1. Introduction
The torque generated at the joint depends on the activation of the motor units on the muscle, as well as the mechanics of muscle fibers and muscle tendons units. These mechanics are influenced by the changes in peripheral limb postures and joint angles, which in turn affect the shape and the length of the muscle [
1]. These variations have been found to have a significant effect on muscle activity [
2] and output torque. It is well known that the change in the muscle shape and length causes lateral oscillations of muscle fibers known as mechanomyography (MMG) signals, which can be measured by accelerometers [
3]. These oscillations are quantified using MMG features such as the root mean square of the amplitude (MMG
RMS), and the frequency domain parameters [
4] like the mean power frequency (
and median power frequency (
).
Previous studies have declared the relationship between the MMG
RMS and muscle length as a function of joint angles [
5]. However, when considering dynamic muscle activity, measuring the effects of a single muscle becomes challenging at the joint receiving the contribution of multiple muscles. For example, the biceps brachii (BB) muscle, brachialis, brachioradialis [
6] and forearm muscles such as the pronator tares and the flexor carpi radialis contribute to the elbow joint torque. Consequently, coactivation of those muscles can mask the behaviours of the BB muscle. It is well known that neuromuscular electrical stimulation (NMES) can selectively activate a muscle of interest [
7], which makes it crucial to further understand the influence of joints on biomechanics at BB muscle [
8] and the torque at the elbow joint. Therefore, understanding the function of muscle(s) of interest may be further enhanced using NMES [
9].
Previous research has demonstrated that contraction properties of muscles can elucidate the effectiveness of NMES on the
and vary with joint angle [
10]. They also found that normalized MMG
RMS increased with knee extension torque as the leg flexion angle increased. This is significant as the change in kneed joint angle is related to biomechanical properties of muscle(s), which affect the motor unit recruitment [
11,
12] and overlapping of cross-bridge elements [
13]. In addition, voluntary contraction of BB revealed a monotonic increase and decrease in MMG
RMS with elbow joint angle [
14]. Typically, MMG
RMS decreased at 90% of the muscle length [
15] and increased at other lengths. This suggests that MMG
RMS reflects the power of the muscle [
16], which is angle specific [
5], and influenced by the motor unit recruitment strategy [
17]. A downshift in
with an increase in elbow joint angle implies the firing rate of motor units [
15,
18] at shorter muscle lengths.
As aforementioned, the length and shape of BB muscle vary with elbow flexion, making it a clear candidate to understand the muscle response to NMES. However, while recent study [
19] investigated the relationship between elbow joint angle and
, and MMG
RMS, the muscle behaviours under the conditions of the forearm postures and elbow joint angles were not studied. Furthermore, the significance of
and
was not assessed. It is worth noting that the variation in the spectral responses of the muscle is influenced by muscle fibers in multiscale movement [
20].
This study aimed to investigate the effects of the neutral (N), pronation (P), and supination (S) positions; and elbow joints of 10°, 30°, 60° and 90° on , , and MMGMPF during a consistent NMES of the BB muscle. We hypothesize that 1) would exhibit a linear increase with certain elbow joint angles and decrease to some extent when the forearm position and elbow joint angle change. Furthermore, we anticipated that 2) and would be consistent or shift downward while the elbow joint angle increased.
2. Materials and Methods
2.1. Subjects
Thirty-six healthy males (age, 22.24 ± 2.94 years; height 172 ± 0.5 cm; weight, 67.01 ± 7.22 kg) participated in this investigation. Subjects had no history of neuromuscular disorders or surgical operations. Before participation, subjects provided written and signed informed consent after disclosure of the study’s purpose and procedures. They were briefed about their rights to withdraw participation at any time. Ethical approval of the experimental protocol, NMRR-20-2613-56796 (IIR) was obtained from the Medical Research Ethics Committee of Malaysia in compliance with the principles outlined in the Declaration of Helsinki. The experiment was conducted in the Laboratory at the Faculty of Electronics and Computer Engineering, Universiti Teknikal Malaysia Melaka (UTeM).
2.2. Experimental protocol
Before participation, all subjects completed a warmup protocol to familiarize themselves with the sensation of NMES intensity. Subjects visited the laboratory on three different occasions separated by at least twenty-four hours. Before preceding experimental trials, the maximum voluntary contraction was determined for the right hand at
of the elbow joint. The trial was repeated once the error between the two trials exceeded 5%. Hence, subjects whose BB muscle failed to provide about 15 % of equivalent NMES were withdrawn from participation [
2].
During the experiment, the forearm was secured in either the neutral, pronation or supination positions, fixed to the rotating custom-made wooden arm at the desired elbow joint angle,
Figure 1. The stimulation protocol consisted of a frequency of 30 Hz, a pulse width of 110 µs, and an amplitude of 30 mA of current, which induced the elbow flexion torque without causing discomfort for any participant.
Following the guidelines of the International Society of Electromyography and Kinesiology (ISEK), a self-adhesive electrode 4 cm × 4 cm (Hercusense TENS /EMS, V2U Healthcare Pte. Ltd, MIDVIEW CITY, Singapore) was placed on the motor point, and the distal electrode was placed at the other end of the BB muscle belly. The stimulation intensity was delivered by Electrical Muscle Stimulation (EMS 7500, V2U Healthcare Pte. Ltd, Midview City, Singapore). An Indelible marker was utilized to ensure the consistent positioning of the stimulation electrodes between the experimental sessions.
The muscle belly was located through palpation when the elbow was flexed at , for proper fixation of the MMG sensor. The BB muscle received NMES in a randomized order of forearm posture (neutral, pronation, supination) and elbow joint angle (10°, 30°, 60° and 90° (Where 0° represents full elbow extension)).
To maintain consistency, the lever arm of the forearm was kept the same for all participants using adjustable hand table vices and screws as shown in
Figure 1. The arm posture and elbow joint angle were maintained as monitored by two assistants present at the site. To ensure the full recovery of the muscle, at least 10 minutes between trials with two different angles, and a five-minute inter-trial were provided. Each trial lasted 30 seconds.
Simultaneous measures of acceleration and torque data were recorded in a customized LabVIEW program (NI LABVIEW 2021 (64-bit) at a sampling rate of 1 kHz. The Arduino Uno R3 Microcontroller was used to interface acceleration and force transducers. Acceleration data was detected using ADXL-313, weight < 2.6 g, low power, high resolution (up to 13-bits) 3-axis accelerometer capable of measuring up to ± 4g (SparkFun, Colorado, USA). ADXL-313 sensor was attached to the muscle belly using adhesive tape (3MTM VHBTM 4920, Center St. Paul, MN, USA). The torque was measured by using a force transducer FS2050 Compression LC1500 GRAM, TE Connectivity (Schaffhausen, Switzerland).
2.3. Signal processing and data analysis
Raw acceleration and torque data were recorded and saved on the personal computer during the data acquisition process. The dataset includes three acceleration signals and one torque component for each posture and angle. MMG data was obtained by filtering acceleration data using a fourth-order Butterworth band pass filter of 5-100 Hz. The force data were filtered using a 4th-order Butterworth low-pass filter with a cutoff frequency of 5 Hz.
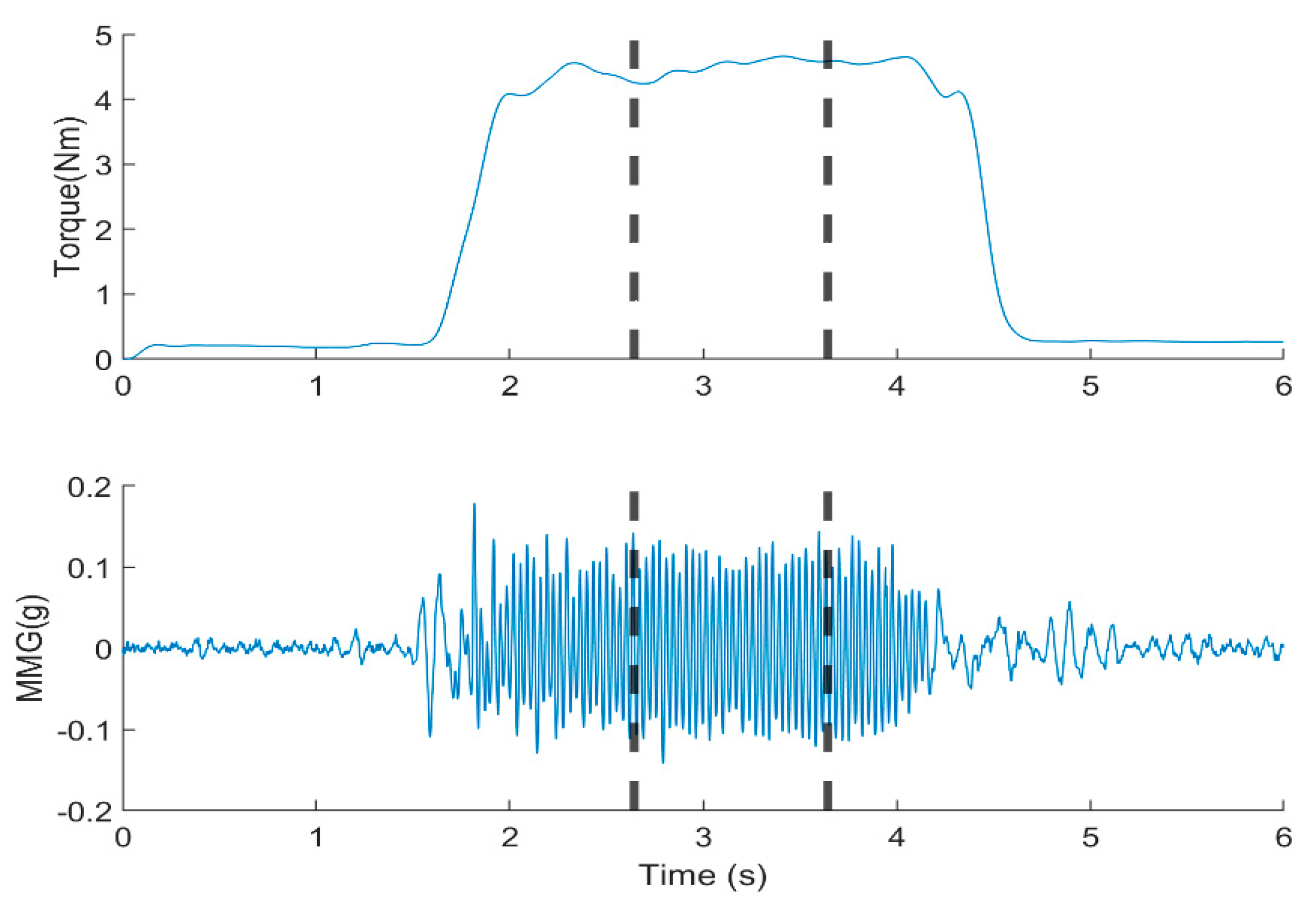
For analysis, the middle plateau of the torque and MMG [
5] was determined using a moving window of 1000s at a threshold of 20 % of the maximum muscle contraction above the baseline. This selection aimed to extract data with no effect on the onset of torque development at the beginning of muscle contraction and offset at the start of muscle relaxation,
Figure 2. A 512-point Short-Time Fourier Transform (STFT), 50 % window overlap was used to compute the MPF and MDF from MMG signals. Thereafter, the
,
,
,
, were calculated. Subsequent analysis was performed using a 100-millisecond window with 50 % overlap.
,
,
,
were normalized to their respective maximum levels among the twelve conditions of four elbow joint angles × three forearm postures [
2]. MATLAB® 2021b was used for signal processing.
2.4. Statistical analysis
The Shapiro-Wilk Test was used to examine the normality of the distribution of and , , obtained at the longitudinal, lateral, and transverse axis of the BB fibers. One-way analysis of variance (ANOVA) with repeated measures was performed to evaluate the effect of the forearm posture on torque and MMG parameters. Similarly, the effect of elbow joint angle on the elbow flexion torque was assessed. Two-way analysis of variance (ANOVA) with repeated measures was utilized to examine the combined effects of posture and elbow joint angle on torque and MMG variables. The Greenhouse-Geisser correction factor was applied whenever the Sphericity assumption was violated. A Bonferroni adjustment was used for the post-Hoc to identify the difference. Investigated variables were significant if the statistical significance (P < 0.05). All variables represent the mean normalized (SD) of measurements. All the statistical tests were conducted using IBM SPSS 25.0 (SPSS Inc., USA).
3. Results
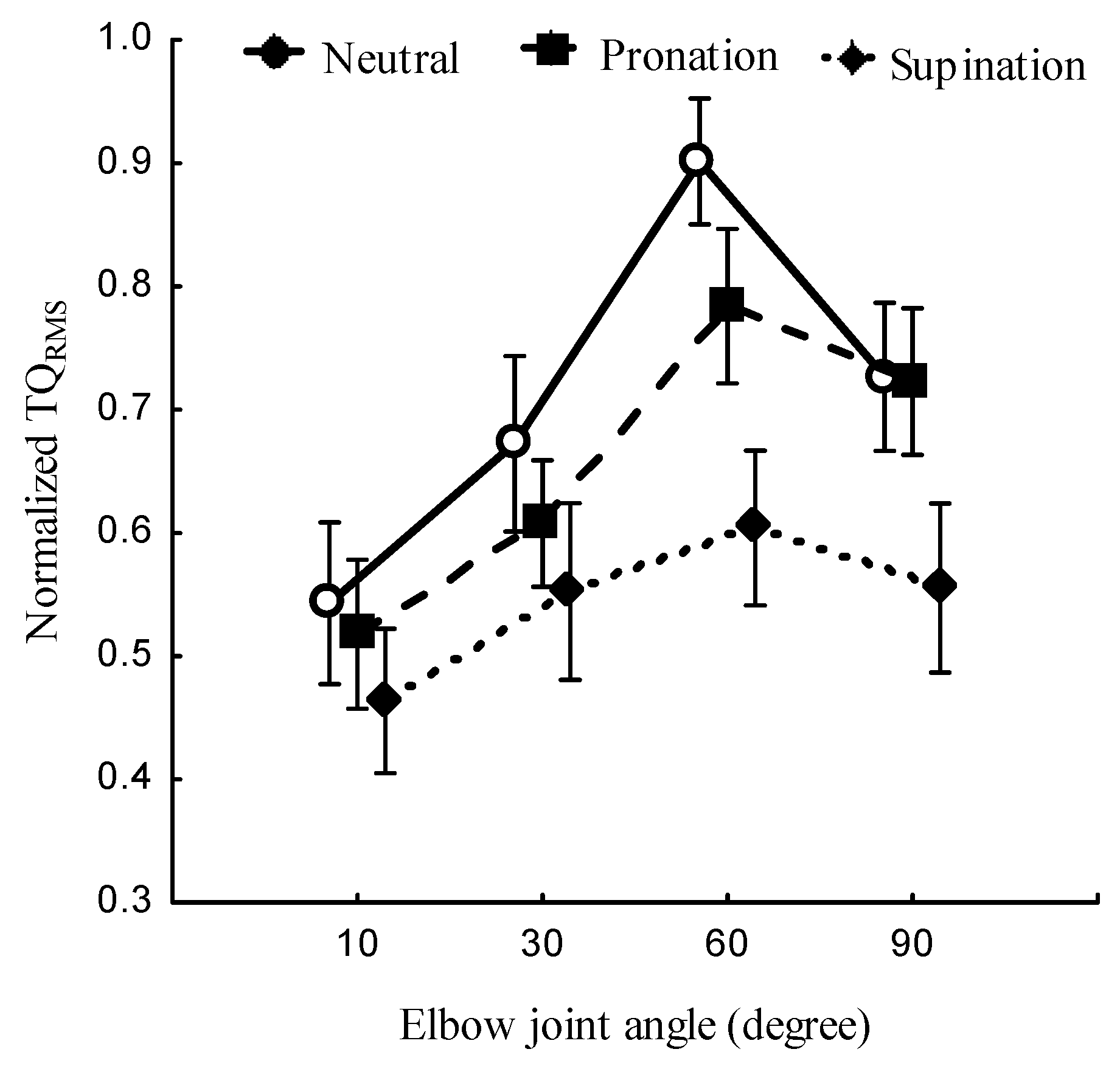
3.1. Effect of forearm posture and joint angle on Torque
Figure 3 and
Table 1 present the relationship between forearm postures, elbow joint angles, and
. The
at the neutral was the highest than pronation and supination,
. Forearm posture had a significant effect on the
,
at all elbow joint angles. The joint angle was found to have a significant effect on the normalized
at the neutral, (
P < 0.05), pronation,
, and the supination (
P < 0.05). The forearm posture and elbow joint angle showed a significant combined main effect on
(
P < 0.05),
Figure 4.
3.2. Effect of elbow joint angle and forearm posture on MMG Responses to NMES
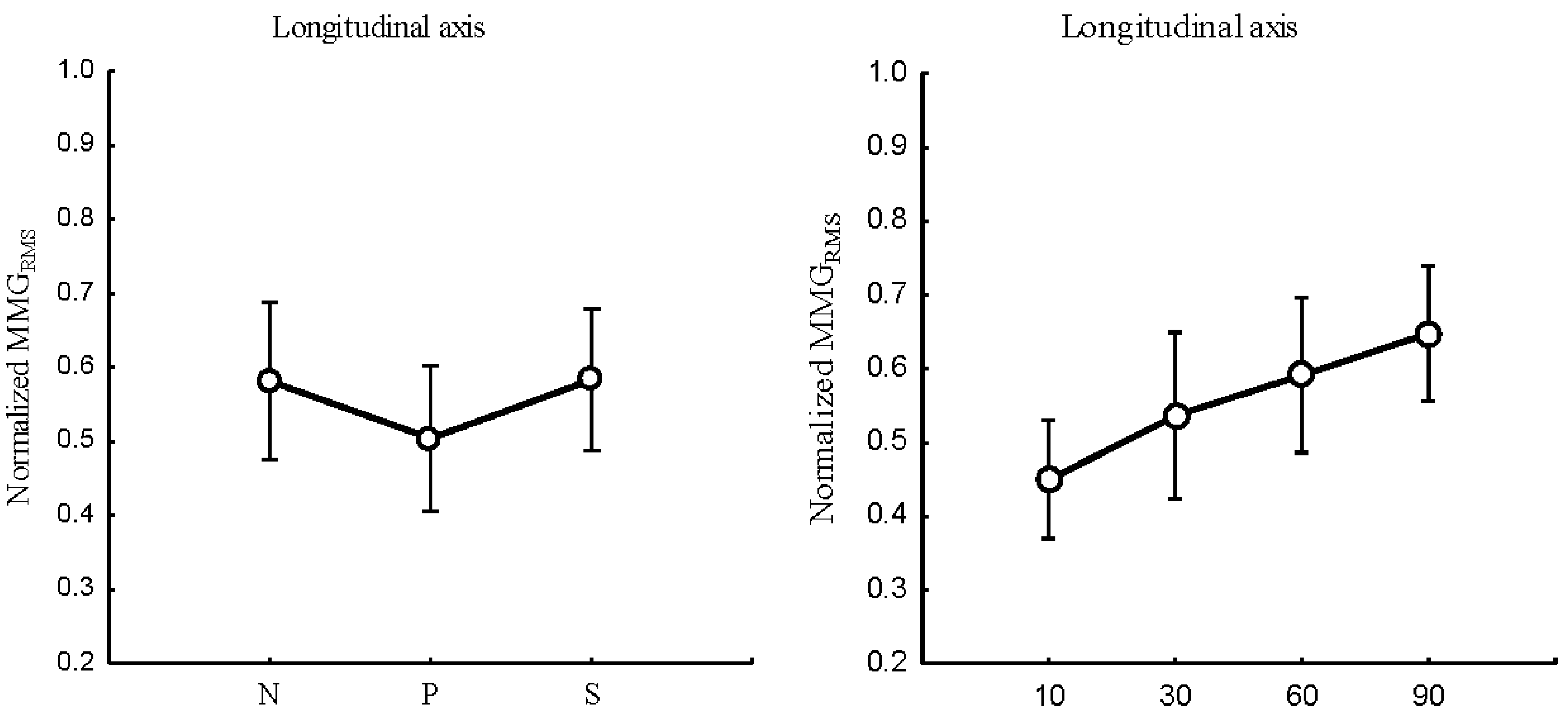
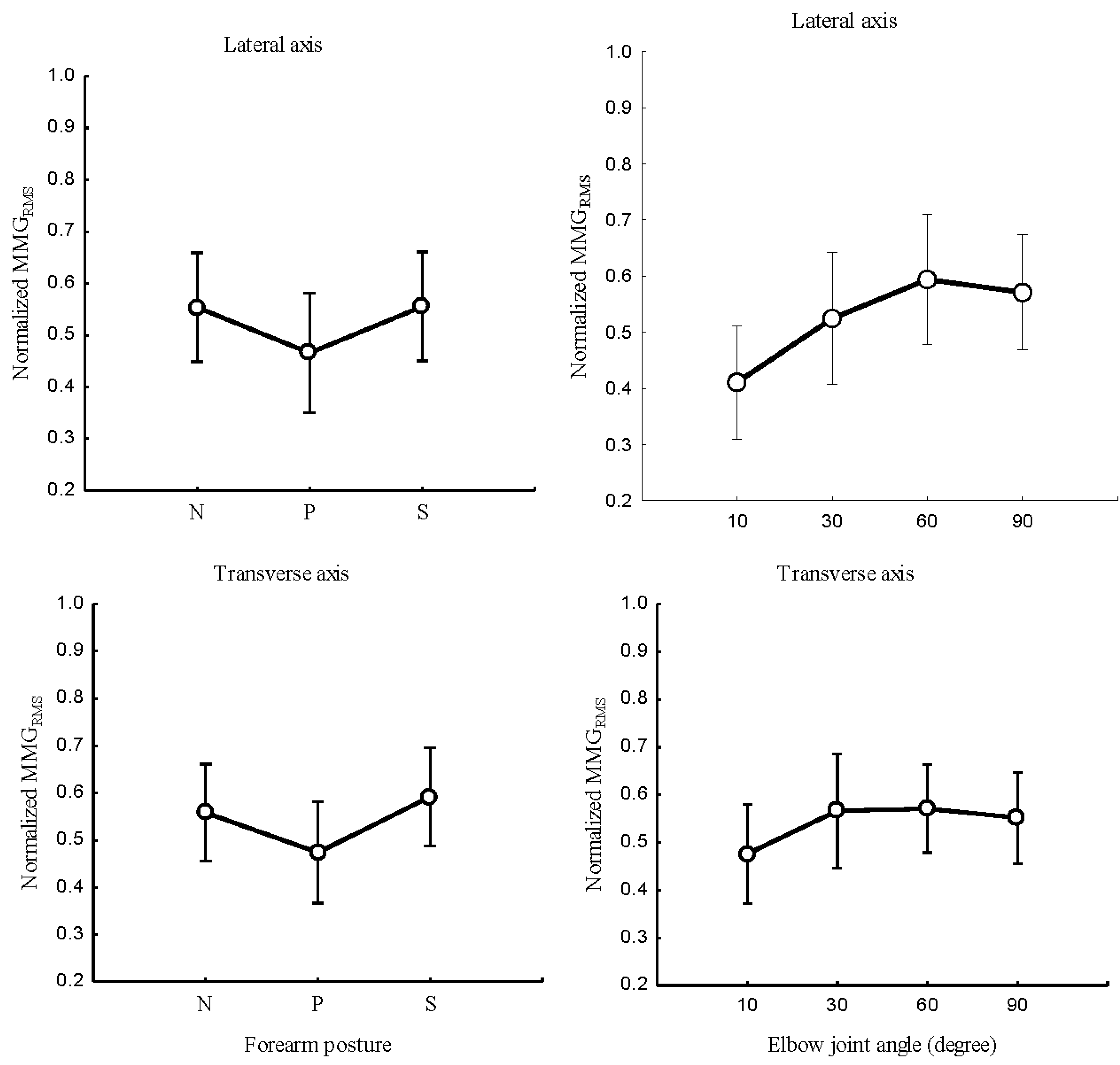
3.2.1. MMG amplitude
The relationship between the forearm posture and
,
Figure 5 (left); elbow joint angles and
,
Figure 5 (right); and the main effect of forearm postures and elbow joints angles on
,
Figure 6 examined the longitudinal, lateral, and transverse axes of BB muscle fibers.
Forearm posture was found to have a significant effect on normalized at the longitudinal axis (P < 0.05), the lateral axis (P <0.05), and the transverse axis (P < 0.05). Elbow joint angles showed significant effects on normalized at the longitudinal, (P < 0.05), lateral (P < 0.05), and transverse (P <0.05) axes of BB muscle fibers. Forearm postures and elbow joint angle interaction were found to have no significant main effects on in the longitudinal (P > 0.05), lateral (P > 0.05) and transverse axis (P > 0.05) of BB muscle fibers.
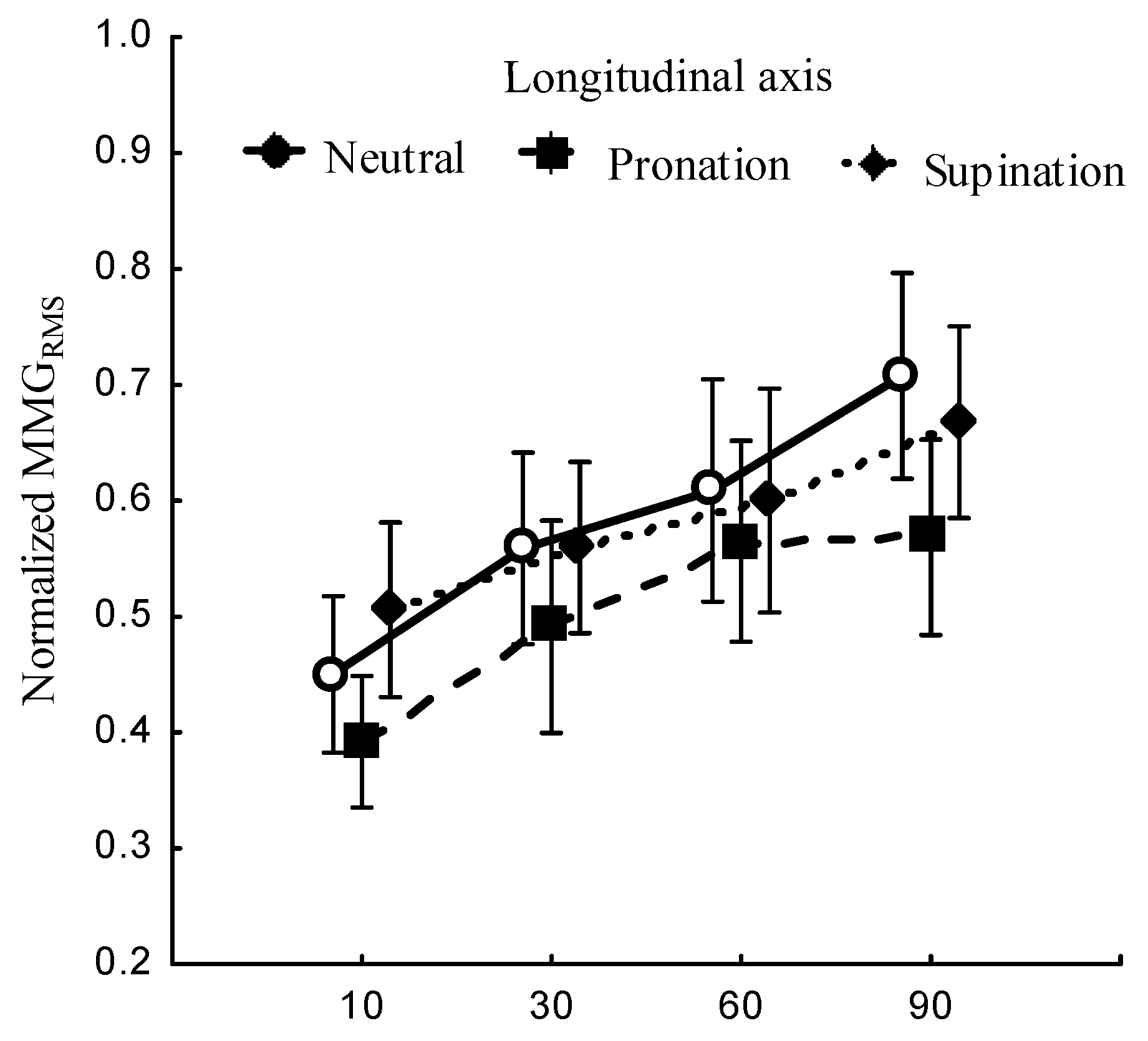
3.2.2. MMG frequency
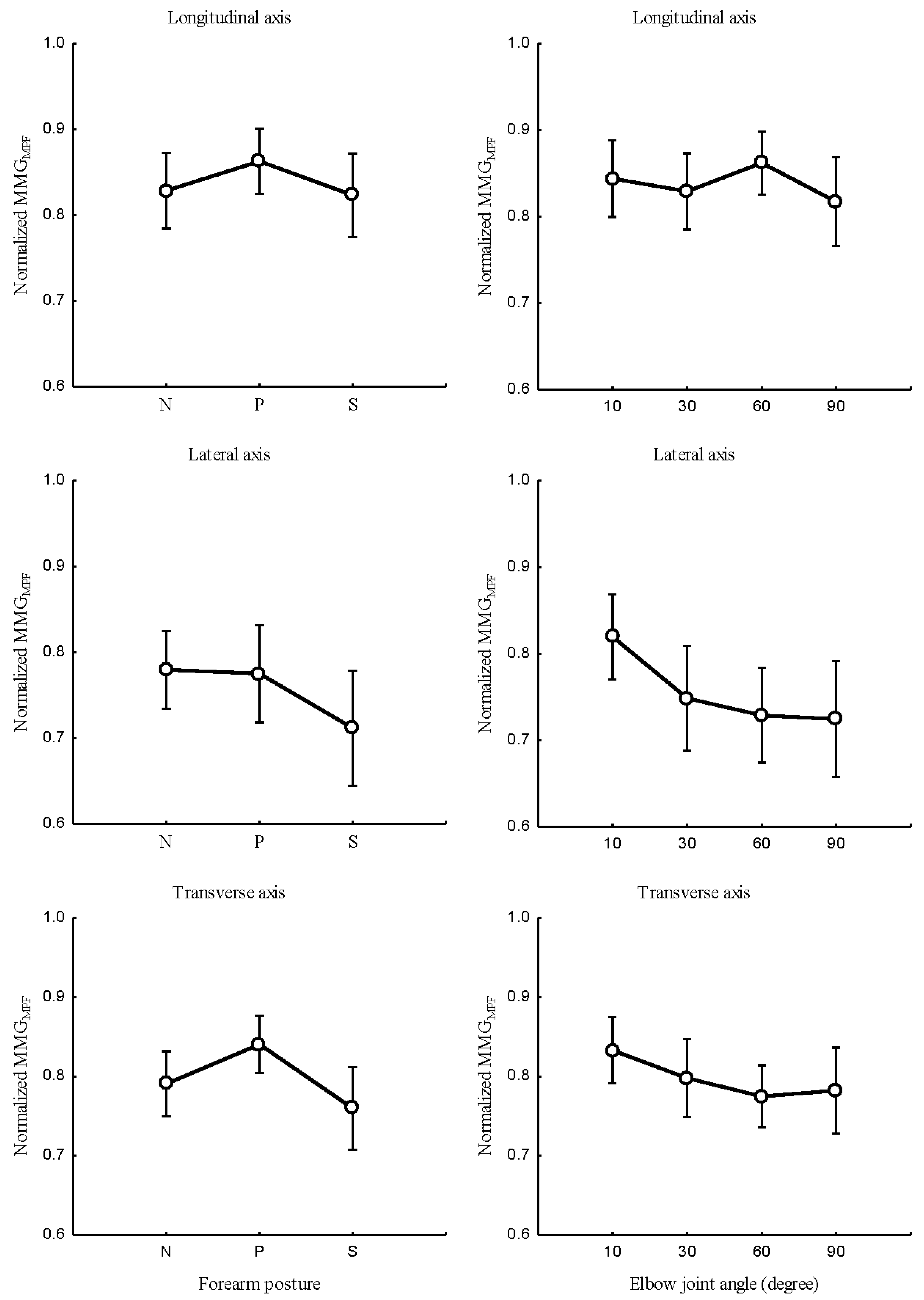
The relationship between the forearm posture and
,
Figure 7 (left); elbow joint angles and
,
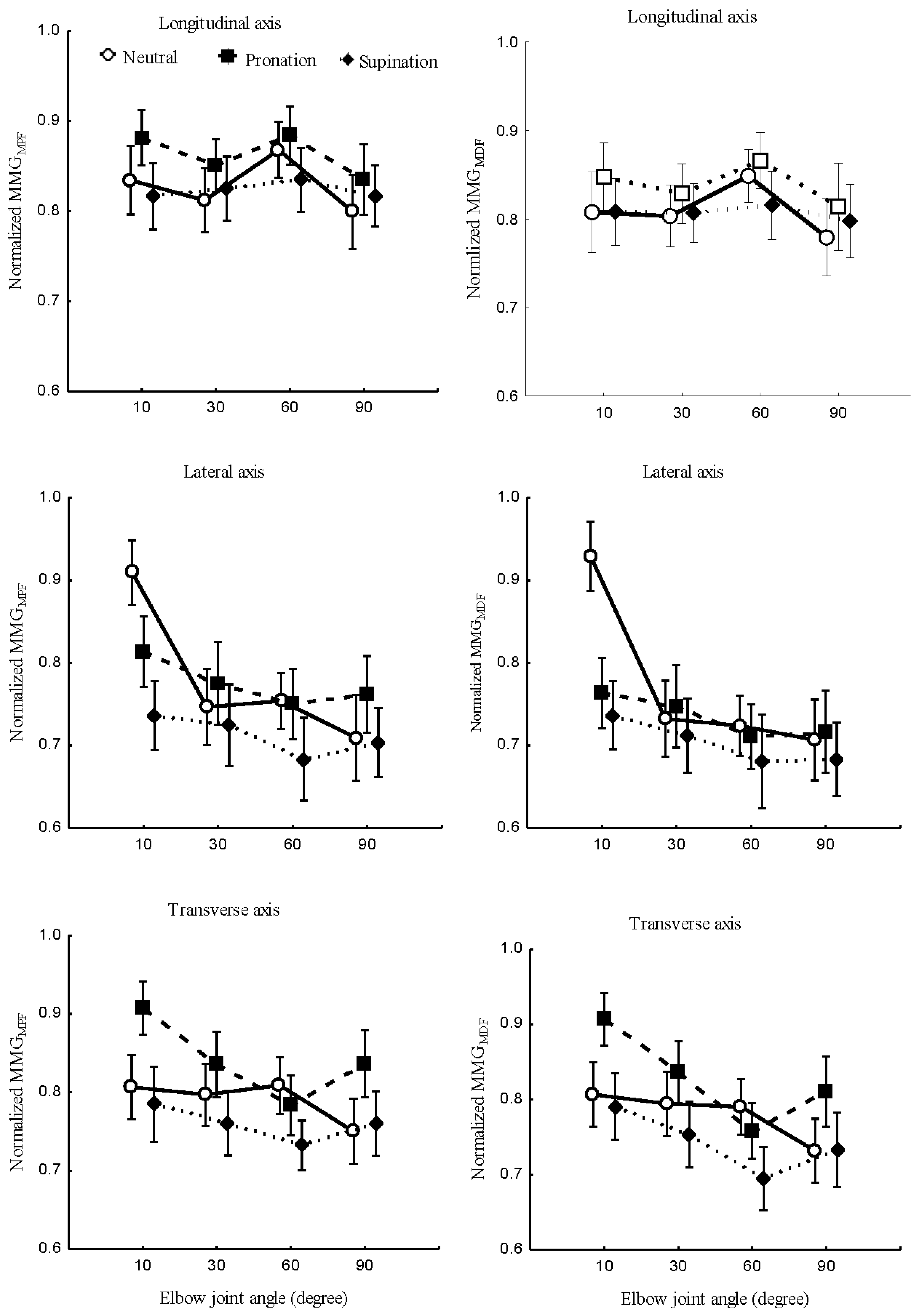
Figure 7 (right); and the main effect of forearm postures and elbow joints angles interaction on
,
Figure 9 (left) were measured at the longitudinal, lateral, and transverse axes of BB muscle fibers.
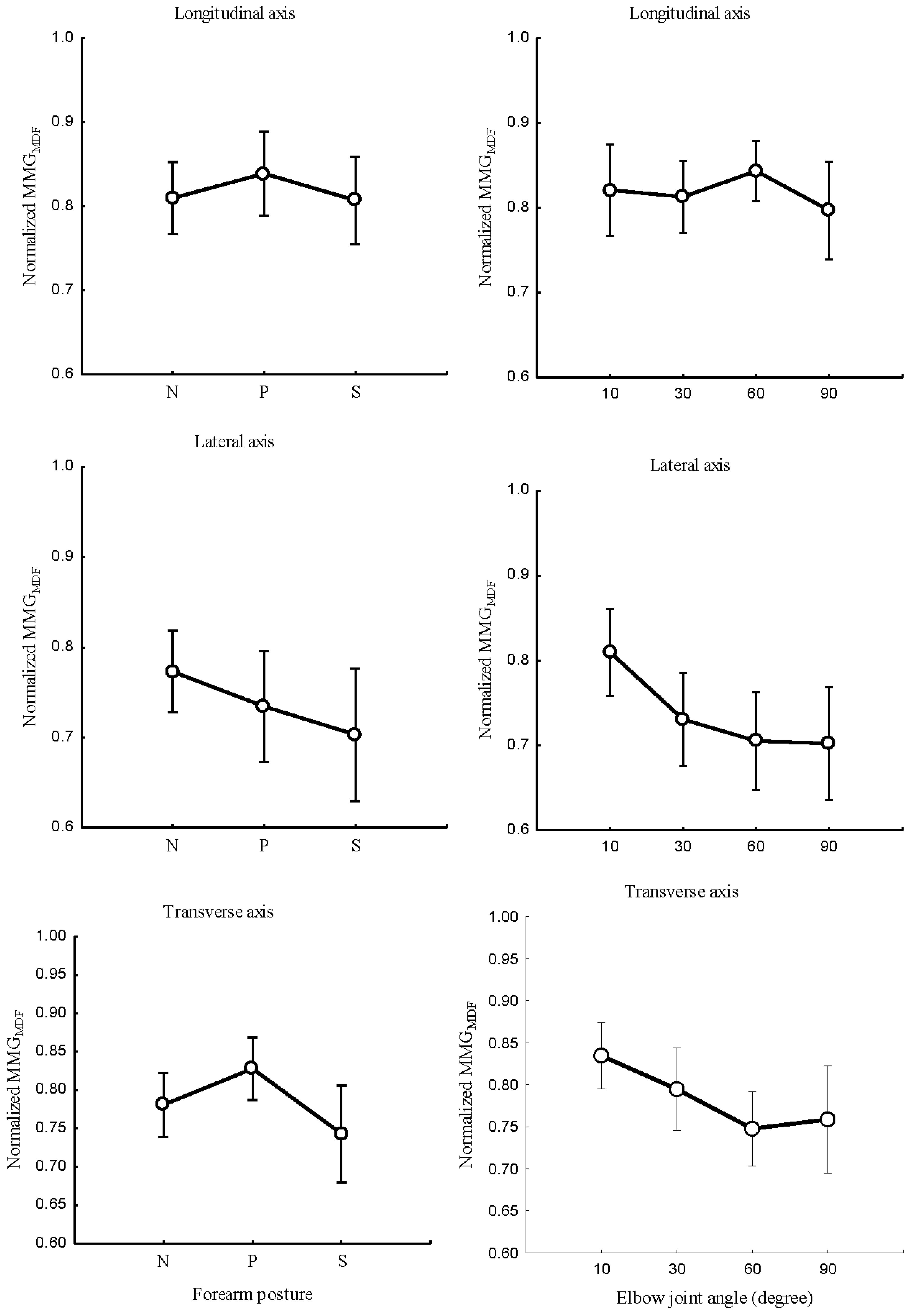
The relationship between the forearm posture and
,
Figure 8 (left); elbow joint angles and
,
Figure 8 (right); and main effect between forearm postures and elbow joints angles on
,
Figure 9 (right) were studied at the longitudinal, lateral, and transverse axes of BB muscle fibers.
Figure 7.
Relationship between MMGMPF of the longitudinal, lateral, and transverse axes of the BB muscle fibers and the forearm posture (neutral, pronation, supination) (left); elbow joint at 10°, 30°, 60° and 90° (right).
Figure 7.
Relationship between MMGMPF of the longitudinal, lateral, and transverse axes of the BB muscle fibers and the forearm posture (neutral, pronation, supination) (left); elbow joint at 10°, 30°, 60° and 90° (right).
Figure 8.
Behaviours of MMGMDF of the longitudinal, lateral, and transverse axes of the BB muscle fibers at the neutral, pronation and supination positions (left) and at the elbow joints at 10°, 30°, 60° and 90° (right).
Figure 8.
Behaviours of MMGMDF of the longitudinal, lateral, and transverse axes of the BB muscle fibers at the neutral, pronation and supination positions (left) and at the elbow joints at 10°, 30°, 60° and 90° (right).
Figure 9.
Behaviours of MMGMPF (left) and MMGMDF (right) of the longitudinal, lateral, and transverse axes of the BB muscle fibers at the neutral, pronation and supination positions (left), and the elbow joint at 10°, 30°, 60° and 90° (right)
Figure 9.
Behaviours of MMGMPF (left) and MMGMDF (right) of the longitudinal, lateral, and transverse axes of the BB muscle fibers at the neutral, pronation and supination positions (left), and the elbow joint at 10°, 30°, 60° and 90° (right)
Forearm posture was found to have significant effects on normalized and at the longitudinal axis (P < 0.05), the lateral axis (P < 0.05), and the transverse axis (P < 0.05). Elbow joint angle revealed significant effects on normalized and at the longitudinal axis, (P < 0.05), the lateral axis (P < 0.05), and the transverse axis (P < 0.05). Forearm postures and elbow joint angle interaction showed insignificant effects on and in the longitudinal axis (P >0.05). For the lateral and transverse axis, forearm posture and elbow joint angle revealed a significant effect on and (P < 0.05).
4. Discussion
This research aimed to investigate the effects of changes in forearm posture(s) and elbow joint angle on NMES-evoked torque and MMG signals. This study indicates that 1) forearm posture and 2) elbow joint angle influence , , , and . We also found that 3) the interaction effect of forearm posture and elbow joint angle on , , and was not significant at the longitudinal axis of BB muscle fibers.
4.1. Effect of forearm posture and elbow joint angle on torque
The results showed significant effects of the neutral, pronation, and supination on the normalized
,
Figure 3 This finding suggests that variation in forearm posture led to variation in muscle length, shape, and size. While a consistent intensity of NMES was maintained, an increase in the depth of the BB with elbow joint angle resulted in a reduction of the current density. Consequently, the level of recruitment of deeper muscle fibers was compromised [
21].
Previous studies have reported that alteration in forearm posture changed the length of the BB muscle, which in turn influenced the spinal excitability of the BB muscle [
22]. This in turn may affect the responsiveness of BB muscle to NMES. Therefore, these results validate the hypothesis that the influence of forearm posture on
is associated with alterations in muscle morphology, which influences the results to deviate from expected outcomes.
Like the findings in the previous studies, the present research also found a significant effect of elbow joint angle on
[
23,
24]. Beyond the elbow flexion angle of 60
, the
exhibited a downward shift. These results indicate that the resting length produced more cross-bridge interaction between actin and myosin filaments. Hence, these are mechanical properties of BB muscle to influence NMES evoked
[
25]. Additionally, as the BB originates from the radial tuberosity, elbow flexion leads to a reduction in the length of BB due to the variation in moment arms [
26]. These changes are associated with the level of neural excitation [
27], and altered fiber type composition [
17,
21,
29,
30,
31]. NMES showed greater torque at intermediate elbow joint angles [
31], which indicates the variation in morphological adaptations of muscle tendons [
32] as well as changes in sarcomere length [
19,
34].
In this study, a consistent intensity of NMES was employed. However, during elbow flexion, some of the deeper motor units were not activated. Consequently, the observed torque decline was influenced by the reduction of the motor units activated per electrode site. This indicates that the changes in muscle depth, because of variation in forearm posture and elbow joint angle impacts electrical excitation of the muscle. While it is known that the motor point of the biceps shifts 1.5 cm to 2 cm over a range of 80° [
34], the size of the electrode of 4 cm × 4 cm used in our experiment is enough to remain fixed at the threshold location of the motor point. Thus, the variations in output measurement of
was strongly influenced by changing biomechanical and mechanical characteristics at the BB muscle.
This research is the first to highlight the NMES-evoked torque when varying the forearm posture and elbow joint angle. These findings suggest that the lever arm, joint structure and myosin were affecte, thus altered the motor output which is length dependent. This is evident, as the muscle length modulates the local circuits neurons [
22], which influence the responsiveness of muscle to NMES, necessary in therapeutic rehabilitation.Additinally, early studies revealed that 20 % torque evoked by NMES provokes a higher degree of muscle activation compared to equivalent 20 % MVC torque in lower limb muscles [
35]. The 15 % NMES-evoked torque found in this study supports the belief that it is beneficial to restore injured muscles and post-surgical procedures [
2]. However, it was noted that the forearm posture influences the spinal pathways but was not evident in MVC. Further investigation should focus on the optimum protocol for achieving an MVC equivalent to 15 % of NMES with changing muscle length.
Inter-individual response to NMES was found to be influenced by body fat, BMI, and sex. Although our experiment involved subjects with a normal BMI range, it is worth noting that upper arm circumference impacts the response of the muscle to NMES [
36]. Therefore, understanding the relationship between muscle length and strength could serve as a valuable direction for future investigations in clinical studies.
4.2. Effect of forearm posture and elbow joint angle on the amplitude of MMG
The alteration in the forearm posture influences the electrophysiological properties of the BB muscle [
37]. These changes are associated with inhibitory and excitatory circuits of the spinal pathways. When the elbow is flexed along with the forearm rotation, the dimensional changes of muscle fibers lead to variations in their firing rates, which are reflected in the form of MMG signals [
38].
In this study, the forearm posture was found to have a significant effect on
in the longitudinal, lateral, and transverse axes. These findings indicate that the forearm posture caused a change in the behaviours of the neural connections that carry signals to the BB muscle [
39]. The insignificant difference in
at
and
° can be attributed to insufficient excitability in deeper muscle fibers due to shorter muscle lesser than the resting length of the BB muscle [
22].
Across investigated elbow joint angles, the pronation posture consistently exhibited the lowest
, (
Figure 5 (left) and
Figure 6). This finding is consistent with a previous study on EMG [
40]. These results can be attributed to the inhibition of the activation of BB fibers under the same synaptic connection with the spinal tracks of the brachioradialis muscle. NMES experiments have shown that isolating the BB from the brachialis and brachioradialis may lead to an additive level of excitation, which can be diminished in the pronation position [
40]. This may be caused by the muscle-tendon complex that experiences a greater stiffness at higher torque output, (
Figure 3 (left) and
Figure 4). Furthermore, it was observed that the magnitude of each of the three axes varied across the forearm postures. These findings suggest that forearm posture can be explored as a proxy for MMG signals.
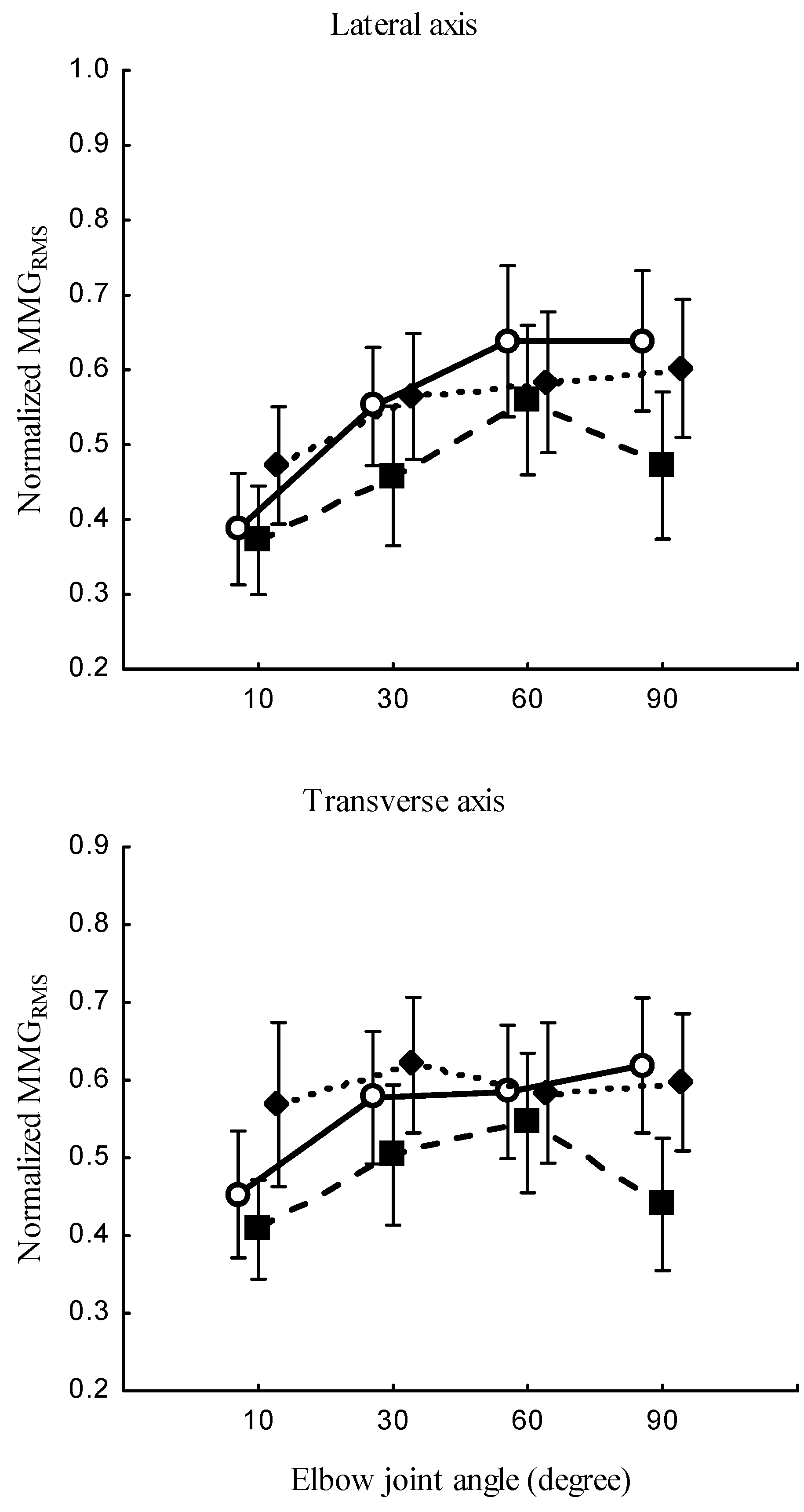
An increase in elbow joint angle showed an increase in the
. This observation complies with the approach taken by Barry [
41], who reported increased MMG amplitude from electrically stimulated gastrocnemius of the flog at shorter muscle lengths. As also supported by previous research [
21], the recruitment of motor units at deeper muscle(s) has dependance on the number of fibers under the stimulation electrodes. Furthermore, the filtering capacity of the tissue between the acceleration sensor and recruited muscle fibers increases with the elbow flexion angle [
42]. This study also showed that the
increases from 10
to 30
, and a decrease from 60
at the lateral and transverse axis,
Figure 5 (right). As also observed in [
15], MMG amplitude increased at 90% of the muscle length and decreased when the muscle was higher or lower than 90%. Hence, it is reasonable to argue that
was influenced by viscosity, thickness, and stiffness, all of which varied with the length of muscle at high elbow joint angles.
The statistical significance difference in the elbow joint across the longitudinal, lateral, and transverse axis can be attributed to the dynamic properties of the muscle. Hence, the finding that the difference in
at the lateral axis, mirrors the resonance frequency of the fiber, and the transverse axis reflects the longitudinal stiffness [
41], is evident.
4.3. Effect of forearm posture and elbow joint angle on the frequency of MMG
This study found that an increase in elbow joint angle is associated with a decrease in
. Evidence of these findings is that, when a muscle is selectively isolated, the decline in
originates from the lengthening or shortening of the sarcomere outside the nominal dimensions of actin and myosin. Additionally, an increase in elbow flexion causes an increase in the muscle diameter and shape which impacts a reduction of muscle fibers recruitment and discharge rate [
43]. This finding agrees with the research reported by Frangioni, that the mean power frequency increased with the muscle length of the gastrocnemius of a frog under electrical stimulation [
44]. Furthermore, studies in voluntary contraction claimed a significant decrease in
at shorter muscle lengths [
45]. Together with the findings in this study, MMG response was independent of the firing patterns but of the contraction and relaxation time properties of the muscle fibers [
15].
A decreased
found in this study is influenced by muscle length [
31]. The patterns of the
were shown to be higher at higher muscle lengths [
46]. The decreasing
with an increased elbow joint angle indicates motor unit synchronization, which was reported to lower the median frequency of the muscle [
47]. The difference in the patterns of
at the longitudinal, lateral, and transverse axes indicate that the levels of activation of muscle fibers vary across the elbow angle and forearm posture. Specifically,
and
of the longitudinal axis
Figure 7 (right) and
Figure 8 (right) increased and decreases with the
. This indicates that the frequency features of MMG signals measured by the longitudinal axis can mirror the patterns of NMES-evoked torque. Conversely,
and
at the lateral and transverse axes exhibited a meaningful decrease as opposed to the
,
Figure 9. This non-linear feature is caused by the muscle architecture, tendon units, and sensory feedback at the muscle spindles. Additionally, the decline in both
and
indicates the discharge rate of Ia afferent inputs, which have been shown to change with the resting length of the muscle. The non-linear relationship of MMG frequency features reveals spectra parameters to be good candidates for the limb torque estimation to be explored in the future.
5. Conclusion
The results of this study support the hypothesis that the muscle vibration elicited by the NMES depends on its biomechanical properties which are determined by the muscle length. Muscle length was found to be influenced by varying the forearm posture and elbow flexion angle. Change in muscle length affects the active and passive contractile elements of skeletal muscle, which in turn influences muscle vibration.
,
and
at the longitudinal axis showed a monotonic increase with elbow joint angles.
and
in the lateral and transverse axis decreased at shorter muscle length
Figure 9.
An increase in elbow flexion angle from 10° to 60° showed a linear increase in the
and
. However, a decrease in
beyond 60° could have been influenced by optimal muscle length and motor neuron adaptation. These findings are consistent with the reported results of the research [
15], that the magnitude of muscle vibration increases with nominal muscle length to 90% and declines at other muscle lengths. These results could provide further clarity by introducing ultrasound to measure the change in muscle dimensions along with the forearm and elbow joint angles during NMES experiments. The outcomes of this study might guide the proper design of rehabilitating programs involving electrical stimulation. Typically, the knowledge about the relationship between NMES and torque and MMG parameters may be helpful in reconstruction surgery, which requires monitoring of post-operative functionality.
Author Contributions
Conceptualization and methodology, investigation, and signal processing, U.R and K.S; statistical analysis U.R and F.S.F; writing; supervision K.S and F.S.; All authors approved the final manuscript.
Funding
This research was funded by the University of Rwanda, the Regional Centre of Excellence for Biomedical Engineering and E-Health (UR-CEBE).
Ethical statement
The study was conducted in compliance with the principles outlined in the Declaration of Helsinki. Ethical approval NMRR-20-2613-56796 (IIR) was provided by the Medical Research and Ethics Committee, Ministry of Health, Malaysia.
Informed Consent
A signed informed consent was obtained from all subjects.
Data availability
Data is available upon reasonable request.
Acknowledgments
Thanks to the subjects who participated in their research. Without their contribution, this project could not have been undertaken.
Conflicts of Interest
The authors declare that they have no competing financial interests that influenced the research work reported in this article.
References
- Akima, H.; Maeda, H.; Koike, T.; Ishida, K. Effect of elbow joint angles on electromyographic activity versus force relationships of synergistic muscles of the triceps brachii. PLoS ONE 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.J.; Downey, R.J.; Rouse, C.A.; Dixon, W.E. Influence of Elbow Flexion and Stimulation Site on Neuromuscular Electrical Stimulation of the Biceps Brachii. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2018, 26, 904–910. [Google Scholar] [CrossRef]
- Islam, M.A.; Sundaraj, K.; Ahmad, R.B.; Ahamed, N.U.; Ali, M.A. Mechanomyography sensor development, related signal processing, and applications: A systematic review. IEEE Sens J 2013, 13, 2499–2516. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Zuniga, J.M.; Abdul Wahab, A.K. Mechanomyography and muscle function assessment: A review of current state and prospects. Clinical Biomechanics 2014, 29, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, M.O.; Hamzaid, N.A.; Hasnan, N.; Khairi, A.; Wahab, A. Torque and mechanomyogram relationships during electrically evoked isometric quadriceps contractions in persons with spinal cord injury. Med Eng Phys 2016, 38, 767–775. [Google Scholar] [CrossRef]
- Kleiber, T.; Kunz, L.; Disselhorst-Klug, C. Muscular coordination of biceps brachii and brachioradialis in elbow flexion with respect to hand position. Front Physiol 2015, 6, 215. [Google Scholar] [CrossRef]
- Uwamahoro, R.; Sundaraj, K.; Subramaniam, I.D. Assessment of muscle activity using electrical stimulation and mechanomyography: A systematic review. Biomed Eng Online 2021, 20. [Google Scholar] [CrossRef] [PubMed]
- Uwamahoro, R.; Sundaraj, K.; Subramaniam, I.D. Analysis of upper limb rehabilitation using muscle mechanics: Current and future perspectives using Mechanomyography signals. In Proceedings of the 12th Biomedical Engineering International Conference, Ubon Ratchathani, Thailand; 2019; pp. 1–5. [Google Scholar]
- Esposito, F.; Cè, E.; Rampichini, S.; Veicsteinas, A. Acute passive stretching in a previously fatigued muscle: Electrical and mechanical response during tetanic stimulation. J Sports Sci 2009, 27, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, K.H.; Maganaris, C.N.; Reeves, N.D.; Sargeant, A.J.; Jones, D.A.; De Haan, A. Influence of knee joint angle on muscle properties of paralyzed and nonparalyzed human knee extensors. Muscle Nerve 2005, 32, 73–80. [Google Scholar] [CrossRef]
- Miyamoto, N.; Oda, S. Mechanomyographic and electromyographic responses of the triceps surae during maximal voluntary contractions. Journal of Electromyography and Kinesiology 2003, 13, 451–459. [Google Scholar] [CrossRef]
- Islam, M.A.; Sundaraj, K.; Ahmad, R.B.; Ahamed, N.U. Mechanomyogram for Muscle Function Assessment: A Review. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, Y.P.; Huang, Q.H.; Chen, X. Continuous monitoring of sonomyography, electromyography and torque generated by normal upper arm muscles during isometric contraction: Sonomyography assessment for arm muscles. IEEE Trans Biomed Eng 2008, 55, 1191–1198. [Google Scholar] [CrossRef]
- Miyamoto, N.; Oda, S. Effect of joint angle on mechanomyographic amplitude during unfused and fused tetani in the human biceps brachii muscle. Eur J Appl Physiol 2005, 95, 221–228. [Google Scholar] [CrossRef]
- Beck, T.W.; Housh, T.J.; Johnson, G.O.; Cramer, J.T.; Weir, J.P.; Coburn, J.W.; Malek, M.H. Does the frequency content of the surface mechanomyographic signal reflect motor unit firing rates? A brief review. Journal of Electromyography and Kinesiology 2007, 17, 1–13. [Google Scholar] [CrossRef]
- Gobbo, M.; Maffiuletti, N.A.; Orizio, C.; Minetto, M.A. Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. Journal of Neuroengineering and Rehabilitation 2014, 11, 1–6. [Google Scholar] [CrossRef]
- Madeleine, P.; Cescon, C.; Farina, D. Spatial and force dependency of mechanomyographic signal features. J Neurosci Methods 2006, 158, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F.; Cheng, S.C.; Lee, M.Y.; Lin, W.Y. Measurement and estimation of muscle contraction strength using mechanomyography based on artificial neural network algorithm. Biomed Eng (Singapore) 2013, 25. [Google Scholar] [CrossRef]
- Miyamoto, N.; Oda, S. Effect of joint angle on mechanomyographic amplitude during unfused and fused tetani in the human biceps brachii muscle. Eur J Appl Physiol 2005, 95, 221–228. [Google Scholar] [CrossRef]
- Garcia-Retortillo, S.; Rizzo, R.; Wang, J.W.; Sitges, C.; Ivanov, P.C. Universal spectral profile, and dynamic evolution of muscle activation. Journal of Applied Physiology 2020, 129, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T. Training based on electrical stimulation superimposed onto voluntary contraction would be relevant only as part of submaximal contractions in healthy subjects. Front Physiol 2018, 9. [Google Scholar] [CrossRef]
- Forman, D.A.; Abdel-Malek, D.; Bunce, C.M.; Holmes, M.W. Muscle length and joint angle influence spinal but not corticospinal excitability to the biceps brachii across forearm postures. J Neurophysiol 2019, 122, 413–423. [Google Scholar] [CrossRef]
- Doheny, E.P.; Lowery, M.M.; FitzPatrick, D.P.; O’Malley, M.J. Effect of elbow joint angle on force-EMG relationships in human elbow flexor and extensor muscles. Journal of Electromyography and Kinesiology 2008, 18, 760–770. [Google Scholar] [CrossRef]
- Nunes, J.P.; Jacinto, J.L.; Ribeiro, A.S.; Mayhew, J.L.; Nakamura, M.; Capel, D.M.; Santos, L.R.; Santos, L.; Cyrino, E.S.; Aguiar, A.F. Placing greater torque at shorter or longer muscle lengths? Effects of cable vs. barbell preacher curl training on muscular strength and hypertrophy in young adults. Int J Environ Res Public Health 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sun, Y.; Sun, L.; Pan, B.; Huang, Z.; Wu, J.; Zhang, Z. A pilot study of individual muscle force prediction during elbow flexion and extension in the neurorehabilitation field. Sensors 2016, 16, 2018. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.J.; Downey, R.J.; Rouse, C.A.; Dixon, W.E. Influence of Elbow Flexion and Stimulation Site on Neuromuscular Electrical Stimulation of the Biceps Brachii. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2018, 26, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Thfipaut-Mathieu, C.; Van Hoecke, J.; Maton, B. Myoelectrical and Mechanical Changes Linked to Length Specificity during Isometric Training. Journal of Applied Physiology 1988, 64, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Han, D. The Relationship between Knee Joint Angle and Knee Flexor and Extensor Muscle Strength. Journal of Physical Therapy Science 2017, 29, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, K.H.; Maganaris, C.N.; Reeves, N.D.; Sargeant, A.J.; Jones, D.A.; De Haan, A. Influence of knee joint angle on muscle properties of paralyzed and nonparalyzed human knee extensors. Muscle Nerve 2005, 32, 73–80. [Google Scholar] [CrossRef]
- Son, J.; Rymer, W.Z. Effects of Changes in Ankle Joint Angle on the Relation Between Plantarflexion Torque and EMG Magnitude in Major Plantar Flexors of Male Chronic Stroke Survivors. Front Neurol 2020, 11. [Google Scholar] [CrossRef]
- Krueger, E.; Scheeren, E.M.; Nogueira-Neto, G.N.; Button, V.L.D.S.N.; Nohama, P. Advances and perspectives of mechanomyography. Revista Brasileira de Engenharia Biomedica 2014, 30, 384–401. [Google Scholar] [CrossRef]
- Kubo, K.; Ohgo, K.; Takeishi, R.; Yoshinaga, K.; Tsunoda, N.; Kanehisa, H.; Fukunaga, T. Effects of isometric training at different knee angles on the muscle-tendon complex in vivo. Scand J Med Sci Sports 2006, 16, 159–167. [Google Scholar] [CrossRef]
- Rassier, D.E.; MacIntosh, B.R.; Herzog, W. Length dependence of active force production in skeletal muscle. Journal of Applied Physiology 1999, 86, 1445–1457. [Google Scholar] [CrossRef]
- Martin, S.; MacIsaac, D. Innervation zone shift with changes in joint angle in the brachial biceps. Journal of Electromyography and Kinesiology 2006, 16, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Flodin, J.; Mikkelsen, C.; Ackermann, P.W. Knee extensor force production and discomfort during neuromuscular electrical stimulation of quadriceps with and without gluteal muscle co-stimulation. Eur J Appl Physiol 2022, 122, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Lee, D.; Kim, Y. Effects of involuntary eccentric contraction training by neuromuscular electrical stimulation on the enhancement of muscle strength. Clinical Biomechanics 2014, 29, 767–772. [Google Scholar] [CrossRef]
- He, L.; Mathieu, P.A. Static hand posture classification based on the biceps brachii muscle synergy features. Journal of Electromyography and Kinesiology 2018, 43, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Talib, I.; Sundaraj, K.; Kiang Lam, C. A Systematic Review of Muscle Activity Assessment of the Biceps Brachii Muscle Using Mechanomyography. Journal of Musculoskeletal & Neuronal Interactions 2018, 18, 446. [Google Scholar]
- Mogk, J.P.M.; Rogers, L.M.; Murray, W.M.; Perreault, E.J.; Stinear, J.W. Corticomotor excitability of arm muscles modulates according to static position and orientation of the upper limb. Clinical Neurophysiology 2014, 125, 2046–2054. [Google Scholar] [CrossRef]
- Kleiber, T.; Kunz, L.; Disselhorst-Klug, C. Muscular coordination of biceps brachii and brachioradialis in elbow flexion with respect to hand position. Front Physiol 2015, 6. [Google Scholar] [CrossRef]
- Barry, D.T. Acoustic signals from frog skeletal muscle. Biophys J 1987, 51, 769–773. [Google Scholar] [CrossRef]
- Beck, T.W.; Housh, T.J.; Cramer, J.T.; Weir, J.P.; Johnson, G.O.; Coburn, J.W.; Malek, M.H.; Mielke, M. Mechnomyographic amplitude and frequency responses during dynamic muscle actions: A comprehensive review. Biomed Eng Online 2005, 4. [Google Scholar] [CrossRef]
- Pasquet, B.; Carpentier, A.; Duchateau, J. Change in muscle fascicle length influences the recruitment and discharge rate of motor units during isometric contractions. Journal of Neurophysiology 2005, 94, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V.; Kwan-Gett, T.S.; Dobrunz, L.E.; Mcmahon, T.A. The Mechanism of Low-Frequency Sound Production in Muscle. Biophysical Journal 1987, 51, 775–783. [Google Scholar] [CrossRef]
- Qi, L.; Wakeling, J.M.; Ferguson-Pell, M. Spectral properties of electromyographic and mechanomyographic signals during dynamic concentric and eccentric contractions of the human biceps brachii muscle. Journal of Electromyography and Kinesiology 2011, 21, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.A.; Herzog, W.; Zhang, Y.T.; Leonard, T.R.; Hlhmnn, H.N. The Effect of Muscle Length on Electrically Elicited Muscle Vibrations in the In-Situ Cat Soleus Muscle. Journal of Electromyography and Kinesiology 1997, 7, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C.; Allen, E.J.; Williams, G.N. Effect of knee position on quadriceps muscle force steadiness and activation strategies. Muscle Nerve 2011, 43, 563–573. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Raw Acceleration and force data acquisition setup: 1) electrical stimulation electrodes, 2) MMG sensor, 3) adjustable elbow rest, 4) support of the force sensor (placed underneath), 5) a fixture of the hand/wrist posture, 6) a fixture for the adjustable elbow joint angle, 7) force and acceleration data acquisition device.
Figure 1.
Raw Acceleration and force data acquisition setup: 1) electrical stimulation electrodes, 2) MMG sensor, 3) adjustable elbow rest, 4) support of the force sensor (placed underneath), 5) a fixture of the hand/wrist posture, 6) a fixture for the adjustable elbow joint angle, 7) force and acceleration data acquisition device.
Figure 2.
Filtered elbow joint torque and MMG signal from the BB. The middle one-second data was used for the calculation of and MMGRMS, MMGMPF, MMGMDF.
Figure 2.
Filtered elbow joint torque and MMG signal from the BB. The middle one-second data was used for the calculation of and MMGRMS, MMGMPF, MMGMDF.
Figure 3.
Normalized TQRMS at neutral, pronation, and supination posture of the forearm (left) and the elbow flexion at 10°, 30°, 60° and 90° (right).
Figure 3.
Normalized TQRMS at neutral, pronation, and supination posture of the forearm (left) and the elbow flexion at 10°, 30°, 60° and 90° (right).
Figure 4.
Normalized TQRMS at the forearm at the neutral, pronation and supination positions and at elbow joint angles of 10°, 30°, 60° and 90°.
Figure 4.
Normalized TQRMS at the forearm at the neutral, pronation and supination positions and at elbow joint angles of 10°, 30°, 60° and 90°.
Figure 5.
Behaviours of the normalized MMGRMS of the longitudinal, lateral, and transverse axes of the BB muscle fibers at the neutral, pronation, and supination (left) and elbow joint at 10°, 30°, 60° and 90° (right).
Figure 5.
Behaviours of the normalized MMGRMS of the longitudinal, lateral, and transverse axes of the BB muscle fibers at the neutral, pronation, and supination (left) and elbow joint at 10°, 30°, 60° and 90° (right).
Figure 6.
MMGRMS of the longitudinal, lateral, and transverse axes of the BB muscle with the forearm in neutral, pronation and supination positions; and the elbow joint at 10°, 30°, 60° and 90°.
Figure 6.
MMGRMS of the longitudinal, lateral, and transverse axes of the BB muscle with the forearm in neutral, pronation and supination positions; and the elbow joint at 10°, 30°, 60° and 90°.
Table 1.
Elicited elbow flexion measured at the elbow joint of 10°, 30°, 60° and 90° in the N, P, and S.
Table 1.
Elicited elbow flexion measured at the elbow joint of 10°, 30°, 60° and 90° in the N, P, and S.
| Elbow joint angles |
|
30° |
|
90° |
| Forearm Posture |
|
Mean ± SD |
Mean ± SD |
Mean ± SD |
Mean ± SD |
| N |
0.4516 |
0.1775 |
0.5707 |
0.1990 |
0.7416 |
0.1839 |
0.6029 |
0.2051 |
| P |
0.4325 |
0.1724 |
0.5108 |
0.1700 |
0.6500 |
0.1704 |
0.5957 |
0.1797 |
| S |
0.4002 |
0.1678 |
0.4524 |
0.1970 |
0.5041 |
0.2033 |
0.4639 |
0.1939 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).