Submitted:
30 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
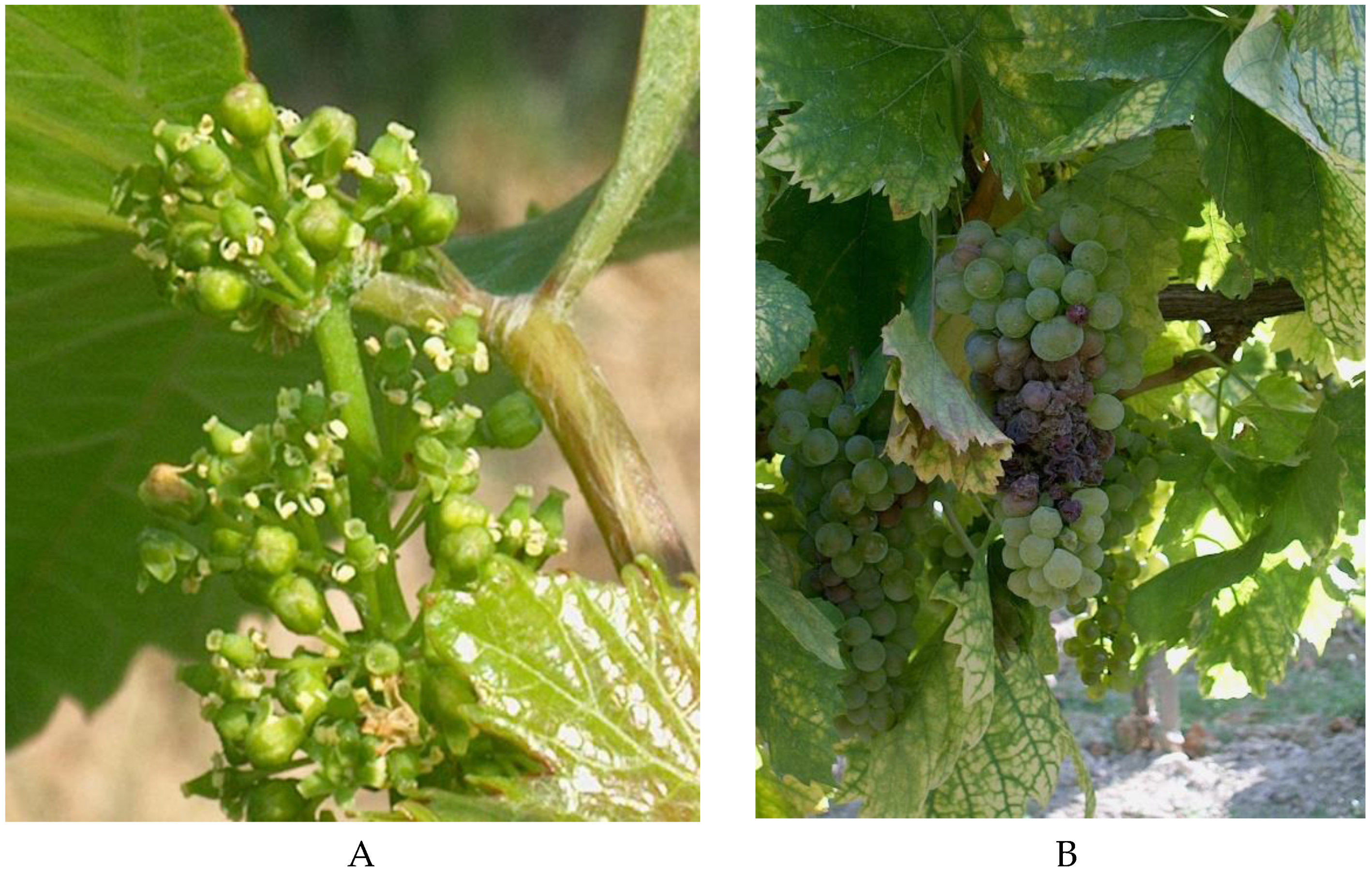
2. Materials and Methods
2.1. Experinemtal site, vineyard and growing conditions
2.2. Experimental harvest, measures
2.3. Calculations, data analyses
3. Results
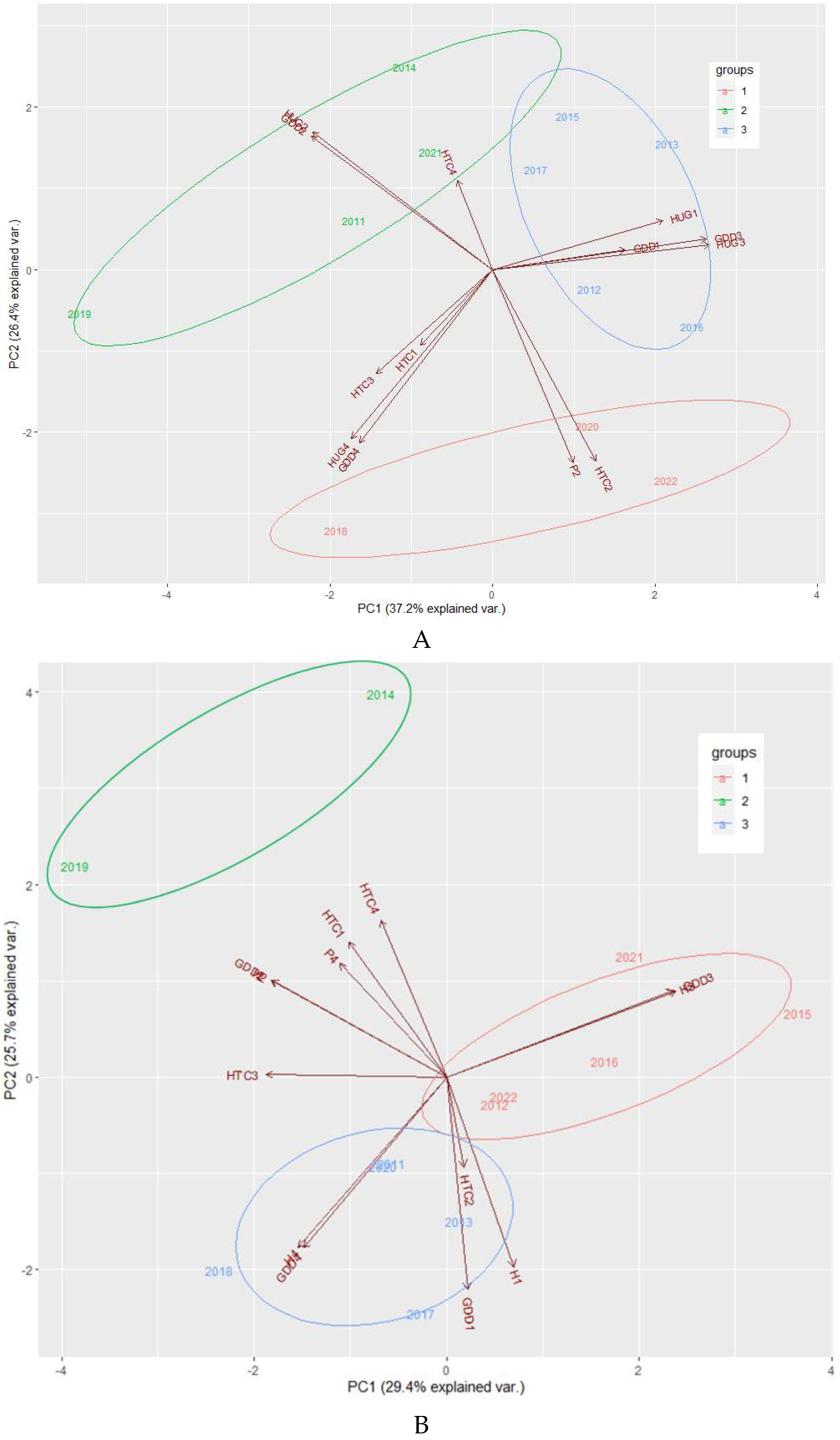
3.1. Evaluation of the meteorological data and indexes
| Year | Budburst | Beginning of flowering | End of flowering | Veraison | Maturity/Harvest |
|---|---|---|---|---|---|
| 2011 | 04. 13. | 05. 30. | 06. 13. | 07. 30. | 09. 21. |
| 2012 | 04. 11. | 05. 29. | 06. 07. | 07. 27. | 09. 06. |
| 2013 | 04. 23. | 06. 07. | 06. 17. | 08. 08. | 10. 01. |
| 2014 | 04. 07. | 06. 04. | 06. 15. | 08. 05. | 09. 22. |
| 2015 | 04. 20. | 06. 04. | 06. 13. | 08. 04. | 09. 12. |
| 2017 | 04. 06. | 06. 08. | 06. 19. | 08. 01. | 09. 20. |
| 2018 | 04. 16. | 05. 21. | 05. 28. | 07. 16. | 09. 20. |
| 2019 | 04. 12. | 06. 06. | 06. 20. | 07. 29. | 10. 02. |
| 2020 | 04. 09. | 06. 04. | 06. 12. | 08. 04. | 09. 22. |
| 2021 | 04. 23. | 06. 13. | 06. 23. | 08. 06. | 09. 30. |
| 2022 | 04. 14. | 06. 02. | 06. 09. | 07. 29. | 09. 22. |
| Year | Budburst | Beginning of flowering | End of flowering | Veraison | Maturity/Harvest |
|---|---|---|---|---|---|
| 2011 | 04. 11. | 05. 27. | 06. 08. | 07. 25. | 09. 15. |
| 2012 | 04. 04. | 05. 24. | 06. 06. | 07. 23. | 09. 10. |
| 2013 | 04. 18. | 06. 04. | 06. 14. | 07. 27. | 09. 27. |
| 2014 | 04. 04. | 05. 27. | 06. 09. | 07. 30. | 09. 16. |
| 2015 | 04. 18. | 06. 02. | 06. 06. | 07. 31. | 09. 09. |
| 2017 | 04. 04. | 06. 06. | 06. 15. | 07. 25. | 09. 13. |
| 2018 | 04. 12. | 05. 18. | 05. 25. | 07. 09. | 09. 05. |
| 2020 | 03. 30. | 05. 29. | 06. 09. | 07. 24. | 09. 10. |
| 2021 | 04. 17. | 06. 11. | 06. 18. | 07. 29. | 09. 09. |
| 2022 | 04. 09. | 05. 25. | 06. 02. | 07. 19. | 09. 01. |
| A | |||||||||||||
| YEAR | GDD1 | GDD2 | GDD3 | GDD4 | HUG1 | HUG2 | HUG3 | HUG4 | HTC1 | HTC2 | HTC3 | HTC4 | P2 |
| 2011 | 298.53 | 165.55 | 543.61 | 651.80 | 450.89 | 217.43 | 734.16 | 877.33 | 0.20 | 0.28 | 0.82 | 0.46 | 8.60 |
| 2012 | 284.50 | 82.69 | 695.46 | 590.05 | 441.66 | 119.49 | 910.64 | 782.54 | 0.85 | 0.42 | 0.75 | 0.04 | 7.20 |
| 2013 | 297.59 | 118.33 | 754.67 | 436.35 | 448.66 | 159.35 | 995.75 | 647.92 | 1.12 | 0.39 | 0.42 | 1.28 | 8.60 |
| 2014 | 274.50 | 151.52 | 604.13 | 414.16 | 441.32 | 198.78 | 807.10 | 591.50 | 1.03 | 0.00 | 1.07 | 3.50 | 0.00 |
| 2015 | 299.63 | 125.32 | 670.72 | 488.73 | 449.31 | 162.34 | 886.58 | 665.45 | 0.98 | 0.00 | 0.32 | 0.81 | 0.00 |
| 2016 | 304.80 | 63.50 | 697.20 | 471.00 | 479.17 | 90.67 | 916.86 | 661.61 | 1.39 | 1.11 | 0.94 | 0.60 | 14.80 |
| 2017 | 350.57 | 124.98 | 592.36 | 547.79 | 516.65 | 169.15 | 787.37 | 750.64 | 0.64 | 0.11 | 0.94 | 0.99 | 2.60 |
| 2018 | 298.11 | 80.03 | 556.93 | 882.49 | 419.64 | 107.75 | 753.81 | 1216.37 | 1.30 | 0.84 | 1.26 | 1.17 | 12.60 |
| 2019 | 237.70 | 201.50 | 513.80 | 708.70 | 362.78 | 259.04 | 683.71 | 992.30 | 2.05 | 0.25 | 1.07 | 0.77 | 8.50 |
| 2020 | 274.50 | 71.70 | 637.00 | 568.80 | 463.10 | 102.80 | 857.69 | 783.14 | 0.74 | 1.58 | 1.18 | 0.62 | 24.00 |
| 2021 | 275.20 | 149.80 | 636.10 | 526.40 | 432.23 | 195.72 | 829.08 | 762.04 | 0.85 | 0.00 | 0.69 | 0.51 | 0.00 |
| 2022 | 300.50 | 80.50 | 688.10 | 613.50 | 443.73 | 106.26 | 906.52 | 830.66 | 1.28 | 2.34 | 0.57 | 0.83 | 35.20 |
| B | |||||||||||||
| YEAR | GDD1 | GDD2 | GDD3 | GDD4 | H1 | H2 | H3 | H4 | HTC1 | HTC2 | HTC3 | HTC4 | P4 |
| 2011 | 280.37 | 135.97 | 551.99 | 645.11 | 427.78 | 182.48 | 742.82 | 863.10 | 0.19 | 0.40 | 0.49 | 0.72 | 83.80 |
| 2012 | 256.88 | 108.36 | 653.20 | 693.47 | 401.71 | 159.26 | 854.12 | 922.30 | 1.06 | 0.33 | 0.72 | 0.11 | 12.80 |
| 2013 | 306.02 | 93.10 | 582.11 | 651.14 | 464.79 | 129.41 | 773.54 | 917.78 | 1.19 | 0.50 | 0.52 | 0.77 | 97.60 |
| 2014 | 239.56 | 113.87 | 608.82 | 448.57 | 387.33 | 163.94 | 811.13 | 625.89 | 1.26 | 0.02 | 1.07 | 3.12 | 289.60 |
| 2015 | 273.06 | 54.75 | 711.22 | 531.04 | 418.80 | 71.01 | 937.93 | 712.71 | 1.03 | 0.00 | 0.31 | 0.75 | 69.60 |
| 2016 | 274.60 | 75.50 | 632.30 | 484.10 | 436.59 | 107.10 | 835.80 | 668.80 | 1.19 | 1.56 | 0.97 | 0.77 | 70.20 |
| 2017 | 337.33 | 97.22 | 541.70 | 615.51 | 498.93 | 133.05 | 721.19 | 830.92 | 0.66 | 0.05 | 0.95 | 0.63 | 70.40 |
| 2018 | 298.68 | 66.44 | 513.95 | 799.28 | 420.36 | 91.89 | 690.87 | 1082.17 | 1.29 | 0.92 | 1.18 | 1.43 | 200.50 |
| 2019 | 234.60 | 187.50 | 515.90 | 643.40 | 356.53 | 238.93 | 688.70 | 870.35 | 2.22 | 0.00 | 1.07 | 0.87 | 102.50 |
| 2020 | 256.40 | 75.50 | 509.80 | 587.90 | 435.12 | 115.03 | 698.62 | 790.76 | 0.64 | 1.44 | 1.06 | 1.01 | 107.40 |
| 2021 | 255.70 | 84.70 | 624.10 | 466.80 | 411.50 | 112.88 | 810.18 | 648.69 | 1.19 | 0.32 | 0.30 | 0.90 | 79.90 |
| 2022 | 249.60 | 58.90 | 599.40 | 630.40 | 377.74 | 83.58 | 794.85 | 819.68 | 1.17 | 1.40 | 0.96 | 0.60 | 64.70 |
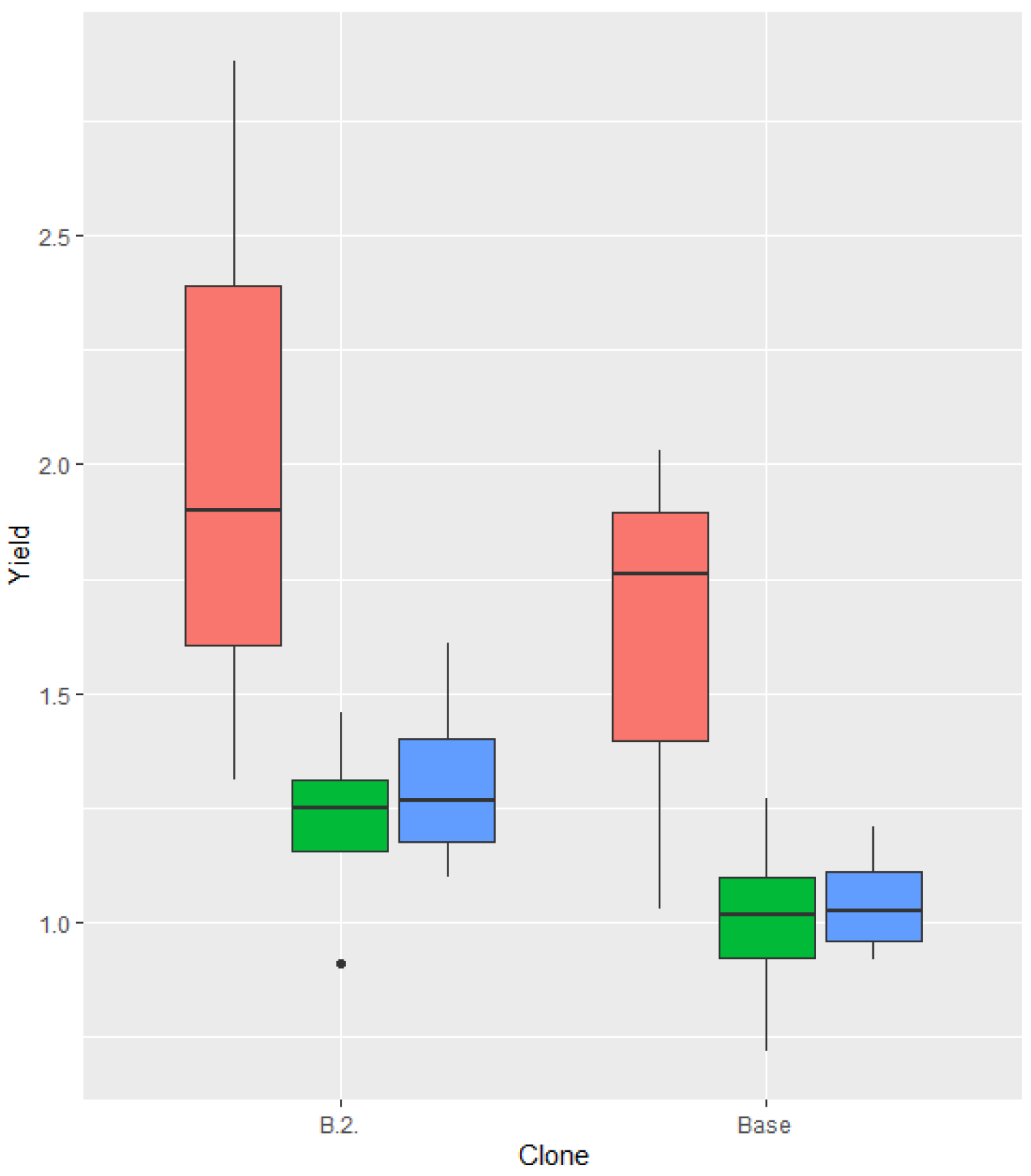
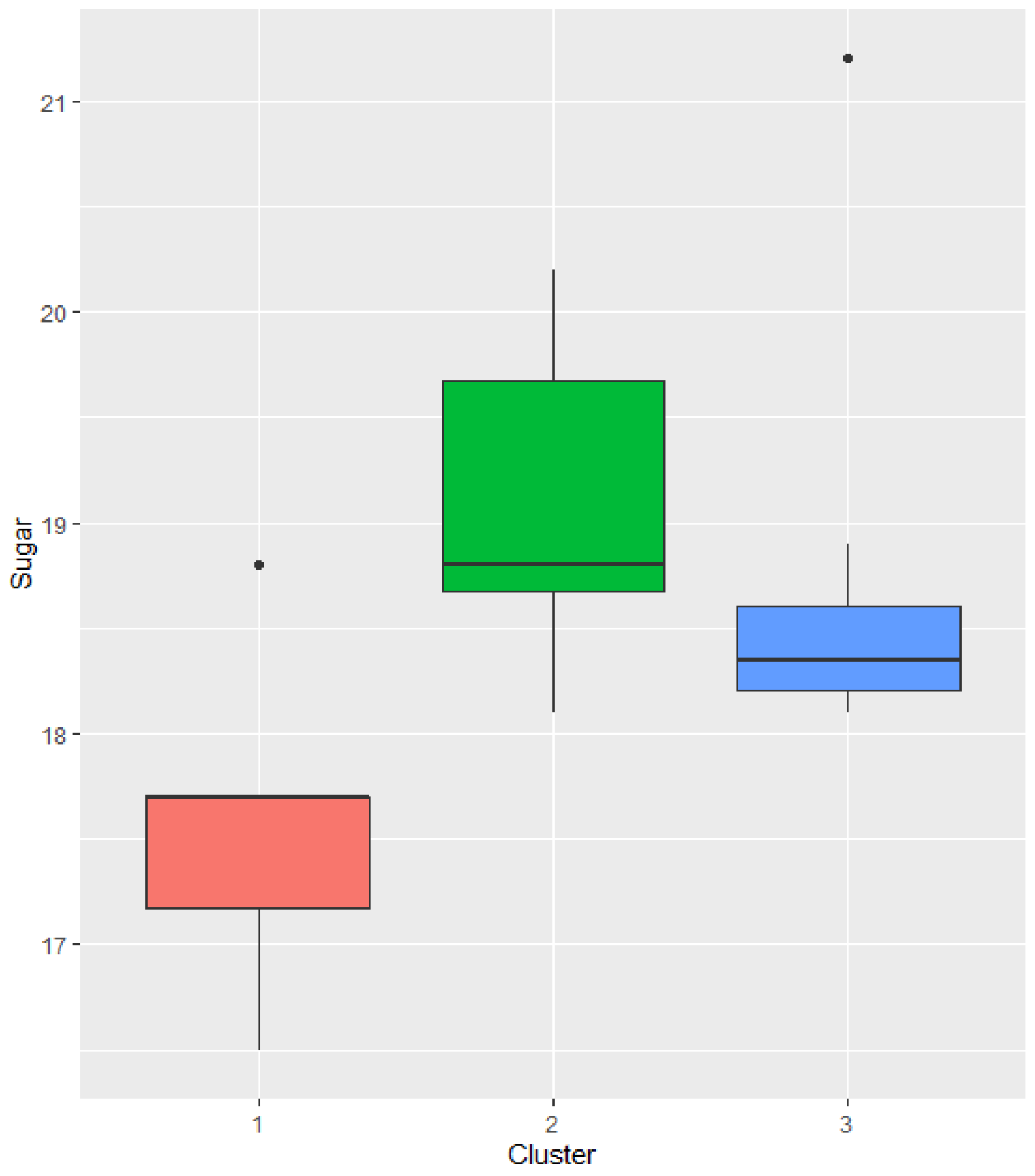
3.2. Evaluation of the harvest results of the ‘Kéknyelű’ variety
| Clone | Yield kg/m2 |
Sugar content of the juice KMW |
Titratable acid contant of the juice g/l |
pH | Botrytis infection % |
Yield kg/m2 |
|---|---|---|---|---|---|---|
| B.1. | 2011 | 0.88 | 20.20 | 7.25 | 3.08 | 10.00 |
| B.2. | 2011 | 1.26 | 17.40 | 7.09 | 3.07 | 10.00 |
| Base | 2011 | 0.99 | 18.60 | 6.59 | 3.38 | 10.00 |
| B.1. | 2012 | 0.95 | 18.30 | 6.52 | 3.55 | 0.00 |
| B.2. | 2012 | 1.10 | 17.50 | 6.36 | 3.67 | 0.00 |
| Base | 2012 | 1.08 | 18.50 | 4.64 | 3.47 | 0.00 |
| B.1. | 2013 | 1.39 | 18.90 | 11.15 | 3.26 | 0.00 |
| B.2. | 2013 | 1.61 | 17.70 | 10.36 | 3.31 | 0.00 |
| Base | 2013 | 1.21 | 21.20 | 9.60 | 3.56 | 0.00 |
| B.1. | 2014 | 1.01 | 18.10 | 16.60 | 3.19 | 30.00 |
| B.2. | 2014 | 0.91 | 18.00 | 17.91 | 3.25 | 30.00 |
| Base | 2014 | 0.72 | 19.50 | 15.57 | 3.24 | 30.00 |
| B.1. | 2015 | 1.07 | 18.40 | 7.82 | 3.39 | 0.00 |
| B.2. | 2015 | 1.20 | 18.00 | 6.83 | 3.40 | 0.00 |
| Base | 2015 | 0.97 | 18.20 | 6.98 | 3.51 | 0.00 |
| B.1. | 2017 | 1.15 | 18.10 | 6.71 | 3.25 | 0.00 |
| B.2. | 2017 | 1.33 | 17.70 | 5.72 | 3.38 | 0.00 |
| Base | 2017 | 0.92 | 18.20 | 6.74 | 3.36 | 0.00 |
| B.1. | 2018 | 1.23 | 17.70 | 8.26 | 3.43 | 3.00 |
| B.2. | 2018 | 1.90 | 18.20 | 6.96 | 3.39 | 0.00 |
| Base | 2018 | 1.76 | 17.70 | 6.85 | 3.55 | 5.00 |
| B.1. | 2019 | 1.39 | 18.70 | 8.70 | 3.28 | 0.00 |
| B.2. | 2019 | 1.46 | 18.60 | 3.29 | 8.00 | 0.00 |
| Base | 2019 | 1.27 | 18.70 | 7.56 | 3.44 | 5.00 |
| B.1. | 2020 | 1.26 | 17.70 | 8.68 | 3.14 | 0.00 |
| B.2. | 2020 | 1.31 | 18.20 | 7.60 | 3.43 | 0.00 |
| Base | 2020 | 1.03 | 18.80 | 8.57 | 3.55 | 0.00 |
| B.1. | 2021 | 1.28 | 18.90 | 8.62 | 3.29 | 0.00 |
| B.2. | 2021 | 1.24 | 20.20 | 7.40 | 3.25 | 0.00 |
| Base | 2021 | 1.04 | 20.20 | 9.38 | 3.37 | 0.00 |
| B.1. | 2022 | 2.79 | 16.50 | 7.40 | 3.19 | 0.00 |
| B.2. | 2022 | 2.88 | 16.30 | 6.10 | 3.41 | 0.00 |
| Base | 2022 | 2.03 | 17.00 | 6.20 | 3.58 | 0.00 |
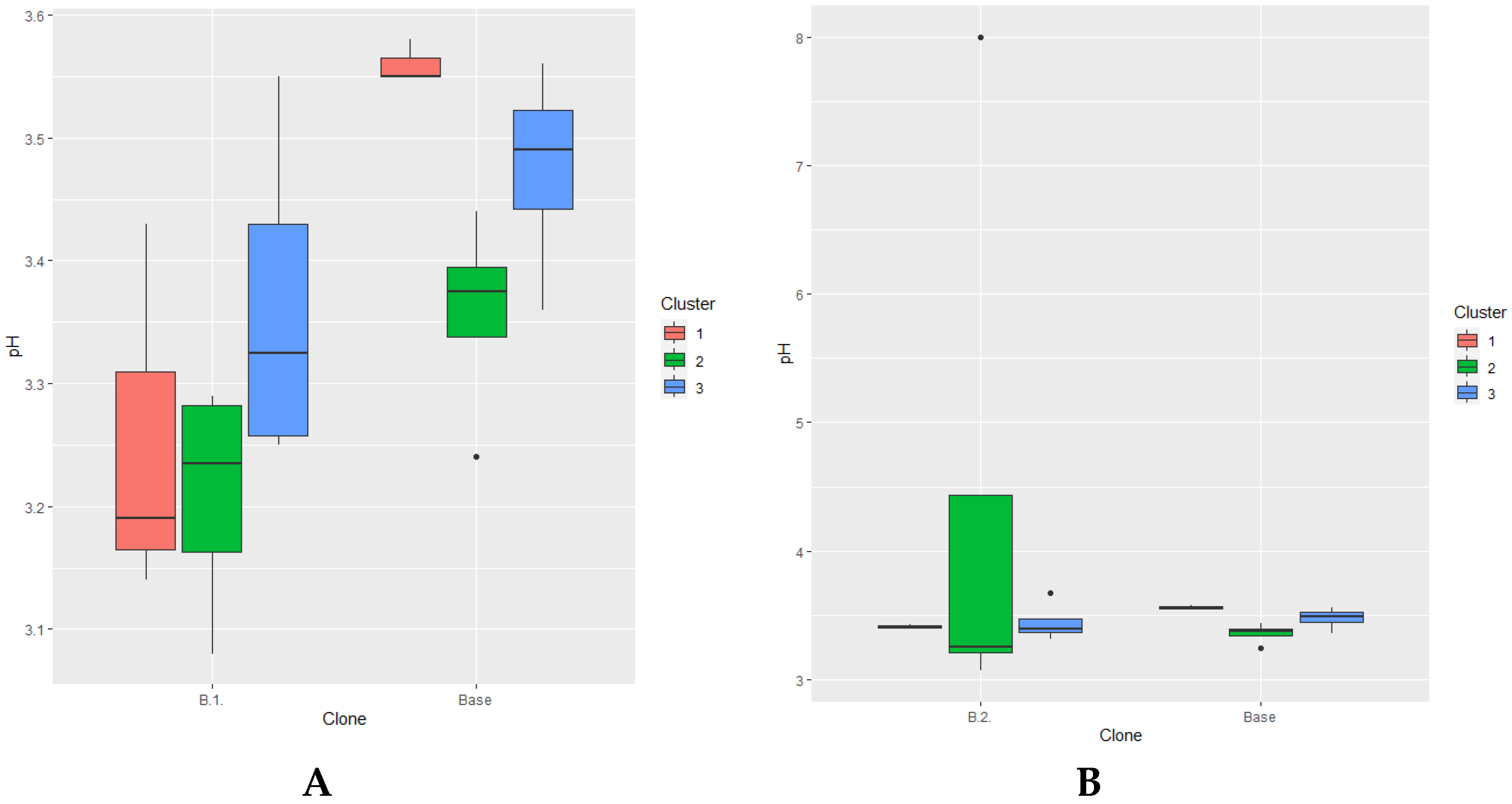
3.3. Evaluation of the results of the harvest of the variety ‘Juhfark’
| Clone | Yield kg/m2 |
Sugar content of the juice KMW |
Titratable acid contant of the juice g/l |
pH | Botrytis infection % |
Yield kg/m2 |
|---|---|---|---|---|---|---|
| B.1. | 2011 | 0.28 | 20.80 | 7.47 | 3.13 | 50.00 |
| B.2. | 2011 | 0.64 | 20.20 | 6.86 | 2.96 | 35.00 |
| Base | 2011 | 0.87 | 20.70 | 8.55 | 2.96 | 30.00 |
| B.1. | 2012 | 1.38 | 17.40 | 8.31 | 3.59 | 0.00 |
| B.2. | 2012 | 1.34 | 17.40 | 7.21 | 3.42 | 0.00 |
| Base | 2012 | 1.06 | 20.10 | 7.16 | 3.55 | 0.00 |
| B.1. | 2013 | 1.35 | 20.70 | 12.07 | 3.37 | 3.00 |
| B.2. | 2013 | 1.56 | 19.90 | 10.82 | 3.33 | 2.00 |
| Base | 2013 | 1.08 | 19.80 | 11.50 | 3.21 | 5.00 |
| B.1. | 2014 | 0.73 | 17.40 | 19.84 | 3.28 | 60.00 |
| B.2. | 2014 | 0.21 | 16.50 | 17.52 | 3.18 | 80.00 |
| Base | 2014 | 0.21 | 15.10 | 16.41 | 3.20 | 85.00 |
| B.1. | 2015 | 1.01 | 19.40 | 8.62 | 3.52 | 3.00 |
| B.2. | 2015 | 1.21 | 18.20 | 8.04 | 3.47 | 5.00 |
| Base | 2015 | 1.46 | 17.80 | 9.91 | 3.50 | 10.00 |
| B.1. | 2017 | 1.31 | 17.70 | 7.71 | 3.36 | 5.00 |
| B.2. | 2017 | 1.28 | 18.40 | 7.45 | 3.33 | 5.00 |
| Base | 2017 | 1.57 | 17.50 | 9.44 | 3.28 | 5.00 |
| B.1. | 2018 | 2.26 | 18.00 | 7.29 | 3.46 | 7.00 |
| B.2. | 2018 | 1.99 | 17.40 | 8.48 | 3.47 | 5.00 |
| Base | 2018 | 2.02 | 17.90 | 9.94 | 3.35 | 10.00 |
| B.1. | 2019 | nd. | nd. | nd. | nd. | nd. |
| B.2. | 2019 | nd. | nd. | nd. | nd. | nd. |
| Base | 2019 | 1.43 | 20.80 | 12.55 | 3.42 | 40.00 |
| B.1. | 2020 | 1.17 | 20.40 | 11.88 | 3.41 | 20.00 |
| B.2. | 2020 | 1.06 | 19.90 | 11.08 | 3.34 | 25.00 |
| Base | 2020 | 1.55 | 17.20 | 12.40 | 3.29 | 20.00 |
| B.1. | 2021 | 1.12 | 19.60 | 14.20 | 3.14 | 20.00 |
| B.2. | 2021 | 1.09 | 19.00 | 12.40 | 3.18 | 20.00 |
| Base | 2021 | 1.48 | 15.10 | 18.80 | 3.01 | 20.00 |
| B.1. | 2022 | 0.96 | 17.70 | 7.47 | 3.37 | 10.00 |
| B.2. | 2022 | nd. | nd. | nd. | nd. | nd. |
| Base | 2022 | 2.25 | 17.10 | 10.54 | 3.14 | 10.00 |
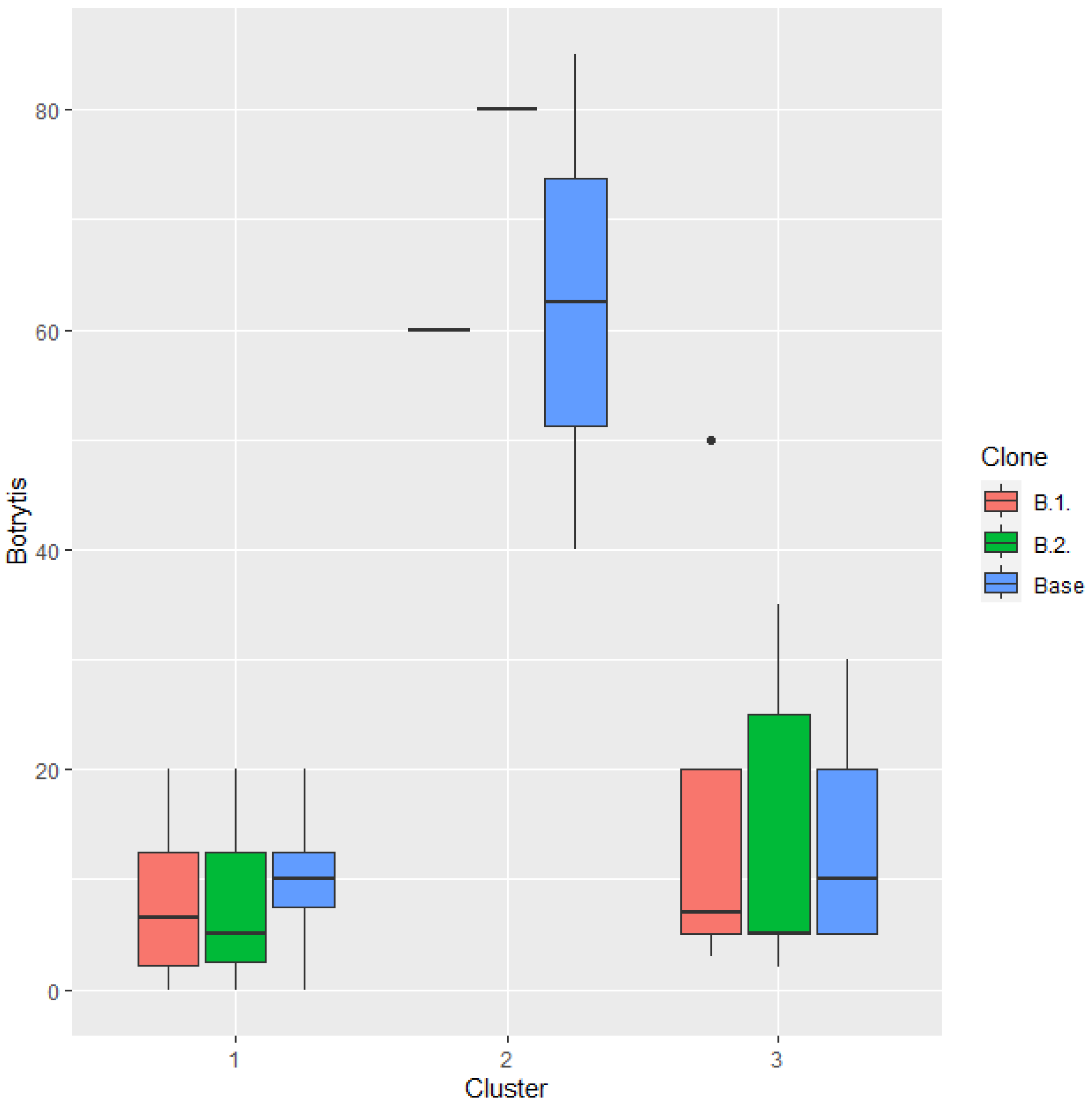
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- van Leeuwen, C.; Darriet, P. The Impact of Climate Change on Viticulture and Wine Quality. Journal of Wine Economics 2016, 11, 150–167. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. Oeno One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Ollat, N.; van Leeuwen, C.; de Cortazar-Atauri, I.G.; Touzard, J.M. The Challenging Issue of Climate Change for Sustainable Grape and Wine Production. Oeno One 2017, 51, 59–60. [Google Scholar] [CrossRef]
- Németh, K. Klímaváltozás Hatása a Borszőlő Biológiai Jellemzőire, Termésmennyiségére, Minőségére. ÉRTÉKÁLLÓ ARANYKORONA 2012, 12, 6–7. [Google Scholar]
- Németh, K. Száraz Termőhelyen. Kertesz Szolesz 2015, 20–21. [Google Scholar]
- Tkhakushinov, E.K.; Hachemizova, E.A. Development Problems of the Regional Agro-Industrial Complex Management System; 2018; Volume 7.
- Ostroukhova, E.; Levchenko, S.; Vasylyk, I.; Volynkin, V.; Lutkova, N.; Boyko, V. COMPARISON OF THE PHENOLIC COMPLEX OF CRIMEAN AUTOCHTHONOUS AND CLASSIC WHITE-BERRY GRAPE CULTIVARS. [CrossRef]
- Májer, J.; Győrffyné Jahnke, G. Autochton Szőlőfajták Optimális Termesztéstechnológiáját Megalapozó Kísérletek Eredményei Badacsonyban. Borászati Füzetek 2005, 17, 4–9. [Google Scholar]
- Somogyi, N.; Németh, K. A Kárpát-Medence Kincsei Határon Innen És Túl. MEZŐHÍR: ORSZÁGOS AGRÁRINFORMÁCIÓS SZAKLAP 2018, 22, 44–46. [Google Scholar]
- Goethe, H. Handbuch Der Ampelographie; Verlag Parely: Berlin, 1887. [Google Scholar]
- Németh, M. Ampelográfiai Album, I. (Termesztett Borszőlőfajtáink 1.); Mezőgazdasági Kiadó: Budapest, 1967. [Google Scholar]
- Jahnke, G.; Korbuly, J.; Májer, J.; Györffyné Molnár, J. Discrimination of the Grapevine Cultivars “Picolit” and “Kéknyelu” with Molecular Markers. Sci Hortic 2007, 114. [Google Scholar] [CrossRef]
- Györffyné Jahnke, G.; Májer, J.; Korbuly, J. Distinguishing the Grapevine Cultivars “Picolit” and “Kéknyelü” with Isozymes and Microsatellite Markers. Acta Hortic 2009, 827, 159–162. [Google Scholar] [CrossRef]
- Bakonyi, L.; Bényei, F.; Fazekas, I.; Hajdu, E.; Korbuly, J.; Lőrincz, A.; Marcinkó, F.; Pernesz, Gy.; Romenda, R.; Zanathy, G. Borszőlőfajták, Csemegeszőlő-Fajták És Alanyok; Bényei, F., Lőrincz, A., Eds.; Mezőgazda Kiadó: Budapest, 2005; ISBN 9789632865362. [Google Scholar]
- Varga, Zs.; Bényei, F.; Lőrincz, A.; Ulz, A. Yields and Quality of Former Vitis-Cultivars at Tokaj-Hegyalja. Borászati Füzetek 2006, 16, 1–8. [Google Scholar]
- Varga, Z.; Ferenczy, A.; Bényei, F.; Zanathy, G. EXAMINATION OF THE RELATIONS OF ORIGIN OF OLD GRAPE VINE CULTIVARS WITH CLUSTER ANALYSIS IN TOKAJ-HEGYALJA. Acta Hortic 2009, 169–176. [Google Scholar] [CrossRef]
- Halász, G.; Veres, A.; Kozma, P.; Kiss, E.; Balogh, A.; Galli, Z.; Szőke, A.; Hoffmann, S.; Heszky, L. Microsatellite Fingerprinting of Grapevine (Vitis Vinifera L.) Varieties of the Carpathian Basin; 2005; Volume 44.
- Lencsés, A.K.; Szőke, A.; Kozma, P.; Halász, G.; Katuláné Debreceni, D.; Veres, A.; Györffyné Jahnke, G.; Kiss, E. Mikroszatellit Markerek Alkalmazása a Magyarországi Szőlő Génforrások Megőrzésére. Kertgazdasag - Horticulture 2010, 42, 58–67. [Google Scholar]
- Jahnke, G.; Májer, J.; Lakatos, A.; Molnár, J.G.; Deák, E.; Stefanovits-Bányai, E.; Varga, P. Isoenzyme and Microsatellite Analysis of Vitis Vinifera L. Varieties from the Hungarian Grape Germplasm. Sci Hortic 2009, 120. [Google Scholar] [CrossRef]
- Jahnke, G.; Májer, J.; Varga, Z.; Deák, E.; Varga, P. An Acid Phosphatase Isoenzyme Pattern Is Characteristic for the Pontican Cultivars. Cereal Res Commun 2009, 37. [Google Scholar] [CrossRef]
- Bodor, P.; Varga, Zs.; Deák, T.; Pedryc, A.; Bisztray, Gy.D. Old Hungarian Grapevine Cultivars and Their Relations Characterized with Microsatellite Markers. Int J Hortic Sci 2008, 14. [Google Scholar] [CrossRef]
- Györffyné Jahnke, G.; Májer, J. Results of the Experiments for the Improvement of the Fertilisation of the Functional Female Flowered Grapevine Cultivar “Kéknyelű”. Acta Hortic 2003, 603, 767–773. [Google Scholar] [CrossRef]
- Kozma, P. A Szőlő Nemesítése. (Breeding of the Vine); Mezőgazdasági Kiadó: Budapest, 1951. [Google Scholar]
- Hajdu, E. Grapevine Breeding in Hungary. Grapevine Breeding Programs for the Wine Industry 2015, 103–134. [Google Scholar] [CrossRef]
- Németh, M. A Szőlő Klónszelekciós Nemesítéséről. (About the Clonal Selection of the Vine). Agrártudomány 1958, 43–49. [Google Scholar]
- Lunz, O. A Klónszelekcióü Hazai Helyzete És Eredményei (National Situataion and Results of Clonal Selection). Szőlőtermesztés és Borászat 1990, 12, 2–7. [Google Scholar]
- Hajdu, E.; Korać, N.; Cindrić, P.; Medić, M. Genetical Variations in Vine (Mutation) The Importance of Clonal Selection of Grapevine and the Role of Selected Clones in Production of Healthy Propagating Stocks. Int J Hortic Sci 2011, 17, 15–24. [Google Scholar] [CrossRef]
- Hajdu, E. A Kertészeti Növények Nemesítése a Szőlő Példáján. (The Horticultural Plant Breeding on the Example of Grape. Gradus 2016, 3, 378–383. [Google Scholar]
- Szűgyi-Reiczigel, Z.; Ladányi, M.; Bisztray, G.D.; Varga, Z.; Bodor-Pesti, P. Morphological Traits Evaluated with Random Forest Method Explains Natural Classification of Grapevine (Vitis Vinifera L.) Cultivars. Plants 2022, 11, 3428. [Google Scholar] [CrossRef] [PubMed]
- Cousins, P.; Garris, A. Quality Improvement in “Vignoles” through Clonal Selection. Acta Hortic 2014, 1046, 287–290. [Google Scholar] [CrossRef]
- Stover, E.; Aradhya, M.; Dangl, J.; Prins, B.; Cousins, P. Grape Genetic Resources and Research at the Davis California National Clonal Germplasm Repository. Acta Hortic 2009, 827, 193–196. [Google Scholar] [CrossRef]
- Győrffyné Jahnke, G.; Knolmájerné Szigeti, G.; Németh, C.; Nagy, Z.A.; Májer, J. Kéknyelű És Juhfark Fajták Klónszelekciója Badacsonyban. Agrofórum Extra 2018, 46, 26–28. [Google Scholar]
- Jahnke, G.; Májer, J.; Varga, P.; SzÖke, B. Analysis of Clones of Pinots Grown in Hungary by SSR Markers. Sci Hortic 2011, 129, 32–37. [Google Scholar] [CrossRef]
- Lemos, A.M.; Machado, N.; Egea-Cortines, M.; Barros, A.I. ATR-MIR Spectroscopy as a Tool to Assist ‘Tempranillo’ Clonal Selection Process: Geographical Origin and Year of Harvest Discrimination and Oenological Parameters Prediction. Food Chem 2020, 325, 126938. [Google Scholar] [CrossRef]
- Pejić, I.; Šimon, S.; Preiner, D.; Žulj Mihaljević, M.; Maletić, E.; Zdunić, G.; Petric, I. v.; Anhalt, U.; Forneck, A.; Ruehl, E. Estimate of Intravarietal Genetic Variation as a Prerequisite for Successful Clonal Selection in Grapevine. Acta Hortic 2015, 1082, 105–112. [Google Scholar] [CrossRef]
- Doulati Baneh, H. Molecular Markers to Detect Genetic Variation in Superior Clones of Grapevine (Vitis Vinifera L. ’Keshmeshi’). Acta Hortic 2015, 1082, 183–188. [Google Scholar] [CrossRef]
- Vezzulli, S.; Leonardelli, L.; Malossini, U.; Stefanini, M.; Velasco, R.; Moser, C. Assessing the Genetic Variability of Grape Clones. Acta Hortic 2014, 1046, 357–362. [Google Scholar] [CrossRef]
- Anhalt, U.C.M.; Crespo Martínez, S.; Rühl, E.; Forneck, A. An AFLP-Marker Study of the Vitis Vinifera L. Cultivar “White Riesling” Comprising 86 Clones to Investigate the Stability of Clones. Acta Hortic 2014, 1046, 681–684. [Google Scholar] [CrossRef]
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. Conference on Human Factors in Computing Systems - Proceedings 2011, 143–146. [Google Scholar] [CrossRef]
- Elkin, L.A.; Kay, M.; Higgins, J.J.; Wobbrock, J.O. An Aligned Rank Transform Procedure for Multifactor Contrast Tests. UIST 2021 - Proceedings of the 34th Annual ACM Symposium on User Interface Software and Technology 2021, 754–768. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.r-project.org/.
- Bonada, M.; Sadras, V.O. Review: Critical Appraisal of Methods to Investigate the Effect of Temperature on Grapevine Berry Composition. Aust J Grape Wine Res 2015, 21, 1–17. [Google Scholar] [CrossRef]
- Boulton, R. The Relationships between Total Acidity, Titratable Acidity and PH in Grape Tissue. VITIS - Journal of Grapevine Research 1980, 19, 113–113. [Google Scholar] [CrossRef]
- Neethling, E.; Petitjean, T.; Quénol, H.; Barbeau, G. Assessing Local Climate Vulnerability and Winegrowers’ Adaptive Processes in the Context of Climate Change. Mitig Adapt Strateg Glob Chang 2017, 22, 777–803. [Google Scholar] [CrossRef]
- Neethling, E.; Barbeau, G.; Coulon-Leroy, C.; Quénol, H. Spatial Complexity and Temporal Dynamics in Viticulture: A Review of Climate-Driven Scales. Agric For Meteorol 2019, 276–277. [Google Scholar] [CrossRef]
- Szenteleki, K.; Horváth, L.; Ladányi, M. Climate Risk and Climate Analogies in Hungarian Viticulture. International Conference on Future Environment and Energy 2012, 28, 250–254. [Google Scholar]
- Szenteleki, K.; Ladányi, M.; Gaál, M.; Zanathy, G.; Bisztray, G.Y. Climatic Risk Factors of Central Hungarian Grape Growing Regions. Appl Ecol Environ Res 2011, 10, 87–105. [Google Scholar] [CrossRef]
- Navrátilová, M.; Beranová, M.; Severová, L.; Šrédl, K.; Svoboda, R.; Abrhám, J. The Impact of Climate Change on the Sugar Content of Grapes and the Sustainability of Their Production in the Czech Republic. Sustainability (Switzerland) 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Carámbula, C.; Moreno, M.T.; Riera, D.; Cretazzo, E.; Tomás, M.; A 1; Medrano, H. Selección Clonal de Las Principales Variedades Autóctonas de Baleares.
- Roby, J.P.; Leeuwen, C. van About the Need of Maintaining Simultaneously Mass and Clonal Selection for Conservation of Genetic Diversity in Both International and Autochtoneous Varieties. In Proceedings of the 2nd International Symposium. Exploitation of autochthonous and more common vine varieties. Genetic pedigree and phenotyping, tolerance and stress, diseases to control, rootstocks. OENOVITI INTERNATIONAL network; Geisenheim, Germany, November 3 2014; pp. 93–97. [Google Scholar]
- Roby, J.-P.; Leeuwen, C. van; Gonçalves, E.; Graça, A.; Martins, A. The Preservation of Genetic Resources of the Vine Requires Cohabitation between Institutional Clonal Selection, Mass Selection and Private Clonal Selection. BIO Web Conf 2014, 3, 01018. [Google Scholar] [CrossRef]
- Prusky, D. PATHOGEN QUIESCENCE IN POSTHARVEST DISEASES. Annual review of Phytopathology 2003, 34, 413–434. [Google Scholar] [CrossRef]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and Necrotrophic Lifestyle Choice During Postharvest Disease Development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Padgett, M.; Morrison, J.C. Changes in Grape Berry Exudates during Fruit Development and Their Effect on Mycelial Growth of Botrytis Cinerea. Journal of the American Society for Horticultural Science 1990, 115, 269–273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





