Submitted:
29 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
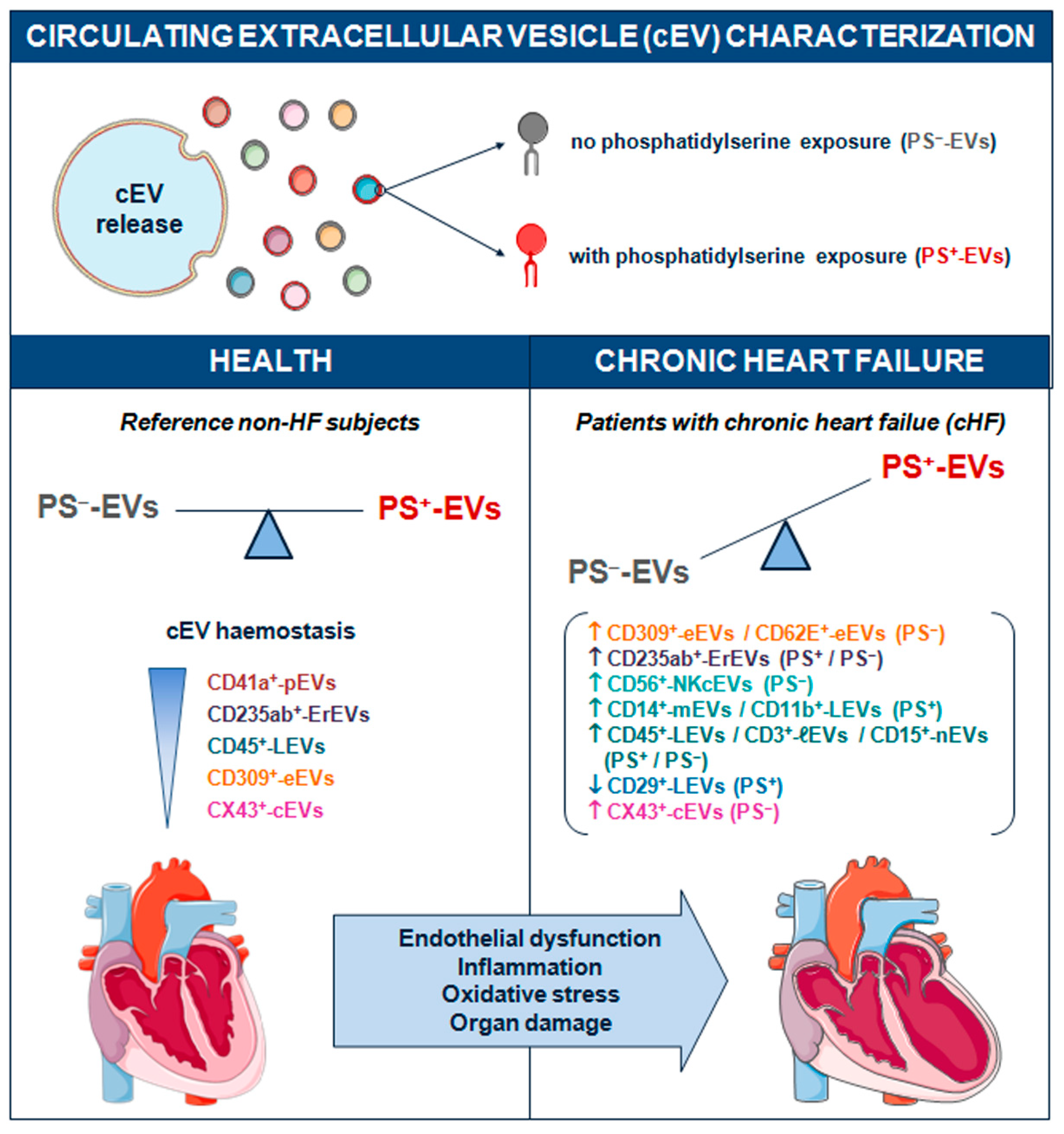
2.1. Circulating Extracellular Vesicles with and Without Phosphatidylserine Membrane Exposure
2.2. Differential Patterns between Phosphatidylserine Positive and Negative Circulating Extracellular Vesicles in Non-Heart Failure Reference Group and Patients with Chronic Heart Failure
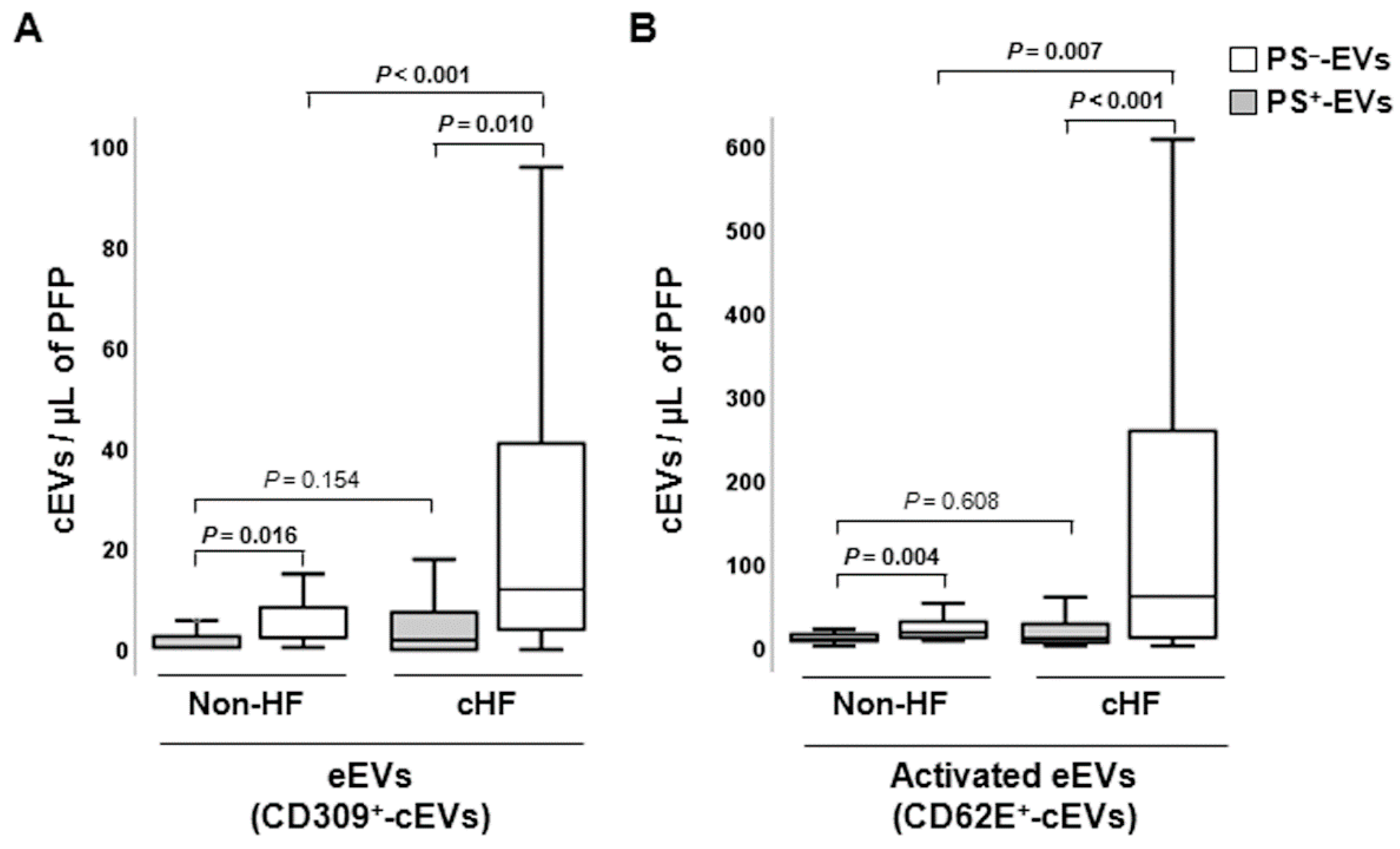
2.2.1. Endothelial-Derived Circulating Extracellular Vesicles
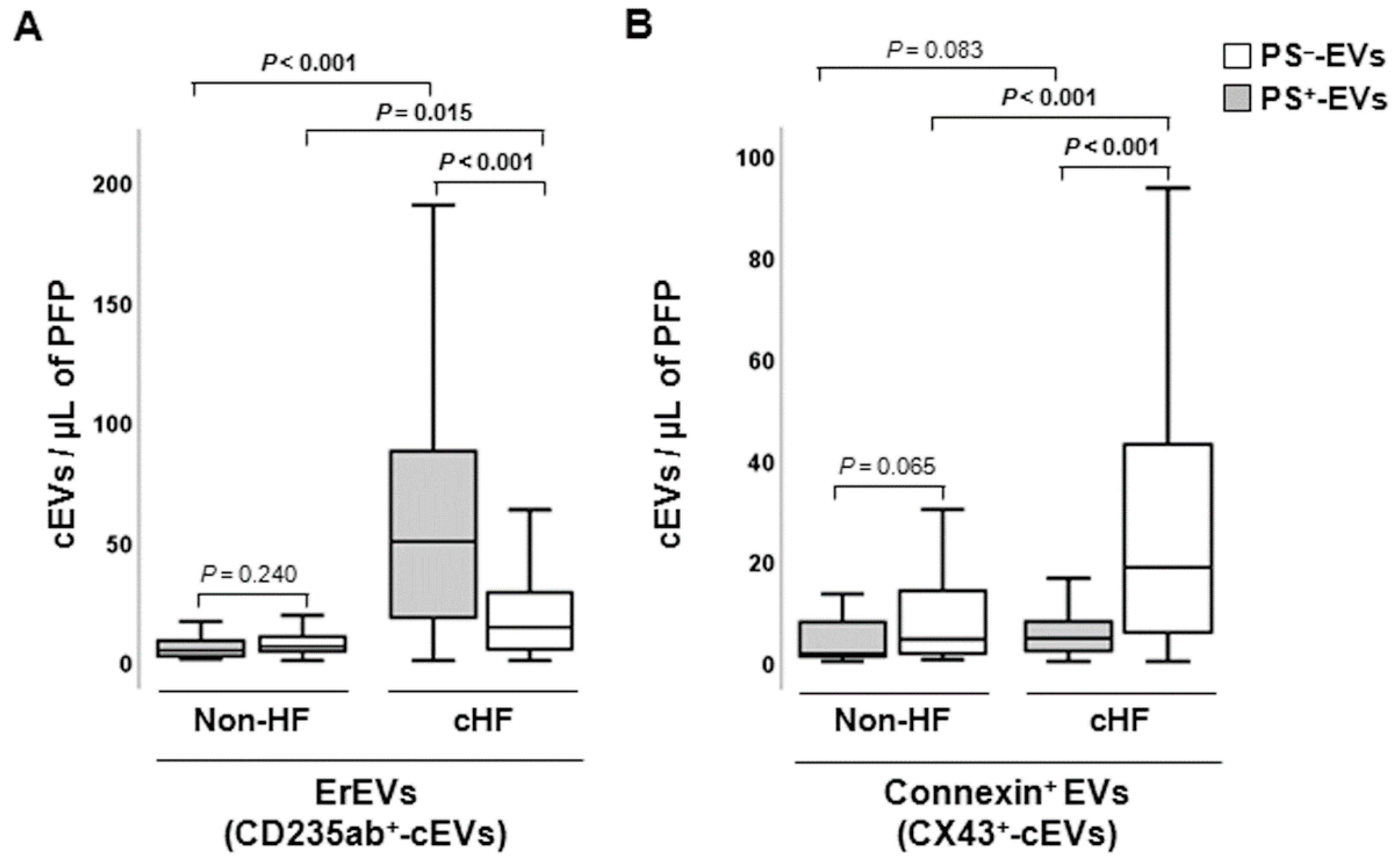
2.2.2. Erythrocyte-Derived Circulating Extracellular Vesicles
2.2.3. Connexin-43-Carrying Circulating Extracellular Vesicles
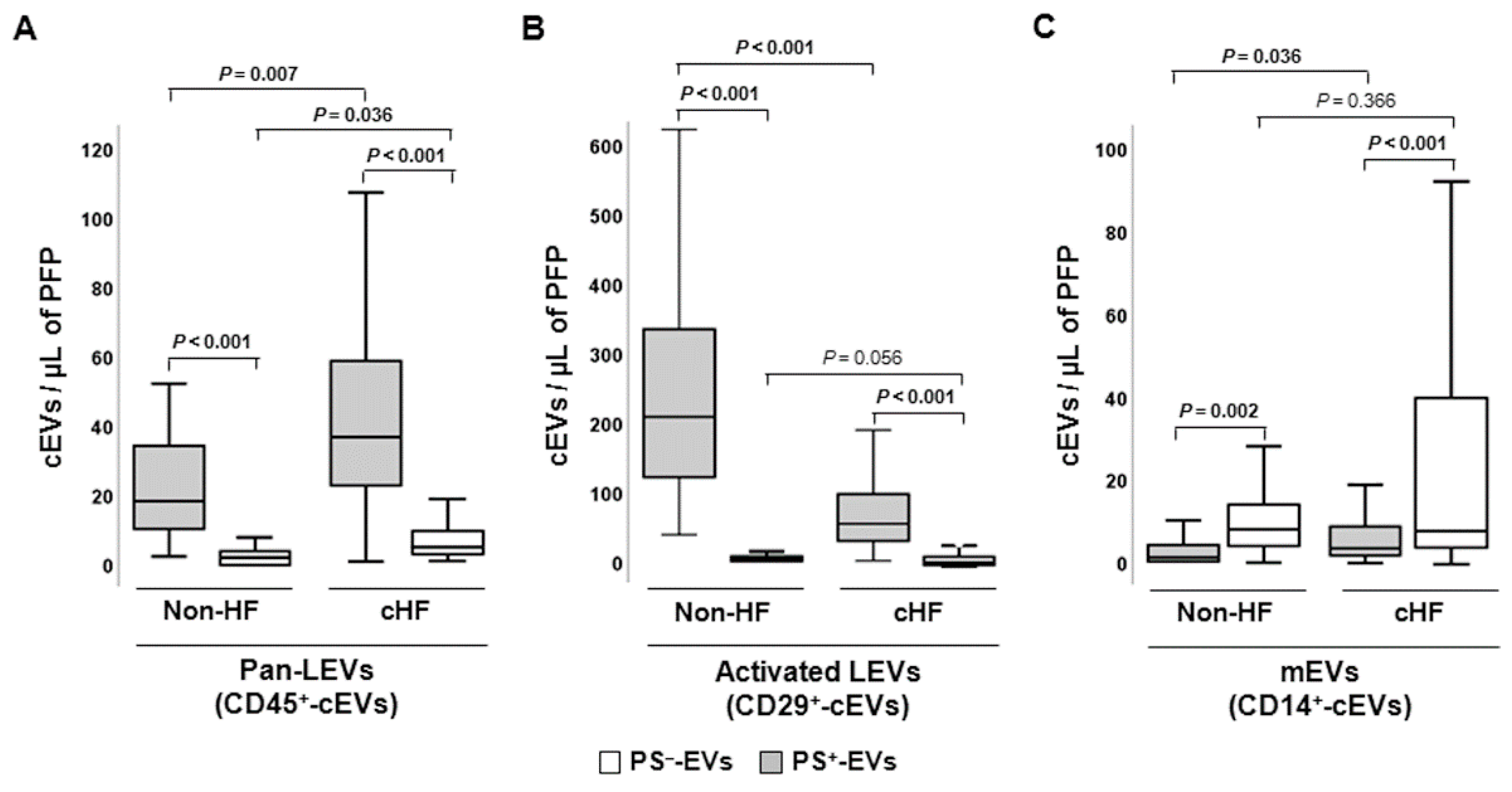
2.2.4. Platelet and Inflammatory Cell-Derived Circulating Extracellular Vesicles
3. Discussion
4. Materials and Methods
4.1. Healthy and Diseased Blood Donors
4.2. Blood Sampling and Circulating Extracellular Vesicle Isolation
4.3. Flow Cytometric Analysis of Circulating Extracellular Vesicles
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badimon, L.; Padro, T.; Arderiu, G.; Vilahur, G.; Borrell-Pages, M.; Suades, R. Extracellular vesicles in atherothrombosis: From biomarkers and precision medicine to therapeutic targets. Immunol Rev 2022, 312, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Greco, M.F.; Padro, T.; Badimon, L. Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padro, T.; Badimon, L. The Role of Blood-Borne Microparticles in Inflammation and Hemostasis. Semin Thromb Hemost 2015, 41, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 2019, 21, 9–17. [Google Scholar] [CrossRef]
- van Niel, G.; D'Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Badimon, L.; Suades, R.; Fuentes, E.; Palomo, I.; Padro, T. Role of Platelet-Derived Microvesicles As Crosstalk Mediators in Atherothrombosis and Future Pharmacology Targets: A Link between Inflammation, Atherosclerosis, and Thrombosis. Front Pharmacol 2016, 7, 293. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Connor, D.E.; Exner, T.; Ma, D.D.; Joseph, J.E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost 2010, 103, 1044–1052. [Google Scholar] [CrossRef]
- Matsumura, S.; Minamisawa, T.; Suga, K.; Kishita, H.; Akagi, T.; Ichiki, T.; Ichikawa, Y.; Shiba, K. Subtypes of tumour cell-derived small extracellular vesicles having differently externalized phosphatidylserine. J Extracell Vesicles 2019, 8, 1579541. [Google Scholar] [CrossRef]
- Perez-Pujol, S.; Marker, P.H.; Key, N.S. Platelet microparticles are heterogeneous and highly dependent on the activation mechanism: studies using a new digital flow cytometer. Cytometry A 2007, 71, 38–45. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Alonso, R.; Mata, P.; Badimon, L. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb Haemost 2013, 110, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Vilella-Figuerola, A.; Cordero, A.; Mirabet, S.; Munoz-Garcia, N.; Suades, R.; Padro, T.; Badimon, L. Platelet-Released Extracellular Vesicle Characteristics Differ in Chronic and in Acute Heart Disease. Thromb Haemost 2023. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H. Sodium-glucose co-transporter 2 inhibitors and acute heart failure. Eur J Heart Fail 2020, 22, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.D.; Conte, J.V. The pathophysiology of heart failure. Cardiovasc Pathol 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr Physiol 2015, 6, 187–214. [Google Scholar] [CrossRef]
- Vilella-Figuerola, A.; Padro, T.; Roig, E.; Mirabet, S.; Badimon, L. New factors in heart failure pathophysiology: Immunity cells release of extracellular vesicles. Front Cardiovasc Med 2022, 9, 939625. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Joop, K.; Berckmans, R.J.; Nieuwland, R.; Berkhout, J.; Romijn, F.P.; Hack, C.E.; Sturk, A. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost 2001, 85, 810–820. [Google Scholar] [CrossRef]
- Mobarrez, F.; Vikerfors, A.; Gustafsson, J.T.; Gunnarsson, I.; Zickert, A.; Larsson, A.; Pisetsky, D.S.; Wallen, H.; Svenungsson, E. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci Rep 2016, 6, 36025. [Google Scholar] [CrossRef]
- Nielsen, C.T.; Ostergaard, O.; Johnsen, C.; Jacobsen, S.; Heegaard, N.H. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum 2011, 63, 3067–3077. [Google Scholar] [CrossRef]
- Mobarrez, F.; Gunnarsson, I.; Svenungsson, E. Altered beta(2) -glycoprotein I expression on microparticles in the presence of antiphospholipid antibodies. J Thromb Haemost 2017, 15, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Vikerfors, A.; Mobarrez, F.; Bremme, K.; Holmstrom, M.; Agren, A.; Eelde, A.; Bruzelius, M.; Antovic, A.; Wallen, H.; Svenungsson, E. Studies of microparticles in patients with the antiphospholipid syndrome (APS). Lupus 2012, 21, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Bergen, K.; Mobarrez, F.; Jorneskog, G.; Wallen, H.; Tehrani, S. Phosphatidylserine expressing microvesicles in relation to microvascular complications in type 1 diabetes. Thromb Res 2018, 172, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, A.; Mobarrez, F.; Rooth, E.; Thalin, C.; von Arbin, M.; Henriksson, P.; Gigante, B.; Laska, A.C.; Wallen, H. Prognostic Value of Circulating Microvesicle Subpopulations in Ischemic Stroke and TIA. Transl Stroke Res 2020, 11, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padro, T.; Crespo, J.; Sionis, A.; Alonso, R.; Mata, P.; Badimon, L. Liquid Biopsy of Extracellular Microvesicles Predicts Future Major Ischemic Events in Genetically Characterized Familial Hypercholesterolemia Patients. Arterioscler Thromb Vasc Biol 2019, 39, 1172–1181. [Google Scholar] [CrossRef]
- Shet, A.S.; Aras, O.; Gupta, K.; Hass, M.J.; Rausch, D.J.; Saba, N.; Koopmeiners, L.; Key, N.S.; Hebbel, R.P. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 2003, 102, 2678–2683. [Google Scholar] [CrossRef]
- Yang, C.; Ma, R.; Jiang, T.; Cao, M.; Zhao, L.; Bi, Y.; Kou, J.; Shi, J.; Zou, X. Contributions of phosphatidylserine-positive platelets and leukocytes and microparticles to hypercoagulable state in gastric cancer patients. Tumour Biol 2016, 37, 7881–7891. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, X.; Si, Y.; Yao, Z.; Dong, Z.; Novakovic, V.A.; Guo, L.; Tong, D.; Chen, H.; Bi, Y.; et al. Increased blood cell phosphatidylserine exposure and circulating microparticles contribute to procoagulant activity after carotid artery stenting. J Neurosurg 2017, 127, 1041–1054. [Google Scholar] [CrossRef]
- Arauna, D.; Chiva-Blanch, G.; Padro, T.; Fuentes, E.; Palomo, I.; Badimon, L. Frail older adults show a distinct plasma microvesicle profile suggesting a prothrombotic and proinflammatory phenotype. J Cell Physiol 2021, 236, 2099–2108. [Google Scholar] [CrossRef]
- Berezin, A.E. Microparticles in Chronic Heart Failure. Adv Clin Chem 2017, 81, 1–41. [Google Scholar] [CrossRef]
- Kou, Y.; Zou, L.; Liu, R.; Zhao, X.; Wang, Y.; Zhang, C.; Dong, Z.; Kou, J.; Bi, Y.; Fu, L.; et al. Intravascular cells and circulating microparticles induce procoagulant activity via phosphatidylserine exposure in heart failure. J Thromb Thrombolysis 2019, 48, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Matan, D.; Mobarrez, F.; Lofstrom, U.; Corbascio, M.; Ekstrom, M.; Hage, C.; Lynga, P.; Persson, B.; Eriksson, M.; Linde, C.; et al. Extracellular vesicles in heart failure - A study in patients with heart failure with preserved ejection fraction or heart failure with reduced ejection fraction characteristics undergoing elective coronary artery bypass grafting. Front Cardiovasc Med 2022, 9, 952974. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, T.; Sugiyama, S.; Sugamura, K.; Ohba, K.; Matsuzawa, Y.; Konishi, M.; Matsubara, J.; Akiyama, E.; Sumida, H.; Matsui, K.; et al. Prognostic value of endothelial microparticles in patients with heart failure. Eur J Heart Fail 2010, 12, 1223–1228. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Jy, W.; Mauro, L.M.; Horstman, L.L.; Ahn, Y.S. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol 2001, 112, 81–90. [Google Scholar] [CrossRef]
- Zuchi, C.; Tritto, I.; Carluccio, E.; Mattei, C.; Cattadori, G.; Ambrosio, G. Role of endothelial dysfunction in heart failure. Heart Fail Rev 2020, 25, 21–30. [Google Scholar] [CrossRef]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, L.M.; La Rosa, G.; Pedicino, D.; D'Aiello, A.; Galli, M.; Liuzzo, G. Where Does Inflammation Fit? Curr Cardiol Rep 2017, 19, 84. [Google Scholar] [CrossRef]
- Shirazi, L.F.; Bissett, J.; Romeo, F.; Mehta, J.L. Role of Inflammation in Heart Failure. Curr Atheroscler Rep 2017, 19, 27. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Vilahur, G.; Martin-Yuste, V.; Sabate, M.; Sans-Rosello, J.; Sionis, A.; Badimon, L. Growing thrombi release increased levels of CD235a(+) microparticles and decreased levels of activated platelet-derived microparticles. Validation in ST-elevation myocardial infarction patients. J Thromb Haemost 2015, 13, 1776–1786. [Google Scholar] [CrossRef]
- Shlomovitz, I.; Speir, M.; Gerlic, M. Flipping the dogma - phosphatidylserine in non-apoptotic cell death. Cell Commun Signal 2019, 17, 139. [Google Scholar] [CrossRef]
- Dombroski, D.; Balasubramanian, K.; Schroit, A.J. Phosphatidylserine expression on cell surfaces promotes antibody-dependent aggregation and thrombosis in beta2-glycoprotein I-immune mice. J Autoimmun 2000, 14, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Takahashi, Y.; Ogata, K.; Kitamura, S.; Nakagawa, N.; Yamamoto, A.; Ishihama, Y.; Takakura, Y. Phosphatidylserine-deficient small extracellular vesicle is a major somatic cell-derived sEV subpopulation in blood. iScience 2021, 24, 102839. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, Y.; Yu, M.; Li, T.; Tong, D.; Yang, X.; Zhang, C.; Guo, L.; Wang, C.; Kou, Y.; et al. Phosphatidylserine-exposing blood cells and microparticles induce procoagulant activity in non-valvular atrial fibrillation. Int J Cardiol 2018, 258, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Sinauridze, E.I.; Kireev, D.A.; Popenko, N.Y.; Pichugin, A.V.; Panteleev, M.A.; Krymskaya, O.V.; Ataullakhanov, F.I. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost 2007, 97, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Zara, M.; Guidetti, G.F.; Camera, M.; Canobbio, I.; Amadio, P.; Torti, M.; Tremoli, E.; Barbieri, S.S. Biology and Role of Extracellular Vesicles (EVs) in the Pathogenesis of Thrombosis. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- De Lorenzo, F.; Saba, N.; Kakkar, V.V. Blood coagulation in patients with chronic heart failure: evidence for hypercoagulable state and potential for pharmacological intervention. Drugs 2003, 63, 565–576. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Exosomal vesicles enhance immunosuppression in chronic inflammation: Impact in cellular senescence and the aging process. Cell Signal 2020, 75, 109771. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Alonso, R.; Lopez-Miranda, J.; Mata, P.; Badimon, L. Circulating CD45+/CD3+ lymphocyte-derived microparticles map lipid-rich atherosclerotic plaques in familial hypercholesterolaemia patients. Thromb Haemost 2014, 111, 111–121. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Alonso, R.; Mata, P.; Badimon, L. High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thromb Haemost 2015, 114, 1310–1321. [Google Scholar] [CrossRef]
- Nieuwland, R.; Berckmans, R.J.; McGregor, S.; Boing, A.N.; Romijn, F.P.; Westendorp, R.G.; Hack, C.E.; Sturk, A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood 2000, 95, 930–935. [Google Scholar] [CrossRef]
| cEVs | cHF (n = 119) | Non-HF (n = 21) | P-value | |
|---|---|---|---|---|
| AV+-EVs + AV─-EVs | 1079.12 [425.93 - 4702.2] | 803.72 [300.6 - 1104.67] | 0.135 | |
| AV+-EVs | ||||
| Total | n | 159.6 [110.8 - 252.37] | 309.2 [121.14 - 453.31] | 0.022 |
| % | 15.76 [3.76 - 32.47] | 51.48 [28.18 - 78.19] | <0.001 | |
| CD309+ | n | 1.9 [0-7.52] | 0 [0 - 2.90] | 0.154 |
| % | 0.61 [0 - 2.16] | 0 [0 - 0.45] | 0.094 | |
| CD41a+ | n | 124 [76.1 - 180.5] | 406 [110 - 646] | 0.001 |
| % | 53.4 [44.03 - 62.52] | 85.61 [77.11 - 96.76] | <0.001 | |
| CD45+ | n | 36 [21.45 - 58] | 18 [9 - 38] | 0.007 |
| % | 16.49 [11.37 - 24.59] | 9.01 [2.24 - 18.77] | 0.016 | |
| CD235ab+ | n | 49.7 [17.5 - 88.35] | 4.3 [1.75 - 8.98] | <0.001 |
| % | 24.17 [13.91 - 33.10] | 1.33 [0.54 - 2.49] | <0.001 | |
| CX43+ | n | 4.6 [2 - 7.98] | 1.6 [0.8 - 8.0] | 0.083 |
| % | 1.88 [1.05 - 2.98] | 0.56 [0.17 - 1.65] | 0.001 | |
| AV─-EVs | ||||
| Total | n | 787.8 [262.2 - 4576.7] | 253.86 [99.57 - 703.16] | 0.007 |
| % | 84.25 [67.53 - 96.24] | 48.52 [21.80 - 71.82] | <0.001 | |
| CD309+ | n | 12 [4 - 43.32] | 2 [1 - 8.44] | <0.001 |
| % | 23.08 [10.4 - 41.24] | 12.82 [2.03 - 19.05] | 0.012 | |
| CD41a+ | n | 4.16 [2.0 - 9.5] | 5 [0.5 - 14.17] | 0.773 |
| % | 6.76 [1.15 - 13.89] | 20.41 [0 - 38.9] | 0.032 | |
| CD45+ | n | 4 [1.92 - 9.12] | 2.2 [0 - 4.0] | 0.036 |
| % | 5.81 [1.74 - 12.51] | 6.01 [0 - 14.28] | 0.586 | |
| CD235ab+ | n | 14 [4.63 - 28.5] | 6.0 [4.0 - 11.0] | 0.015 |
| % | 19.56 [9.1 - 36.49] | 27.4 [11.11 - 47.62] | 0.245 | |
| CX43+ | n | 18.60 [5.4 - 43.2] | 4.4 [1.6 - 14.0] | 0.001 |
| % | 27.2 [15.23 - 42.81] | 18.06 [9.09 - 30.43] | 0.028 | |
| pEVs | cHF patients | Non-HF subjects | P-value |
|---|---|---|---|
| AV+-pEVs | |||
| CD31+ | 28.5 [16 - 58.9] | 166 [53 - 283.78] | < 0.001 |
| CD41a+ | 124 [80 - 178.6] | 406 [126 - 642] | 0.001 |
| CD31+/CD41a+ | 28.25 [12 - 58.9] | 122 [40 - 220] | 0.001 |
| CD62P+ | 30 [14 - 51.3] | 58 [31 - 92] | 0.010 |
| AV─-pEVs | |||
| CD31+ | 17.1 [6 - 44] | 4 [2 - 10] | 0.002 |
| CD41a+ | 4.16 [2 - 9.5] | 5 [1 - 12.78] | 0.773 |
| CD31+/CD41a+ | 0 [0 - 0] | 0 [0 - 0] | 0.123 |
| CD62P+ | 2 [0 - 6] | 0 [0 - 4] | 0.085 |
| Parameters | Patients with cHF (n=119) |
|---|---|
| Demographic characteristics | |
| Male, n (%) | 81 (68) |
| Female, n (%) | 38 (32) |
| Age, years | 67 ± 11.8 |
| Clinical data | |
| Systolic blood pressure, mmHg | 120.4 ± 19.1 |
| Diastolic blood pressure, mmHg | 73.9 ± 11.1 |
| Left ventricular ejection fraction, % | 45.59 ± 18.98 |
| Comorbidities | |
| Smokers, n (%) | 13 (10.9) |
| Hypertension, n (%) | 82 (68.9) |
| Pulmonary hypertension, n (%) | 49 (41.1) |
| Dyslipidaemia, n (%) | 64 (53.7) |
| Chronic kidney disease, n (%) | 46 (38.6) |
| Diabetes mellitus, n (%) | 53 (44.5) |
| Atrial fibrillation, n (%) | 50 (42) |
| Background medication | |
| Angiotensin-converting-enzyme inhibitors, n (%) | 48 (40.3) |
| Angiotensin II receptor blockers, n (%) | 35 (29.4) |
| Angiotensin receptor neprilysin inhibitors, n (%) | 17 (14.2) |
| Beta-blockers, n (%) | 100 (84) |
| Aldosterone antagonists, n (%) | 66 (55.4) |
| Diureticsa, n (%) | 103 (86.5) |
| Ivabradine, n (%) | 14 (11.7) |
| Statins, n (%) | 77 (64.7) |
| Insulin, n (%) | 16 (13.4) |
| Anti-diabetic drugs, n (%) | 40 (33.6) |
| Anticoagulants, n (%) | 61 (51.2) |
| Antiplatelet agents, n (%) | 46 (38.6) |
| Anti-arrhythmic drugs, n (%) | 26 (21.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





