Submitted:
29 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
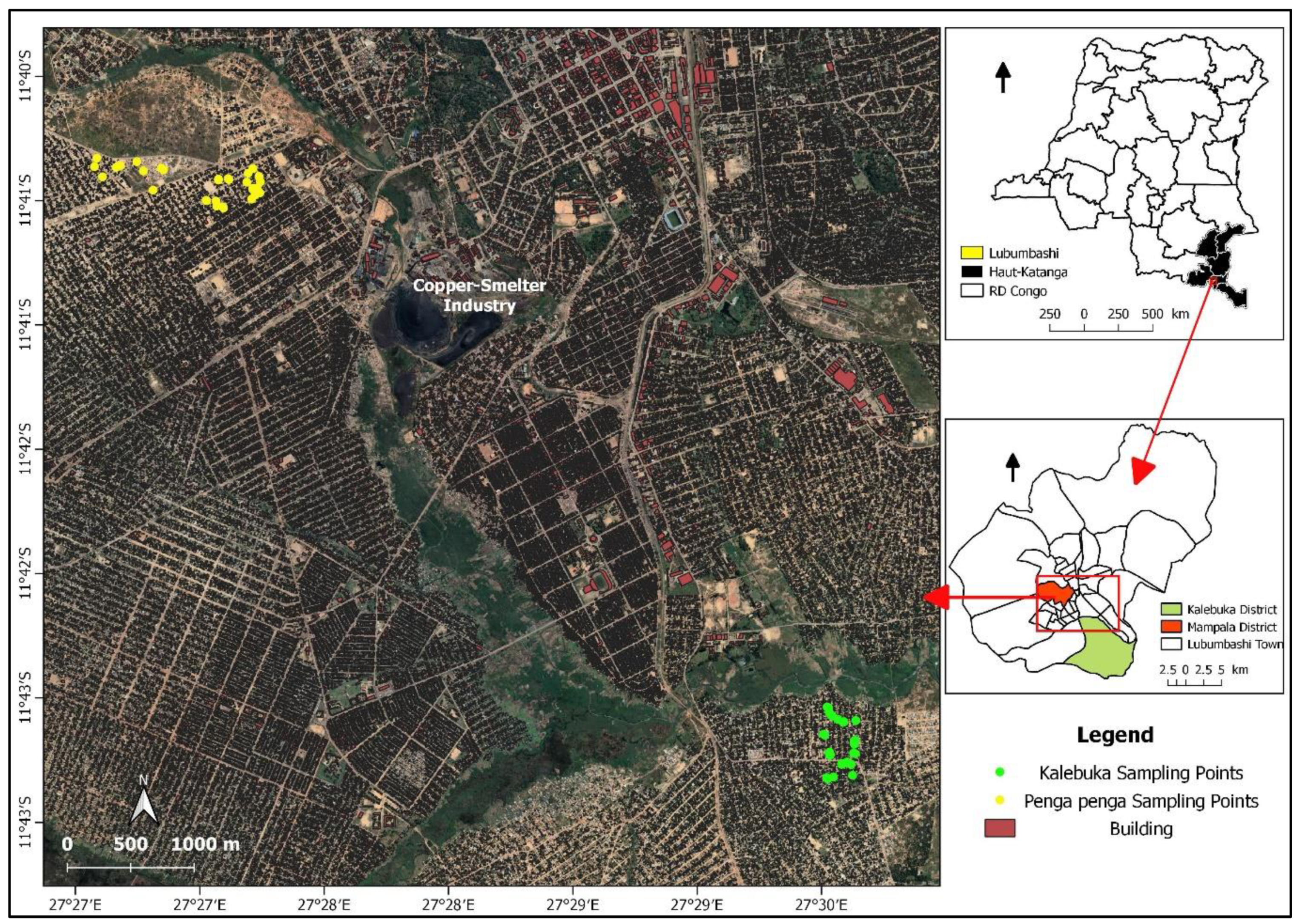
2.1. Study sites.
2.2. Soil and plant sampling
2.3. Chemical analysis of plants and soil
2.4. Calculation of trace metal bioconcentration factors
2.5. Determination of the safe weekly consumption (SWC)
2.6. Statistical analysis
3. Results
3.1. Soil mineral composition in the tree rhizosphere
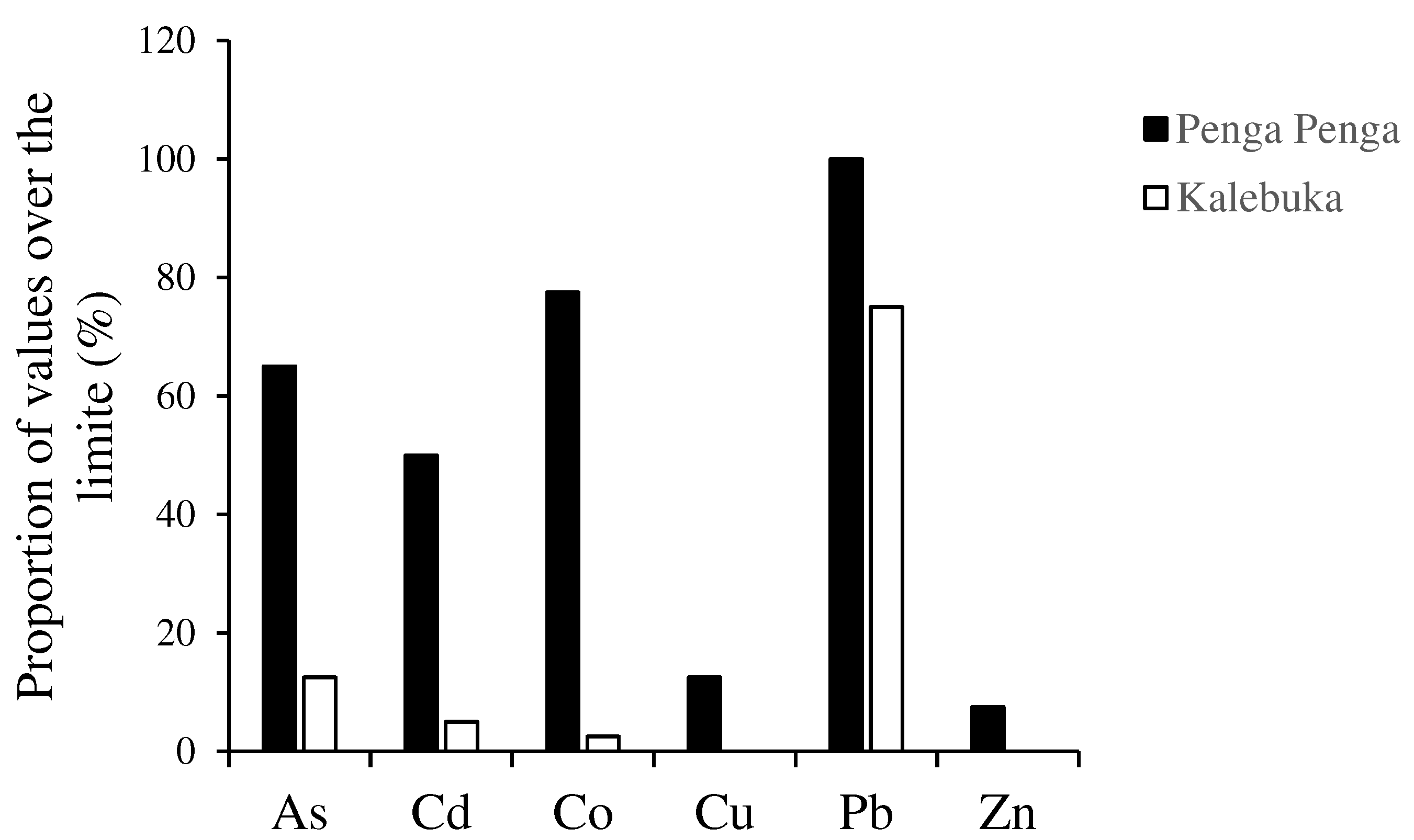
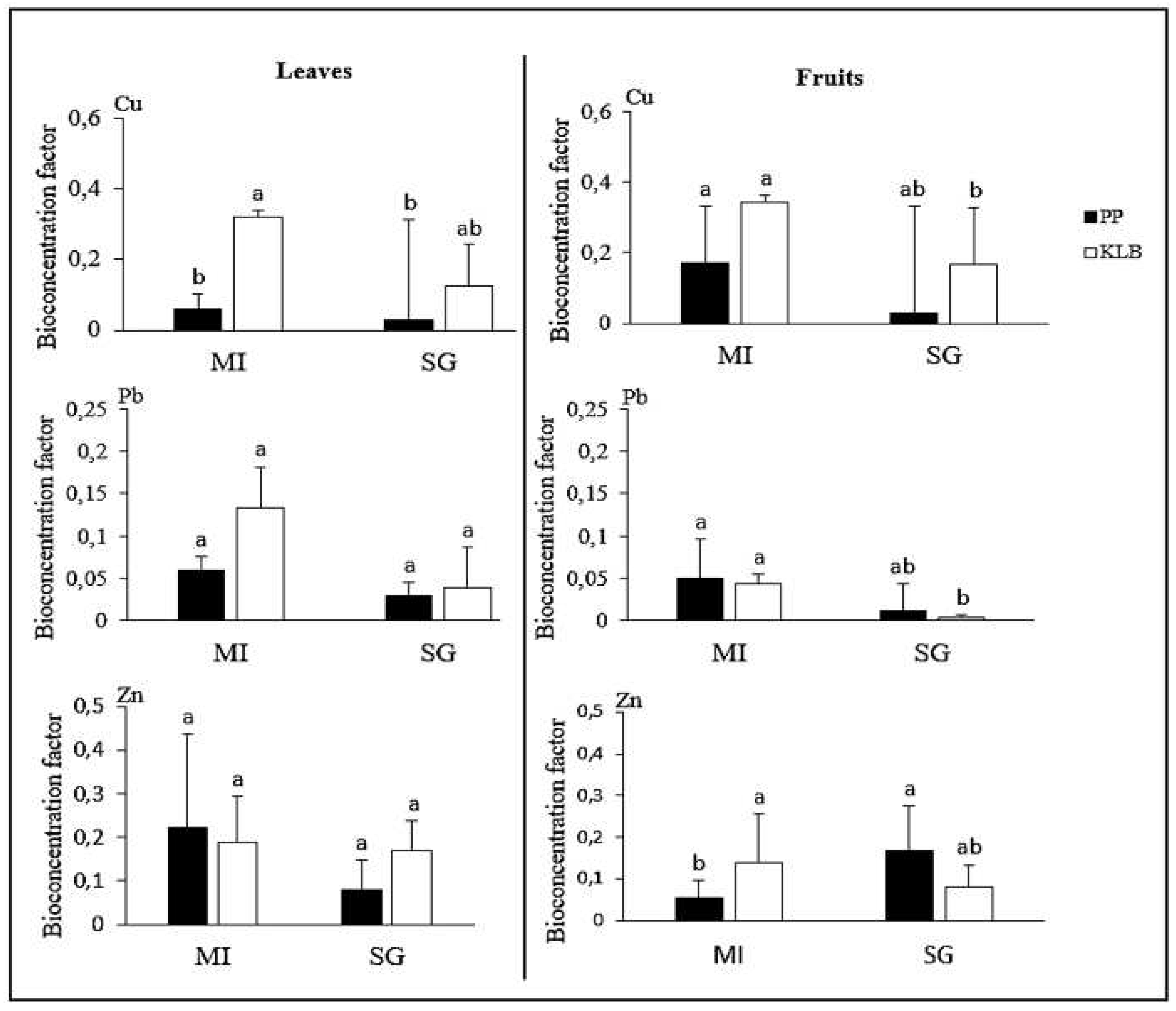
3.2. Accumulation and bioconcentration factors of trace elements in plants in Penga Penga and Kalebuka
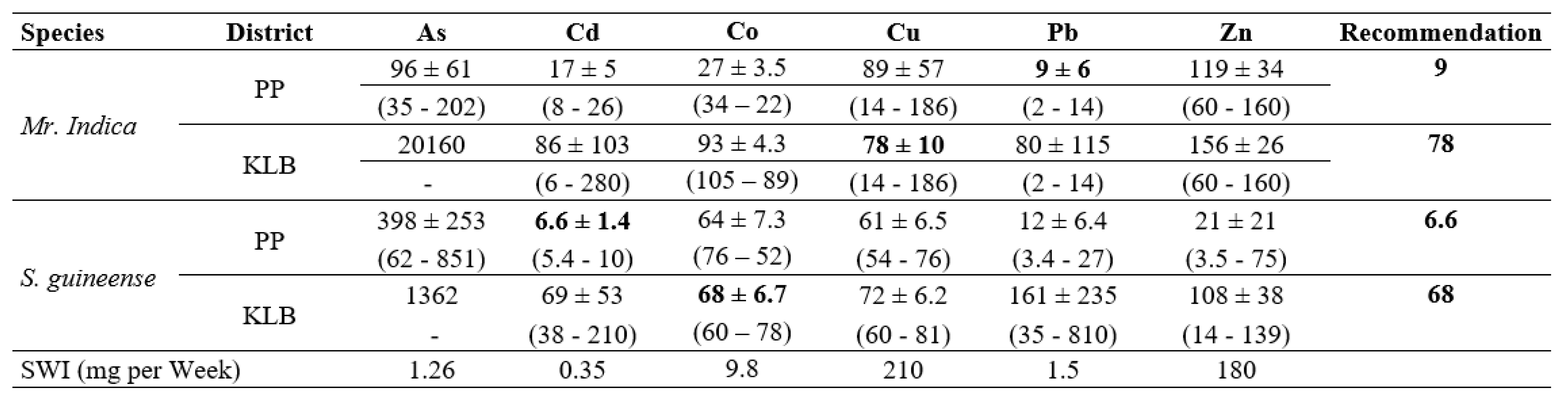
3.3. Safe weekly consumption (SWC)
4. Discussion
4.1. Trace metal concentration in the tree rhizosphere
4.2. Metal accumulation in leaves and fruits
4.3. Human exposure and safe weekly consumption
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mbenza, M., Aloni, K., Muteb, M. Some considerations on air pollution in Lubumbashi (Shaba, Zaire). Geo-eco-trop, 1989, 13 (1-4), 113-125.
- Mees, F., Masalehdani, M.N.N., De Putter, T., D'Hollander, C., Van Biezen, E., Mujinya, B.B., Potdevin, J.L., Van Ranst, E. Concentrations and forms of heavy metals around two ore processing sites in Katanga, Democratic Republic of Congo. J.Afr.Earth Sci. 2013, vol. 77,22-30. [CrossRef]
- Atibu, E.K., Devarajan, N., Thevenon, F., Mwanamoki, P.M., Tshibanda, J.B., Mpiana, P.T., Prabakar, K., Mubedi, J.I., Wildi, W., Poté, J. Concentration of metals in surface water and sediment of Luilu and Musonoie Rivers, Kolwezi-Katanga, Democratic Republic of Congo. Appl. Geochem. 2013, vol. 39, 26-32. [CrossRef]
- Muhaya, B. B., Kunyonga, C. Z., Mulongo, S. C., Mushobekwa, F. Z., & Bisimwa, A. M. Trace metal contamination of sediments in Naviundu river basin, Luano and Ruashi rivers, and Luwowoshi spring in Lubumbashi city, Democratic Republic of Congo. J. Environ. Sci. Eng. 2017, 6(9), 456-464.
- Muimba-Kankolongo, A., Banza Lubaba Nkulu, C., Mwitwa, J., Kampemba, F.M., Mulele Nabuyanda, M., Haufroid, V., Smolders, E., Nemery, B. Contamination of water and food crops by trace elements in the African Copperbelt: A collaborative cross-border study in Zambia and the Democratic Republic of Congo. 2021, Environ. Adv., vol. 6,100103. [CrossRef]
- Pourret, O., Lange, B., Bonhoure, J., Colinet, G., Decrée, S., Mahy, G., Séleck, M., Shutcha, M., Faucon, M.-P. Assessment of soil metal distribution and environmental impact of mining in Katanga (Democratic Republic of Congo). 2016, Appl. Geochem., 64, 43-55. [CrossRef]
- Shutcha, M.N.; Mukobo RP.; Muyumba K.D.; Mpundu M.M.; Faucon M P.; Lubalega K. T.; Ludovic A.; Annabelle J.; Vandenheede N.; Pourret O.; Michel Ngongo L.M.; Colinet G. Fond pédogéochimique et cartographie des pollutions des sols à Lubumbashi, in Anthropisation des paysages Katangais, Presses Universitaires de Liége-Agronomiqe Gembloux., Bogaert J., Gilles C. & Gregory M., 2018, 215-218.
- Mpinda, M.T., Mujinya, B.B., Mees, F., Kasangij, P.K., Van Ranst, E. Patterns and forms of copper and cobalt in Macrotermes falciger mounds of the Lubumbashi area, DR Congo. 2022, J. Geochem. Explor. 2022, 238, 107002. [CrossRef]
- Leblanc et Malaisse, F. Lubumbashi, un écosystème urbain tropical, Université nationale du Zaire, 1978.
- Mpundu Mubemba M. Contaminations des sols en éléments traces métalliques à Lubumbashi (Katanga/R.D. Congo). Évaluation des risques de contamination de la chaîne alimentaire et choix de solutions de remédiation. Thèse de doctorat. Faculté des Sciences Agronomiques, Université de Lubumbashi, Lubumbashi (RDC), 2010.
- Shutcha, M.N., Mubemba, M.M., Faucon, M.-P., Luhembwe, M.N., Visser, M., Colinet, G., Meerts, P. Phytostabilisation of Copper-Contaminated Soil in Katanga: An Experiment with Three Native Grasses and Two Amendments. 2010, Int. J. Phytoremediation, vol. 12, no 6, 616-632. [CrossRef]
- Munyemba, K.F. Quantification et modélisation de la dynamique paysagère dans la région de Lubumbashi : évaluation de l’impact écologique des dépositions issues de la pyrométallurgie. Thèse de doctorat. Faculté des Sciences Agronomiques, Université de Lubumbashi, Lubumbashi (RDC). 2010, p. 284.
- Munyemba, K., Bamba, I., Djibu, K.J.P., Amisi, M., Veroustraete, F., Ngongo, L.M., Bogaert, J. Occupation des sols dans le cône de pollution `à Lubumbashi. Presses Universitaires de Lubumbashi. 2008, Ann Fac Sc Agro. 1(2): 19–22.
- Banza, C.L.N.; Nawrot, T.S.; Haufroid V.; Decree S.; De Putter T.; Smolders E.; Kabyla B.I.; Luboya O.N.; Ilunga A.N.; Mutombo A.M.; Nemery B. 2009. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ Res. 2009, 109 :745–752. [CrossRef]
- Cheyns, K.; Banza, C. L. N.; C., Ngombe L.K.; Asosa, J.N.; Haufroid V.; De Putter, T.; Nawrot, T.; Kimpanga, C.M.; Numbi, O.L.; Ilunga, B.K.; Nemery, B.; Smolders, E. Pathways of human exposure to cobalt in Katanga, a mining area of the D.R. Congo », Sci. Total Environ., 2014, 490,313-321. [CrossRef]
- Mukendi, R. A. M.; Banza C. L. N.; Mukeng C. A. K.; Ngwe J. T. M.; Mwembo A. N. A. N.; Kalenga P. M. K. Exposition de l’homme aux éléments tracés métalliques et altération du sperme : étude menée dans les zones minières du Haut-Katanga en République Démocratique du Congo, Pan Afr. Med. J., 2018, 30. [CrossRef]
- Nkuku C. & Rémon M. Stratégies de survie à Lubumbashi (RD Congo). Enquête sur 14000 ménages urbains. Mémoires lieux de savoir : Archive congolaise, Harmattan, Paris, 2006.
- INS. Annuaire statistique. Ministère de plan et de la révolution de la modernité & PNUD, RD Congo, 2014, p560.
- Shutcha, M.N., Faucon, M.-P., Kamengwa Kissi, C., Colinet, G., Mahy, G., Ngongo Luhembwe, M., Visser, M., Meerts, P. Three years of phytostabilisation experiment of bare acidic soil extremely contaminated by copper smelting using plant biodiversity of metal-rich soils in tropical Africa (Katanga, DR Congo) », Ecol. Eng. 2015, 82, 81-90. [CrossRef]
- Mwanasomwe, K.L. Amélioration du procédé de phytostabilisation avec les espèces ligneuses pour la production des services écosystémiques en milieux pollués urbains et périurbains de l’arc cuprifère Katangais. Thèse de doctorat, Gembloux Agro Bio-Tech, Université de Liège. Belgique. 2022, p217.
- Säumel, I., Kotsyuk, I., Hölscher, M., Lenkereit, C., Weber, F., Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin. Germany. Environ. Pollut., 2012, 165, 124-132. [CrossRef]
- Cao, L.T.T., Bourquin, L.D. Relationship of Arsenic and Lead in Soil with Fruit and Leaves of Apple Trees at Selected Orchards in Michigan. J. Food Prot., 2020, 83, 6, 935-942. [CrossRef]
- Liu, G., Chen, T., Cui, J., Zhao, Y., Li, Z., Liang, W., Sun, J., Liu, Z., Xiao, T. Trace Metal(loid) Migration from Road Dust to Local Vegetables and Tree Tissues and the Bioaccessibility-Based Health Risk: Impacts of Vehicle Operation-Associated Emissions », Int. J. Environ. Res. Public. Health, 2023, 20, 3, 2520. [CrossRef]
- 24. FAO and WHO. CF/10 INF/1. Working document for information and use in discussions related to contaminants and toxins in the GSCTFF. http://www.fao.org/fao-who-codexalimentarius, file: cf10_INF1e.pdf. 2016, p150.
- Tauqeer, H.M., Turan, V., Iqbal, M. Correction to: Production of Safer Vegetables from Heavy Metals Contaminated Soils: The Current Situation, Concerns Associated with Human Health and Novel Management Strategies, in Advances in Bioremediation and Phytoremediation for Sustainable Soil Management, J. A. Malik, Éd., Cham: Springer International Publishing, 2022, C1-C1. [CrossRef]
- Kavusi, E., Shahi Khalaf Ansar, B., Ebrahimi, S., Sharma, R., Ghoreishi, S.S., Nobaharan, K., Abdoli, S., Dehghanian, Z., Asgari Lajayer, B., Senapathi, V., Price, G.W., Astatkie, T. Critical review on phytoremediation of polyfluoroalkyl substances from environmental matrices: Need for global concern », Environ. Res., 2023, 217, 114844. [CrossRef]
- Langunu, S. 2022. Efficacité à long terme d’une phytostabilisation assistée in situ sur un sol fortement contaminé par les éléments traces métalliques à Lubumbashi. Mémoire d’études approfondie. Faculté des sciences Agronomiques, Université de Lubumbashi. RD Congo. 2022, p 65.
- Faucon, M. P., Shutcha, M. N., & Meerts, P. Revisiting copper and cobalt concentrations in supposed hyperaccumulators from SC Africa: influence of washing and metal concentrations in soil. Plant Soil, 2007, 301, 1-2, 29-36. [CrossRef]
- Miller, D. A., and White, R. A. A Conterminous United States Multilayer Soil Characteristics Dataset for Regional Climate and Hydrology Modeling. Earth Interact., 1998, 2, 2, 1-26. [CrossRef]
- Maiti, S. K., Kumar, A. and Ahirwal, J. Bioaccumulation of metals in timber and edible fruit trees growing on reclaimed coal mine overburden dumps, Int. J. Min. Reclam. Environ., 2016, 30, 3, 231-244. [CrossRef]
- Ali, H., Khan, E., Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications », Chemosphere, 2013, 91, 7, 869-881, mai 2013. [CrossRef]
- Yang, Y.; Liang, Y.; Ghosh, A.; Song, Y.; Chen, H.; Tang, M. Assessment of arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead–zinc mine area: potential applications for phytoremediation, Environ. Sci. Pollut. Res., 2015, 22, 17, 13179-13193. [CrossRef]
- Pelkonen, R., Alfthan, G., Järvinen, O. Element Concentrations in Wild Edible Mushrooms in Finland. The Finnish Environment. 2008, 25:1-42. http://hdl.handle.net/10138/38380.
- Muyumba, K. D. ; Olivier P. ; Amandine L. ; Michel-P F. ; Gregory M.; Michel NL.; Gilles, C. Évaluation expérimentale de la phytodisponibilité du cuivre et du cobalt dans les sols des écosystèmes métallifères de l’Arc cuprifère katangais, in Anthropisation des paysages Katangais, Presses Universitaires de Liége-Agronomiqe Gembloux., Gembloux: Bogaert J., Gilles C. & Gregory M, 2018, 192-213.
- Mujinya, B.B., Van Ranst, E., Verdoodt, A., Baert, G., Ngongo, L.M. Termite bioturbation effects on electro-chemical properties of Ferralsols in the Upper Katanga (D.R. Congo), Geoderma, 2010, 158, 3-4, 233-241. [CrossRef]
- Hale, B., Evans, L., Lambert, R. Effects of cement or lime on Cd, Co, Cu, Ni, Pb, Sb and Zn mobility in field-contaminated and aged soils, J. Hazard. Mater., 2012, 199-200, 119-127. [CrossRef]
- Tang, J., Cao, C., Gao, F., Wang, W. Effects of biochar amendment on the availability of trace elements and the properties of dissolved organic matter in contaminated soils, Environ. Technol. Innov., 2019, 16,100492. [CrossRef]
- Lubalega K.T. Metal contamination and termite mounds around Lubumbashi. DEA thesis, University of Lubumbashi, DR Congo p55, 2009.
- Mwanasomwe, J. K., Langunu, S., Shutcha, M. N., Colinet, G. Effects of 15-Year-Old Plantation on Soil Conditions, Spontaneous Vegetation, and the Trace Metal Content in Wood Products at Kipushi Tailings Dam, Front. Soil Sci., 2022, 2, 934491. [CrossRef]
- Simon, L. Potentially Harmful Elements in Agricultural Soils, in PHEs, Environment and Human Health, C. Bini et J. Bech, Éd., Dordrecht: Springer Netherlands, 2014, 85-150. [CrossRef]
- Adamczyk-Szabela, D., Wolf, W.M. The Impact of Soil pH on Heavy Metals Uptake and Photosynthesis Efficiency in Melissa officinalis, Taraxacum officinalis, Ocimum basilicum, Molecules, 2022, 27,15, 4671. [CrossRef]
- Podar, D., Maathuis, F.J.M. The role of roots and rhizosphere in providing tolerance to toxic metals and metalloids », Plant Cell Environ., 2022, 45,3,719-736. [CrossRef]
- Boim, A.G.F., Melo, L.C.A., Moreno, F.N., Alleoni, L.R.F. Bioconcentration factors and the risk concentrations of potentially toxic elements in garden soils, J. Environ. Manage., 2016, 170, 21-27. [CrossRef]
- Oti, W. O. Bioaccumulation Factors and Pollution Indices of Heavy Metals in Selected Fruits and Vegetables from a Derelict Mine and Their Associated Health Implications, Int. J. Environ. Sustain., 2015, 4,1. [CrossRef]
- Mpundu, M.M., Useni, S.Y., Mwamba, M.T., Kateta, M.G., Mwansa, M., Ilunga, K., Kamengwa, K.C., Kyungu, K., and Nyembo K.L., 2013. Trace metal levels in soils from different vegetable gardens in the mining town of Lubumbashi and risks of contamination of vegetable crops. J.of Applied Bios., 2013, 65: 4957-4968.
- Mununga Katebe, F., Raulier, P., Colinet, G., Ngoy Shutcha, M., Mpundu Mubemba, M., Jijakli, M.H. Assessment of Heavy Metal Pollution of Agricultural Soil, Irrigation Water, and Vegetables in and Nearby the Cupriferous City of Lubumbashi, (Democratic Republic of the Congo), Agronomy, 2023, 13, 2, 357. [CrossRef]
- WHO (World Health Organization). Report Fruit and Vegetable Initiative, Report of the meeting, Geneva, 2003, 25-27.
- Hung, HC., Joshipura, KJ. Fruit and vegetable intake and risk of major chronic disease. Journal of the National Cancer Institute, 2004, 96:1577-84.
- WHO. Food safety, decision of the thirteenth World Health Assembly, New York, NY, 2020.
- Ogunkunle, A. T. J.; Bello, O. S.; Ojofeitimi, O. S. Determination of heavy metal contamination of street-vending fruits and vegetables in Lagos state, Nigeria, Int. Food Res. J., 2014, 21, 2115-2120.
- S. Study of heavy metals in mango (Mangifera indica L.) in Perlis, Malaysia. Indian Res. J.Pharm, 2015, 2, 299–303.
- Širić, I., Eid, E.M., El-Morsy, M.H.E., Osman, H.E.M., Adelodun, B., Abou Fayssal, S., Mioč, B., Goala, M., Singh, J., Bachheti, A., Arya, A.K., Choi, K.S., Kumar, V., Kumar, P. Health Risk Assessment of Hazardous Heavy Metals in Two Varieties of Mango Fruit (Mangifera indica L. var. Dasheri and Langra), Horticulturae, 2022, 8, 9, 832. [CrossRef]
- Mangambu, M., Mushagalusa, K., Kadima, N. Contribution à l’étude photochimique de quelques plantes médicinales antidiabétiques de la ville de Bukavu et ses environs (Sud-Kivu, R.D.Congo)., J. Appl. Biosci., 2014, 75,1,6211. [CrossRef]
- Badou, R.B., Yedomonhan, H., Tossou, M., Diversité d’usages et Statut de conservation de Syzygium guineense (Willd.) DC. subsp. macrocarpum (Engl.) F. White (Myrtaceae) au Bénin, Int. J. Environ. Stud., 2019, 76, 5, 827-842. [CrossRef]
- Sferrazzo, G., Palmeri, R., Vanella, L., Parafati, L., Ronsisvalle, S., Biondi, A., Basile, F., Li Volti, G., Barbagallo, I. Mangifera indica L. Leaf Extract Induces Adiponectin and Regulates Adipogenesis, Int. J. Mol. Sci., 2019, 20, 13, 3211. [CrossRef]
- Drabo, C., Nikiema, Z.S., Dianda, O.Z., Dao, A., Sanou, J., Sawadogo, M. Usage thérapeutique du manguier (Mangifera indica L., Anacardiaceae) au Burkina Faso, VertigO, 2023. [CrossRef]
| Parameters | Penga Penga | Kalebuka |
|---|---|---|
| pH KCl | 6.3 (4.8-7.8) | 6.8 (5.4 - 8.2) |
| TOC (%) | 1.6 (0.5-3.0) | 1.2 (0.4 - 0.2) |
| Co (mg.kg-1) | 21 (4.6-90) | 1.9 - 2.5 |
| Cu (mg.kg-1) | 2966 (213-17096) | 48 (45.3 – 50) |
| Zn (mg.kg-1) | 202 (21-736) | 18.2 (0 - 36.4) |
| Trace metals | SWI (mg per week) |
| As | 1.26 |
| Cd | 0.35 |
| Co | 9,8 |
| Cu | 210 |
| Pb | 1.5 |
| Zn | 180 |
| Parameters | Penga Penga | Kalebuka | References |
| pH | 7.7 (6.7-8.4) a | 6.4 (4.5-7.7) b | 4.9 - 6.8 |
| Al2O3 (%) | 2.5 (1.4-5.0) a | 3.0 (1.4-4.6) b | 1.9-10.7 |
| Fe2O3 (%) | 4.3 (2.7-9.1) a | 4.5 (2.5-6.5) a | 0.9-7.4 |
| Ca (%) | 0.7 (0.1-2.3) a | 0.3 (0.1-1.0) b | - |
| K (%) | 0.9 (0.4-2.0) a | 1.0 (0.5-1.6) a | - |
| Mn (mg kg-1) | 251 (89-679) a | 292 (77-717) a | - |
| AsT (mg kg-1) | 12.8 (3-81) a | 7.6 (3-16) b | - |
| CuT (mg kg-1) | 1379 (60-4670) a | 189 (22-695) b | 20 - 456 |
| PbT (mg kg-1) | 142 (17-547) a | 33 (2.0-110) b | 7 - 82 |
| ZnT (mg kg-1) | 467 (129-1236) a | 115 (31-275) b | 26 - 180 |
| AsS (mg kg-1) | <0.01 | <0.01 | - |
| CdS (mg kg-1) | 0.07 (<0.01-0.5) a | 0.13 (<0.01-1.05) a | - |
| CoS (mg kg-1) | 0.31 (0.013-3.8) a | 0.17 (0.01-1.7) a | - |
| CuS (mg kg-1) | 1.4 (0.2-7.4) a | 0.7 (0.04-11.8) b | - |
| PbS (mg kg-1) | 0.11 (0.03-0.4) a | 0.1 (0.06-0.4) a | - |
| ZnS (mg kg-1) | 0.4 (0.01-6.9) a | 1.7 (0.007-12.3) a | - |
| Species | Leaves | Fruit | FAO/WHO limits | |||
|---|---|---|---|---|---|---|
| Penga Penga | Kalebuka | Penga Penga | Kalebuka | |||
| As | M. Indica | 0.2 a | 0.1 b | 0.1 | < 0.001 | 0.1 |
| (0.08-0.3) | (0.07-0.24) | (0.05-0.2) | ||||
| S. guineense | 0.1 a | 0.05 b | 0.02 | < 0.001 | ||
| (0.07-0.2) | (0.04-0.08) | (0.00-0.1) | ||||
| Cd | M. Indica | 0.1 a | 0.14 a | 0.19a | 0.09 b | 0.2 |
| (0.07-0.3) | (0.05-0.22) | (0.1-0.3) | (0.01-0.4) | |||
| S. guineense | 0.2 a | 0.09 a | 0.2 a | 0.03 b | ||
| (0.06-0.8) | (0.03-0.3) | (0.1-0.3) | (0.02-0.05) | |||
| Co | M. Indica | 3.3 a | 0.3 b | 2.9 a | 0.84 b | 1 |
| (2.5-4.1) | (0.1-1.2) | (2.3-3.6) | (0.75-0.88) | |||
| S. guineense | 2.7 a | 0.7 b | 0.84 a | 0.79 a | ||
| (1.8-4.1) | (0.6-0.82) | (0.7-1.02) | (0.68-0.91) | |||
| Cu | M. Indica | 22.2 a | 13 b | 29.3 a | 22.0 b | 40 |
| (15-42) | (9-16) | (9-64) | (19-27) | |||
| S. guineense | 18.4 a | 19.8 a | 18.9 a | 15.9 b | ||
| (13-26) | (17-24) | (15-21) | (14-19) | |||
| Pb | M. Indica | 3.3 a | 1.0 b | 2.2 a | 0.31 b | 0.3 |
| (1.2-5) | (0.7-1.5) | (0.5-6) | (0.03-0.6) | |||
| S. guineense | 2.2 a | 0.8 b | 0.8 a | 0.11 b | ||
| (1.1-4.3) | (0.3-1.2) | (0.3-2.4) | (0.01-0.2) | |||
| Zn | M. Indica | 47.8 a | 19.7 b | 13.3 a | 9.5 b | 60 |
| (19-137) | (12-25) | (9-24) | (7-13) | |||
| S. guineense | 20.3 a | 13.9 b | 39.1 a | 14.7 b | ||
| (12-45) | (11-16) | (28-47) | (7-70) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





