov, M.; Paolino, M.; Pierron, R.; Cappelli, A.; Giorgi, G.; Léonard, J.; Huix-Rotllant, M.; Ferré, N.; Yang, X.; Kaliakin, D.; Blanco-González, A.; Olivucci, M. Towards the engineering of a photon-only two-stroke rotary molecular motor. Nature Communications 2022, 13, 6433. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Saletti, M.; Reale, A.; Licciardi, M.; Varvarà, P.; Marquette, A.; Léonard, J.; Bonechi, C.; Donati, A.; Giorgi, G.; Giuliani, G.; Carlotti, B.; Ortica, F.; Latterini, L.; Gentile, M.; Paccagnini, E.; Olivucci, M.; Cappelli, A. Chem. Eur. J. 2022, 28, e2022014.
- de Candia, M.; Zaetta, G.; Denora, N.; Tricarico, D.; Majellaro, M.; Cellamare, S.; Altomare, C.D. New azepino[4,3-b]indole derivatives as nanomolar selective inhibitors of human butyrylcholinesterase showing protective effects against NMDA-induced neurotoxicity. Eur. J. Med. Chem. 2017, 125, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Purgatorio, R.; de Candia, M.; Catto, M.; Carrieri, A.; Pisani, L.; De Palma, A.; Toma, M.; Ivanova, O.A.; Voskressensky, L.G.; Altomare, C.D. Investigating 1,2,3,4,5,6-hexahydroazepino[4,3-b]indole as scaffold of butyrylcholinesterase-selective inhibitors with additional neuroprotective activities for Alzheimer’s disease. Eur. J. Med. Chem. 2019, 177, 414–424. [Google Scholar] [CrossRef]
- Purgatorio, R.; Gambacorta, N.; de Candia, M.; Catto, M.; Rullo, M.; Pisani, L.; Nicolotti, O.; Altomare, C.D. First-in-Class Isonipecotamide-Based Thrombin and Cholinesterase Dual Inhibitors with Potential for Alzheimer Disease. Molecules 2021, 26, 5208. [Google Scholar] [CrossRef]
- Paolino, M.; Rullo, M.; Maramai, S.; de Candia, M.; Pisani, L.; Catto, M:; Mugnaini, C. ; Brizzi, A.; Cappelli, A.; Olivucci, M.; Corelli, F.; Altomare, C.D. Design, synthesis and biological evaluation of light-driven on-off multitarget AChE and MAO-B inhibitors. RSC Med. Chem. 2022, 13, 873–883. [Google Scholar] [CrossRef]
- Sheng, R.; Xu, Y.; Hu, C.; Zhang, J.; Lin, X.; Li, J.; Yang, B.; He, Q.; Hu, Y. Design, synthesis and AChE inhibitory activity of indanone and aurone derivatives. Eur. J. Med. Chem. 2009, 44, 7–17. [Google Scholar] [CrossRef]
- Petermayer, C.; Thumser, S.; Kink, F.; Mayer, P.; Dube, H. Hemiindigo: highly bistable photoswitching at the biooptical window. J. Am. Chem. Soc. 2017, 139, 15060–15067. [Google Scholar] [CrossRef]
- Lazinski, L.M.; Royal, G.; Robin, M.; Maresca, M.; Haudecoeur, R. Bioactive aurones, indanones, and other hemiindigoid scaffolds: medicinal chemistry and photopharmacology perspectives. J. Med. Chem. 2022, 65, 12594–12625. [Google Scholar] [CrossRef] [PubMed]
- Salum, M.L.; Arroyo Mañez, P.; Luque, F.J.; Erra-Balsells, R. Combined experimental and computational investigation of the absorption spectra of E- and Z-cinnamic acids in solution: The peculiarity of Z-cinnamics. J. Photochem. Photobiol. B Biol. 2015, 148, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Purgatorio, R.; de Candia, M.; Catto, M.; Rullo, M.; Pisani, L.; Denora, N.; Carrieri, A.; Nevskaya, A.A.; Voskressensky, L.G.; Altomare, C.D. Evaluation of Water-Soluble Mannich Base Prodrugs of 2,3,4,5-Tetrahydroazepino[4,3-b]indol-1(6H)-one as Multitarget-Directed Agents for Alzheimer's Disease. ChemMedChem 2021, 16, 589–598. [Google Scholar] [CrossRef]
- Purgatorio, R.; Gambacorta, N.; Samarelli, F.; Lopopolo, G.; de Candia, M.; Catto, M.; Nicolotti, O.; Altomare, C.D. Assessing the Role of a Malonamide Linker in the Design of Potent Dual Inhibitors of Factor Xa and Cholinesterases. Molecules 2022, 27, 4269. [Google Scholar] [CrossRef]
- Nel, M.S.; Petzer, A.; Petzer, J.P.; Legoabe, L.J. 2-Benzylidene-1-indanone derivatives as inhibitors of monoamine oxidase. Bioorg. Med. Chem. Lett. 2016, 26, 4599–4605. [Google Scholar] [CrossRef]
- Affini, A.; Hagenow, S.; Zivkovic, A.; Marco-Contelles, J.; Stark, H. Novel indanone derivatives as MAO-B/H3R dual-targeting ligands for treatment of Parkinson’s disease. Eur. J. Med. Chem. 2018, 148, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Pourshojaei, Y.; Abiri, A.; Eskandari, K.; Haghighijoo, Z.; Edraki, N.; Asadipour, A. Phenoxyethyl Piperidine/Morpholine Derivatives as PAS and CAS Inhibitors of Cholinesterases: Insights for Future Drug Design. Sci. Rep. 2019, 9, 19855. [Google Scholar] [CrossRef] [PubMed]
- Alagöz, M.A.; Oh, J M. ; Zenni, Y.N.; Özdemir, Z.; Abdelgawad, M.A.; Naguib, I.A.; Ghoneim, M.M.; Gambacorta, N.; Nicolotti, O.; Kim, H.; Mathew, B. Development of a Novel Class of Pyridazinone Derivatives as Selective MAO-B Inhibitors. Molecules 2022, 27, 3801. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, H.; Li, H.; Misal Castro, L.C.; Zheng, J.; Roisnel, T.; Dorcet, V.; Sortais, J.-B.; Darcel, C. Phosphane-Pyridine Iron Complexes: Synthesis, Characterization and Application in Reductive Amination through the Hydrosilylation Reaction. Eur. J. Inorg. Chem. 2012, 3546–3550. [Google Scholar] [CrossRef]
- Kuwano, R.; Kondo, Y.; Matsuyama, Y. Palladium-Catalyzed Nucleophilic Benzylic Substitutions of Benzylic Esters. J. Am. Chem. Soc. 2003, 125, 12104–12105. [Google Scholar] [CrossRef]
- Das, S.; Karmakar, H.; Bhattacharjee, J.; Panda, T.K. Aluminium complex as an efficient catalyst for the chemo-selective reduction of amides to amines. Dalton Trans. 2019, 48, 11978–11984. [Google Scholar] [CrossRef]
- Lator, A.; Gaignard Gaillard, Q.; Mérel, D.S.; Lohier, J.-F.; Gaillard, S.; Poater, A.; Renaud, J.-L. Room-Temperature Chemoselective Reductive Alkylation of Amines Catalyzed by a Well-Defined Iron(II) Complex Using Hydrogen. J. Org. Chem. 2019, 84, 6813–6829. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Nagata, M.; Kitagawa, M.; Akahane, K.; Uoto, K. Discovery of novel thieno[2,3-d]pyrimidin-4-yl hydrazone-based inhibitors of cyclin D1-CDK4: synthesis, biological evaluation and structure-activity relationships. Part 2. Bioorg. Med. Chem. 2009, 17, 7850–7860. [Google Scholar] [CrossRef] [PubMed]
- Claffey, J.; Müller-Bunz, H.; Tacke, M. Benzyl-substituted titanocene dichloride anticancer drugs: From lead to hit. J. Organometal. Chem. 2010, 695, 2105–2117. [Google Scholar] [CrossRef]
- Rizzo, S.; Bartolini, M.; Ceccarini, L.; Piazzi, L.; Gobbi, S.; Cavalli, A.; Recanatini, M.; Andrisano, V.; Rampa, A. Targeting Alzheimer's disease: Novel indanone hybrids bearing a pharmacophoric fragment of AP2238. Bioorg. Med. Chem. 2010, 18, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Purgatorio, R.; de Candia, M.; De Palma, A.; De Santis, F.; Pisani, L.; Campagna, F.; Cellamare, S.; Altomare, C.D.; Catto, M. Insights into Structure-Activity Relationships of 3-Arylhydrazonoindolin-2-One Derivatives for Their Multitarget Activity on β-Amyloid Aggregation and Neurotoxicity. Molecules 2018, 23, 1544. [Google Scholar] [CrossRef]
- Purgatorio, R.; de Candia, M.; Catto, M.; Rullo, M.; Pisani, L.; Denora, N.; Carrieri, A.; Nevskaya, A.A.; Voskressensky, L.G.; Altomare, C.D. Evaluation of Water-Soluble Mannich Base Prodrugs of 2,3,4,5-Tetrahydroazepino[4,3-b]indol-1(6H)-one as Multitarget-Directed Agents for Alzheimer's Disease. ChemMedChem 2021, 16, 589–598. [Google Scholar] [CrossRef]
- Purgatorio, R.; Kulikova, L.N.; Pisani, L.; Catto, M.; Candia, M.; Carrieri, A.; Cellamare, S.; De Palma, A.; Beloglazkin, A.A.; Reza Raesi, G.; et al. Scouting around 1,2,3,4-tetrahydrochromeno[3,2-c]pyridin-10-ones for single- and multitarget ligands directed towards relevant Alzheimer’s targets. ChemMedChem 2020, 15, 1947–1955. [Google Scholar] [CrossRef]
- Titov, A.A.; Purgatorio, R.; Obydennik, A.Y.; Listratova, A.V.; Borisova, T.N.; de Candia, M.; Catto, M.; Altomare, C.D.; Varlamov, A.V.; Voskressensky, L.G. Synthesis of isomeric 3-Benzazecines decorated with endocyclic allene moiety and exocyclic conjugated double bond and evaluation of their anticholinesterase activity. Molecules 2022, 27, 6276. [Google Scholar] [CrossRef]

| Cmpdsa | IC50 (μM) or % inhibition at 10 μMb | |||
|---|---|---|---|---|
| AChEc | BChEc | MAO-Ac | MAO-Bc | |
| E-1a | 0.105±0.005f | 10.0±0.15f | (33±3)f | 0.260±0.012f |
| E/Z-1a | 0.780±0.013f | (28±6%)f | (45±5)f | 1.59±0.04f |
| E-1b | 0.155±0.010 | n.a. | (33±1) | 0.686±0.059 |
| E/Z-1b | 0.915±0.138 | (25±10%) | (32±2) | 0.839±0.123 |
| E-1c | 0.174±0.017 | (28±7%) | (15±1) | 0.479±0.081 |
| E/Z-1c | 0.295±0.045 | (20±7%) | 2.98±0.08 | 0.743±0.048 |
| E-1d | 0.990±.050 | (28±9%) | (12±4) | 0.338±0.036 |
| E/Z-1d | 2.28±0.21 | n.a. | (37±2) | 0.382±0.006 |
| E-1e | 0.102±0.019 | 5.00±0.45 | (20±5) | 0.178±0.086 |
| E/Z-1e | 1.36±0.06 | n.a. | (25±5) | 0.651±0.061 |
| E-1f | 0.261±0.014 | 10.0±0.85 | (22±2) | 0.178±0.086 |
| E/Z-1f | 1.41±0.09 | (20±3%) | 3.19±0.10 | 0.241±0.026 |
| E-1g | 0.115±0.004 | 9.90±0.11 | (39±7) | 0.292±0.060 |
| E/Z-1g | 0.135±0.062 | (40±2%) | (49±1) | 0.354±0.070 |
| E-1h | 0.039±0.001 | 7.00±0.01 | (13±6) | 0.355±0.134 |
| E/Z-1h | 0.053±0.006 | 10.0±0.32 | (39±5) | 0.358±0.008 |
| Donepezild | 0.021±0.005 | 4.80±1.00 | ||
| Tacrined | 0.090±0.005 | 0.025±0.003 | ||
| Clorgylinee | 0.0022±0.0002 | 2.48±0.43 | ||
| Safinamidee | (18±3%) | 0.028±0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





