1. Introduction
The fast development of nanoscience and material chemistry has increased interest in researching new and innovative synthesis methods to produce new nanomaterials with unique catalytic activity [1, 2], unique optical properties [
3], high active area [
4], antibacterial properties [
5], and high biocompatibility [
6]. The new field of nanozyme-based catalysis, which has been introduced as an alternative to enzyme-based catalysis, is called nanozyme chemistry. On the other hand, nanozymes are known as nanomaterials with high enzyme-like activity and can be used to simulate enzymatic reactions in harsh environmental conditions (for example, higher temperature or wider pH range) [7-10]. In fact, natural enzymes show several weaknesses as follows [11, 12]; (I) low stability (narrow thermal range and pH range), (II) difficulty in recovery, and (III) inability to reuse the enzyme in reactions, especially industrial reactions. Commonly, to overcome these problems, enzyme immobilization has been considered [13, 14]. Although enzyme immobilization can enhance enzyme stability, however, the immobilized enzymes reveal very lower activity than the native enzymes due to the enzyme inactivation during the immobilization process [
15]. Hence to solve these difficulties, the design and development of low-cost nanozymes with high stability and quasi-enzymatic activity were considered an interesting way for performing enzyme-catalyzed reactions in harsh conditions [16-18]. Recently, nanozyme-based systems had been used for several applications in the field of catalysis [19, 20], biomedical imaging [
21], tumor therapy [22, 23], and sensing and detection [24-26].
Manganese dioxide (MnO2) has been utilized for developing different catalytic systems, for instance, chemical, electro-catalytical, and photocatalytic systems due to its higher catalytic activity than other transition metal oxides as well as its low cost, high stability, and nontoxicity [27, 28]. However, the MnO2 nanoparticles are known for their high enzyme-like activity with dual oxidase- and peroxidase-like activity which make them suitable nanozymes for sensing applications [29-33].
Considering the above-mentioned literature, MnO2 nanoparticles are considered nanozymes with intrinsic peroxidase-like activity and there are several reports on their application in the field of nanozyme-based sensing and detection, however, up to now, there is no report on the investigation of their biochemical and kinetics properties toward enzyme-mediated oxidations. Hence, in this work, the MnO2 nanozymes were synthesized and their biochemical properties including pH stability, thermal stability, and salt stability were evaluated toward n-electron irreversible oxidation of 3,3’-diaminobezedine. Besides, the kinetics performances of the as-prepared nanozymes toward DAB oxidation were also investigated.
2. Methods
2.1. Synthesis of MnO2 nanozymes
To synthesize MnO2 nanozymes, 150.0 mg KMnO4 was dissolved in 15.0 mL of deionized water. Then, 150.0 μL of 30% hydrogen peroxide and 75.0 μL of 80% hydrazinium hydroxide were introduced into the solution followed by 5 min stirring. After 2.0 min, the brown precipitate was collected and then washed five times with deionized water.
2.2. Nanozyme activity assay
A mixture of 40 µL hydrogen peroxide, 0.5 mL of DAB (final concentration of 2.8 mM), and 40 µL of MnO
2-nanozymes (final concentration of 0.015 mg mL
̶ 1 in the mixture) was added to 2.0 mL of 0.4 M acetate buffer (pH= 4.0). After 30 min, the UV-Vis spectra against a reagent blank were recorded at 460 nm. It should be noted that the specific activity of the as-mentioned nanozymes (nM s
-1) was calculated using the absorption coefficient of the oxidation product at 460 nm (ɛ=5500 M
-1 cm
-1). Afterward, the relative activity of the MnO
2 nanozymes was calculated using equation 1 [
34]:
2.3. pH and thermal effect
The effect of pH on the nanozyme activity was determined by probing their activity over a pH range of 2.0-9.0. Afterward, the relative activity was calculated for each pH using equation 1, and the plot of activity as a function of pH was used as an index for pH stability measurements. Besides, the thermal stability of the as-mentioned nanozymes was investigated by calculating their activity after incubation at different temperatures for 30.0 min.
2.4. Salt stability
The nanozyme stability against high salt concentrations as a serious problem of native enzymes was evaluated by recording their nanozymatic activity in reaction media with high salt concentration over 3-7 M. It is notable that NaCl was used as a model salt for this experiment.
2.5. Kinetics studies
The kinetics studies were performed by measuring the activity of the as-mentioned nanozymes as a function of DAB concentrations based on the Michaelis–Menten model. Afterward, the kinetic parameters, Vmax and Km were estimated by using the linear plot of Lineweaver–Burk.
3. Results and discussion
3.1. Characterization of MnO2 nanozymes
The size and morphological properties of MnO
2 nanoparticles were determined using DLS and scanning electron microscopy imaging methods, respectively. In this regard, the SEM image of the prepared MnO
2 nanozymes was recorded. The results shown in
Figure 1, revealed that the as-prepared MnO
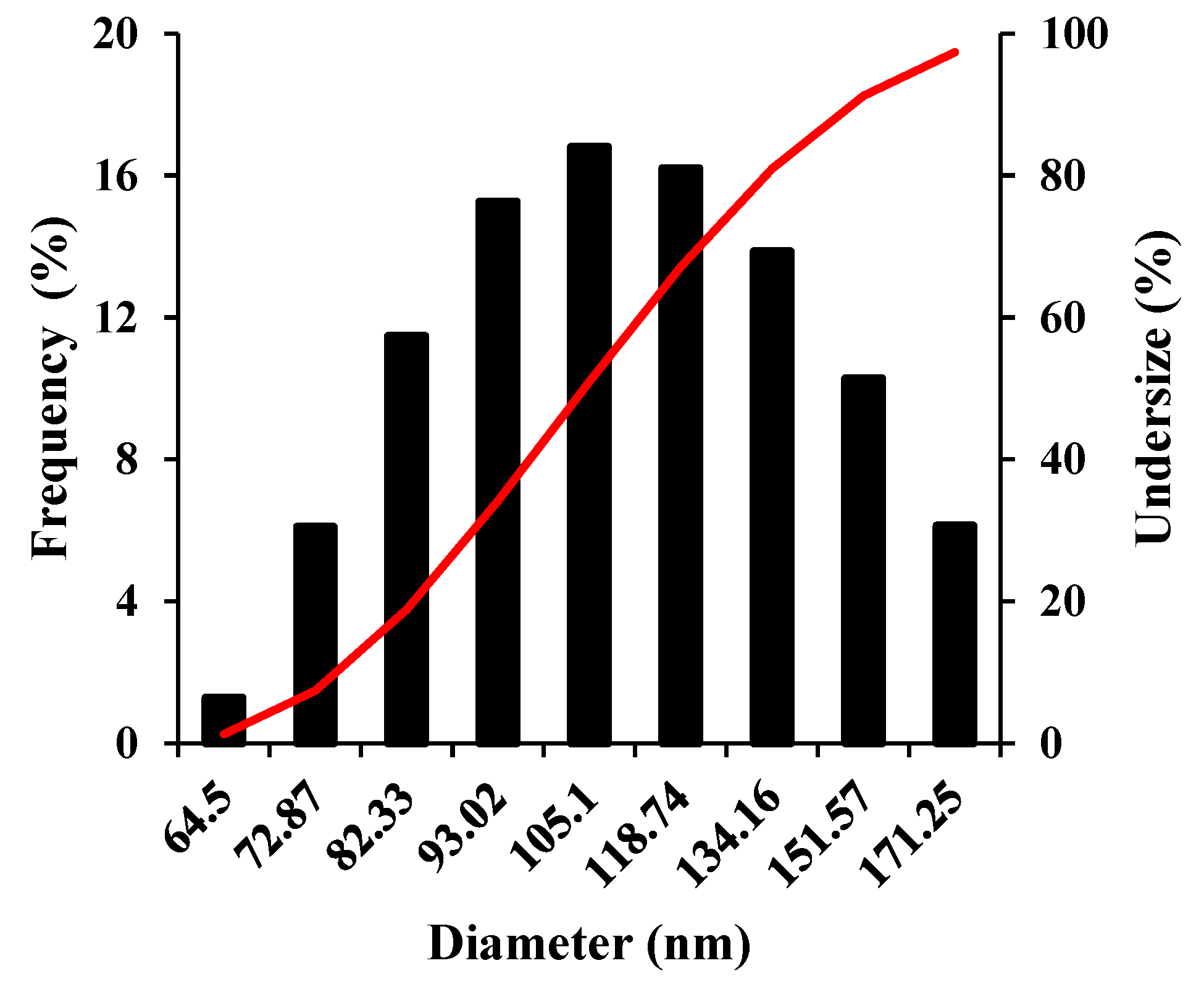
2 nanozymes have uniform and small size particles. However, the SEM image cannot provide any useful information on their size distribution. Hence, for the estimation of size distribution and calculation of the average size of these nanozymes, the DLS analysis was performed. The results shown in
Figure 2, revealed that the as-prepared nanozymes have a size distribution over 64-171 nm with an average size of 109 nm.
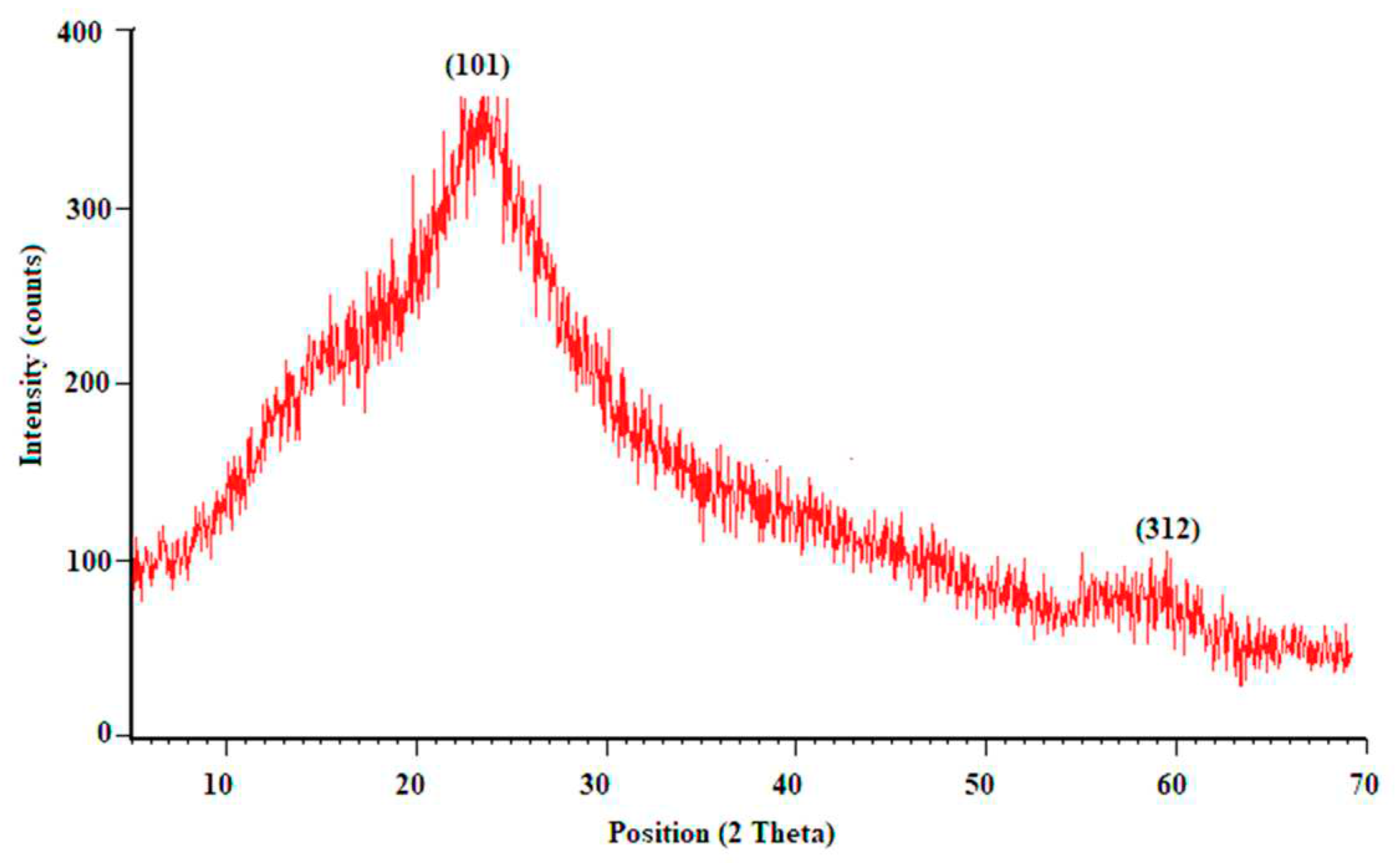
The crystalline properties of the synthesized MnO
2 nanozymes were investigated using XRD analysis. The results of this analysis are shown in
Figure 3. As seen in this figure, the results of X-ray diffraction analysis indicate the presence of two characteristic peaks of MnO2 at the diffraction angles of 23.66 and 60.11 which are assigned to (101) and (312) plans of MnO
2, in order.
3.2. Investigation of nanozymatic behavior
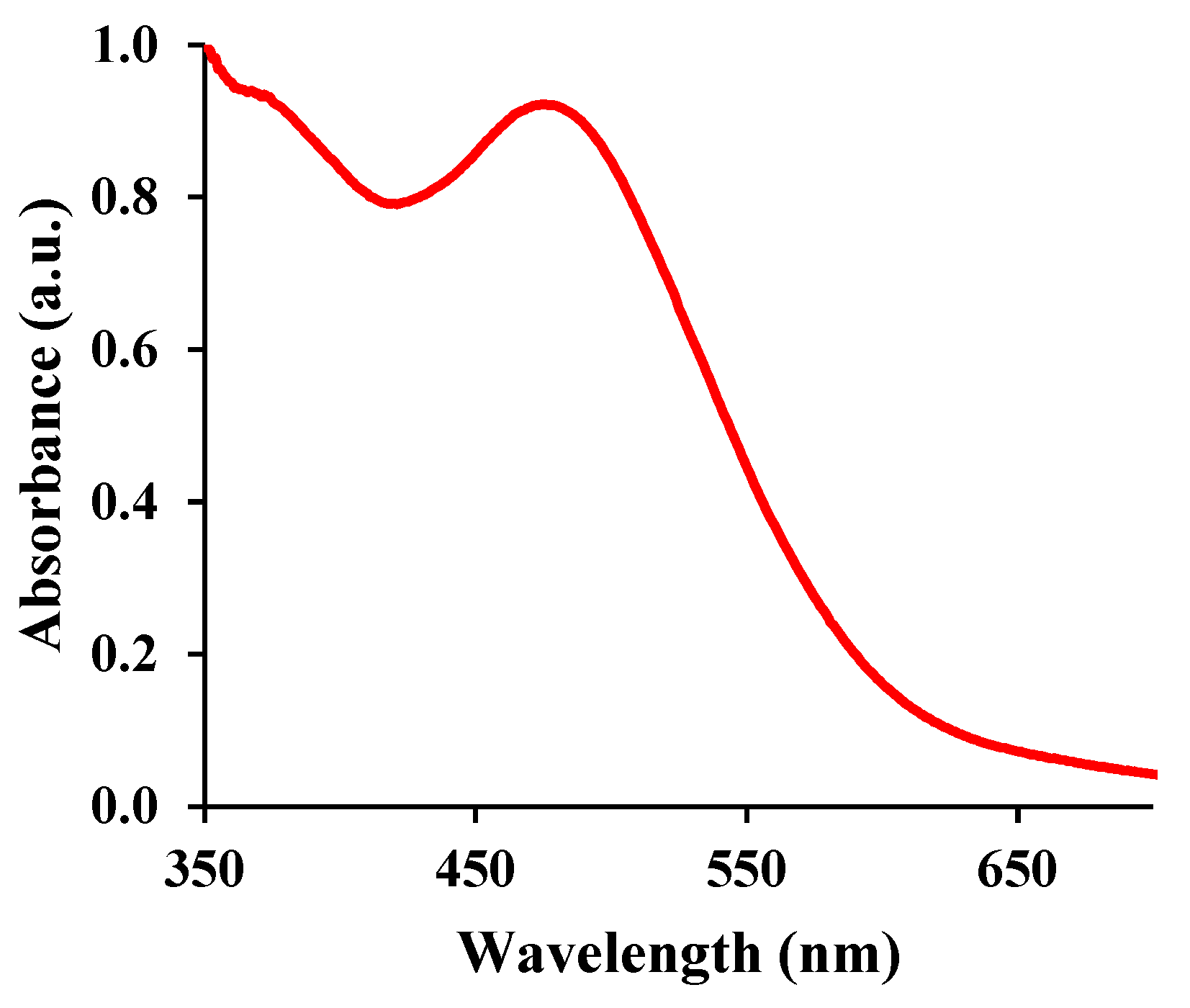
The pseudo-peroxidase activity of MnO
2 nanoparticles was investigated using DAB as a peroxidase substrate and its brown-colored oxidation product (i.e. polyDAB) as an analytical probe system (
Figure 4). As seen in
Figure 4, in the presence of DAB, the synthesized MnO
2 nanozymes catalyze the oxidation process of DAB with hydrogen peroxide to form its corresponding brown-colored indamine polymer (polyDAB) with a maximum absorbance at 460 nm. In fact, during the oxidation of DAB, MnO
2 nanozymes act on hydrogen peroxide molecules and produce active hydroxyl radicals [24, 26, 31, 33]. Then the generated radicals react with DAB molecules to produce the DAB cation (DAB
+). The DAB
+ then reacts with a DAB molecule to produce a DAB dimer ((DAB)
2). By proceeding with this cycle, finally, an indamine polymer was produced as the final product of DAB oxidation, as reported [24, 26].
3.3. pH stability
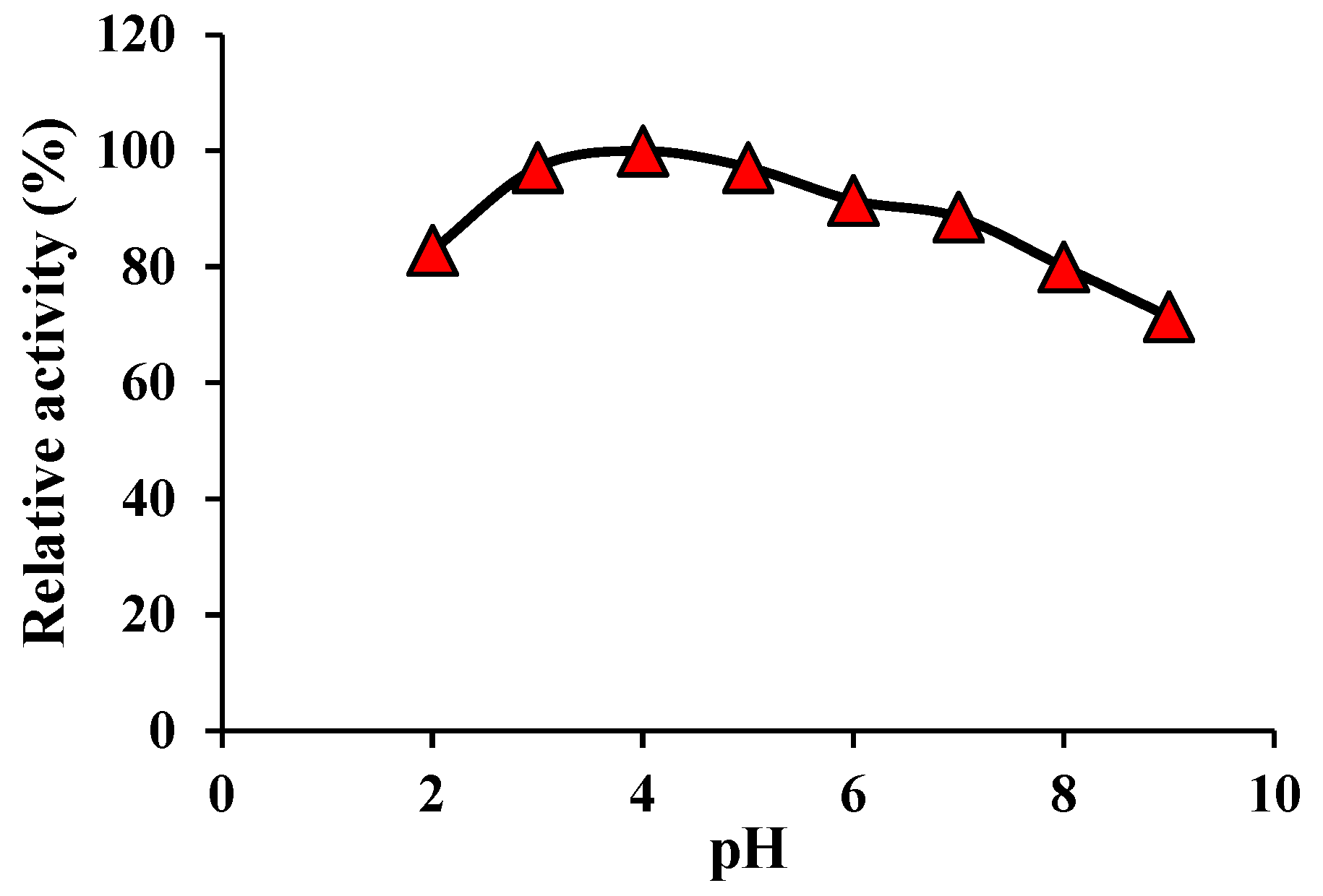
The effect of pH on the nanozyme activity was determined by probing their activity over a pH range of 2.0-9.0. In fact, this experiment can provide insights into the stability of these nanozymes against environmental pH changes. The results are shown in
Figure 5. According to these results, the maximum nanozyme activity of these nanozymes was estimated over a wide pH range of 3.0-6.0. It should be noted that at harsh acidic conditions (pH=2) and harsh basic conditions (pH=9.0), the as-mentioned nanozymes saved 82% and 71% of their maximal activity, in turn, pointing to their high pH stability.
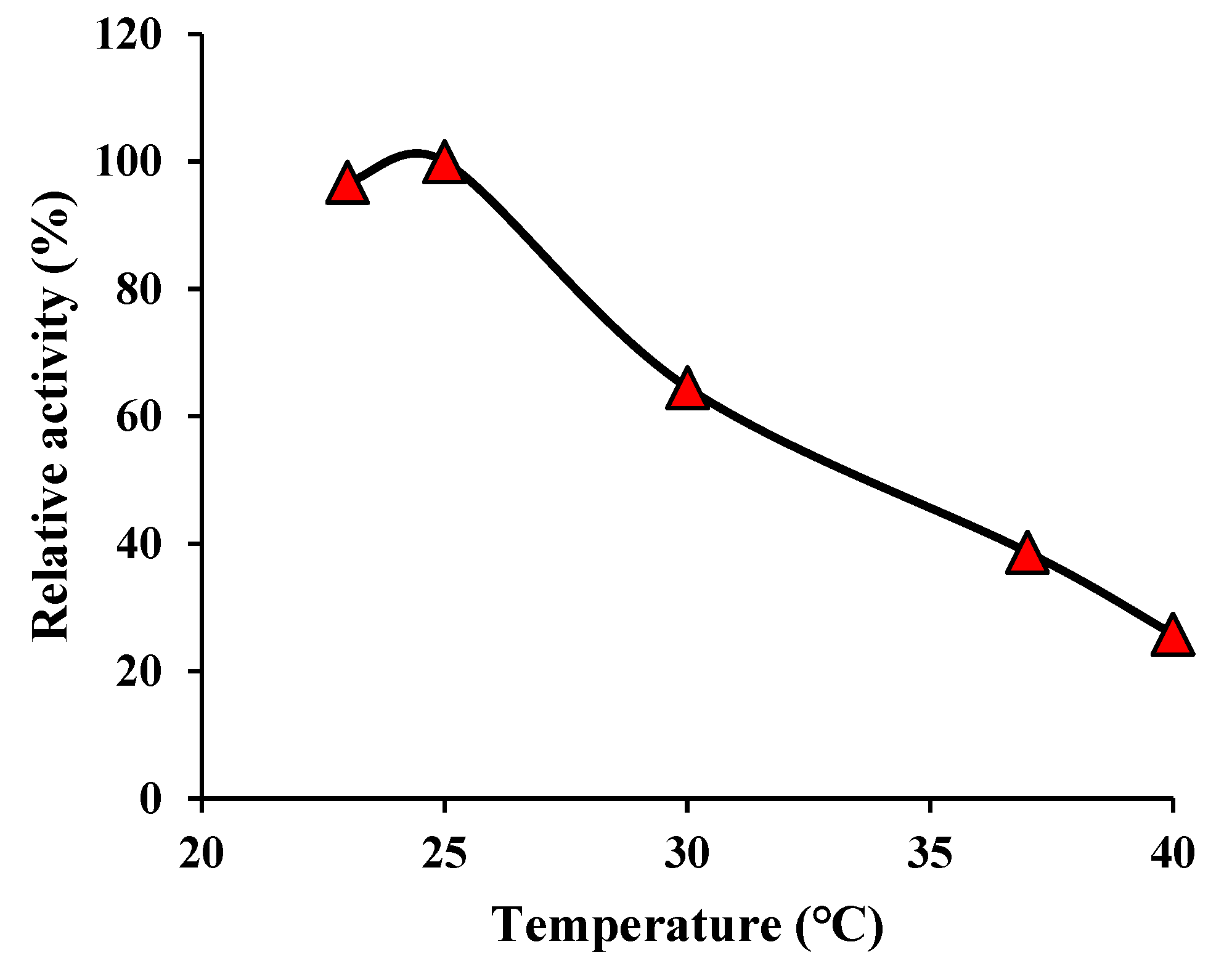
3.4. Thermal stability
The thermal stability of the as-mentioned nanozymes was evaluated by measuring the relative activity of nanozymes over the temperature range of 23-40 ℃. The results can obtain useful information about both the optimal temperature range of MnO
2 nanozymes and their stability against environmental temperature variations. The results are shown in
Figure 6, according to this figure, the maximum nanozyme activity was estimated at a temperature range of 23-25 ℃ and then it was decreased by increasing the temperature.
3.5. Salt stability
The nanozyme stability against high salt concentrations as a serious problem of native enzymes was evaluated by recording their nanozymatic activity in reaction media with high salt concentration over 3-7 M of NaCl. The results shown in
Figure 7, revealed that the as-mentioned nanozymes can save their maximal activity over a wide range of high salt concentrations over 3-7 M of NaCl. Based on the above results it can be concluded that the as-prepared MnO
2 nanozymes can be used for catalyzing the peroxidase-mediated oxidation reactions at high salt concentrations without any decrease in catalytic efficiency and nanozymatic activity instead of the unstable native peroxidase.
3.6. Kinetics studies
Kinetic studies were carried out to estimate the kinetic parameters (i.e., K
m and V
max) of the as-prepared MnO
2 nanozyme as pseudo-peroxidase nanoenzyme toward n-electron irreversible oxidation of 3,3’-diaminobezedine. It is well known that the V
max value reflects the intrinsic properties of the enzyme/nanozyme and is defined as the highest possible rate of the enzyme/nanozyme-catalyzed reaction (i.e., catalytic efficiency) when all enzyme molecules or all nanozyme particles are saturated with the substrate [34, 35]. The higher value of V
max is assigned to the higher catalytic efficiency of the enzyme/nanozyme. In contrast, the affinity of the substrate of an enzyme/nanozyme to interact with its active site is represented by the K
m value, the lower values indicate a higher affinity of the substrate for binding to the enzyme/nanozyme [34, 35]. The estimation of the kinetic parameters of MnO
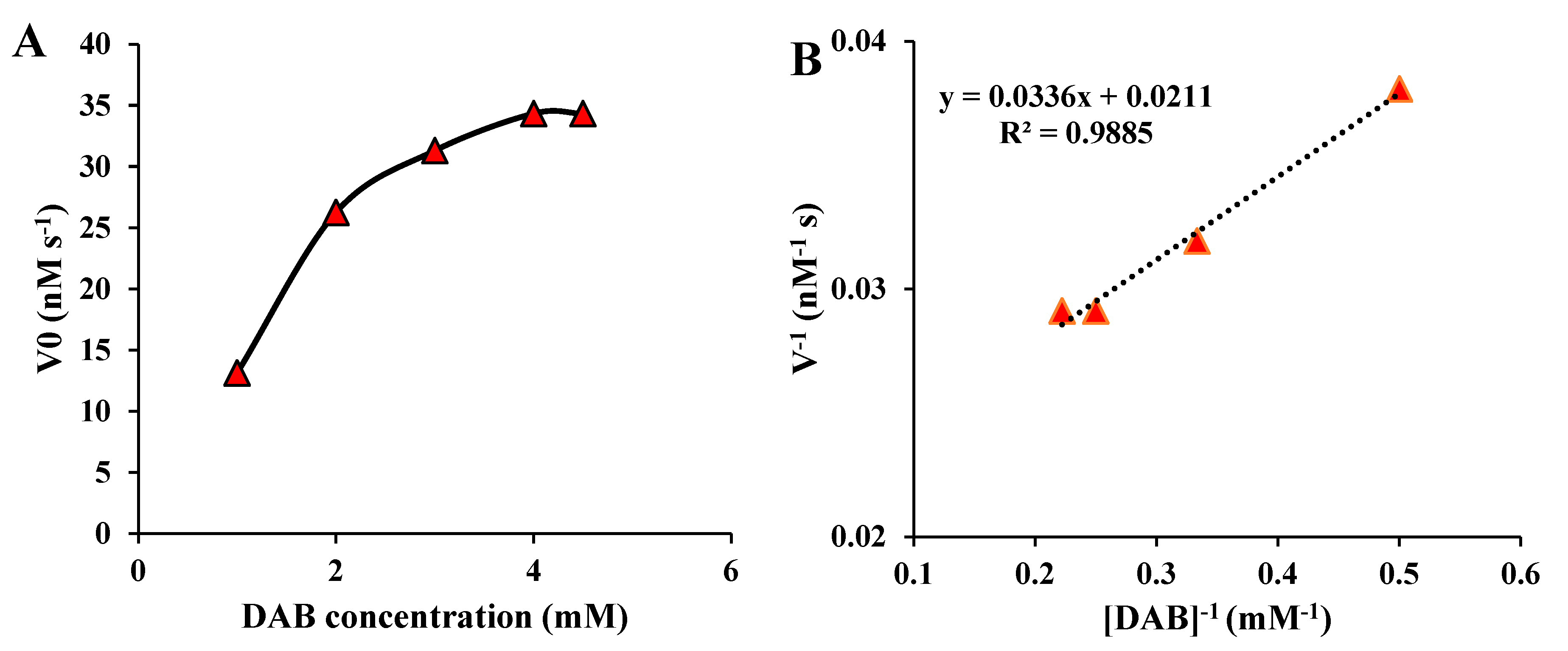
2 nanozymes was performed by measuring the initial velocity of the nanozyme-mediated reaction as a function of the DAB concentration. The Michaelis-Menten saturation curve and the Lineweaver-Burk linear plot for the as-mentioned nanozymes were shown in
Figure 8. As seen in
Figure 8A, the reaction rate was increased by increasing the DAB concentration and then reached a saturation state after a certain substrate concentration. Besides, Lineweaver-Burk linear plot (
Figure 8B) provided a K
m as low as 1.6 mM and a V
max as high as 47 nM sec
-1 for the MnO
2 nanozymes toward irreversible oxidation of DAB to produce brown-colored polyDAB.
4. Conclusions
It is well-known that MnO2 nanoparticles are nanozymes with intrinsic peroxidase-like activity. There are several reports on the application of these nanozymes for sensor development, however, up to now, there is no report on the investigation of their biochemical and kinetics properties toward enzyme-mediated oxidations. Hence, in this work, the MnO2 nanozymes were synthesized and their biochemical properties including pH stability, thermal stability, and salt stability were evaluated. The results showed that the as-prepared nanozymes reveal their maximal enzyme-like activity over a wide pH range of 3.0-6.0 at a temperature of 23-25 ℃ toward oxidation of 3,3’-diaminobezedine as the peroxidase substrate. Besides, the salt stability studies exhibited that the as-mentioned nanozymes can save their maximal activity over a wide range of high salt concentrations over 3-7 M of NaCl. Moreover, the kinetics studies revealed a Km as low as 1.6 mM and a Vmax as high as 47 nM sec-1 for the MnO2 nanozymes toward irreversible oxidation of DAB to produce brown-colored polyDAB.
Acknowledgments
The authors gratefully thank the Hormozi Laboratory of Chemistry and Biochemistry for the support of this work.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hormozi Jangi S. R.; Akhond M. (2020). High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. Journal of Chemical Sciences, 132, 1-8.
- Hormozi Jangi, S. R. (2023). Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@ Al-MCM nanocatalyst: a new approach toward reuse instead of common mineralization. Chemical Papers, 1-15.
- Hormozi Jangi, S. R., & Akhond, M. (2021). Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sensors and Actuators B: Chemical, 339, 129901. [CrossRef]
- He, L., Chang, C., Xue, Q., Zhong, X., Zhao, X., Liu, Y., ... & Ding, X. (2023). Bismuth nanoparticles decorated vertically arranged graphene as flexible electrodes for highly efficient detection of Pb (II) ions in water. Microchemical Journal, 108433. [CrossRef]
- Zheng, Z., Liang, W., Lin, R., Hu, Z., Wang, Y., Lu, H., ... & Pan, Y. (2023). Facile synthesis of zinc indium oxide nanofibers distributed with low content of silver for superior antibacterial activity. Small Structures, 4(4), 2200291. [CrossRef]
- Hormozi Jangi, S. R. (2023). Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. Chemical Research and Nanomaterials, 1(4), 1-9.
- Zhang, S., Ruan, H., Xin, Q., Mu, X., Wang, H., & Zhang, X. D. (2023). Modulation of the biocatalytic activity and selectivity of CeO 2 nanozymes via atomic doping engineering. Nanoscale, 15(9), 4408-4419. [CrossRef]
- Hormozi Jangi, S. R., & Dehghani, Z. (2023). Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. Chemical Research and Nanomaterials. https://crn.shiraz.iau.ir/article_701960.html?lang=en.
- Yang, D., Wang, L., Jia, T., Lian, T., Yang, K., Li, X., ... & Xue, C. (2023). Au/Fe 3 O 4-based nanozymes with peroxidase-like activity integrated in immunochromatographic strips for highly-sensitive biomarker detection. Analytical Methods, 15(5), 663-674. [CrossRef]
- Akhond, M., Hormozi Jangi, S. R., Barzegar, S., & Absalan, G. (2020). Introducing a nanozyme-based sensor for selective and sensitive detection of mercury (II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chemical Papers, 74, 1321-1330. [CrossRef]
- Cardoso, A. G., Ahmed, S. R., Keshavarz-Motamed, Z., Srinivasan, S., & Rajabzadeh, A. R. (2023). Recent advancements of nanomodified electrodes-towards Point-of-Care detection of cardiac biomarkers. Bioelectrochemistry, 108440. [CrossRef]
- Cao, L. (2005). Immobilised enzymes: science or art?. Current opinion in chemical biology, 9(2), 217-226. [CrossRef]
- Homaei, A. A., Sariri, R., Vianello, F., & Stevanato, R. (2013). Enzyme immobilization: an update. Journal of chemical biology, 6, 185-205. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2021). High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process Biochemistry, 105, 79-90. [CrossRef]
- Klibanov, A. M. (1979). Enzyme stabilization by immobilization. Analytical biochemistry, 93, 1-25. [CrossRef]
- Jangi, S. R. H. (2023). Determining kinetics parameters of bovine serum albumin-protected gold nanozymes toward different substrates. Qeios. [CrossRef]
- Hormozi Jangi, S. R. (2023). Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios. 10. [CrossRef]
- Hormozi Jangi, A. R., Hormozi Jangi, M. R., & Hormozi Jangi, S. R. (2020). Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chinese Journal of Chemical Engineering, 28(6), 1492-1503. [CrossRef]
- Bittencourt, G. A., de Souza Vandenberghe, L. P., Martínez-Burgos, W. J., Valladares-Diestra, K. K., de Mello, A. F. M., Maske, B. L., ... & Soccol, C. R. (2023). Emerging contaminants bioremediation by enzyme and nanozyme-based processes–a review. Iscience. [CrossRef]
- Ahmadi-Leilakouhi, B., Hormozi Jangi, S. R., & Khorshidi, A. (2023). Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chemical Papers, 77(2), 1033-1046. [CrossRef]
- Liu, C., Fan, W., Cheng, W. X., Gu, Y., Chen, Y., Zhou, W., ... & Luo, Q. Y. (2023). Red emissive carbon dot superoxide dismutase nanozyme for bioimaging and ameliorating acute lung injury. Advanced Functional Materials, 2213856. [CrossRef]
- Zeng, X., Ruan, Y., Chen, Q., Yan, S., & Huang, W. (2023). Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chemical Engineering Journal, 452, 138422. [CrossRef]
- Han, J., & Yoon, J. (2020). Supramolecular nanozyme-based cancer catalytic therapy. ACS Applied Bio Materials, 3(11), 7344-7351. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2020). Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchemical Journal, 158, 105328. [CrossRef]
- Yu, R., Wang, R., Wang, Z., Zhu, Q., & Dai, Z. (2021). Applications of DNA-nanozyme-based sensors. Analyst, 146(4), 1127-1141. [CrossRef]
- Hormozi Jangi, S. R., Davoudli, H. K., Delshad, Y., Hormozi Jangi, M. R., & Hormozi Jangi, A. R. H. (2020). A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surfaces and Interfaces, 21, 100771. [CrossRef]
- Xia, H. Y., Li, B. Y., Zhao, Y., Han, Y. H., Wang, S. B., Chen, A. Z., & Kankala, R. K. (2022). Nanoarchitectured manganese dioxide (MnO2)-based assemblies for biomedicine. Coordination Chemistry Reviews, 464, 214540. [CrossRef]
- Tang, Y., Zheng, S., Cao, S., Xue, H., & Pang, H. (2020). Advances in the application of manganese dioxide and its composites as electrocatalysts for the oxygen evolution reaction. Journal of Materials Chemistry A, 8(36), 18492-18514. [CrossRef]
- Wu, J., Yang, Q., Li, Q., Li, H., & Li, F. (2021). Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Analytical Chemistry, 93(8), 4084-4091. [CrossRef]
- Zou, N., Wei, X., Zong, Z., Li, X., Meng, F., & Wang, Z. (2021). Preparation of manganese dioxide nanozyme as catalyst for electrochemical sensing of hydrogen peroxide. Int. J. Electrochem. Sci, 16(210324), 10-20964.
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Analytica Chimica Acta, 1127, 1-8. [CrossRef]
- Yin, Z., Ji, Q., Wu, D., Li, Z., Fan, M., Zhang, H., ... & Zeng, L. (2021). H2O2-responsive gold nanoclusters@ mesoporous silica@ manganese dioxide nanozyme for “off/on” modulation and enhancement of magnetic resonance imaging and photodynamic therapy. ACS Applied Materials & Interfaces, 13(13), 14928-14937. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3, 3′-diaminobenzidine. Microchimica Acta, 187, 431. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Dehghani, Z. (2020). High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochemistry, 90, 102-112. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2022). Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochemistry, 120, 138-155. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).