3. Results
The reactive annealing is performed in compliance with both suggested reaction path and structural features in [
12]. In conformity of the proposed reaction sequence the synthesis and structure formation of CZTS from binaries Cu
2S and SnS
2 trough reaction of Cu
2SnS
3 with ZnS completes at temperatures higher than 600 °C. In this sense the temperature profile configuration is directed onto modification of the part where the direct formation of the quaternary CZTS takes place.
Detailed analysis of results for features of rapid thermal annealing consists of scrupulous phase analysis and assessment of the influence of parameters of annealing process on the crystal cell parameters of the target phase – Kesterite (Cu
2ZnSnS
4). In contrast to Stannite where [
13,
14] fine difference between some close disposed reflexes of Cu
2ZnSnSe
4 and either ZnSe or other binaries could become apparent trough more precise angle-resolved analysis, the case for Kesterite is rather more complicated [
15] and additional method as Raman shift is essential for a correct evaluation of phase distribution.
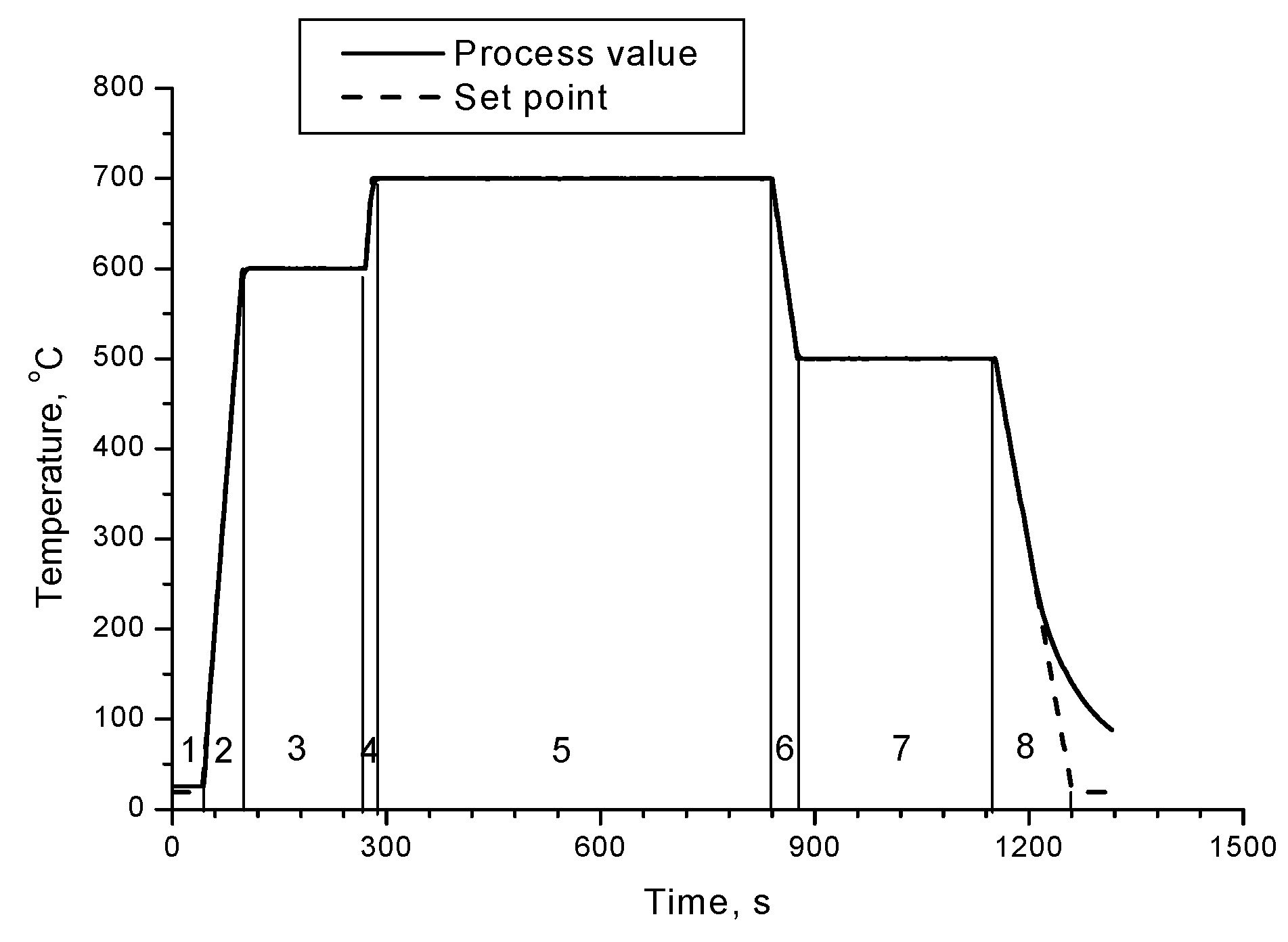
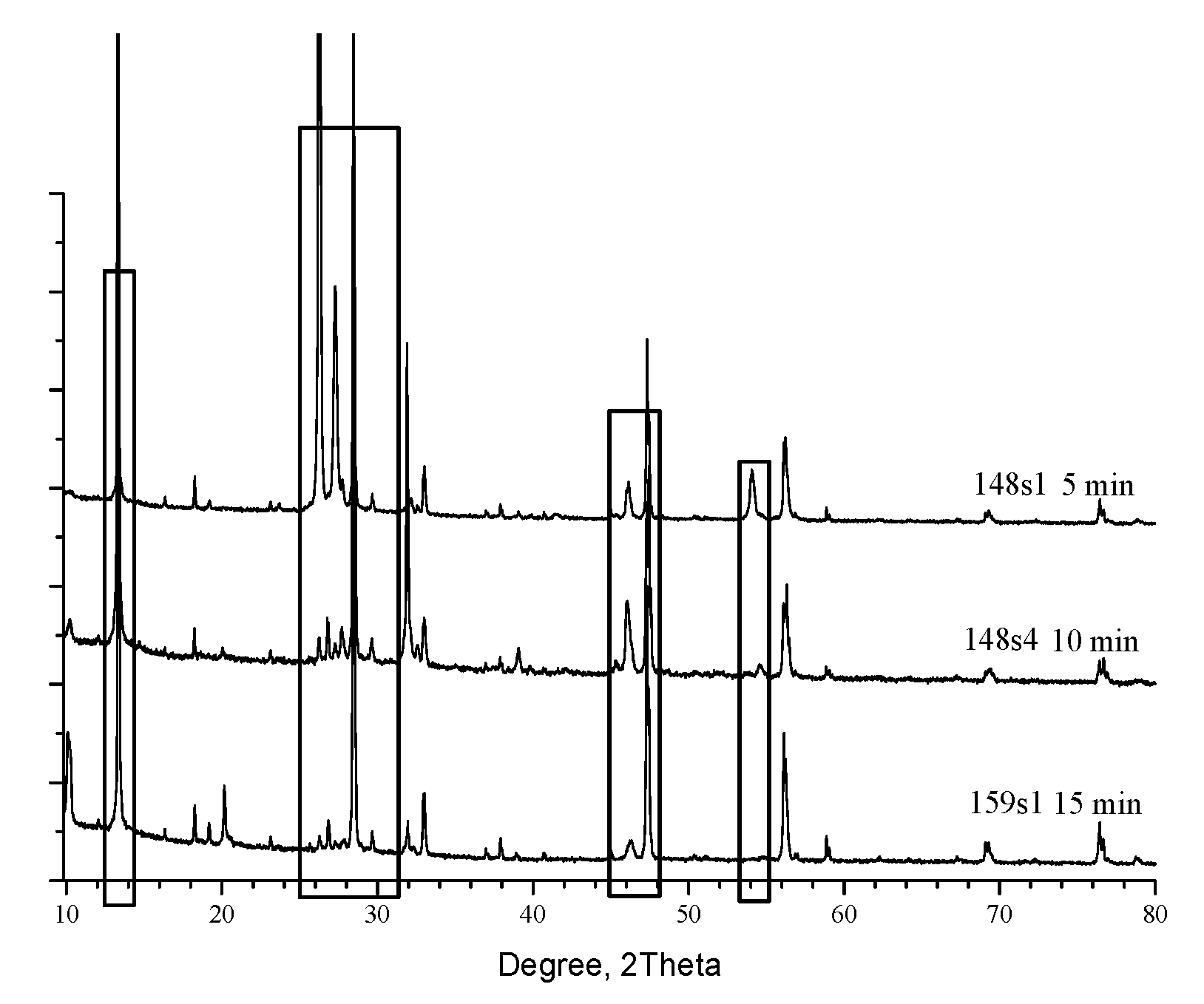
On the
Figure 2 are presented XRD patterns of samples 1, 2 and 3 annealed at different durations (15 min, 10 min and 5 min respectively) of the Segment 5 at 700 °C – according to
Figure 1.
The patterns are dominated of characteristic reflexes for the Kesterite phase [1-01-075-4122] at 28.4° (1,1,2), 47.3° (2,2,0) and 56.1° (3,1,2) and its minorities at 16.32°, 18.23°, 23.13°, 29.6°, 32.9°, 36.9°, 37.9°, 40.7°, 44.9°, 56.9°, 58.8°, 67.3°, 69.1°, 76.4° and 78.8° in 2Θ scale. Additional appreciable reflexes are detected near 10.2°, 13.3°, 26.2° 54° and 66.1° and confirm that the system is definitely not monophase. The formal phase analysis performed through [
11] and presented in Table.2 have specified for Sample 1-Kesterite, ɑ-SnS [1:01-083-1758]; Cu
7S
4 – [1:00-024-0061] and CuS – [1:03-065-3928].
Table 2.
Phase composition according to XRD [
11] and Raman shift analyses.
Table 2.
Phase composition according to XRD [
11] and Raman shift analyses.
| Sample |
process |
Cu2ZnSnS4
Kesterite-4122
|
Zn
h
|
Sn
|
Cu
Covellite
|
Cu7S4
Roxbyite
|
Cu2
digenite
|
Zn
h
|
Zn
h
|
ZnS(W)
0688h
|
| 1-159s1 |
Base
15min, 700 °C |
●+ |
●2201+ |
◊ |
◊ |
◊ |
◊- |
●2424
|
●2195
|
◊ |
| 2-148s4 |
Time-10 min |
●+ |
●2347
|
●+ |
● |
● |
◊- |
◊ |
◊ |
● |
| 3-148s1 |
Time – 5 min |
●+ |
◊ |
●+ |
● |
● |
◊- |
◊ |
◊ |
◊ |
| 4-158s1 |
Speed-fast |
●+ |
●4998+ |
● |
◊ |
◊ |
ױ |
◊ |
◊ |
◊ |
| 5-150s1 |
Speed-slow |
●+ |
●6022+ |
◊ |
◊ |
◊ |
◊ |
◊ |
◊ |
◊ |
| 8-149s1 |
Temp-750oC |
●+ |
●2140
|
◊- |
◊ |
◊ |
●9133/-
|
◊ |
◊ |
◊ |
| 7-148s3 |
Temp-650oC |
●+ |
●4989
|
●+ |
◊ |
◊ |
ױ |
◊ |
◊ |
◊ |
| 6-162s1 |
Temp-600oC |
●+ |
●6009
|
●+ |
◊ |
◊ |
◊ |
◊ |
◊ |
◊ |
| 1-159s1 |
Base-700oC |
●+ |
●2201+ |
◊ |
◊ |
◊ |
ױ |
●2424
|
●2195
|
◊ |
For Sample 2 (10 min) are recorded the same phases but SnS is in other structure configuration [1:01-073-1859] in addition with ZnS [1:01-089-2347&00-012-0688]. Sample 3 (15min) is common for all other RTP configurations and consisted only of Kesterite and Hexagonal ZnS [1:01-089-2201, 01-089-2424& 01-089-2195].
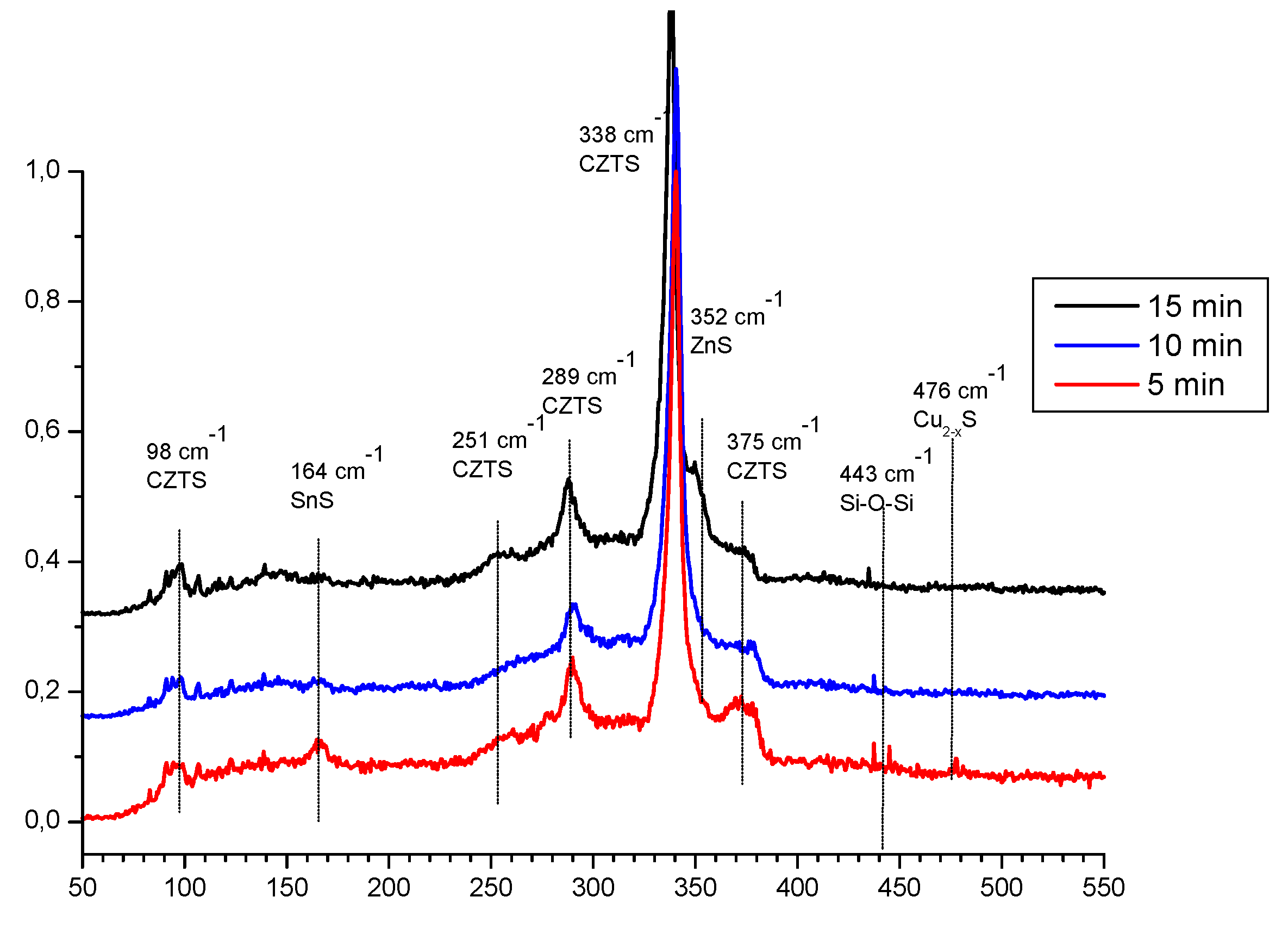
On the next
Figure 3 are presented Raman spectra of the same films. In accordance of [
16,
17,
18] could be concluded that the spectra are dominated again of signals for Kesterite at 338, 287, 252, 374 and 97 cm
-1. After [
19,
20] disposition of the shoulder near 350 cm
-1 in conjunction with signals at 98 cm-1 and possibly near 160 cm-1 could give an idea for the distribution of ZnS. On the same way the shift at 164 cm
-1 and shoulder near 311cm
-1 could be recognized as confirmation for presence of SnS [
21,
22,
23]. Vibration at 476 cm
-1 is characteristic for the bong Cu-S in nonstoichiometric unsaturated copper sulphides [
20]. This comparative analysis confirms as possible suggested phase distribution but full identification passes through of more detailed view on the XRD patterns.
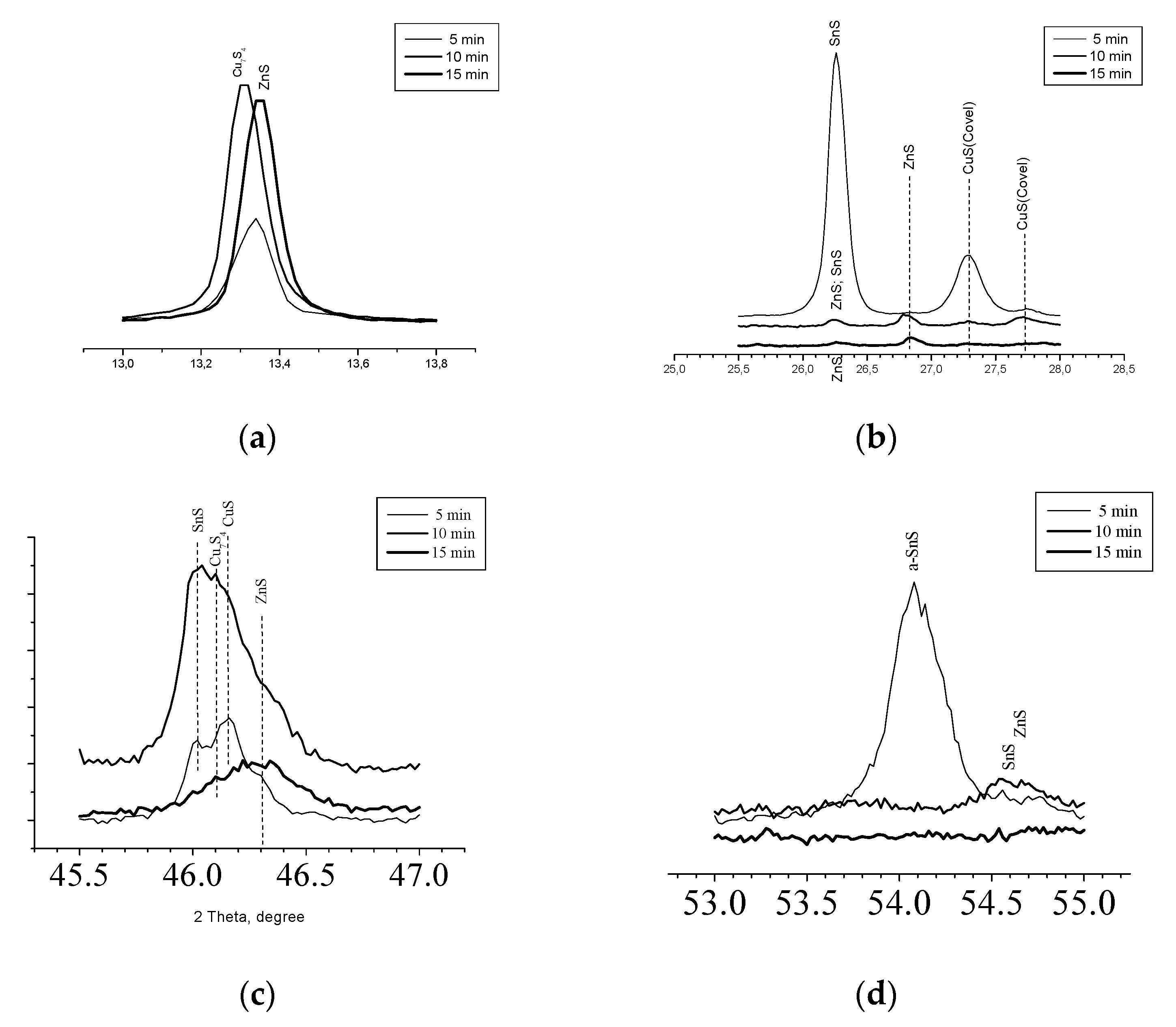
As was mentioned before the main phases in concomitant with the Kesterite dispose reflexes quite close to those for main phase but more precise analysis could reveal the fine structure of the films. At
Figure 4 is presented part of pattern of
Figure 2 in the vicinity of 13.0 – 14.0 ° 2Θ. The picture presents fine disposition of reflexes which allowed to distinguish the one for ZnS at 13.35° (Sample 3) from the other at 13.28° (Sample 2) belonging to Cu
7S
4.
On
Figure 4(b) is presented pattern from
Figure 2 at the interval of 25.5° - 28.0° 2Θ. In accordance of performed phase recognition the reflex at 26.24° for Sample 1 should be assumed of coming from SnS only, for Sample 3 the small reflex at the same angle should be assumed of ZnS only but for Sample 2 the reflex should be consistent of ZnS and SnS.
At 26.82° are disposed reflexes only for Samples 2 and 3, recognized as from ZnS whereas at 27.28° and 27.75° are seen single signals from CuS for Samples 1 and 2. Further at
Figure 4(c) are disposed pattern between angles 45.5°– 47.0°. Here the large signal from Sample 3 is due to ZnS. For Samples 1 and 2 the reflex at 46.02° is proving for SnS whereas those at 46.11° and 46.18° are caused of Cu
7S
4 and CuS respectively.
Figure 4(d) shows in details the field near 54°. The reflex at 54.05° for Sample 1 comes from ɑ-SnS [1:01-083-1758] whereas from Sample 2 the reflex at 54.5° is due to SnS [1:01-073-1859] accompanied with Wurtzite [1:00-012-0688].
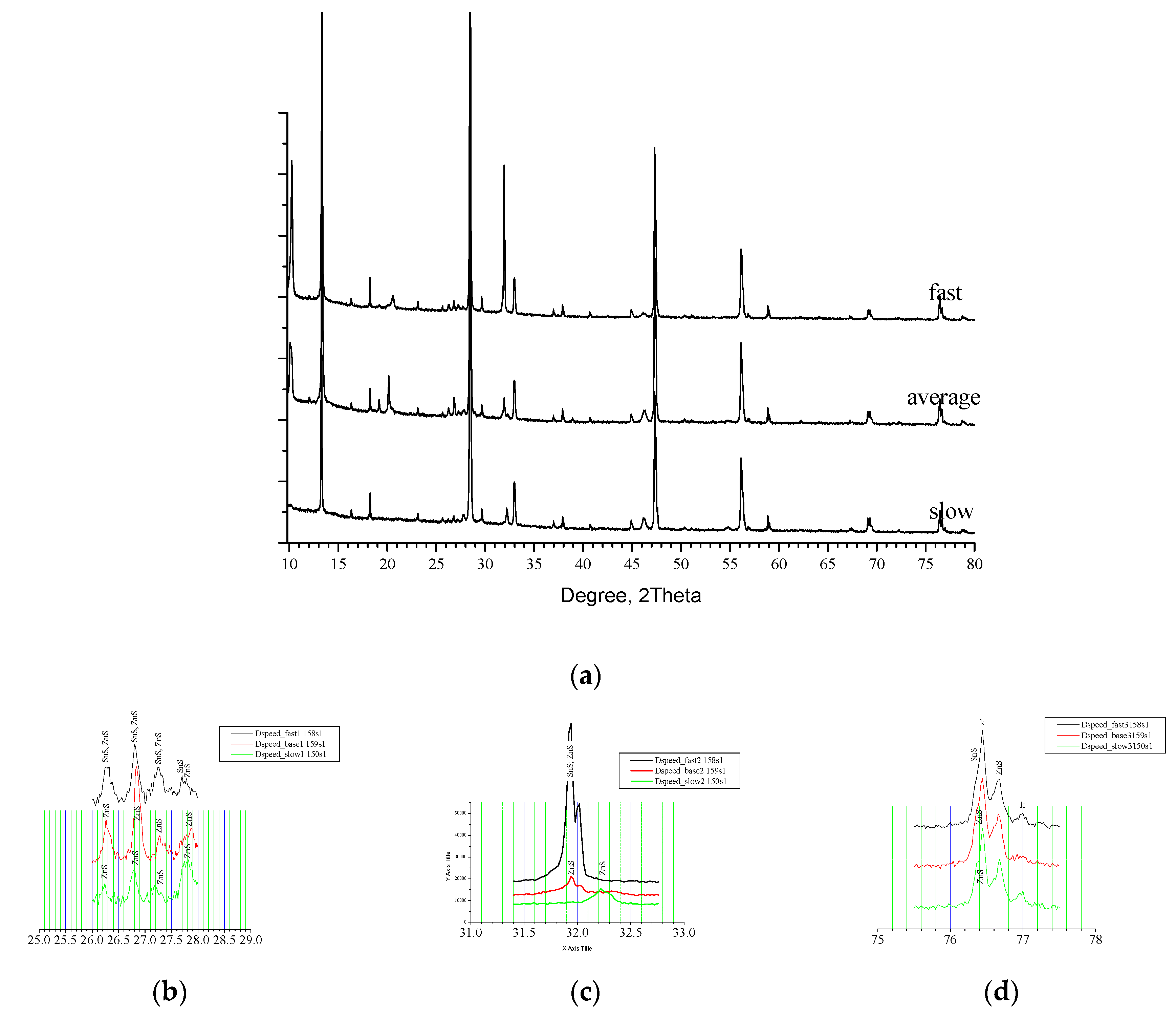
On
Figure 5(a) are shown XRD patterns for Sample 4 (fast) and Sample 5(slow) that on comparison with Sample 1(base) present RTP configuration with different speeds of rising and decreasing of temperature for the dynamic Segments No2, No4, No6 and No8 (see
Figure 1).
According to [
21] phase distribution of the samples is summarised at
Table 2. Sample 4, annealed at higher speed of rising of temperature contains mainly of Kesterite but pair of SnS phases – orthorhombic [1:01-072-8499] and [1:00-001-0984] is registered as well as a Hexagonal ZnS [1:01-074-4998]. For Sample 5, annealed at slow rate are found Kesterite and Hexagonal ZnS [1:01-075-6022]. As was mentioned before the average rate Sample 1 contained Kesterite and Hexagonal zinc sulphides. Comparative phase analysis has shown the Kesterite and zinc sulphides as common structures in the three samples whereas Sample 4 contained in addition two forms of Herzenbergite [1:01-072-8499& 00-001-0984]. They have similar layered orthorhombic structure and differ in orientation. Obviously both of them are result of non-equilibrium interaction in the chain of formation of CZTS caused by the high speed of change of the temperature. Apparently, in more soft conditions - as longer reaction time (previous case) or slower dynamic parameters of the process, according with [
22] this intermediate interact further on related scheme. On
Figure 5(b) are shown XRD pattern of the samples in vicinity of 26 ° up to 28 ° 2Θ. Reflexes near 26.2 ° are assumed for all samples to be from ZnS but for Sample 4 it appears to be in convolution with signal from SnS. On the same way could be spell out the reflexes at 26.8 ° and at 27.3 °. Near 27.75 ° the disposition is quite similar but the complex reflex for Sample 4 looks well divided for both SnS and ZnS. On the next
Figure 5(c) is presented interval 31.5 ° - 32.5 °. Sample 5 disposes here an individual reflex at 32.23 ° whereas the other Samples 1 and 4 present similar twin reflex at 31.94 ° and 32.02 °. [
21] spelt out the twin for Sample 1 as came from ZnS only while for Sample 4 assumed pair of ZnS and SnS. This could be explained with obvious difference in the height (intensity) of the reflexes. Between 76 ° and 77 ° on
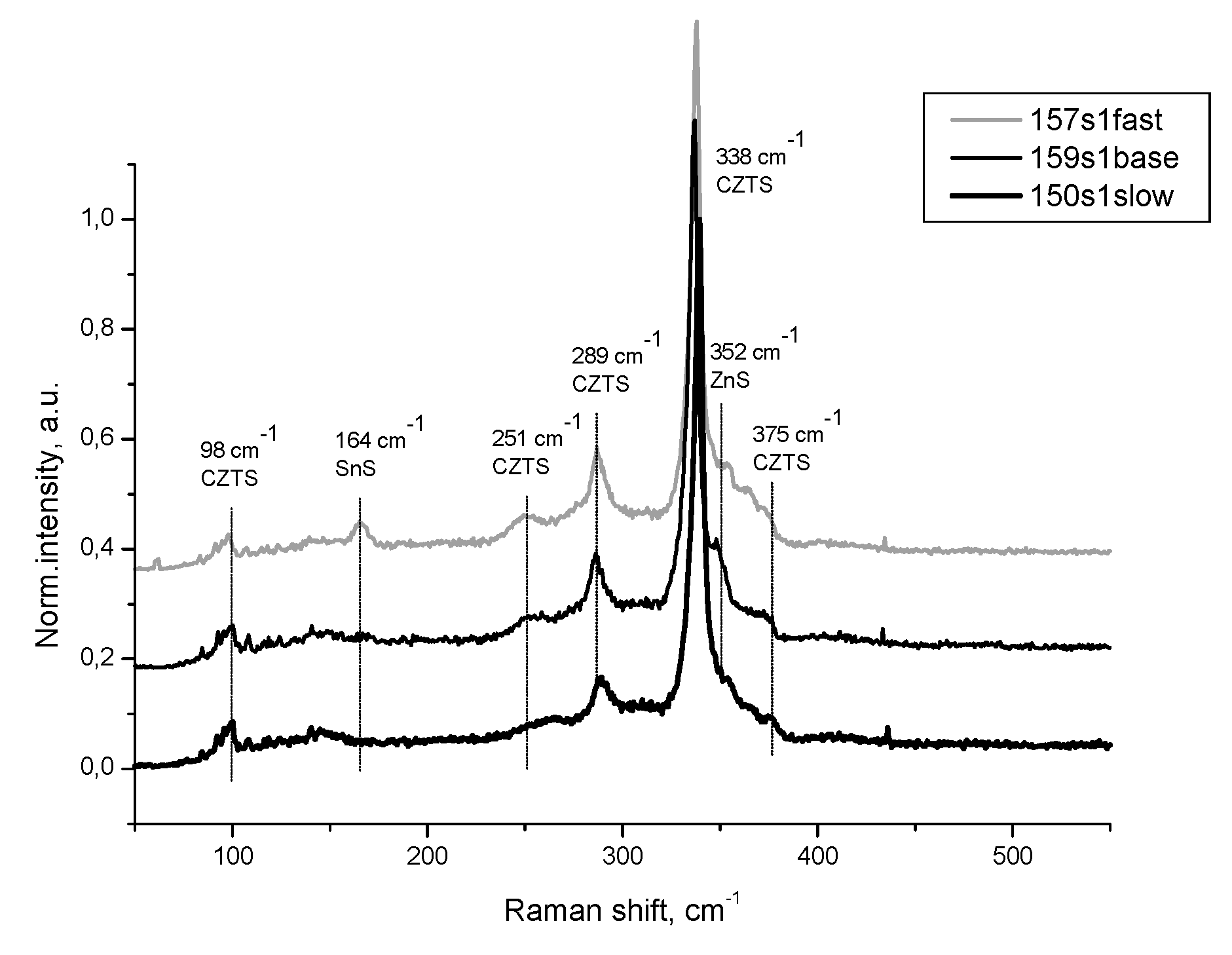
Figure 5(d) are disposed reflexes at 74.4 ° and 77 ° assumed of Kesterite. ZnS is presented here with reflex at 76.68 ° and a shoulder at the first Kesterite reflex. For Sample 4 is distinguished here a twin shoulder caused of both ZnS and SnS. Raman spectra presented at
Figure 6 are in good agreement with the supposed phase composition of the Samples 4,5 and 1. In all samples the Kesterite is well defined with the majority of its characteristic vibrations at 338 cm
-1, 289 cm
-1, 251 cm
-1, 98 cm
-1 and 375 cm
-1. For all of samples is seen shoulder at 352 cm
-1, near the most intensive signal for Kesterite attributed to ZnS. Only for Sample 4 is registered resonant vibration at 164 cm
-1, attributed to SnS in confirmation with established XRD phase distribution.
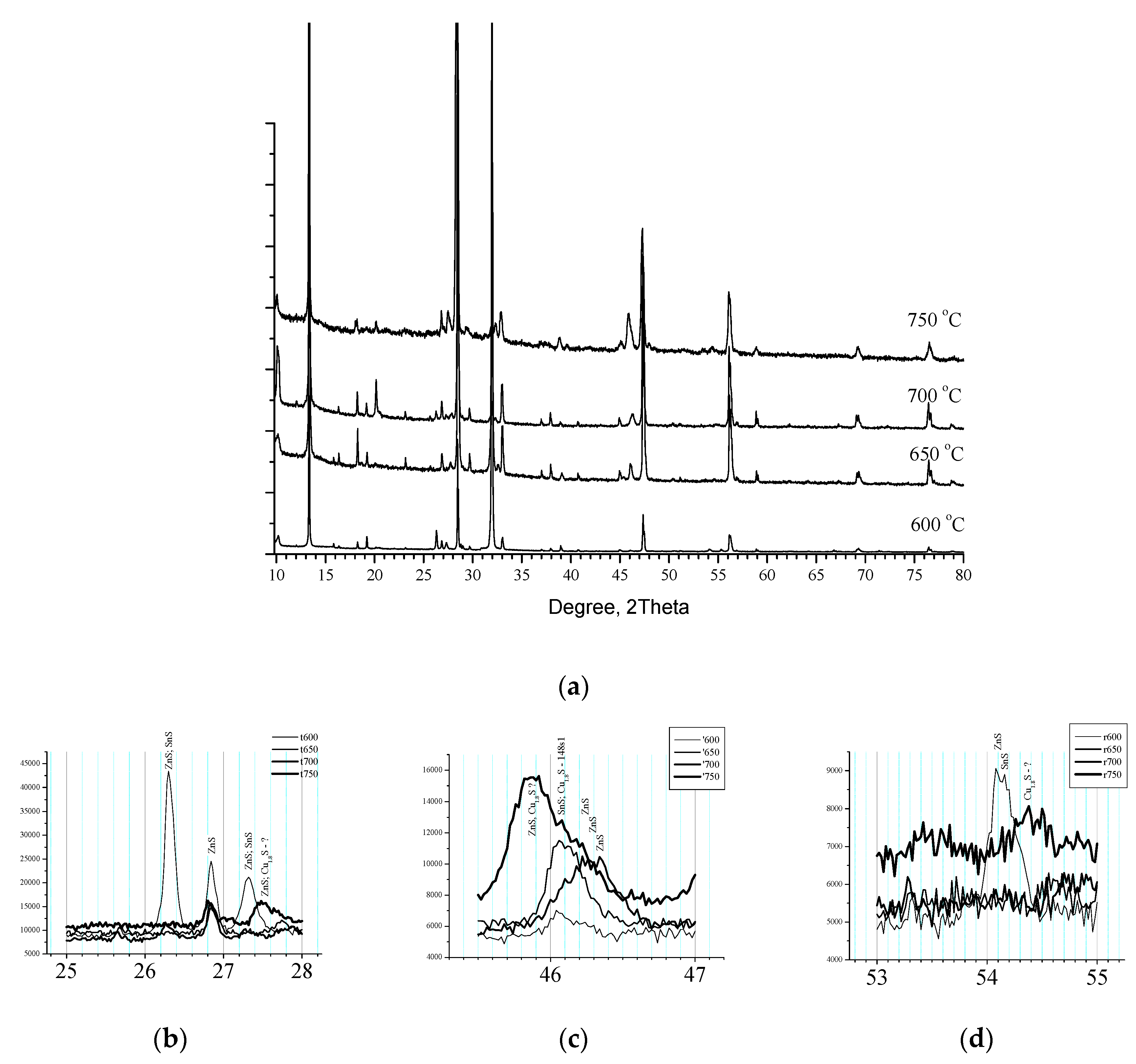
At the next
Figure 7 is presented XRD pattern of Samples 6, 7 and 8 annealed in different temperature (level of Segment 5,
Figure 1) respectively 600 °C, 650 °C and 750 °C which together with Sample 1 (700 °C for level of Segment 5) are the process set configuration for investigation of influence of the temperature. The Pattern present a well-crystallized Kesterite in all samples accompanied with different ZnS phases in almost the same structure [1:01-073-6009, 01-074-4989, 01-089-2201, 01-089-2424, 01-089-2195, 01-089-2140].
For low temperature Samples 6 and 7, respectively annealed at 600 °C and 650 °C are registered hexagonal tin sulphides while for high temperature Sample 8 (750 °C) is attributed Digenite [1:01-070-9133]. Detailed XRD analysis at the interval 26 ° – 28 ° is shown at
Figure 7(b). Sample 6 disposed three reflexes (at 26.30 °, 26.83 ° and 27.32 °) attributed to ZnS/SnS, ZnS and ZnS/SnS respectively. The reflex at 26.83 ° is common for the all samples whereas Sample 8 disposed a peak at 27.5 ° identified to ZnS and may be Cu
1.8S. On the next
Figure 7(c) are shown interval of angles between 45.5 ° - 47 °. Samples 5 and 6 (600 °C and 650 °C) showed reflex at 46.08 ° recognized as caused from SnS. Between 46.2 ° and 46.4 ° is seen large reflex for Sample 1 attributed to ZnS. Sample 8 showed a peak between 45.80 ° and 45.82 ° attributable to ZnS and may be Digenite and a shoulder at 46.08 ° recognized as caused from SnS and may be Digenite. Further in
Figure 7(d) at 53 ° - 55 ° Sample 5 (600 °C) disposed a twin at 54.09 ° and 54.16 ° whereas for Sample 8 (750 °C) at 54.38 ° was recognized a signal from Digenite.
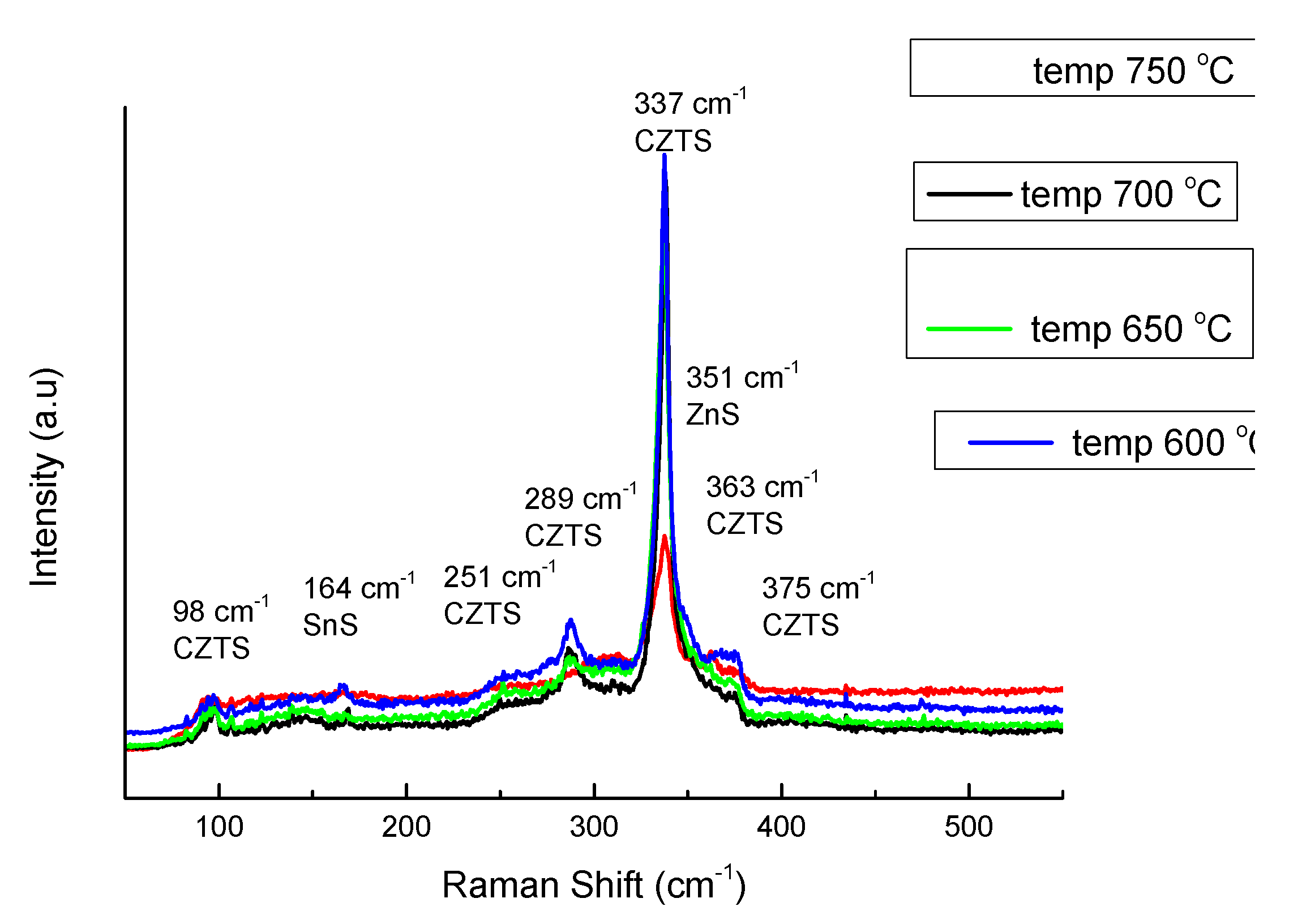
On the next
Figure 8 is presented set of pattern for Raman shift of the same samples. Raman spectra showed presence of Kesterite, ZnS and SnS for Sample 5 and Sample 6, Kesterite and ZnS for Sample 1 and Sample 8, but there were no seen confirmation for Cu-S bond. In sequence the features for phase composition recognized from XRD [
21] analysis is confirmed by Raman shift, but only with exception of the phase Cu
1.8S. XRD reflexes for it was not defined uniquely and absence of specific Raman resonant vibration give reasons to consider this phase as non-existent.
On the next
Figure 9 are presented dependences of crystallite size and lattice strain, calculated by Williamson-Hall method [
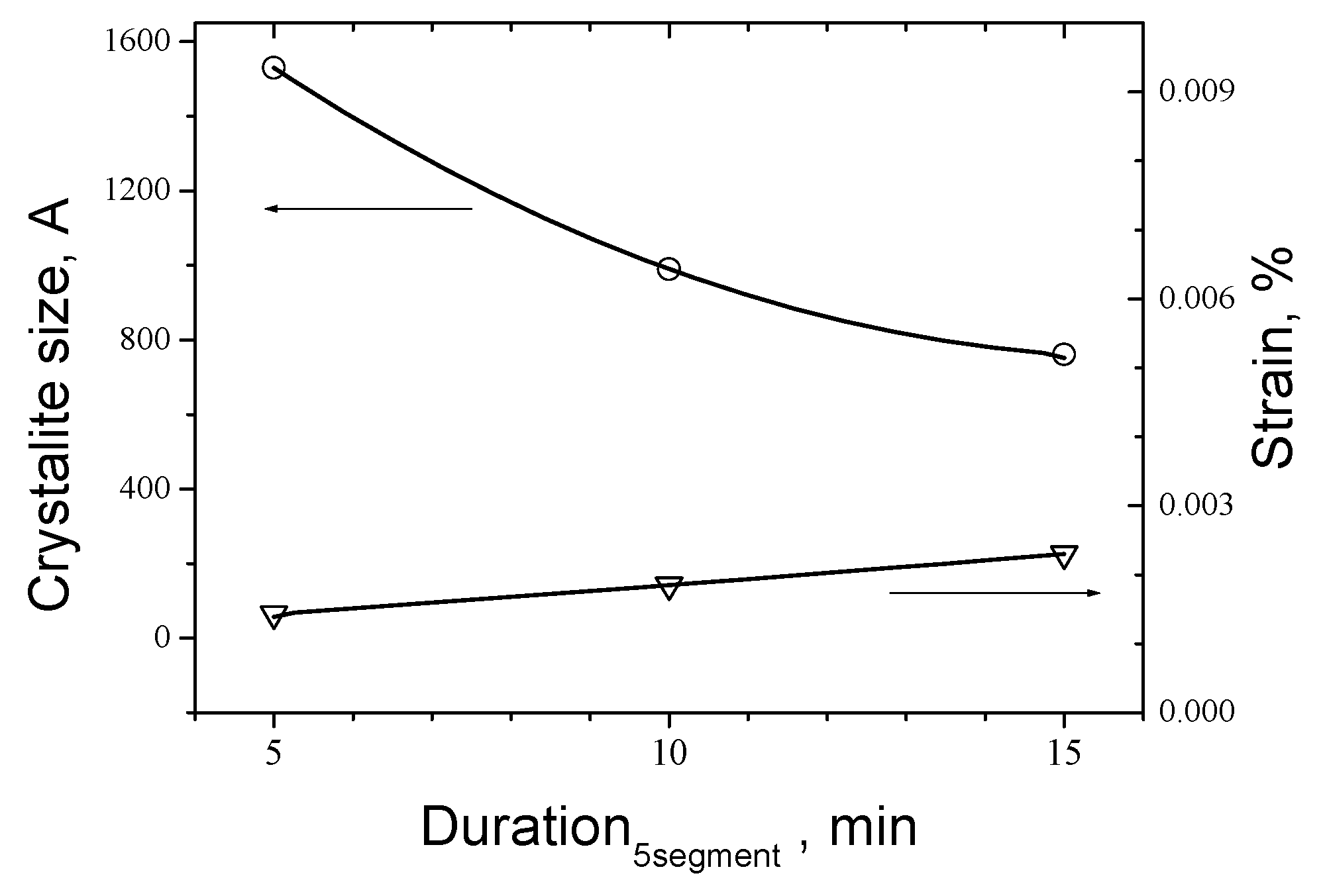
21] on dependence of process parameters for sets of investigating of influence of Duration of the Segment 5 (Samples 1, 2 and 3); Speed of change of temperature at dynamic segments 2, 4, 6 and 8 (Samples 4,1 and 5) and Temperature level of Segment 5 (Samples 6, 7, 1 and 8) respectively for 600 °C, 650 °C, 700 °C and 750 °C.
As can be seen that the trend of crystallite size with rising of duration of Segment 5 from 5 to 15 min is decreasing from 1530 Å down to near 760 Å whereas the strain of the cell rises from 0.00143 % up to 0.00230 %. The same trend is seen for the temperature where the crystallite size drops from 1193 Å for 600 ºC to 609 Å for 750 ºC respectively and strain rises from 0.00133 % up to 0,0036 %.
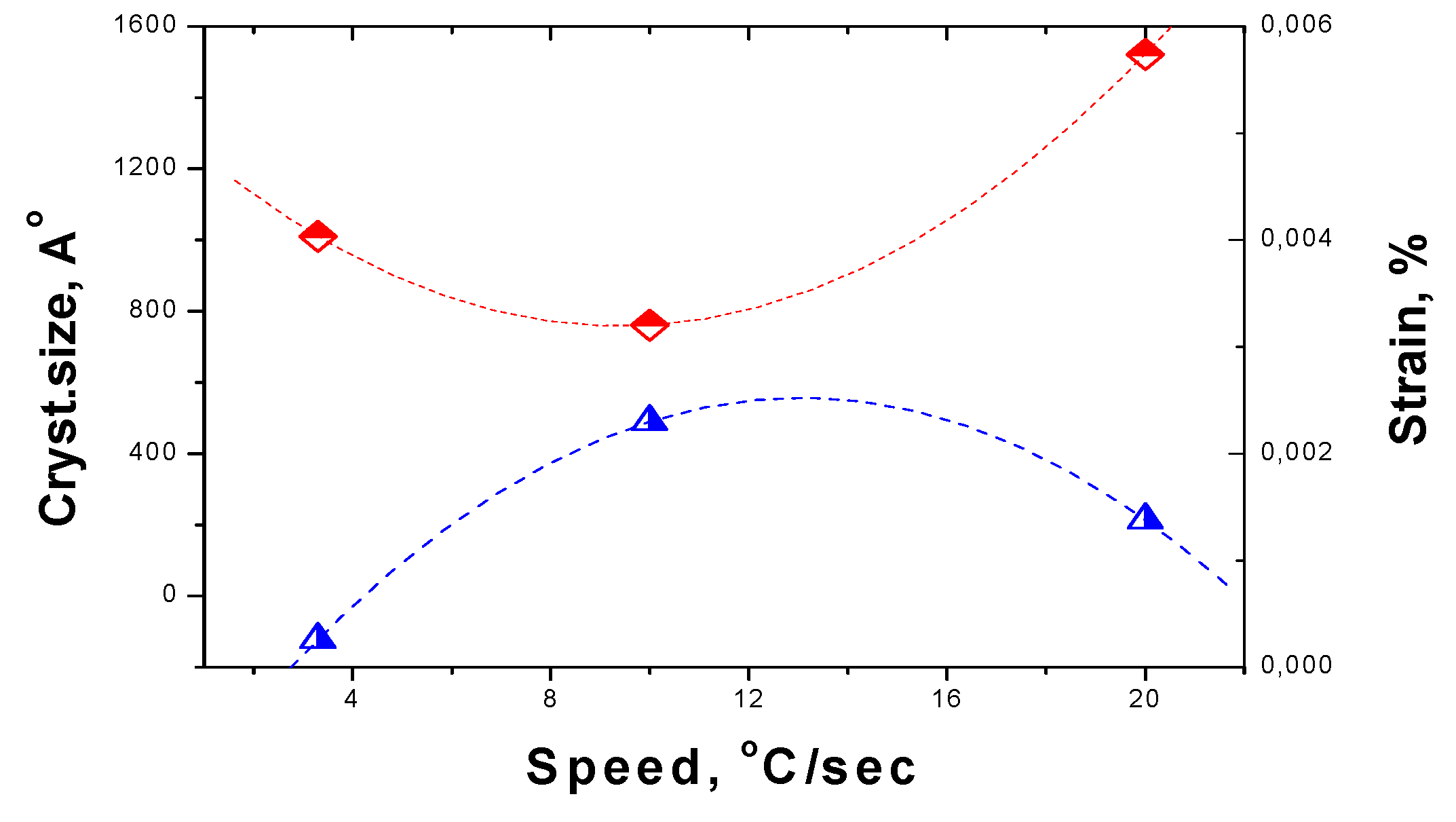
For dependence of crystallite size and strain on the speed of change of the temperature the general trend is the same, but curves are not monotonous, as can be seen by
Figure 10. The crystallite size is 1521 Å for the fast process and decrease down to 1010 Å for the slowest while at average speed is as low as 761 Å. The strain drops from 0.00139 % up to 0.00026 % while at average speed is at the maximum of 0,0023 %.
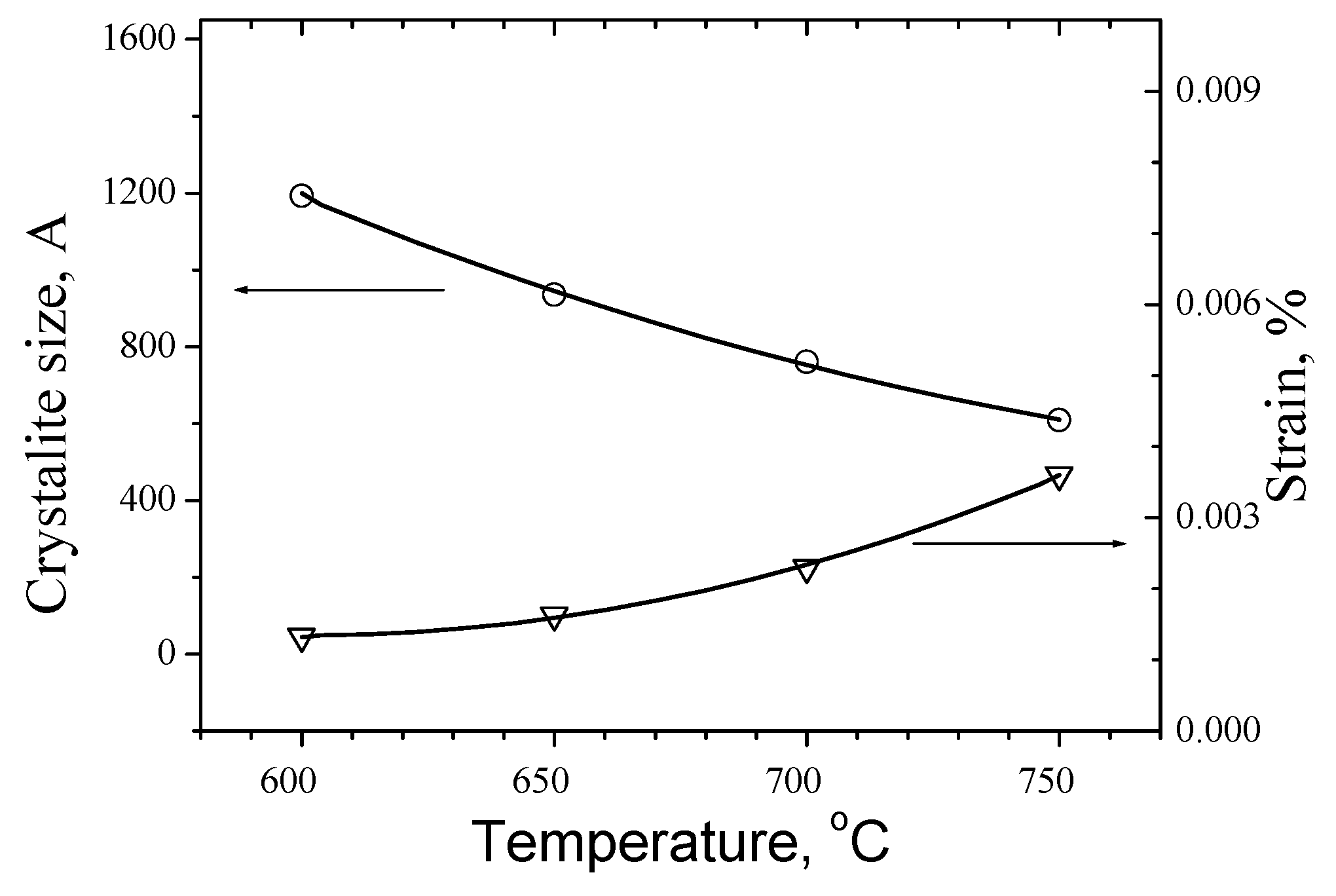
Regarding the influence of temperature, as is shown in
Figure 11, in comparison with the last data at [24-RenartCP] it is seen a remarkable difference. While the cell parameters for 700 °C at [
24] are respectively a=5.6177 Å and c = 11.2232 Å in our case they are a = 5.4262 Å and c=10.8519 Å. This is the reason in consequence to register a difference in the cell volume at 600 °C from 335 Å
3[
24] whereas cell volume in our case is near 320 Å
3.
Generally, could be concluded that slower and longer process at higher temperatures give from copper-poor zinc-rich near stoichiometric substrates a preferable phase composition of Kesterite and zinc sulphides, whereas the cell strain increase with rise of temperature and increase of duration of Segment 5, but decreases when the speed of the process decrease. Results are in agreement with [
25] and the older one [
26] but in some details are quite different from the newer works [
24]. For example, the shown difference in cell parameters [
24] at 700 °C is easy to explain having in mind the dynamic of the process – at [
24] the process is near the equilibrium whereas in our case we demonstrate time resolved (faster) process which in slower stages coincides with previous results [
24,
25,
26].
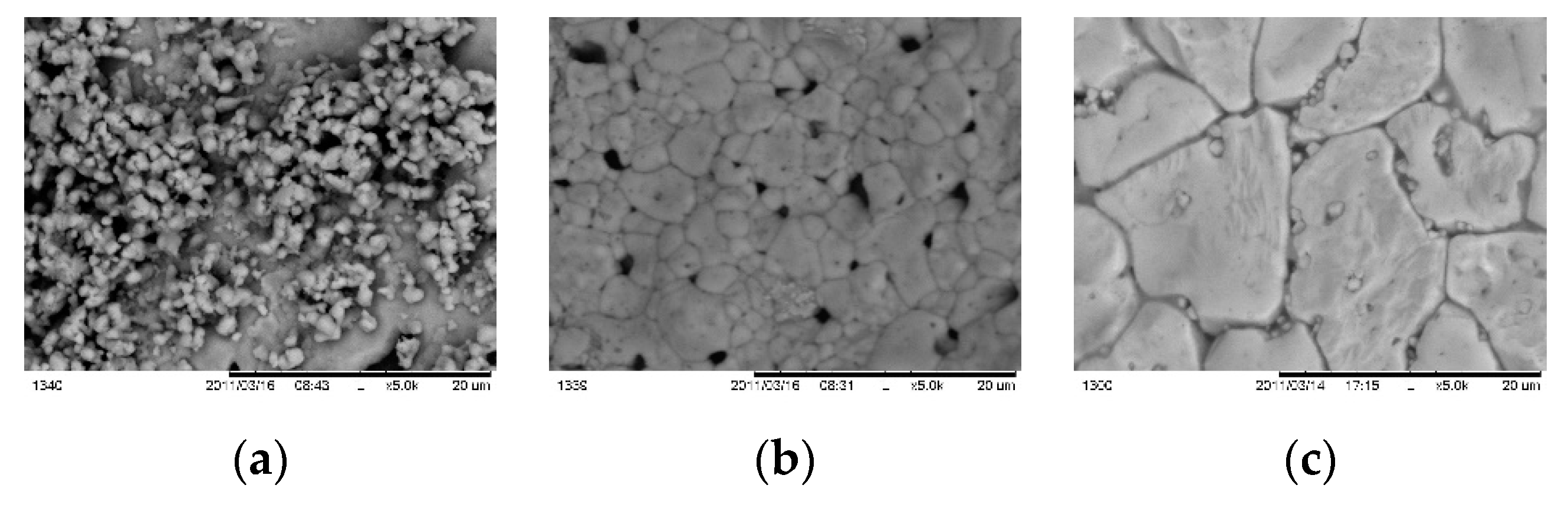
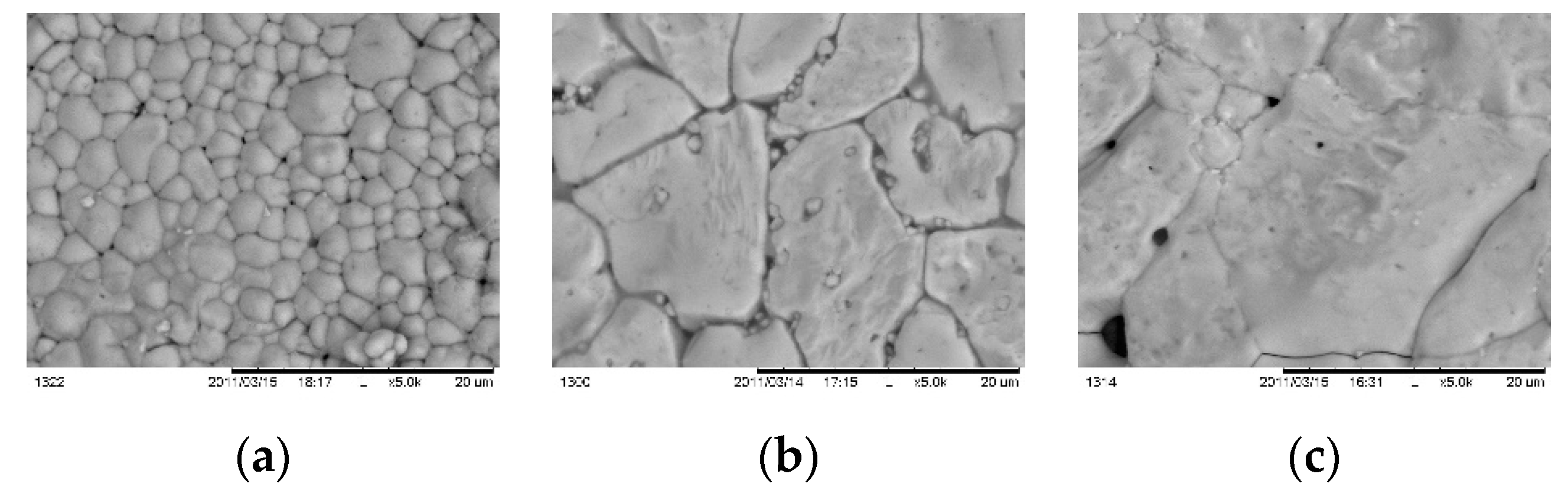
Figure 12 presents SEM micrographs of samples, annealed in different duration.
For short time of annealing small part of as deposited layer crystallized on the substrate area, leaving un-reacted mass on the top surface. For 10 min (
Figure 12 b) is formed pin-hols layer with grains sized between 1 – 4 μm whereas at 15 min (
Figure 12 c) the grains are much larger sizing 4 - 5 μm at thickness near 1 μm.
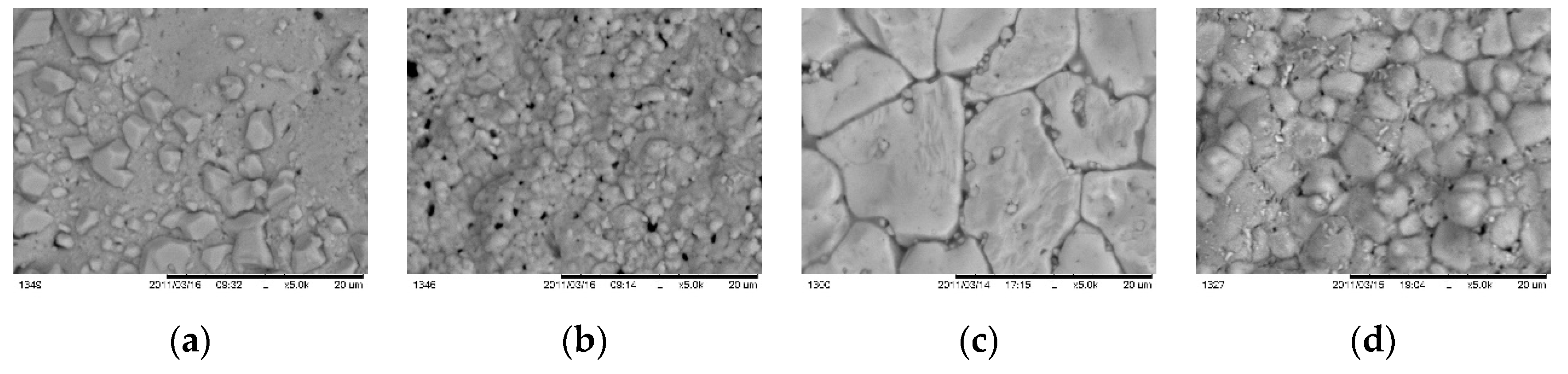
Figure 13 presents morphology issues for samples annealed at different speed. It is seen enlarging of grain sizes from 1-2 μm for fast (
Figure 13 a) annealing through 4-5 μm for average (
Figure 13 b) going up to 8 – 10 μm for samples annealed at slow process (
Figure 13 c).
Figure 14 shows footprints of morphology for samples processed at different temperature.
Figure 14(a) presents CZTS layer with single independent grains with size near 1 – 2 μm. At temperature 650 °C surface concentration of the grains raised and pinholes are seen as well. At 700 °C the grain sizes reach maximum 4-5 μm while at higher temperature 750 ºC got smaller with heterogeneous residuals on the grain borders.
SEM observations gave slightly different imagination for the crystallite size from the values, calculated.
Figure 9 ,
Figure 10 and
Figure 11 shows, derived from XRD analysis, crystallites sizes from 0.05 up to 0.15 μm whereas SEM present sizes near 1 – 8 μm at thickness near 1 μm.
Here we can conclude that tendences for change of the crystallite sizes, derived by XRD data by Williamson-Hall method [1-arvo] differs by an order of magnitude and in addition, are opposite of the tendences of change of grain sizes, derived by SEM. In fact, Williamson-Hall method gives sizes of short-ordered crystal unit of the Kesterite that is quite different from the visible grain structure of the layers.
As was reported elsewhere [RTAJScrag2012 and CZTS2014], the Raman spectroscopy could be used for characterizing of Cu/Zn disorders and distinguish the origin of the substitution in slow-cooled Kesterite thin films. Here is present a rough idea about dependences in time resolved RTA process.
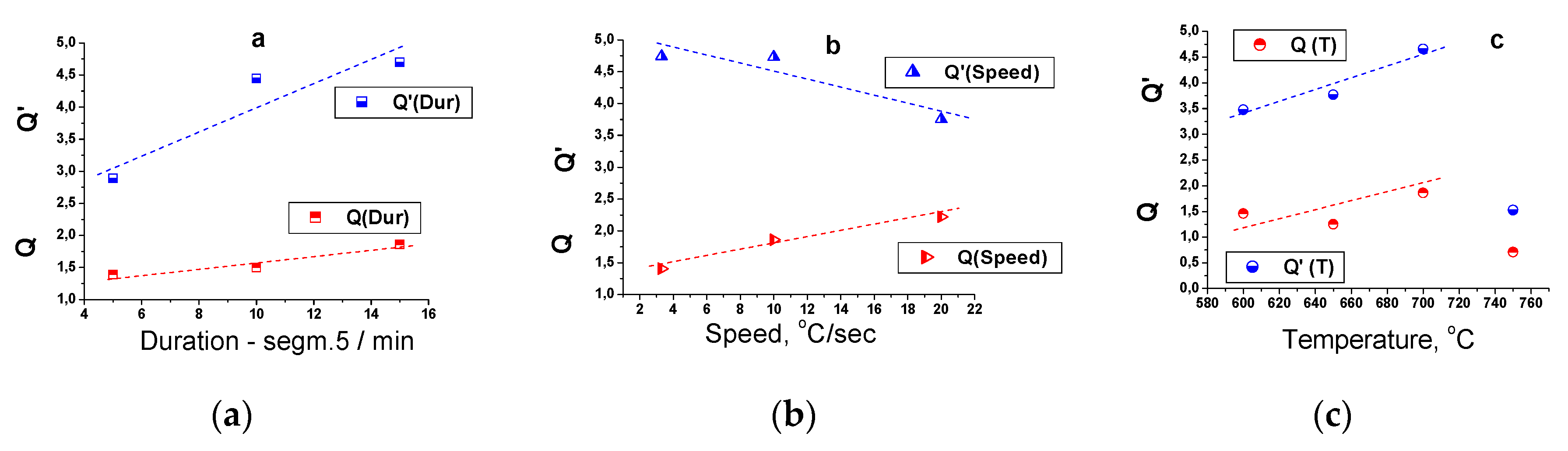
On
Figure 15(a) are presented dependences of Q and Q’ on the duration of the high-temperature annealing step of the process. It is seen that the both Q and Q’ increase with increasing of the duration. The order of magnitudes of increase of the Q is quite small – from 1.39 through 1.5 up to 1.86, whereas the Q’ is quite higher and increases near twice from 2.9 up to 4.7. The same trend is observed at the dependence of Q and Q’ on the temperature (level of segment 5), but at higher level of 750 °C the both Q and Q’ drops sharply. This effect could be interpreted as an increase in the disorder in the Kesterite structure. The increase of the speed of the increase and decrease of the temperature influence in opposite way the Q and Q’. This effect could be explained with increase in the disorder of the structure at high speed in the dynamic stages bringing the process closer to the case of quenching in the experiments in [CZTS2014].