Submitted:
28 June 2023
Posted:
29 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Methods for Creating and Studying Membrane Materials
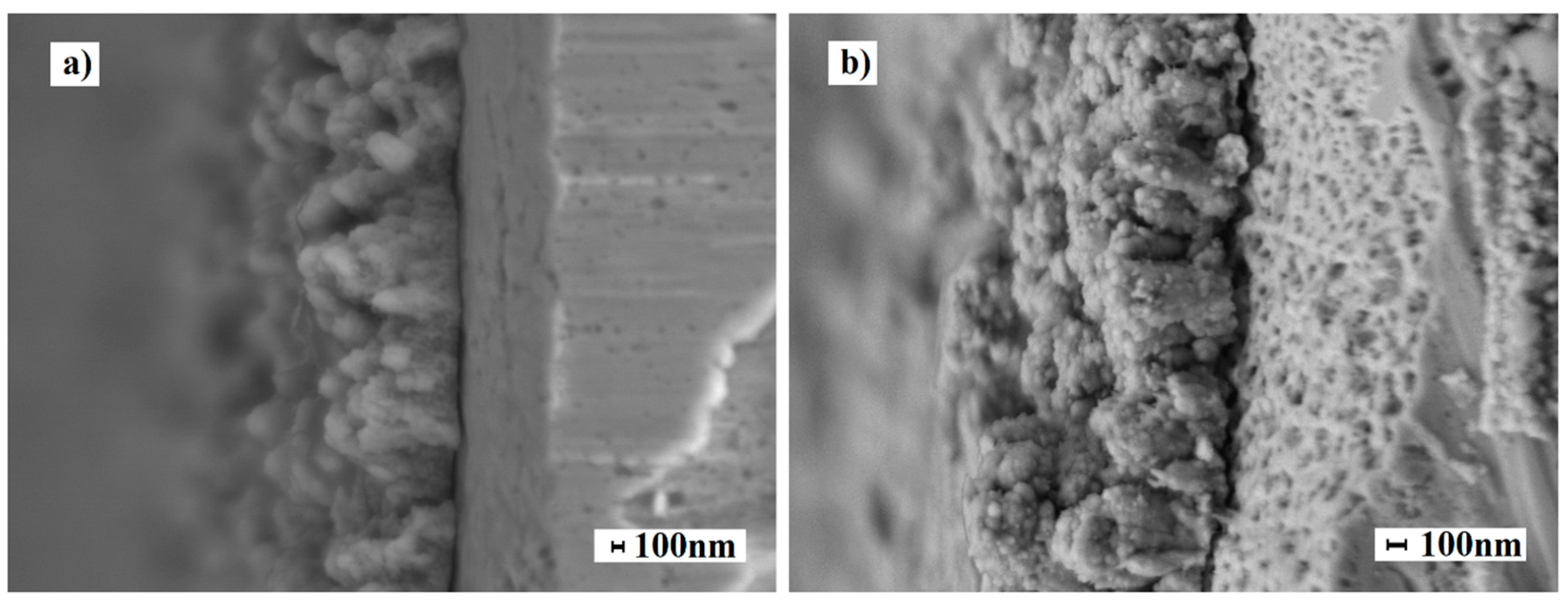
2.2. Synthesis and Study of the Morphology of Nanostructured Coatings
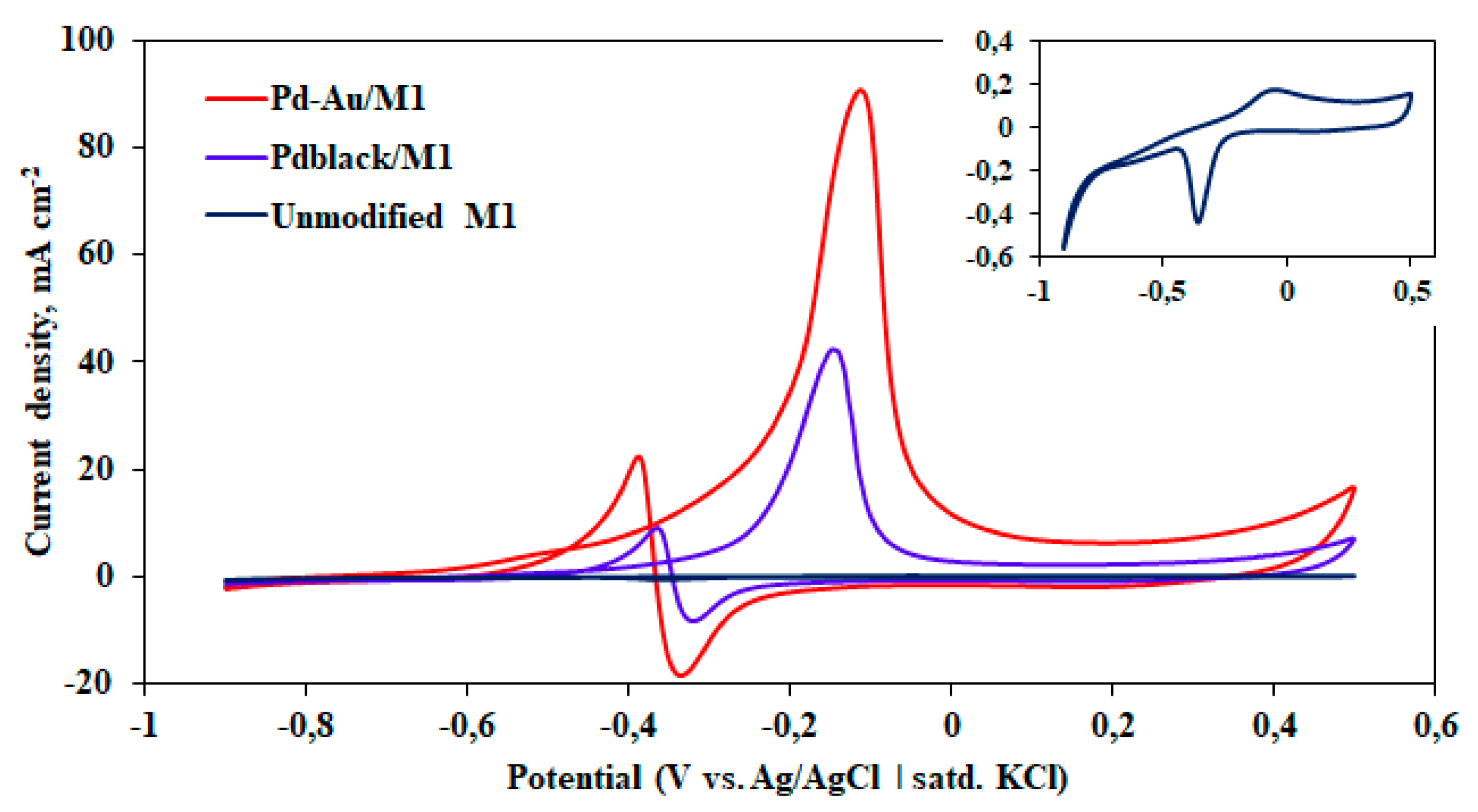
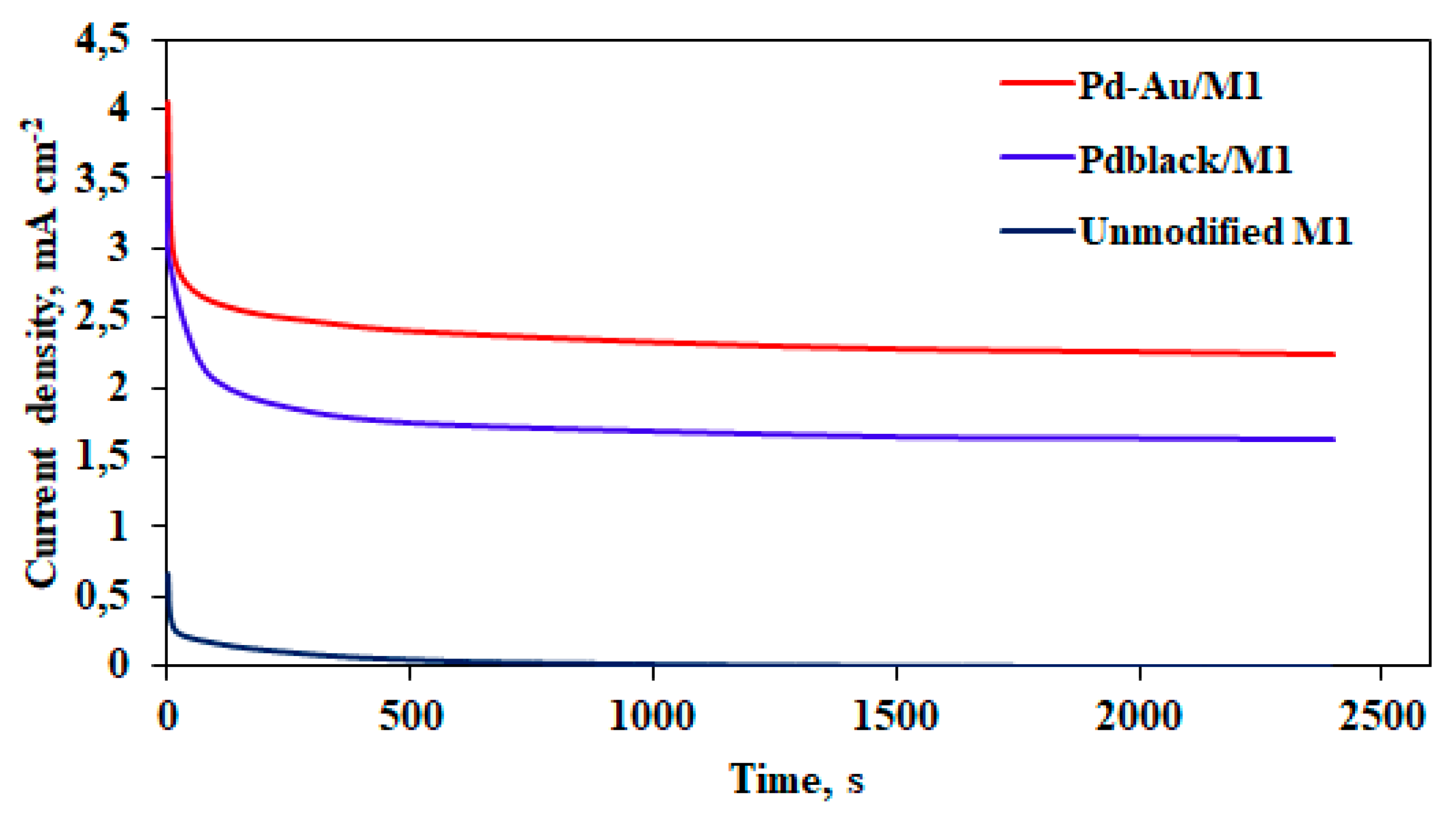
2.3. Study of Developed Materials in Catalytic and Membrane Applications
3. Results and Discussion
3.1. Structural Characteristics of the Developed Membrane Materials
2.2. Morphology of Nanostructured Coatings and Catalytic Characteristics of Modified Films
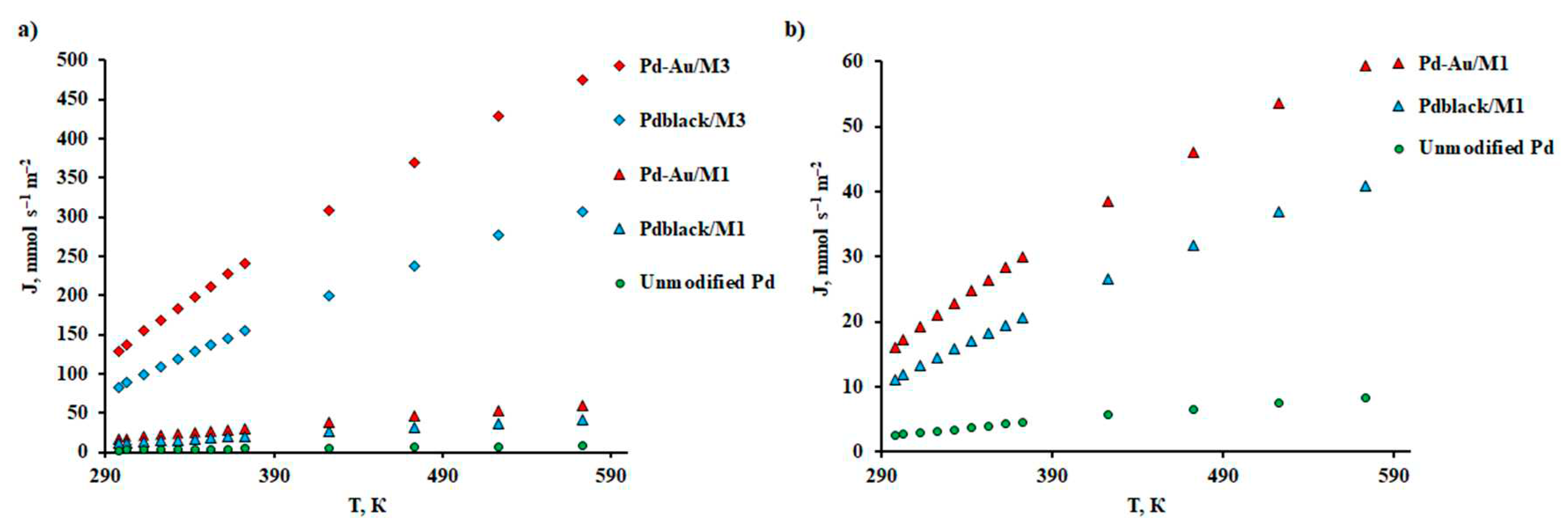
2.3. Investigation of Modified Membrane Materials in Hydrogen Transport Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ongis, M.; Di Marcoberardino, G.; Baiguini, M.; Gallucci, F.; Binotti, M. Optimization of Small-Scale Hydrogen Production with Membrane Reactors. Membranes 2023, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Stenina, I.; Yurova, P.; Achoh, A.; Zabolotsky, V.; Wu, L.; Yaroslavtsev, A. Improvement of Selectivity of RALEX-CM Membranes via Modification by Ceria with a Functionalized Surface. Polymers 2023, 15, 647. [Google Scholar] [CrossRef] [PubMed]
- Falina, I.; Kononenko, N.; Timofeev, S.; Rybalko, M.; Demidenko, K. Nanocomposite Membranes Based on Fluoropolymers for Electrochemical Energy Sources. Membranes 2022, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Butylskii, D.; Troitskiy, V.; Chuprynina, D.; Kharchenko, I.; Ryzhkov, I.; Apel, P.; Pismenskaya, N.; Nikonenko, V. Selective Separation of Singly Charged Chloride and Dihydrogen Phosphate Anions by Electrobaromembrane Method with Nanoporous Membranes. Membranes 2023, 13, 455. [Google Scholar] [CrossRef]
- Achoh, A.R.; Pribytkov, F.B.; But, A.Yu.; Zabolotsky, V.I. Exchange sorption and electrical conductivity of heterogeneous anion-exchange membranes in mixed sodium hydroxide/sodium naphthenate and sodium sulfate/sodium nitrate nlectrolyte solutions. Petroleum Chemistry 2018, 58, 1159–1164. [Google Scholar] [CrossRef]
- Shkirskaya, S.A.; Kononenko, N.A.; Timofeev, S.V. Structural and Electrotransport Properties of Perfluorinated Sulfocationic Membranes Modified by Silica and Zirconium Hydrophosphate. Membranes 2022, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Kozmai, A.E.; Mareev, S.A.; Butylskii, D.Yu.; Ruleva, V.D.; Pismenskaya, N.D.; Nikonenko, V.V. Low-frequency impedance of ion-exchange membrane with electrically heterogeneous surface. Electrochim. Acta 2023, 451, 142285. [Google Scholar] [CrossRef]
- Fedotov, A.S.; Tsodikov, M.V.; Yaroslavtsev, A.B. Hydrogen Production in Catalytic Membrane Reactors Based on Porous Ceramic Converters. Processes 2022, 10, 2060. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambra, S.; Hayakawa, Y. Performance evaluation of hydrogen permeation through pd/cu membrane at different plasma system conditions. S. Afr. J. Chem. Eng. 2021, 35, 118–125. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Shostak, N.; Baryshev, M. Gas-Transport Characteristics of PdCu–Nb–PdCu Membranes Modified with Nanostructured Palladium Coating. Int. J. Mol. Sci. 2022, 23, 228. [Google Scholar] [CrossRef]
- Ryu, S.; Badakhsh, A.; Oh, J.G.; Ham, H.C.; Sohn, H.; Yoon, S.P.; Choi, S.H. Experimental and Numerical Study of Pd/Ta and PdCu/Ta Composites for Thermocatalytic Hydrogen Permeation. Membranes 2023, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Fasolin, S.; Barison, S.; Agresti, F.; Battiston, S.; Fiameni, S.; Isopi, J.; Armelao, L. New Sustainable Multilayered Membranes Based on ZrVTi for Hydrogen Purification. Membranes 2022, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Yang, Z.; Du, M.; Mi, J.; Hao, L.; Tong, Y.; Feng, Y.; Li, S. Effect of annealing process on the hydrogen permeation through Pd–Ru membrane. J. Membr. Sci. 2022, 654, 120572. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Lutsenko, I.S.; Baryshev, M.G. The influence of a crystallographically atypical pentagonal nanostructured coating on the limiting stage of low-temperature hydrogen transport through Pd–Cu membranes. Doklady Physics 2021, 66, 209–213. [Google Scholar] [CrossRef]
- Nam, S.-E.; Lee, K.-H. Hydrogen separation by Pd alloy composite membranes: introduction of diffusion barrier. J. Membr. Sci. 2001, 192, 177–185. [Google Scholar] [CrossRef]
- Nam, S.-E.; Lee, K.-H. Preparation and Characterization of Palladium Alloy Composite Membranes with a Diffusion Barrier for Hydrogen Separation. Ind. Eng. Chem. Res. 2005, 44, 100–105. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, M.M.; Ilias, S. Characterization of Pd–Cu membranes fabricated by surfactant induced electroless plating (SIEP) for hydrogen separation. Int. J. Hydrog. Energy 2012, 37, 3477–3490. [Google Scholar] [CrossRef]
- Kim, D.-W.; Park, Y.J.; Moon, J.-W.; Ryi, S.-K.; Park, J.-S. The effect of Cu reflow on the Pd–Cu–Ni ternary alloy membrane fabrication for infinite hydrogen separation. Thin Solid Films 2008, 516, 3036–3044. [Google Scholar] [CrossRef]
- Knapton, A.G. Palladium alloys for hydrogen diffusion membranes. Platinum Metals Review 1977, 21, 44–50. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=ad97e592b7172231570bd8b7f71f4c52064c6cf1.
- Pati, S.; Ashok, J.; Dewangan, N.; Chen, T.; Kawi, S. Ultra-thin (~1 μm) Pd–Cu membrane reactor for coupling CO2 hydrogenation and propane dehydrogenation applications. J. Membr. Sci. 2020, 595, 117496. [Google Scholar] [CrossRef]
- Nooijer, N.d.; Arratibel Plazaola, A.; Meléndez Rey, J.; Fernandez, E.; Pacheco Tanaka, D.A.; Sint Annaland, M.v.; Gallucci, F. Long-Term Stability of Thin-Film Pd-Based Supported Membranes. Processes 2019, 7, 106. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Zhu, H.; Feng, J.; Jiang, H.; An, F.; Jin, Y.; Xu, W.; Yang, Z.; Sun, B. Hydrophobically modified Pd membrane for the efficient purification of hydrogen in light alcohols steam reforming process. J. Membr. Sci. 2022, 647, 120326. [Google Scholar] [CrossRef]
- Petriev, I.S.; Lutsenko, I.S.; Pushankina, P.D.; Frolov, V.Yu.; Glazkova, Yu.S.; Malkov, T.I.; Gladkikh, A.M.; Otkidach, M.A.; Sypalo, E.B.; Baryshev, P.M.; Shostak, N.A.; Kopytov, G.F. Hydrogen Transport through Palladium-Coated Niobium Membranes. Russ. Phys. J. 2022, 65, 312–316. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Michienzi, V.; Calles, J.A.; Sanz, R.; Caravella, A.; Alique, D. Versatile and Resistant Electroless Pore-Plated Pd-Membranes for H2-Separation: Morphology and Performance of Internal Layers in PSS Tubes. Membranes 2022, 12, 530. [Google Scholar] [CrossRef] [PubMed]
- Bosko, M.L.; Fontana, A.D.; Tarditi, A.; Cornaglia, L. Advances in hydrogen selective membranes based on palladium ternary alloys. Int. J. Hydrog. Energy 2021, 46, 15572–15594. [Google Scholar] [CrossRef]
- Zhu, K.; Li, X.; Zhang, Y.; Zhao, X.; Liu, Z.; Guo, J. Tailoring the hydrogen transport properties of highly permeable Nb51W5Ti23Ni21 alloy membrane by Pd substitution. Int. J. Hydrog. Energy 2022, 47, 6734–6744. [Google Scholar] [CrossRef]
- Alrashed, F.S.; Paglieri, S.N.; Alismail, Z.S.; Khalaf, H.; Harale, A.; Overbeek, J.P.; van Veen, H.M.; Hakeem, A.S. Steam reforming of simulated pre-reformed naphtha in a PdAu membrane reactor. Int. J. Hydrog. Energy 2021, 46, 21939–21952. [Google Scholar] [CrossRef]
- Sazali, N. A comprehensive review of carbon molecular sieve membranes for hydrogen production and purification. Int. J. Adv. Manuf. Technol. 2020, 107, 2465–2483. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, S.; Zhang, M.; Liao, N. Selective and efficient hydrogen separation of Pd–Au–Ag ternary alloy membrane. Int. J. Hydrog. Energy 2022, 47, 13054–13061. [Google Scholar] [CrossRef]
- Han, Z.; Xu, K.; Liao, N.; Xue, W. Theoretical investigations of permeability and selectivity of Pd–Cu and Pd–Ni membranes for hydrogen separation. Int. J. Hydrog. Energy 2021, 46, 23715–23722. [Google Scholar] [CrossRef]
- Agnolin, S.; Melendez, J.; Di Felice, L.; Gallucci, F. Surface roughness improvement of Hastelloy X tubular filters for H2 selective supported Pd–Ag alloy membranes preparation. Int. J. Hydrog. Energy 2022, 47, 28505–28517. [Google Scholar] [CrossRef]
- Wei, W.; Liu, L.C.; Gong, H.R.; Song, M.; Chang, M.L.; Chen, D.C. Fundamental mechanism of BCC-FCC phase transition from a constructed PdCu potential through molecular dynamics simulation. Comput. Mater. Sci. 2019, 159, 440–447. [Google Scholar] [CrossRef]
- Zhao, C.; Goldbach, A.; Xu, H. Low-temperature stability of body-centered cubic PdCu membranes. J. Membr. Sci. 2017, 542, 60–67. [Google Scholar] [CrossRef]
- Moon, D.-K.; Han, Y.-J.; Bang, G.; Kim, J.-H.; Lee, C.-H. Palladium-copper membrane modules for hydrogen separation at elevated temperature and pressure. Korean J. Chem. Eng. 2019, 36, 563–572. [Google Scholar] [CrossRef]
- Howard, B.H.; Killmeyer, R.P.; Rothenberger, K.S.; Cugini, A.V.; Morreale, B.D.; Enick, R.M.; Bustamante, F. Hydrogen permeance of palladium–copper alloy membranes over a wide range of temperatures and pressures. J. Membr. Sci. 2004, 241, 207–218. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Speight, J.; Book, D. Effects of low Ag additions on the hydrogen permeability of Pd–Cu–Ag hydrogen separation membranes. J. Membr. Sci. 2014, 451, 216–225. [Google Scholar] [CrossRef]
- Martin, M.H.; Galipaud, J.; Tranchot, A.; Roué, L.; Guay, D. Measurements of hydrogen solubility in CuxPd100−x thin films. Electrochim. Acta 2013, 90, 615–622. [Google Scholar] [CrossRef]
- Yuan, L.; Goldbach, A.; Xu, H. Segregation and H2 Transport Rate Control in Body-Centered Cubic PdCu Membranes. J. Phys. Chem. B 2007, 37, 10952–10958. [Google Scholar] [CrossRef]
- Gao, M.C.; Ouyang, L.; Doğan, Ö.N. First principles screening of B2 stabilizers in CuPd-based hydrogen separation membranes: (1) Substitution for Pd. J. Alloys Compd. 2013, 574, 368–376. [Google Scholar] [CrossRef]
- Yuan, L.; Goldbach, A.; Xu, H. Permeation Hysteresis in PdCu Membranes. J. Phys. Chem. B 2008, 112, 12692–12695. [Google Scholar] [CrossRef]
- Opalka, S.M.; Huang, W.; Wang, D.; Flanagan, T.B.; Løvvik, O.M.; Emerson, S.C.; She, Y.; Vanderspurt, T.H. Hydrogen interactions with the PdCu ordered B2 alloy. J. Alloys Compd. 2007, 583–587. [Google Scholar] [CrossRef]
- Shinoda, Y.; Takeuchi, M.; Dezawa, N.; Komo, Y.; Harada, T.; Takasu, H.; Kato, Y. Development of a H2-permeable Pd60Cu40-based composite membrane using a reverse build-up method. Int. J. Hydrog. Energy 2021, 46, 36291–36300. [Google Scholar] [CrossRef]
- Roa, F.; Block, M.J.; Way, J.D. The influence of alloy composition on the H2 flux of composite PdCu membranes. Desalination 2002, 147, 411–416. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Liang, X.; Chen, R.; Guo, J.; Fu, H.; Liu, D. A two-step electroless plating method for Pd composite membranes with enhanced hydrogen selectivity and superior high-temperature stability. J. Environ. Chem. Eng. 2022, 10, 108477. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Alique, D.; Calles, J.A.; Sanz, R. Pd-thickness reduction in electroless pore-plated membranes by using doped-ceria as interlayer. Int. J. Hydrog. Energy 2020, 45, 7278–7289. [Google Scholar] [CrossRef]
- Melendez, J.; Fernandez, E.; Gallucci, F.; van Sint Annaland, M.; Arias, P.L.; Pacheco Tanaka, D.A. Preparation and characterization of ceramic supported ultra-thin (~1 µm) Pd-Ag membranes. J. Membr. Sci. 2017, 528, 12–23. [Google Scholar] [CrossRef]
- Gao, H.; Lin, J.Y.S.; Li, Y.; Zhang, B. Electroless plating synthesis, characterization and permeation properties of Pd–Cu membranes supported on ZrO2 modified porous stainless steel. J. Membr. Sci. 2005, 265, 142–152. [Google Scholar] [CrossRef]
- Dube, S.; Gorimbo, J.; Moyo, M.; Okoye-Chine, C.G.; Liu, X. Synthesis and application of Ni-based membranes in hydrogen separation and purification systems: A review. J. Environ. Chem. Eng. 2023, 11, 109194. [Google Scholar] [CrossRef]
- Hoang, H.T.; Tong, H.D.; Gielens, F.C.; Jansen, H.V.; Elwenspoek, M.C. Fabrication and characterization of dual sputtered Pd–Cu alloy films for hydrogen separation membranes. Mater. Lett. 2004, 58, 525–528. [Google Scholar] [CrossRef]
- Ryi, S.-K.; Park, J.-S.; Kim, S.-H.; Cho, S.-H.; Kim, D.-W.; Um, K.-Y. Characterization of Pd–Cu–Ni ternary alloy membrane prepared by magnetron sputtering and Cu-reflow on porous nickel support for hydrogen separation. Sep. Purif. Technol. 2006, 50, 82–91. [Google Scholar] [CrossRef]
- Jokar, S.M.; Farokhnia, A.; Tavakolian, M.; Pejman, M.; Parvasi, P.; Javanmardi, J.; Zare, F.; Gonçalves, M.C.; Basile, A. The recent areas of applicability of palladium based membrane technologies for hydrogen production from methane and natural gas: A review. Int. J. Hydrog. Energy 2023, 48, 6451–6476. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Bolotin, S.; Lutsenko, I.; Kukueva, E.; Baryshev, M. The influence of modifying nanoflower and nanostar type Pd coatings on low temperature hydrogen permeability through Pd-containing membranes. J. Membr. Sci. 2021, 620, 118894. [Google Scholar] [CrossRef]
- Vicinanza, N.; Svenum, I.-H.; Peters, T.; Bredesen, R.; Venvik, H. New Insight to the Effects of Heat Treatment in Air on the Permeation Properties of Thin Pd77%Ag23% Membranes. Membranes 2018, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Vielstich, W. Brennstoffelemente. Moderne Verfahren zur elektrochemischen Energlegewfnming; Verlag Chemie: Weinheim, Deutschland, 1965; p. 388. [Google Scholar]
- Basov, A.; Dzhimak, S.; Sokolov, M.; Malyshko, V.; Moiseev, A.; Butina, E.; Elkina, A.; Baryshev, M. Changes in Number and Antibacterial Activity of Silver Nanoparticles on the Surface of Suture Materials during Cyclic Freezing. Nanomaterials 2022, 12, 1164. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Glazkova, Y.; Andreev, G.; Baryshev, M. Investigation of the Dependence of Electrocatalytic Activity of Copper and Palladium Nanoparticles on Morphology and Shape Formation. Coatings 2023, 13, 621. [Google Scholar] [CrossRef]
- Pushankina, P.; Baryshev, M.; Petriev, I. Synthesis and Study of Palladium Mono- and Bimetallic (with Ag and Pt) Nanoparticles in Catalytic and Membrane Hydrogen Processes. Nanomaterials 2022, 12, 4178. [Google Scholar] [CrossRef]
- Petriev, I.S.; Pushankina, P.D.; Lutsenko, I.S.; Baryshev, M.G. Anomalous Kinetic Characteristics of Hydrogen Transport through Pd–Cu Membranes Modified by Pentatwinned Flower-Shaped Palladium Nanocrystallites with High-Index Facets. Tech. Phys. Lett. 2021, 47, 803–806. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Lutsenko, I.; Shostak, N.; Baryshev, M. Synthesis, Electrocatalytic and Gas Transport Characteristics of Pentagonally Structured Star-Shaped Nanocrystallites of Pd-Ag. Nanomaterials 2020, 10, 2081. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, J.-J.; Jiang, K.; Wang, J.-Q.; Cai, W.-B. Pd–Cu/C electrocatalysts synthesized by one-pot polyol reduction toward formic acid oxidation: Structural characterization and electrocatalytic performance. Int. J. Hydrog. Energy 2015, 40, 1726–1734. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, W.; Chen, W.; Wu, Y.; Xu, Q.; Yan, Y.; Zhang, H.; Wu, J.; Yang, D. PdCu alloy nanodendrites with tunable composition as highly active electrocatalysts for methanol oxidation. RSC Adv. 2017, 7, 5800–5806. [Google Scholar] [CrossRef]
- Shan, S.; Petkov, V.; Prasai, B.; Wu, J.; Joseph, P.; Skeete, Z.; Kim, E.; Mott, D.; Malis, O.; Luoa, J.; Zhong, C.-J. Catalytic activity of bimetallic catalysts highly sensitive to the atomic composition and phase structure at the nanoscale. Nanoscale 2015, 7, 18936–18948. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Nayebossadri, S.; Speight, J.D.; Bujalski, W.; Steinberger-Wilckens, R.; Book, D. Effects of thin film Pd deposition on the hydrogen permeability of Pd60Cu40 wt% alloy membranes. J. Membr. Sci. 2015, 493, 580–588. [Google Scholar] [CrossRef]
- Yeon, S.; Lee, S.J.; Chinnadurai, D.; Yu, Y.; Lee, Y.W.; Choi, M.Y. Rapid alloying of Au–Pd nanospheres by a facile pulsed laser technique: Insights into a molar-dependent electrocatalytic methanol oxidation reaction. J. Alloys Compd. 2022, 891, 162011. [Google Scholar] [CrossRef]
- Woo, S.; Lee, J.; Park, S.-K.; Kim, H.; Chung, T. D.; Piao, Y. Electrochemical codeposition of Pt/graphene catalyst for improved methanol oxidation. Current Applied Physics 2015, 15, 219–225. [Google Scholar] [CrossRef]
- Huang, W.; Kang, X.; Xu, C.; Zhou, J.; Deng, J.; Li, Y.; Cheng, S. 2D PdAg Alloy Nanodendrites for Enhanced Ethanol Electroxidation. Adv. Mater. 2018, 30, 1706962. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chi, M.; Zhu, Q.; Ma, D.; Sune, J.; Bao, X. Supported bimetallic PdAu nanoparticles with superior electrocatalytic activity towards methanol oxidation. J. Mater. Chem. A 2013, 1, 9157–9163. [Google Scholar] [CrossRef]
- Pacheco Tanaka, D.A.; Llosa Tanco, M.A.; Okazaki, J.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Preparation of “pore-fill” type Pd–YSZ–γ-Al2O3 composite membrane supported on α-Al2O3 tube for hydrogen separation. J. Membr. Sci. 2008, 320, 436–441. [Google Scholar] [CrossRef]
- Arratibel, A.; Astobieta, U.; Pacheco Tanaka, D.A.; van Sint Annaland, M.; Gallucci, F. N2, He and CO2 diffusion mechanism through nanoporous YSZ/γ-Al2O3 layers and their use in a pore-filled membrane for hydrogen membrane reactors. Int. J. Hydrog. Energy 2016, 41, 8732–8744. [Google Scholar] [CrossRef]
- Nair, B.K.R.; Choi, J.; Harold, M.P. Electroless plating and permeation features of Pd and Pd/Ag hollow fiber composite membranes. J. Membr. Sci. 2007, 288, 67–84. [Google Scholar] [CrossRef]
- Balachandran, U. (Balu); Lee, T.H.; Park, C.Y.; Emerson, J.E.; Picciolo, J.J.; Dorris, S.E. Dense cermet membranes for hydrogen separation. Sep. Purif. Technol. 2014, 121, 54–59. [Google Scholar] [CrossRef]
- Sato, K.; Nishioka, M.; Higashi, H.; Inoue, T.; Hasegawa, Y.; Wakui, Y.; Suzuki, T.M.; Hamakawa, S. Influence of CO2 and H2O on the separation of hydrogen over two types of Pd membranes: Thin metal membrane and pore-filling-type membrane. J. Membr. Sci. 2012, 415–416, 85–92. [Google Scholar] [CrossRef]
- Nair, B.K.R.; Harold, M.P. Pd encapsulated and nanopore hollow fiber membranes: Synthesis and permeation studies. J. Membr. Sci. 2007, 290, 182–195. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ghasemzadeh, K.; Marelli, M.; Evangelisti, C. A supported Pd-Cu/Al2O3 membrane from solvated metal atoms for hydrogen separation/purification. Fuel Process. Technol. 2019, 195, 106141. [Google Scholar] [CrossRef]
- Pan, X.; Kilgus, M.; Goldbach, A. Low-temperature H2 and N2 transport through thin Pd66Cu34Hx layers. Catal. Today 2005, 104, 225–230. [Google Scholar] [CrossRef]
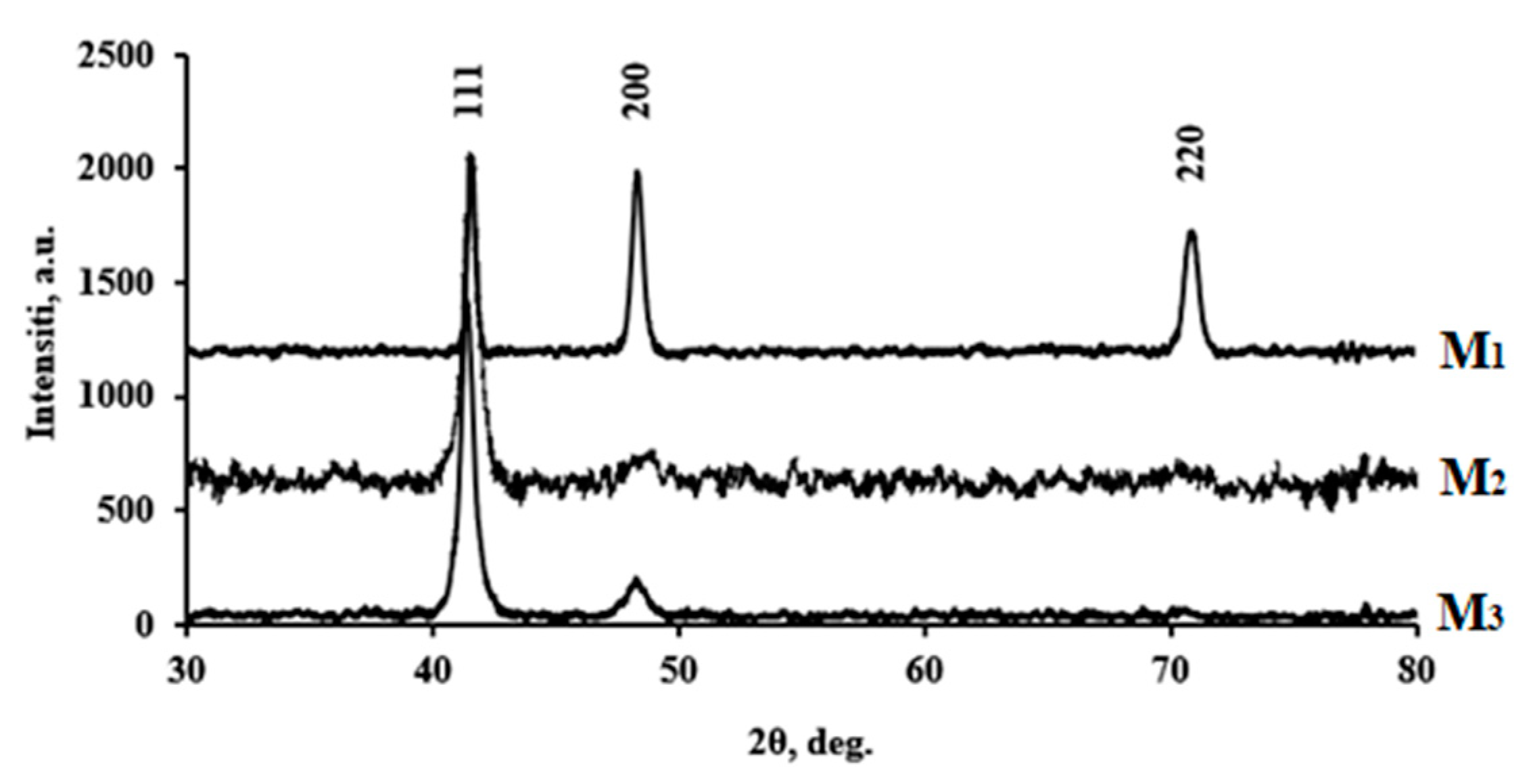
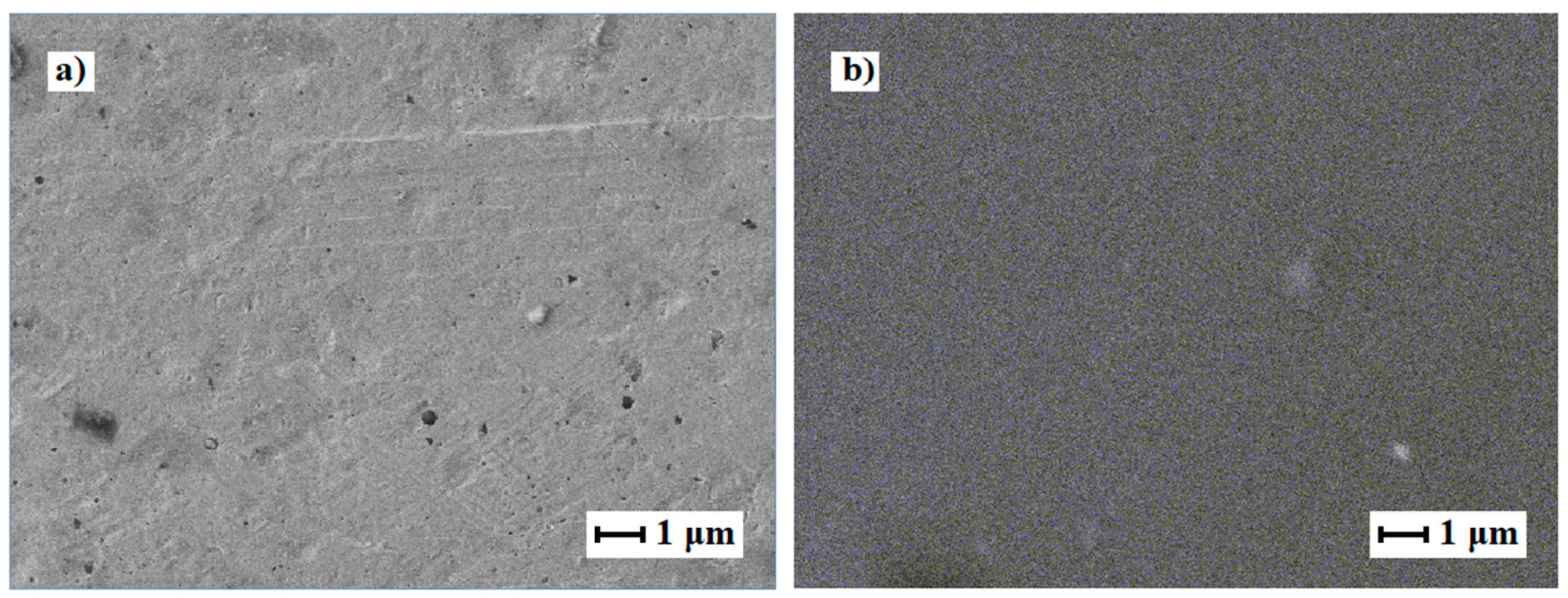
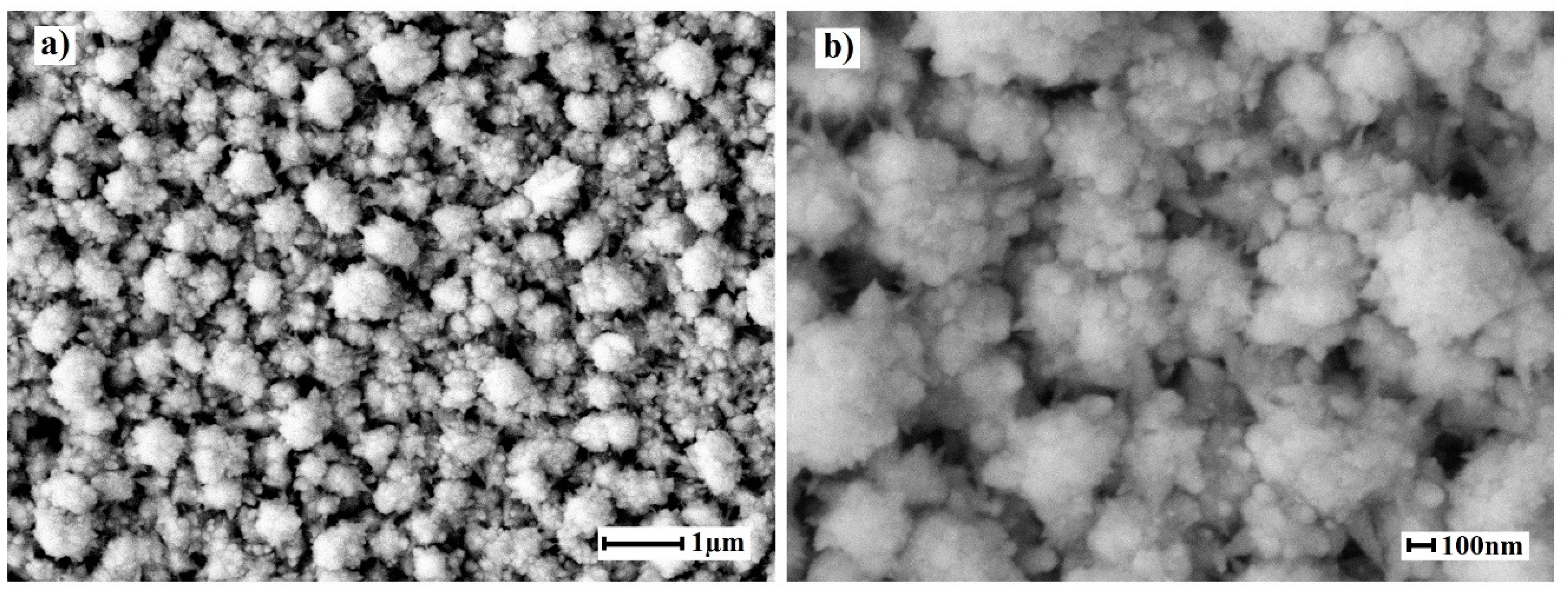
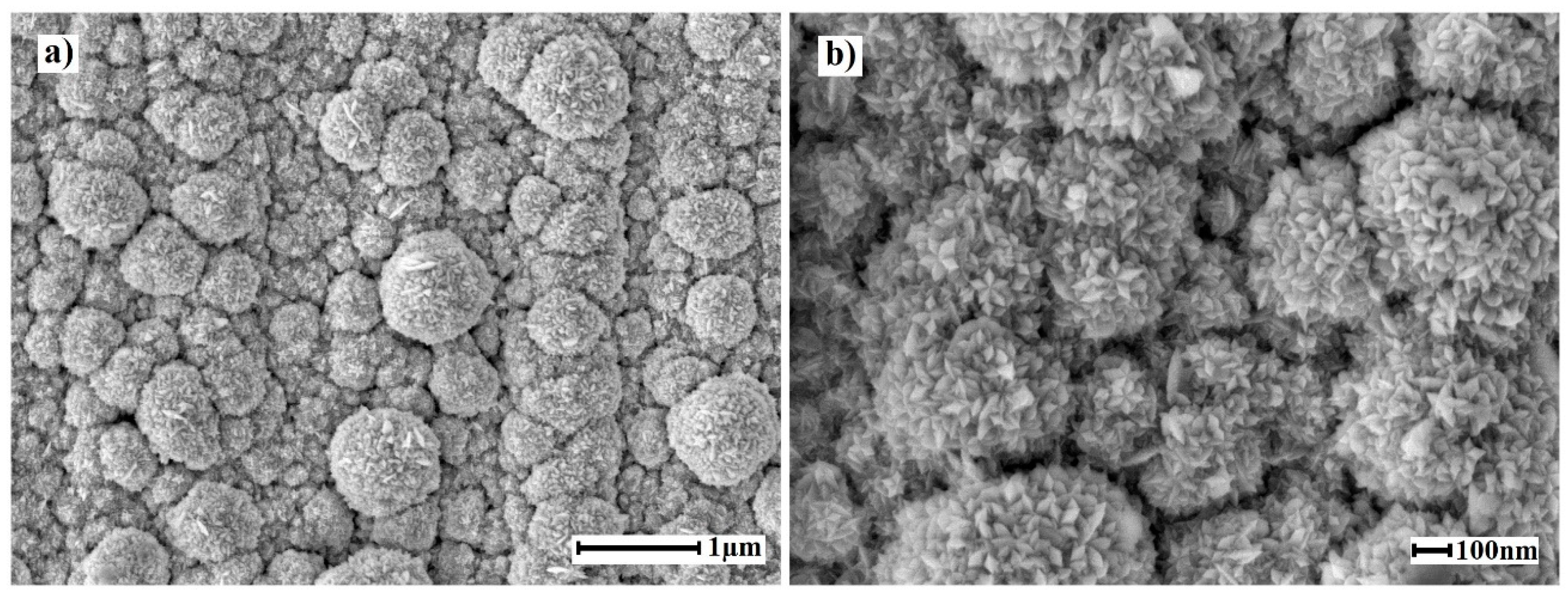

| Film | Elemental composition | a, Å | D, nm | Reference |
|---|---|---|---|---|
| Pd-Cu M1 | Pd 60.08 Cu 39.92 |
3.76±0.002 | 30.5 | This work |
| Pd-Cu M2 | Pd 59.96 Cu 40.04 |
3.76±0.06 | 21.1 | This work |
| Pd-Cu M3 | Pd 60.31 Cu 39.69 |
3.77±0.003 | 22.1 | This work |
| Pd-Cu | Pd 50 Cu 50 |
3.77 | – | [11] |
| Pd-Cu | Pd 55.31 Cu 44.69 |
3.867 | – | [17] |
| Pd-Cu | Pd 53.1±0.4 Cu 46.9±0.4 |
3.775 | – | [36] |
| Pd-Cu | Pd 51.9±0.4 Cu 48.1±0.4 |
3.763 | – | [38] |
| Pd-Cu | Pd 46 Cu 54 |
3.782 | – | [47] |
| Pd-Cu | Pd 59.2±0.8 Cu 40.8±0.8 |
3.757±0.003 | – | [63] |
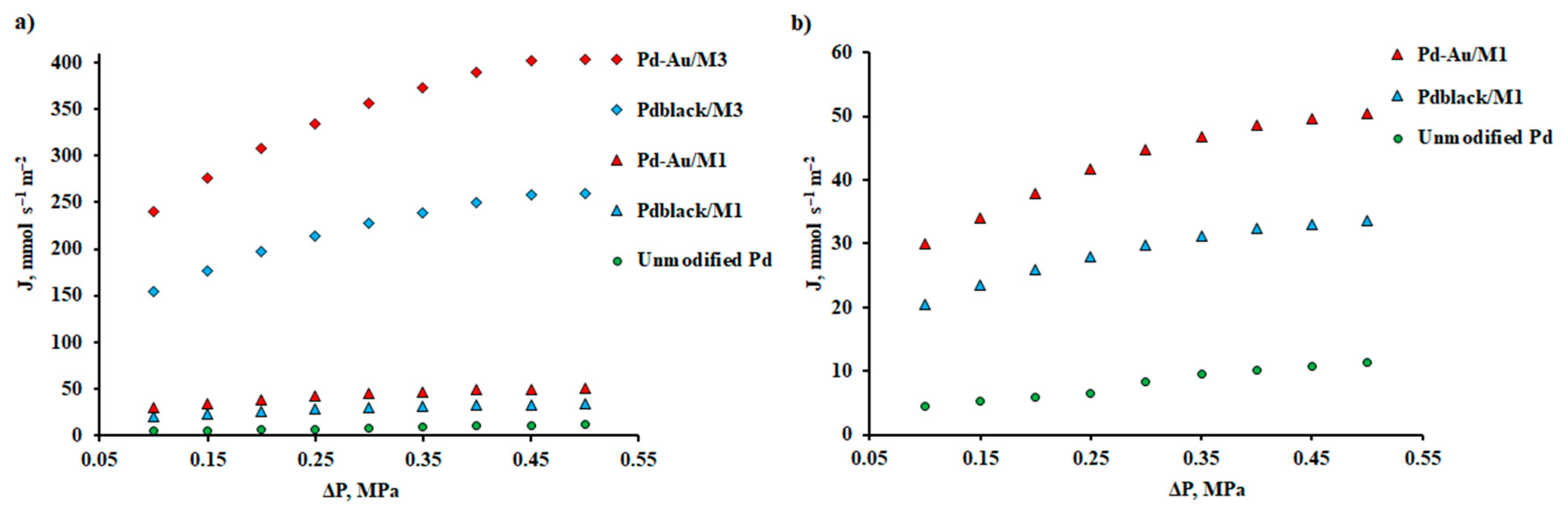
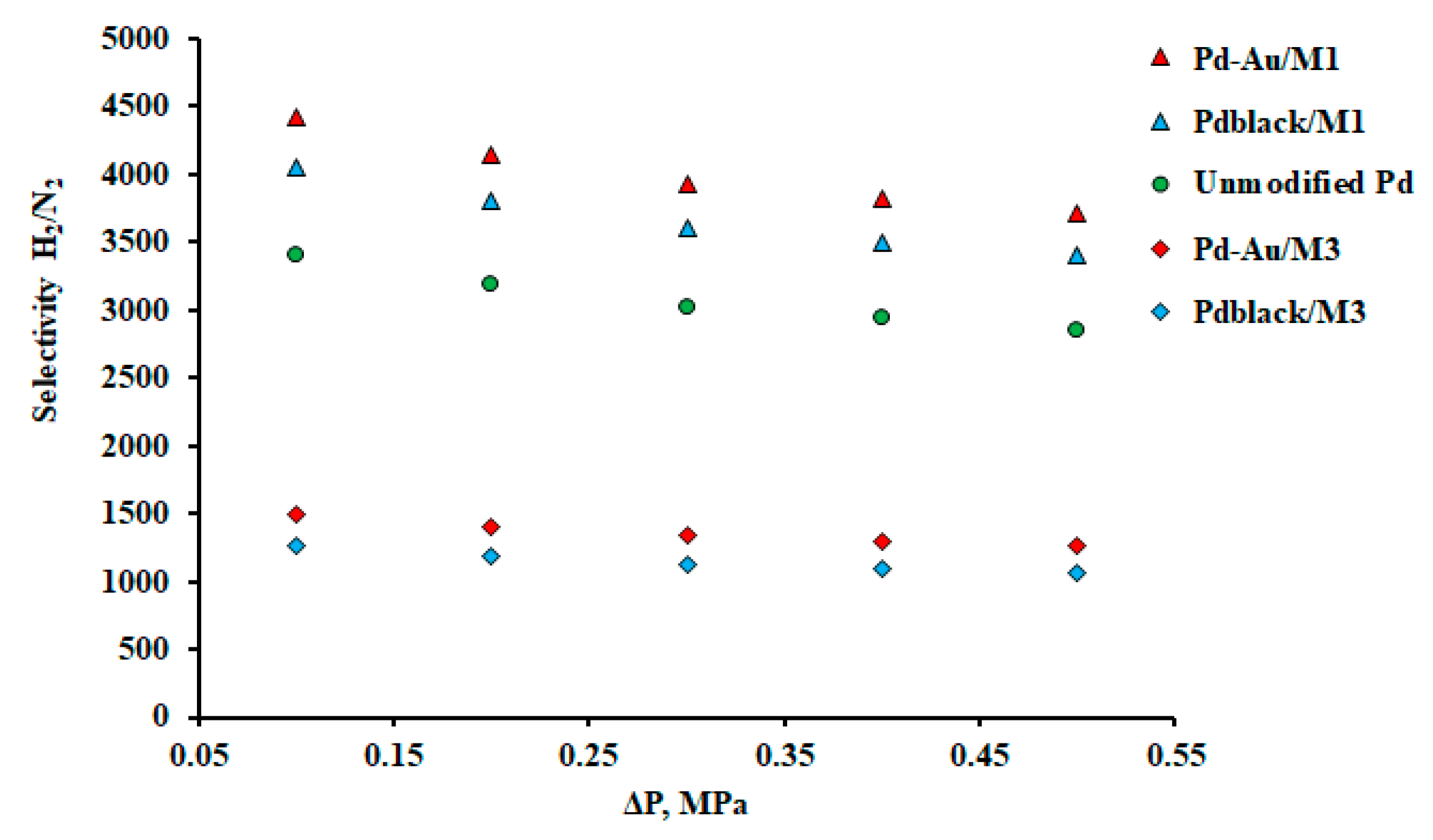
| Membrane | Support | Thickness, μm | J, mmol s–1 m–2 | Temperature, К | ∆p, kPa | Selectivity H2/N2 |
Reference |
|---|---|---|---|---|---|---|---|
| Pd | YSZ – Al2O3 | 5 | 450 | 623 | 390 | 350 | [68] |
| Pd | Al2O3 | 10 | 95 | 573 | 200 | 176 | [69] |
| Pd88Ag12 | Al2O3 | 11 | 200 | 623 | 160 | 2073 | [70] |
| PdCu/Ta | – | 250 | 5.2 | 673 | 100 | – | [11] |
| 50 vol. % Pd-GDC | – | 282 | 41 | 1173 | <100 | – | [71] |
| 50 vol. % Pd-CZY | – | 500 | 17 | 1173 | <100 | – | [72] |
| 50 vol. % Pd-YSZ | – | 218 | 24 | 1173 | <100 | – | [73] |
| Pd70Cu30 | Al2O3 | 20 | 105 | 573 | 100 | 1500 | [74] |
| Pd66Cu34 | Al2O3 | 4 | 190 | 783 | 350 | 5000 | [75] |
| Pd47Cu53 | Al2O3 – ZrO2 | 3.5 | 220 | 573 | 500 | 100 | [33] |
| Pd | – | 20 | 0.11 | 373 | 500 | 3399 | This work |
| Pd-Au / М1 | – | 20 | 59.41 | 573 | 100 | 4419 | This work |
| Pdblack / М1 | – | 20 | 40.97 | 573 | 100 | 4055 | This work |
| Pd-Au / М3 | Al2O3 | 0.3 | 475.28 | 573 | 100 | 1503 | This work |
| Pdblack / М3 | Al2O3 | 0.3 | 307.29 | 573 | 100 | 1263 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





