1. Introduction
Degenerative aortic stenosis is the most common valvular heart disease in developed countries (1). It is associated with ageing and cardiovascular risk factors, confers high morbidity and mortality risk (2, 3), and yields severe physical impairment due to heavy symptom burden and exercise intolerance (4). On the one hand, exercise intolerance can be explained by changes in cardiovascular hemodynamics: altered hemodynamic is a hallmark of aortic stenosis, with reduced stroke volume and impaired pulse wave contributing to increased arterial stiffness, decreased wall shear stress and impaired endothelial function (5) — all of which have an impact the individuals’ response to exercise (6). On the other hand, physical limitation may be a direct result of severe aortic stenosis symptoms and frequent episodes of physical immobilisation (e.g., during hospital admissions) (7), which may lead to a vicious circle of increasing physical inactivity and decreasing exercise tolerance.
Transcatheter aortic valve implantation (TAVI) provides an effective and safe treatment option for severe aortic stenosis, especially in vulnerable patients with multiple co-morbidities (8). As compared to traditional surgical aortic valve replacement (sAVR), TAVI predominantly caters older populations with multiple risk factors and co-morbidities (9, 10). Hence, TAVI corrects the central hemodynamic derangement of aortic stenosis (i.e., replaces the dysfunctional valve causing left ventricular obstruction) and improves survival, but does not address other aspects of the aortic stenosis syndrome, such as patients’ frailty and deconditioning (11).
Exercise-based cardiac rehabilitation is a pivotal intervention for several cardiovascular diseases, and improves exercise capacity, vascular function and quality of life (12-15). Retrospective observations of patients after TAVI who were selected for, and agreed to participate in, cardiac rehabilitation suggest that participation in rehabilitation programs is associated with improved event-free survival (16). Prospective comparisons of TAVI and sAVR have also shown that cardiac rehabilitation improves walking distance (on the 6-minute walk test, 6MWT) and daily living activities (17, 18). Disappointingly, however, randomized trials of exercise-based cardiac rehabilitation in patients after TAVI are scarce. One seminal randomized pilot study has shown that 8 weeks of exercise training improved change in peak oxygen uptake (VO2peak), muscular strength and quality of life, when compared to usual care (19). The effects of exercise training on other indicators of cardiovascular health, such as vascular function, remained elusive.
In the present study, we sought to compare the effects of a supervised centre-based exercise training programme and an unsupervised home-based exercise routine on exercise capacity, vascular function, and quality of life in patients after TAVI.
2. Materials and Methods
2.1. Study population
We included consecutive patients after a successful TAVI implantation at the national TAVI referral centre, the University Medical Centre (UMC) Ljubljana, Slovenia. Indications for TAVI were endorsed by the local Heart Team according to current ESC guidelines for valvular heart disease (20).
Inclusion criteria were: TAVI implantation within 3 to 6 months, mobility (defined as more than 100 meters on 6MWT), ability to attend at 8-12 weeks of outpatient cardiac rehabilitation program and optimal medical treatment.
Exclusion criteria were: patients’ choice for TAVI albeit sAVR has been indicated by the local Heart Team, non-cardiac physical disability that would impede cardiac rehabilitation, TAVI access site complication that required surgical treatment, unstable cardiovascular disease (e.g. New York Heart Association (NYHA) class IV, decompensated heart failure) or recent (<3 months prior to inclusion) cardiovascular event, active malignancy, severe chronic obstructive pulmonary disease or poorly managed asthma, severe peripheral artery, musculoskeletal or central nervous disease that would impede bicycle exercise, and echocardiographic signs of bioprosthetic valve dysfunction (maximal velocity of more than 3 m/s, mean aortic gradient equal or more than 20 mmHg, aortic valve area under 1.2 cm2 or at least moderate paravalvular regurgitation (21).
2.2. Study design
This prospective, single-centre randomized controlled pilot study was conducted at the Center for Preventive Cardiology, Department of Vascular Diseases, UMC Ljubljana, Slovenia from June 2019 to July 2020. Patient enrolment was terminated prematurely due to the coronavirus-19 (COVID-19) pandemic outbreak. Participants were randomly allocated into 2 groups (1 interventional and 1 control group) in 1:1 ratio. Randomization was done by the recruiting investigator (who was not the recruiting/treating physician to ensure concealed allocation) using adaptive (urn) randomization with sealed envelopes. All measurements were performed twice: at baseline and after the study period (8-12 weeks).
2.3. Intervention
Supervised group underwent centre-based cardiac rehabilitation, which consisted of 8 to 12-week outpatient exercise training program with two visits per week (12-24 visits in total). Peak oxygen uptake (VO2peak) at baseline cardiopulmonary exercise test (CPET) was used as principal intensity parameter. Exercise sessions consisted of a 10-minute warm up, moderate endurance training (20 minutes of cycling at 40% VO2peak with gradual progression towards a target intensity 75% VO2peak and 40-minute duration), low-to-moderate intensity training resistance training (free weights and resistance bands), balance training, and a 10-minute cool down. Individual adjustments were permitted according to patient preferences and training progression under cardiovascular physical therapist supervision.
Unsupervised (control) group underwent thorough baseline cardiovascular assessment, including CPET, and a 30-minute informative session with a physical therapist. Regular exercise was recommended for at least 150 minutes per week (with timing and durations 20-45 minutes at patients’ discretion) with intensity based on CPET baseline results (target heart rate reserve 40-75% and the adequate rate of perceived exertion). Follow-up was carried out monthly to appraise progression and/or recommend adjustment.
Our primary endpoint was defined as change in VO2peak assessed with CPET after intervention or after 8 weeks of home-based exercise routine respectively.
2.4. Exercise capacity and muscle strength
Aerobic exercise capacity was assessed using CPET. CPET was performed on a cycle ergometer Schiller CS-200. An adjusted ramp protocol was used with 3-minute warm-up without workload, followed by gradual increase in workload by one tenth of maximal estimated workload per minute, calculated on basis of gender, age and height.
Patients were ECG monitored throughout the test and blood pressure measurement were taken every 2 minutes. During exercise, oxygen (O2) and carbon dioxide (CO2) flow was measured (VO2 and VCO2, respectively) using a mouthpiece connected to the device. We defined the ventilatory anaerobic threshold (AT) at the ration between VO2 and VCO2 of 1.0. Participants were encouraged to give their maximal effort before they stopped cycling.
The following data was obtained from the test: total time of exercise test, peak work load in Watts (W), % of expected peak work load (based on age, gender and BMI), VO2peak, VO2 at AT, time of AT, respiratory exchange ratio (RER) calculated as the ratio of peak CO2 exhalation and VO2peak, O2 pulse, ventilatory equivalents for CO2 (VE/VCO2), basal heart rate (HR), maximal HR, heart rate reduction (HRR) at 1 and 3 minutes after exercise termination and maximal systolic and diastolic blood pressure.
In addition, a 6MWT was performed using a standardised protocol with patients asked to walk between two marks on a 30-meter distance under medical personnel supervision for 6 minutes.
Muscular strength was assessed with a hand grip test using a hydraulic dynamometer SH5001 (Seahan Corporation, Korea). Patients were asked to sit and use their stronger hand abducted and flexed in the elbow to 90 degrees. The grip of the dynamometer was adjusted according to the participants hand size. 3 consecutive measurements were obtained and the highest was taken as final result for further analysis.
2.5. Vascular function
All vascular function measurements were assessed using an Aloka Prosound a7 ultrasound machine. Flow-mediated dilatation (FMD), a marker of endothelial function, was measured on the right brachial artery and performed according to standardized practice by the same experienced investigator. Patients were fasted and advised to abstained from coffee, smoking or exercise 3-hours prior to measurements. After visualizing the brachial artery approximately 1 to 2 cm above the antecubital fossa, 3 arterial diameter measurements were obtained (d
baseline). A forearm cuff was then inflated with the pressure of 50 mmHg above patient’s systolic blood pressure to maintain limb ischemia for 270 seconds. After 60 seconds of cuff deflation, 3 hyperaemic brachial artery diameter measurements were obtained (d
hyperaemia). Finally, FMD was calculated as the percentage change in diameter using the following formula:
Non-endothelial-dependent vasodilatation with nitro-glycerine was not assessed due to safety reasons in this frail population.
Carotid artery stiffness was measured on the right commune carotid artery through pulse-wave analysis using echo-tracking ultrasound. In case of carotid artery murmurs or prior surgical or percutaneous procedures, measurements were not performed. The following parameters were included in the analysis: beta (β) coefficient, pulse wave velocity (PWV) and augmentation index (AI).
Intra and interobserver variability were assessed on 20 healthy subjects. Intraclass correlation coefficient for FMD measurements was 0.95.
2.6. Self-reported Health Status
Self-reported health status was assessed using a validated 36-item Short Form Survey (SF-36) and EuroQol 5 dimensions, 5 levels (EQ-5D-5L) questionnaire. SF-36 was developed at the RAND Corporation as a part of the Medical Outcomes Study. It consists of 11 questions referring to mental and physical functions generating two combined parameters: mental component summary (MCS) and physical component summary (PCS). Calculations were made using the German normative (22) with higher scores indicating improved quality of life. EuroQol 5D (EQ-5D) is a preference-based measures questionnaire developed by the EuroQol Group (23). It has been formally translated and validated into Slovenian language (24, 25). It consists of a 5 dimensions descriptive system, each containing 5 levels, and a final visual analogue scale (VAS). Calculated values range from 0 to 1, with higher scores indicating better quality of life.
2.7. Laboratory biomarkers
All patients venous blood samples were taken from the cubital vein after 30 minutes of rest in supine position. Measurements include glomerular filtration rate and B-type natriuretic peptides as safety markers to detect possible deteriorations in renal and/or cardiac function.
2.8. Statistical analysis
Our primary end-point was change of functional capacity measured with VO2peak after cardiac rehabilitation program. Power calculations suggest 36 patients would be required to detect a 1 ml/kg/min change in VO2peak (a = 0.05, b = 0.2). Accounting for a dropout rate of 10% in this elderly population we decided to include 40 patients. Due to COVID-19 restrictions, inclusion was abruptly stopped after enrolling 39 patients.
Normality of data distribution was appraised visually and by the Shapiro Wilk test. Descriptive statistics are expressed as means (with standard deviations) for normally distributed continuous variables, medians (with interquartile range) for non-normally distributed continuous variables, and as numbers (with percentages) for categorical variables. Between-group comparisons were carried out with t-test for normally distributed continuous variables, Fishers’ Exact test for non-normally distributed continuous variables, or χ2 tests for proportions. Statistical significance was set at p < 0.05.
Between-group differences in change of outcomes (dependent variables) over the study period were estimated using linear mixed effects models (accounting for repeated measurements in each patient); patients were assigned as random effects, and time (pre vs. post), group (supervised vs. unsupervised exercise training) and time*group interaction were assigned as fixed effects. In the context of randomised trials, a significant time*group interaction suggests a significant effect of group allocation on outcome change over time (i.e., treatment effect). However, given the randomisation failure resulting in a significant between-group difference in baseline body mass index (BMI), secondary/sensitivity analyses were carried out using multivariate adjustment with BMI as a fixed covariate. Statistical analyses were performed with R version 4.2.1 (The R foundation for statistical computing, 2022).
3. Results
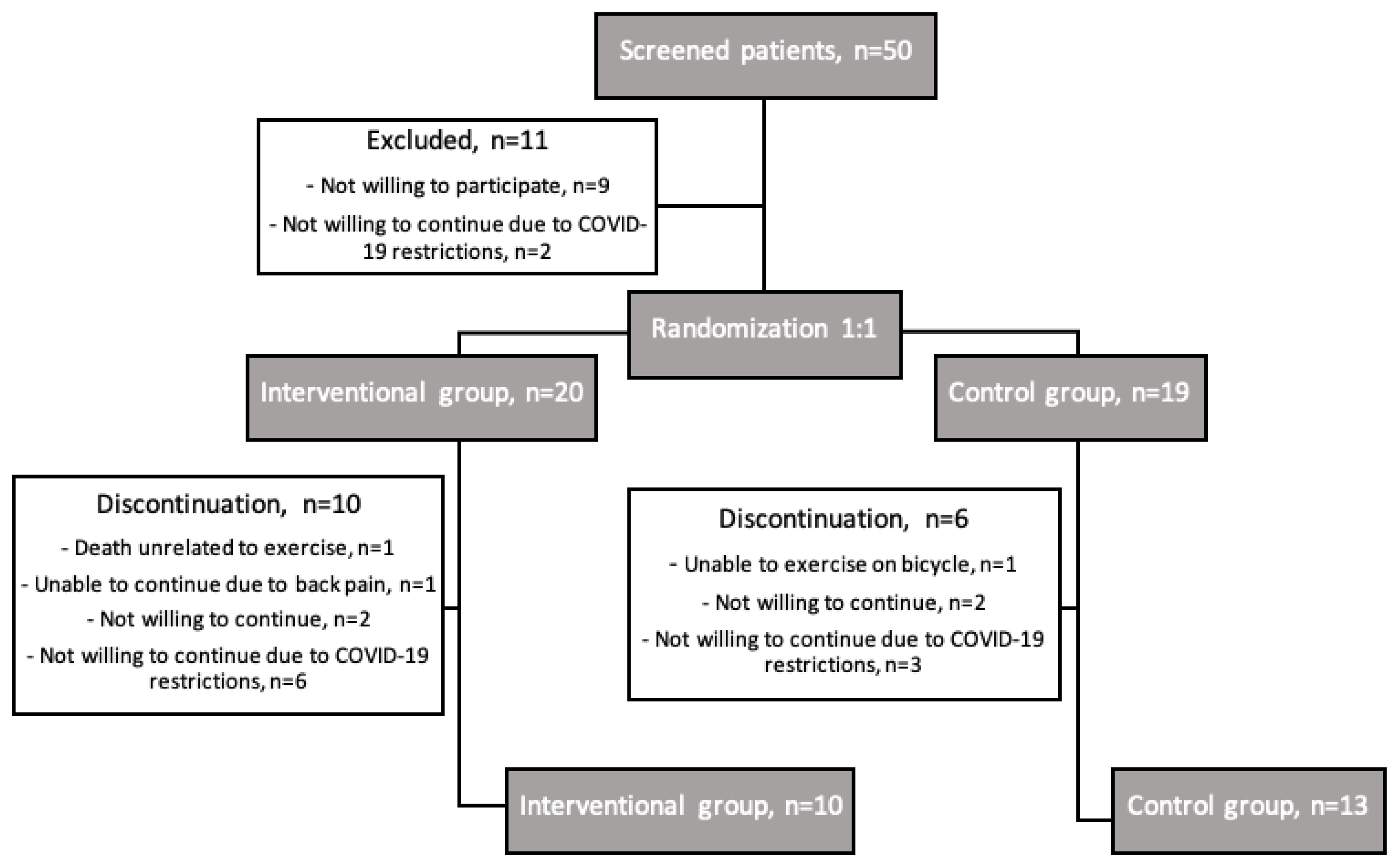
After screening 50 consecutive patient after TAVI, 39 were included in the study. Out of 20 patients randomized in the interventional group and 19 in the control group, 10 and 13 completed the study respectively (
Figure 1). We experienced a high drop-out rate of 41%, mostly related to the COVID-19 pandemic outbreak and restrictions.
Patients were, on average, 81 ± 1 years old, 14 (61%) were women, mean time from TAVI to inclusion was 110 ± 4 days. Baseline characteristics were not statistically significant between groups, except for BMI, which was significantly higher in the interventional group (p=0.043). See
Table 1.
Adherence to rehabilitation training was 100%, with 5 patients (50%) completing the program with 24 visits and 5 patients (50%) with 12 visits. All training sessions were safe, without reported side effects (e.g., dyspnoea, dizziness, palpitations, cardiac arrhythmias, chest pain). One death was reported in the interventional group during the study due septic shock, which was deemed unrelated to exercise training (as reviewed by three treating physicians and one investigator). Laboratory safety endpoints — renal function and natriuretic peptides — did not differ significantly between groups: -8.1 mL/min/1.73m2 (95% CI [-18.7; 2.6]; p=0.136) for estimated glomerular filtration rate (eGFR) and -0.14 ng/l (95% CI [-0.67; 0.39]; p=0.606) for log NT-proBNP levels, respectively.
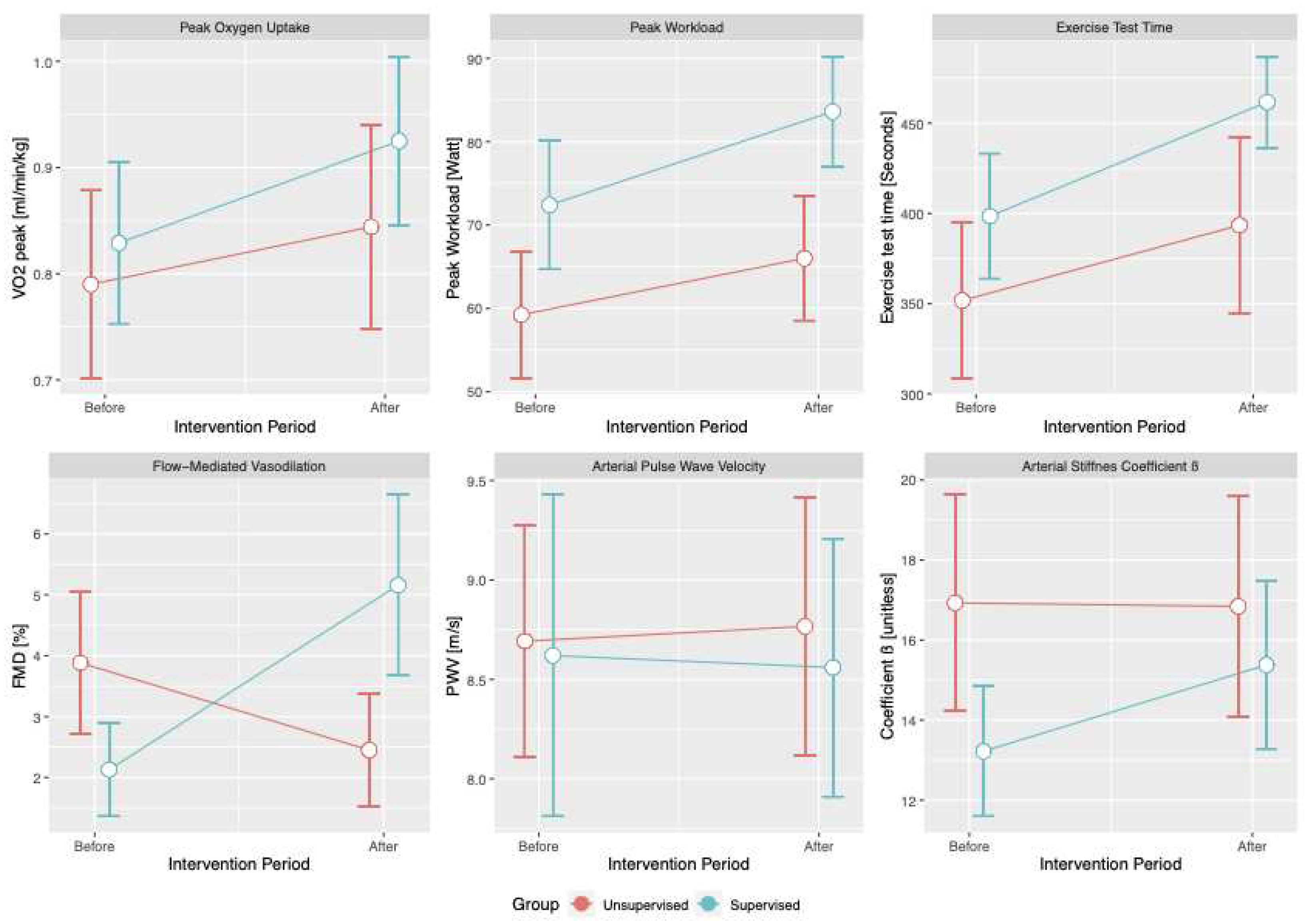
Both groups improved their exercise capacity and vascular function over the study period. Cumulative exercise time significantly increased by 47 seconds (95% CI [5.0;89.6]; p=0.029), exercise workload by 8.2 Watts (95% CI [0.6;15.8]; p=0.034), and VO2peak by 0.09 ml/min/kg (95% CI [0.01;0.16]; p=0.02) during the study period. A significant between-group difference in change over the study period was detected only for FMD (4.49%; 95% CI [2.35;6.63]; p < 0.001)); statistical significance was retained after adjusting for BMI (4.56%; 95% CI [2.44;6.71]; p < 0.001). See
Table 2 and
Figure 2.
Health status scores did not change significantly with respect to time or group. Study intervention was associated with a non-significant between-group difference in change of EQ-5D-5L TTO (trade time off) health values — namely, an increase of 0.15 (95% CI [-0.01;0.31]; p=0.066) for the intervention group; the association was statistically strengthened after BMI adjustment (95% CI [0.0002;0.31]; p=0.05).
4. Discussion
Our study has shown that patients after TAVI undergoing either supervised centre-based exercise training or unsupervised home-based exercise routines improve exercise capacity and vascular function. We detected a between-group difference only for change in vascular function over the study period, as determined by improved FMD in the supervised training group, and no discernible change in health-related quality of life parameters over time or with respect to intervention. While marred by a higher-than-expected drop-out rate due to the COVID-19 pandemic, our findings may add insight into physiologic and clinical adaptations in post-TAVI patients — namely, an increase in exercise capacity over time, irrespective of centre- or home-based exercise training, and a possible added effect of supervised cardiac rehabilitation on vascular function.
Exercise training improves exercise capacity, which may be one of the strongest predictors of cardiovascular prognosis (26). In our study, two approaches were employed: a supervised centre-based exercise training program, and an unsupervised exercise routine with detailed baseline appraisal and exercise recommendations with follow-up. Both approaches yielded significant improvements in exercise capacity parameters — namely, an 8.2 Watt increase in workload, a 0.09 ml/min/kg increase in VO2peak, and a 47 seconds increase in exercise testing time. However, our findings did not corroborate our initial hypothesis that supervised training would yield detectable differences in exercise capacity improvements. One randomized controlled trial has previously reported that only patients in the exercise group significantly improved their exercise capacity and muscular strength, but the comparator — usual care — did not employ a structured recommendation (19). Hence, our results may imply that exercise capacity in post-TAVI patients improves because a major limitation (i.e., severe aortic stenosis) is removed, thus allowing patients to improve physical activity and, in turn, exercise capacity. Additionally, the effect of home-base exercise routine should not be underestimated and could have had a strong impact on our results. A recent observational pilot study showed it improves physical health and well-being, suggesting a fair solution to overcome the barrier of transportation to the medical facility (27).
Conversely, we showed that supervised training improved vascular function (as determined by FMD) when compared to unsupervised exercise routines. FMD is a sensitive marker of endothelial function and a hallmark of vascular alterations in cardiovascular diseases (28); as such, it has been extensively addressed in exercise trials in diverse populations (29-33), and has been shown to improve upon exercise training (6). Several pathophysiological explanations have been proposed, such as exercise-induced improvements of endothelial function as a result of shear stress-derived adaptations and increased nitric oxide (NO) bioavailability (33, 34). Our study findings therefore add to the growing body of evidence that FMD and endothelial function play a central role in the favourable cardiovascular effects of exercise training in diverse cardiovascular diseases. Parameters of arterial stiffness, on the other hand, did not change significantly. Arterial stiffness — as opposed to FMD — reflects morphological rather than functional changes, and may be less sensitive to the effects of exercise, especially in populations with advanced impairments of vascular morphology, such as elderly individuals or patients with high burden of risk factors and/or co-morbidities (35).
Health status, as appraised with the SF-36 and EQ-5D-5L instruments, did not change significantly over time and/or with respect to intervention group. Our results, while suggesting stability of health status over time, should be interpreted with caution. Previous studies have shown that participation to a cardiac rehabilitation programme is associated with improved health-related quality of life and increased health status measures (19, 36, 37). In our study, however, the impact of the COVID-19 pandemic outbreak may have overshadowed any improvements brought about by the intervention, as several studies suggest that the pandemic has affected the quality of life in the general population (38-42). Also, several health status measures have shown estimates directions, which are in line with existing evidence, but could not be statistically corroborated. Time trade-off scores for quality of life, for instance, decreased by –0.08 points (p=0.167) over time, but increased by +0.15 points (p=0.061) if patients were randomized to supervised training. While the null hypothesis must be rejected in the present study, we urge further studies (not marred by unexpected shocks, such as the COVID-19 pandemic) to be undertaken and provide adequate answers with appropriate statistical certainty.
Our study has several limitations — first and foremost, the smaller-than-anticipated sample size due to the COVID-19 pandemic. The study was undertaken right before the outbreak of the COVID-19 pandemic, which reduced the accessibility of cardiac rehabilitation programmes and introduced safety challenges for patients at high risk for COVID-19 complications, such as those after TAVI. As a result, we experienced a lower-than-anticipated inclusion rate and a much higher-than-anticipated drop-out rate. This resulted in at least four methodological issues. Firstly, the study was underpowered to detect smaller magnitudes of difference in exercise capacity. Secondly, the likelihood of randomization failure increased (i.e., BMI was higher in the intervention group and needed statistical adjustment). Thirdly, reasons for drop-out were very specific (COVID-19 pandemic) and likely affected the characteristics of patients who continued with the study as opposed to those who chose to withdraw. Fourthly, the heavily promoted local public health guidance for outdoors exercise during the pandemic has likely increased the exercise routines in the unsupervised group. These limitations notwithstanding, we believe our results merit reporting, as they add to the growing body of evidence on management options for the increasing post-TAVI patient population.
5. Conclusions
Both supervised and unsupervised exercise training improve exercise capacity and vascular function in patients after TAVI. In our study, only improvements in vascular function were significantly larger with supervised—as compared to unsupervised—exercise training. Our findings suggest that exercise training — be it supervised centre-based or unsupervised home-based — improves indicators of cardiovascular health in patients after TAVI. Our study, however, should be regarded as a hypothesis-generating pilot study, as it was conducted during the COVID-19 pandemic and, as a consequence, included a smaller-than-intended and selected post-TAVI patient population.
Author Contributions
LV contributed to drafting the work, acquisition, analysis, and interpretation of the data for the work, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MB performed all TAVI implantations. BJ and MB contributed to drafting the work, revising it critically for important intellectual content, and provided approval for the final manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the National Ethics Committee (reference number: 0120-215//2019/4) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study is registered in ClinicalTrial.gov (NCT03966417).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be available on request to the corresponding author.
Acknowledgments
We would like to thank all the participants in the study. We specially thank all nurses, physical therapists and administrators from the Centre of Preventive Cardiology, Department of Vascular Diseases, UMC Ljubljana for generously helping us in this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. 2014, 56, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014, 371, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Pohle K, Maffert R, Ropers D, Moshage W, Stilianakis N, Daniel WG, et al. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation. 2001, 104, 1927–1932. [Google Scholar] [CrossRef]
- Rodriguez-Pascual C, Paredes-Galan E, Ferrero-Martinez AI, Baz-Alonso JA, Duran-Munoz D, Gonzalez-Babarro E, et al. The frailty syndrome and mortality among very old patients with symptomatic severe aortic stenosis under different treatments. Int J Cardiol. 2016, 224, 125–131. [Google Scholar] [CrossRef]
- Poggianti E, Venneri L, Chubuchny V, Jambrik Z, Baroncini LA, Picano E. Aortic valve sclerosis is associatedwith systemic endothelial dysfunction. Journal of the American College of Cardiology. 2003, 41, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sports Med. 2009, 39, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Wald DS, Williams S, Bangash F, Bestwick JP. Watchful Waiting in Aortic Stenosis: The Problem of Acute Decompensation. Am J Med. 2018, 131, 173–177. [Google Scholar] [CrossRef]
- De Backer O, Sondergaard L. Challenges When Expanding Transcatheter Aortic Valve Implantation to Younger Patients. Front Cardiovasc Med. 2018, 5, 45. [Google Scholar] [CrossRef]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012, 5, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Horn P, Stern D, Veulemans V, Heiss C, Zeus T, Merx MW, et al. Improved endothelial function and decreased levels of endothelium-derived microparticles after transcatheter aortic valve implantation. EuroIntervention. 2015, 10, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Anayo L, Rogers P, Long L, Dalby M, Taylor R. Exercise-based cardiac rehabilitation for patients following open surgical aortic valve replacement and transcatheter aortic valve implant: a systematic review and meta-analysis. Open Heart. 2019, 6, e000922. [Google Scholar]
- Bellmann B, Lin T, Greissinger K, Rottner L, Rillig A, Zimmerling S. The Beneficial Effects of Cardiac Rehabilitation. Cardiol Ther. 2020, 9, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Eichler S, Salzwedel A, Reibis R, Nothroff J, Harnath A, Schikora M, et al. Multicomponent cardiac rehabilitation in patients after transcatheter aortic valve implantation: Predictors of functional and psychocognitive recovery. Eur J Prev Cardiol. 2017, 24, 257–264. [Google Scholar] [CrossRef]
- Butter C, Gross J, Haase-Fielitz A, Sims H, Deutsch C, Bramlage P, et al. Impact of Rehabilitation on Outcomes after TAVI: A Preliminary Study. J Clin Med. 2018, 7. [Google Scholar]
- Ribeiro GS, Melo RD, Deresz LF, Dal Lago P, Pontes MR, Karsten M. Cardiac rehabilitation programme after transcatheter aortic valve implantation versus surgical aortic valve replacement: Systematic review and meta-analysis. Eur J Prev Cardiol. 2017, 24, 688–697. [Google Scholar] [CrossRef]
- Russo N, Compostella L, Tarantini G, Setzu T, Napodano M, Bottio T, et al. Cardiac rehabilitation after transcatheter versus surgical prosthetic valve implantation for aortic stenosis in the elderly. Eur J Prev Cardiol. 2014, 21, 1341–1348. [Google Scholar] [CrossRef]
- Pressler A, Christle JW, Lechner B, Grabs V, Haller B, Hettich I, et al. Exercise training improves exercise capacity and quality of life after transcatheter aortic valve implantation: A randomized pilot trial. Am Heart J. 2016, 182, 44–53. [Google Scholar] [CrossRef]
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011, 32, 205–217. [Google Scholar] [CrossRef]
- Ellert U, Kurth BM. [Methodological views on the SF-36 summary scores based on the adult German population]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004, 47, 1027–1032.
- EuroQol. (2022). EQ-5D, an instrument to describe and value health. https://euroqol.org/euroqol/ [accessed November 10, 2022].
- Rupel V, Ogorevc M. The EQ-5D health states value set for Slovenia. Slovenian Journal of Public Health. 2012, 51, 128–140. [Google Scholar]
- Prevolnik Rupel V, Ogorevc M. EQ-5D-5L Slovenian population norms. Health Qual Life Outcomes. 2020, 18, 333. [Google Scholar] [CrossRef] [PubMed]
- Novakovic M, Novak T, Vizintin Cuderman T, Krevel B, Tasic J, Rajkovic U, et al. Exercise capacity improvement after cardiac rehabilitation following myocardial infarction and its association with long-term cardiovascular events. Eur J Cardiovasc Nurs. 2022, 21, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bhattal GK, Park KE, Winchester DE. Home-Based Cardiac Rehabilitation (HBCR) In Post-TAVR Patients: A Prospective, Single-Center, Cohort, Pilot Study. Cardiol Ther. 2020, 9, 541–548. [Google Scholar] [CrossRef]
- Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Vasic D, Novakovic M, Bozic Mijovski M, Barbic Zagar B, Jug B. Short-Term Water- and Land-Based Exercise Training Comparably Improve Exercise Capacity and Vascular Function in Patients After a Recent Coronary Event: A Pilot Randomized Controlled Trial. Front Physiol. 2019, 10, 903. [Google Scholar] [CrossRef]
- Novakovic M, Prokselj K, Rajkovic U, Vizintin Cuderman T, Jansa Trontelj K, Fras Z, et al. Exercise training in adults with repaired tetralogy of Fallot: A randomized controlled pilot study of continuous versus interval training. Int J Cardiol. 2018, 255, 37–44. [Google Scholar] [CrossRef]
- Trsan J, Kosuta D, Rajkovic U, Fras Z, Jug B, Novakovic M. Vascular Function in Patients After Myocardial Infarction: The Importance of Physical Activity. Front Physiol. 2021, 12, 763043. [Google Scholar] [CrossRef]
- Montero D, Walther G, Benamo E, Perez-Martin A, Vinet A. Effects of exercise training on arterial function in type 2 diabetes mellitus: a systematic review and meta-analysis. Sports Med. 2013, 43, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Pearson MJ, Smart NA. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int J Cardiol. 2017, 231, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Green DJ, Smith KJ. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb Perspect Med. 2018, 8. [Google Scholar]
- Janić M, Lunder M, Šabovič M. Arterial Stiffness and Cardiovascular Therapy. BioMed Research International. 2014, 2014, 621437. [Google Scholar]
- Izawa K, Hirano Y, Yamada S, Oka K, Omiya K, Iijima S. Improvement in physiological outcomes and health-related quality of life following cardiac rehabilitation in patients with acute myocardial infarction. Circ J. 2004, 68, 315–320. [Google Scholar] [CrossRef]
- Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, et al. Impact of Exercise Rehabilitation on Exercise Capacity and Quality-of-Life in Heart Failure: Individual Participant Meta-Analysis. J Am Coll Cardiol. 2019, 73, 1430–1443. [Google Scholar] [CrossRef]
- Poudel AN, Zhu S, Cooper N, Roderick P, Alwan N, Tarrant C, et al. Impact of Covid-19 on health-related quality of life of patients: A structured review. PLoS One. 2021, 16, e0259164. [Google Scholar]
- Kulnik ST, Sareban M, Hoppchen I, Droese S, Egger A, Gutenberg J, et al. Outpatient Cardiac Rehabilitation Closure and Home-Based Exercise Training During the First COVID-19 Lockdown in Austria: A Mixed-Methods Study. Front Psychol. 2022, 13, 817912. [Google Scholar] [CrossRef]
- Campbell D, Davison J. The Impact of COVID-19 on the Health-Related Quality of Life of Individuals Living in Scottish Communities with High Infection Rates. European Journal of Environment and Public Health. 2022, 6. [Google Scholar]
- Ferreira LN, Pereira LN, da Fe Bras M, Ilchuk K. Quality of life under the COVID-19 quarantine. Qual Life Res. 2021, 30, 1389–1405. [Google Scholar] [CrossRef]
- Ping W, Zheng J, Niu X, Guo C, Zhang J, Yang H, et al. Evaluation of health-related quality of life using EQ-5D in China during the COVID-19 pandemic. PLoS One. 2020, 15, e0234850. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).