Introduction
The use of leadless pacemaker technology has remarkably increased over the last years (1). The most diffuse technology is the Micra transcatheter pacemaker system (TPS; Medtronic, MN; US), a small capsule-shaped device that is implanted into the wall of the right ventricle from through the femoral vein via a 27 F transvenous sheath. Presently, TPS functions as a single-chamber device in either VVI or VDD modalities. Registry data have repeatedly shown that this system reduces device-related peri-procedural and long-term morbidity compared to conventional transvenous lead pacemakers (2-5). According to data from the Micra IDE study as well as the post-approved Micra registry, the incidence of major periprocedural bleeding events ranges between 1.4-3% (2,6). Importantly, a recent contribution by Piccini and colleagues (5) reported that pericardial effusion during TPS positioning was associated with the following characteristics: BMI ≤ 20 kg/m2, age> 85 years, female gender, chronic obstructive pulmonary disease (COPD), and a history of AF. Given the concern for major bleeding complications, especially in high-risk patients, adequate peri-procedural anticoagulation management during TPS positioning is of utmost relevance.
In a single-center, retrospective study, Kiani and colleagues (7) reported TPS implantation under uninterrupted vitamin K antagonist. In this limited series (26 patients), no increased bleeding events were found with this strategy. In another retrospective study (8) including a higher number of patients under DOAC therapy, in most patients (89%) the DOAC was stopped prior to the procedure. In 5 patients the implantation procedure was performed without interrupting the DOAC. The safety and feasibility of uninterrupted anticoagulation peri-procedural approach has been further supported by a large multicenter cohort that included 1210 patients under chronic anticoagulation (9). This study showed no difference between interrupted or uninterrupted ACO management strategies. However, in this large study, the specific type of anticoagulation therapy, whether VKA or DOAC, was not specified.
Considering specifically peri-procedural management of DOACs, EHRA recommendations (10,11) advise that in patients undergoing an elective invasive low bleeding risk intervention, such as TPS positioning, DOAC intake should be stopped ≥ 24 hours prior to the procedure and reinitiated 6-24 hours after the procedure. Whether this DOAC peri-procedural management scheme is also adequate for TPS implantation, a procedure involving femoral transvenous catheterization with a large-sized 27F venous sheath, remains an open question.
The present retrospective, two-center study specifically investigates peri-procedural DOAC management during leadless pacemaker implantation. The study objectives are two fold:
To assess the effects of a standardized DOAC management approach, consisting in withholding 1 dose prior to the procedure and reinitiation 6-24 hours, on peri-procedural adverse events;
To identify clinical characteristics associated with this DOAC management strategy.
Materials and methods
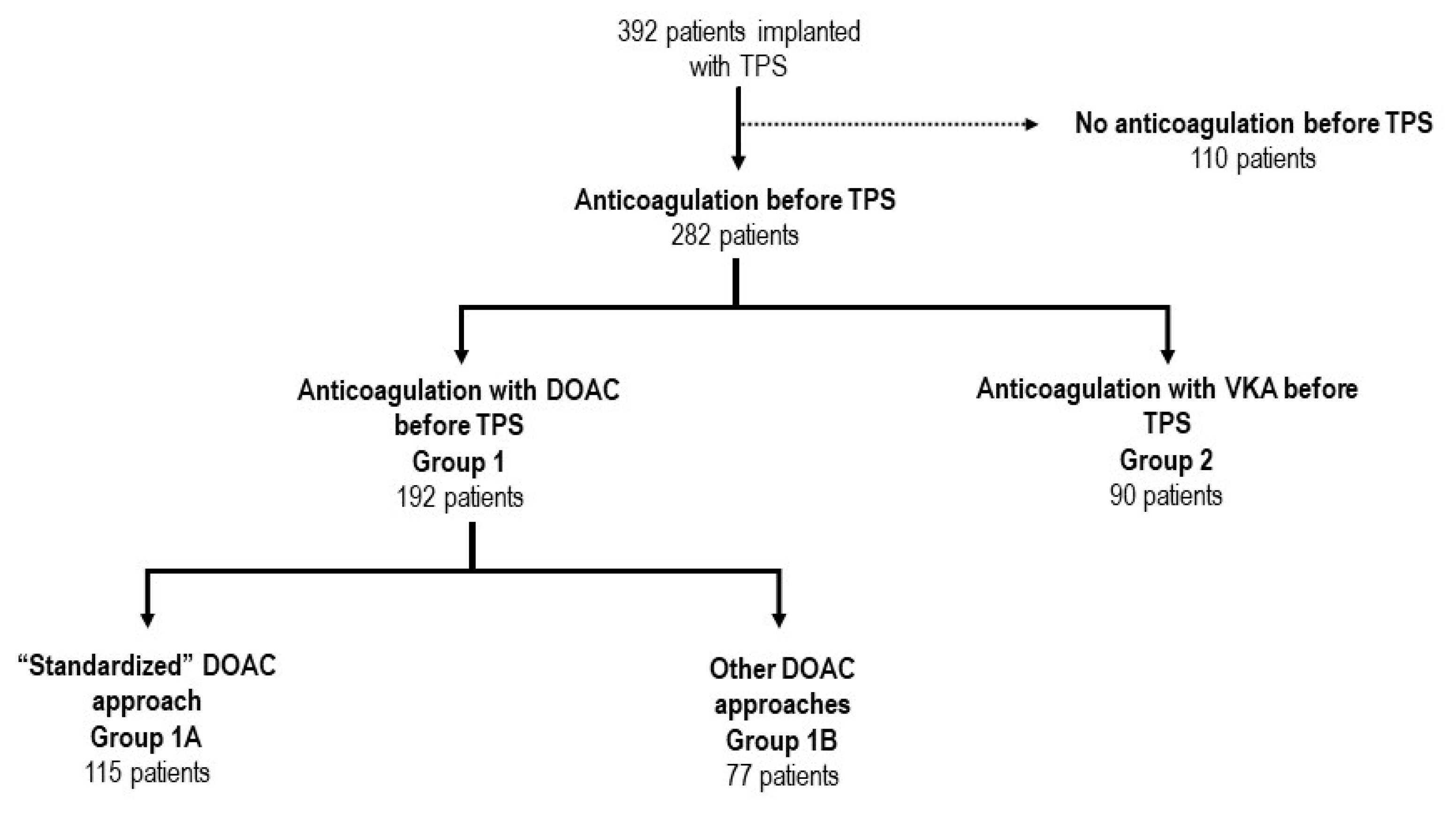
This two-center retrospective study included all consecutive patients undergoing TPS implantation at Cardiocentro Ticino (Lugano) and at the University Hospital of Zurich between September 2015 and December 2021. Study data were included in an institutional pacemaker database, approved by the regional ethics’ review board (KEK-ZH-NR: 2020-00811). Baseline demographic and clinical characteristics were assessed, including indications to single-chamber pacemaker, major comorbidities and ongoing treatments. For each patient taking oral anticoagulants, the peri-procedural management of anticoagulation was assessed (see section below).
Definition of peri-procedural anticoagulation regimens and general patient management
Different anticoagulation therapy regimens were prescribed based on patient bleeding and thromboembolic risk profiles, institutional protocol, as well as operator discretion.
Concerning DOAC (Group 1), two subgroups of peri-procedural management were considered:
- -
Group 1A included patients treated with the standardized approach, which consisted in DOAC interruption of 1 dose prior to the procedure. The therapy was then reinitiated 6-24 hours after the procedure;
- -
Group 1B gathered all other peri-procedural DOAC regimens, including:
Interrupted and delayed reinitiation: DOAC interruption before the procedure of at least 2 consecutive doses for either dabigatran/ apixaban or for rivaroxaban/ edoxaban; DOAC anticoagulation therapy was then reinitiated >24 hours after the procedure;
Uninterrupted: no peri-procedural DOAC interruption was performed;
Interrupted with “Bridging”: in patients treated with DOACs, the oral anticoagulant was interrupted before the procedure for at least 2 consecutive doses when treating with dabigatran/ apixaban or 1 dose for rivaroxaban/ edoxaban, and either fractionated or unfractionated heparin were administered peri-procedurally;
For patients who were under chronic VKA (Group 2), ACO was managed as follows:
- -
Interrupted with “Bridging”: in patients under chronic VKA, this approach consisted in suspending VKA at least 72 hours before, performing TPS positioning with INR<1.5, and either fractionated or unfractionated heparin were administered peri-procedurally;
- -
Interrupted without “Bridging”: in patients under chronic VKA, this approach consisted in suspending VKA at least 72 hours before, performing TPS positioning with INR<1.5, and reinitiating VKA after the procedure without resorting to “bridging” with either fractionated or unfractionated heparin;
- -
Uninterrupted: TPS positioning was performed without discontinuing VKA with INR level < 3 (12).
After TPS positioning, bed rest was prescribed for at least 8 hours, including 6 hours with a groin pressure dressing.
Leadless pacemaker implantation
Implantations were performed in local anesthesia and mild sedation in line with previous reports (13,14). The procedure was performed according to a standardized implantation protocol, which has been presented in detail elsewhere (13). Briefly, through femoral vein access and following appropriate dilation at the level of the right groin, the 27 Fr venous sheath was advanced into the right atrium over a super-stiff guidewire. The steerable delivery system was gently advanced to the right atrium and curved across the atrioventricular junction to reach the right ventricular (RV) septum. Contrast dye injection verified adequate contact to the inferior or mid-septal endocardial boarder of the right ventricle before delivery of the device. After verifying device fixation and confirming adequate electrical measures, the tether was cut and the delivery system removed. Hemostasis at the femoral puncture site was obtained either by applying a figure-of-eight suture or with the use of vascular closure devices (Perclose ProGlide™ Suture-Mediated Closure System ,Abbott, US) (15). The choice of the hemostasis technique was left to the operator's discretion.
Study endpoints and classification of adverse events
Periprocedural adverse events were considered as those events occurring within 30 days of the procedure. Major complications included any intra-procedural and peri-procedural adverse event causing transitory or permanent functional impairment that required additional unplanned measures for resolution. Minor complications included any mild adverse event that caused transient patient discomfort causing a prolongation of hospital stay, but that did not require additional medical measures for resolution.
Statistics
Data are described as mean and standard deviation or median and 25th-75th percentiles if continuous (depending on distribution), and as counts and percent if categorical. For comparison between groups, the Student´s- t-test was performed for normally-distributed continuous variables and the Mann Whitney-U test for non-parametric data. The Fisher´s exact test was used to compare group distributions of categorical variables. A 2-sided p value <0.05 was considered statistically significant. All analyses were performed using Stata, version 15 (StataCorp, College Station, TX, USA).
Results
Patient characteristics and management of anticoagulation during TPS
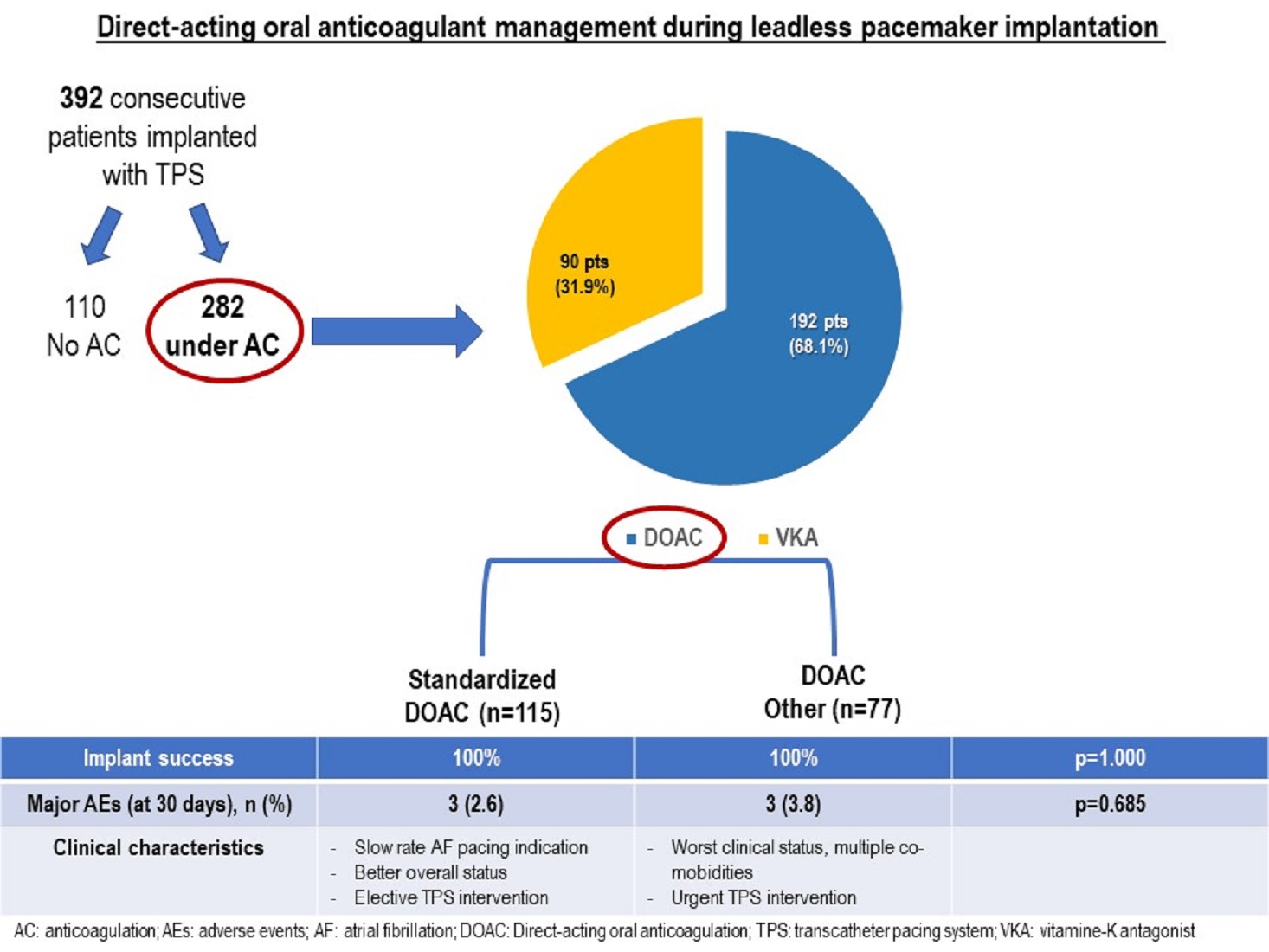
Three-hundred and ninety-two consecutive patients undergoing leadless pacemaker implantation were included in this analysis. The mean age was 81.4± 7.3 years, and the majority of patients were male (66.3%) (
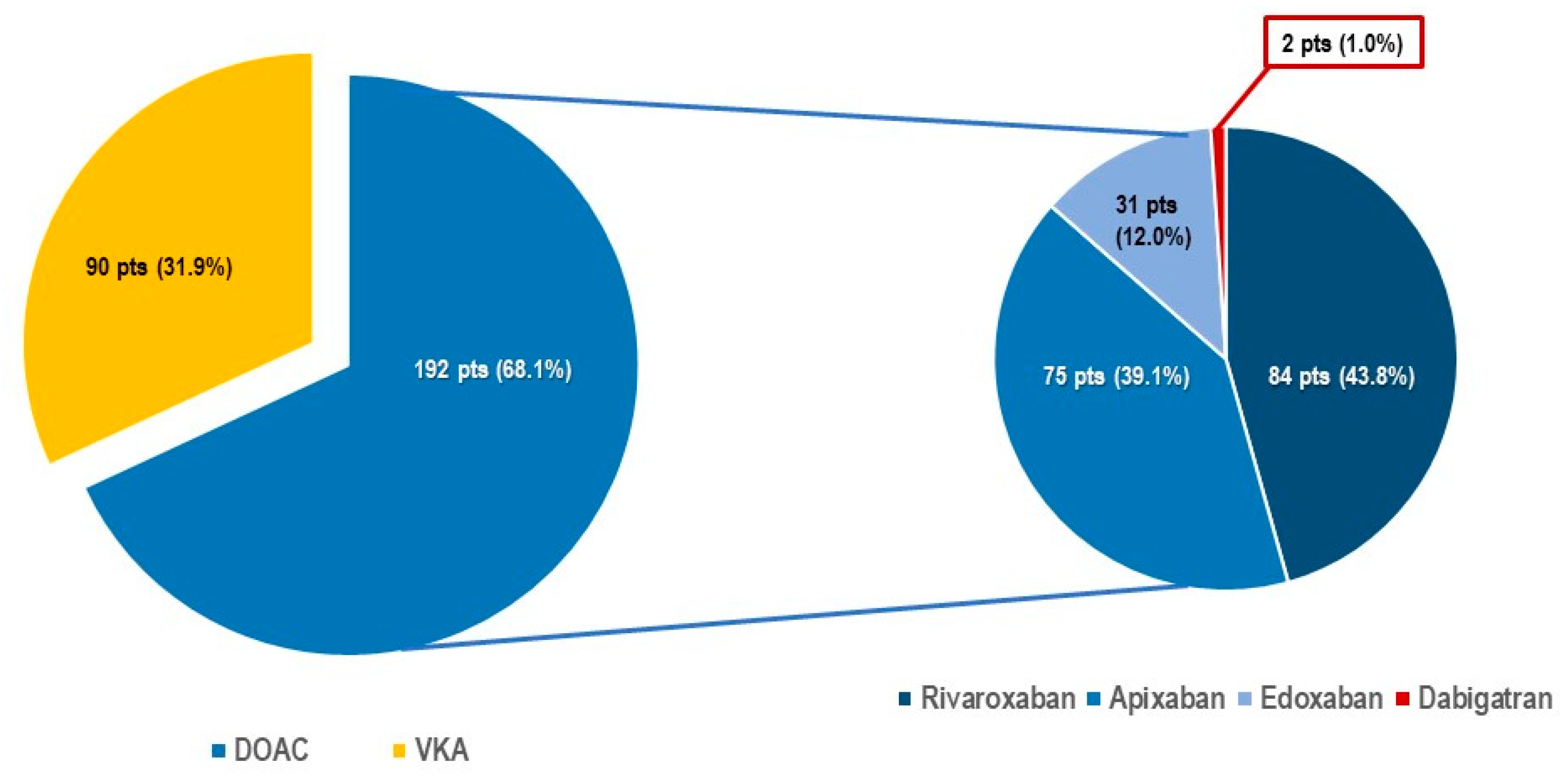
Table 1). Structural heart disease was present in 71.2% of patients and mean left ventricular ejection fraction (55.5 ± 9.6%) was preserved. Most patients suffered from one or more associated co-morbidity including renal impairment (53.1%), diabetes mellitus (19.6%), chronic obstructive lung disease (15.3%), tumoral disease (14.5%), peripheral artery disease (14.5%), and/or high bleeding risk (7.2%). The main indication for device implantation was bradycardic atrial fibrillation (40.3%). Of note, 282 patients (71.9%) were under oral anticoagulation, including 192 (68.1%) patients (Group 1) treated with DOAC (
Figure 1 and
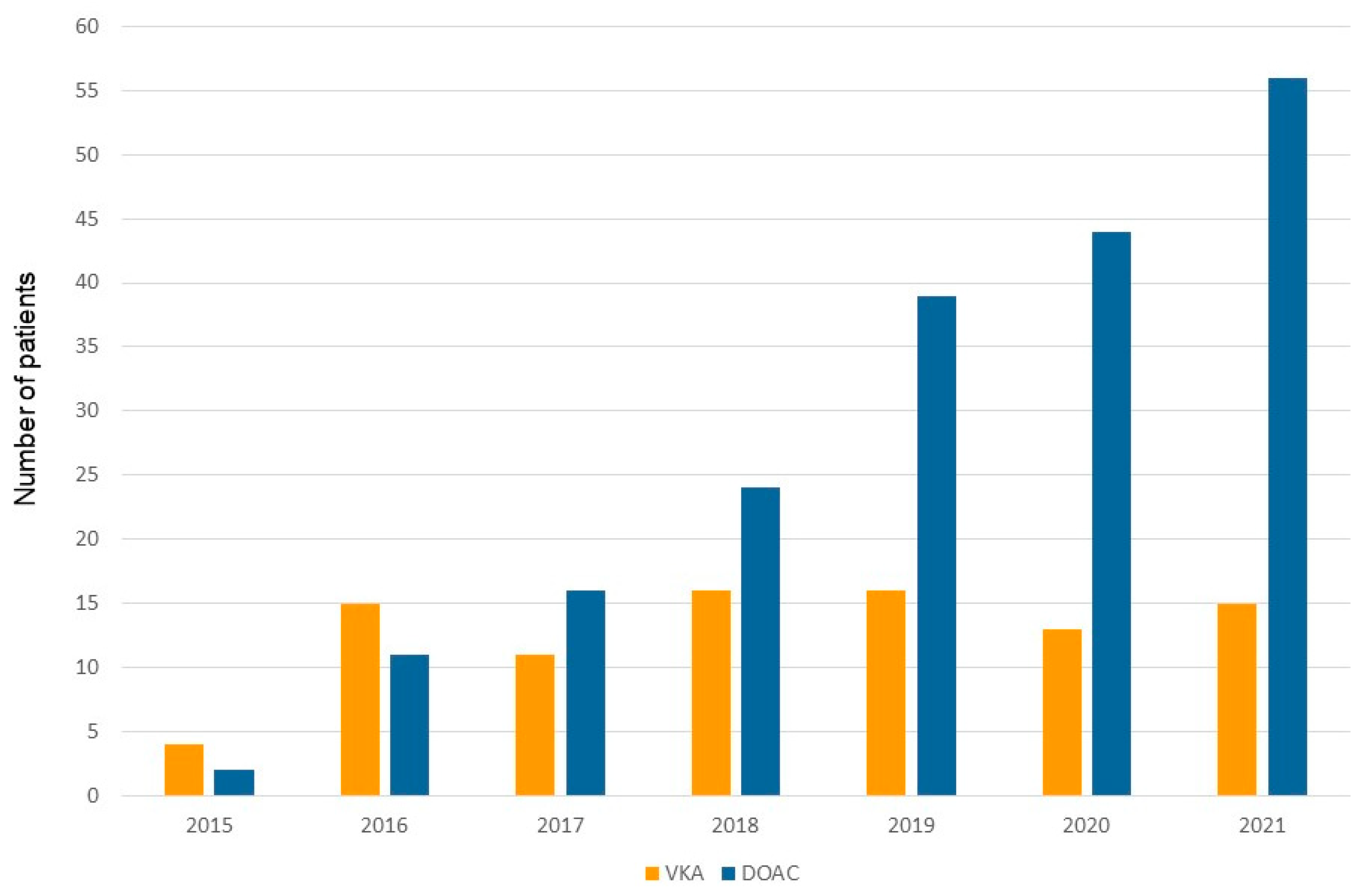
Figure 2) and 90 patients (31.9%) under vitamin K antagonists (VKA) (Group 2). The use of DOAC has gradually and continuously increased over time (
Figure 3). Patients under DOAC less often presented underlying structural heart disease (DOAC: 55.4% vs VKA: 95.3%, p< 0.001) and associated co-morbidities, including diabetes mellitus (DOAC: 16.0% vs VKA: 21.9%, p=0.001), advanced renal failure requiring hemodialysis (DOAC: 0 vs VKA: 7.8%, p<0.001), and higher bleeding risk requiring left appendage closure (DOAC: 1.1% vs VKA 10.9%, p<0.001).
Differing peri-procedural NOAC regimens during TPS procedure
Most patients under DOAC anticoagulation were treated according to the standardized approach (n=115 patients, 59.9%, Group 1A) (
Figure 1). There were some differences in the baseline characteristics between the two groups (
Table 2). Patients in group 1A presented significantly less structural heart disease (1A: 42.6% vs 1B: 68.8%, p<0.001), fewer comorbidities like renal insufficiency (1A: 33.0% vs 1B: 66.2%, p<0.001) and/or peripheral artery disease (1A: 4.3% vs 1B: 20.8%, p<0.001). Furthermore, there were some differences in pacemaker indication between the groups, implantation in Group 1A being more often performed to treat slow-ventricular rate atrial fibrillation (1A: 55.7% vs 1B: 32.5%, p=0.002) and the DOAC most often prescribed in Group 1A patients was rivaroxaban (1A: 54.7% vs 1B: 27.2%, p<0.001), while apixaban was more often prescribed in group 1B patients (apixaban use: 1A 31.3% vs 1B: 50.6%, p=0.010). For other differences in pacemaker indication refer to
Table 2. Noteworthy, the implantation procedure was performed more often in the setting of a planned hospitalization for Group 1A patients compared to Group 1B (1A: 57.4% vs 1B: 23.4%, p<0.001).
In Group 1 A the DOAC was stopped with a mean of 25.7±8.5 hours before and re-initiated 7.4±5.9 hours after the procedure (
Table 3). In patients from group 1B, peri-procedural management of NOAC varied considerably, and included “
interruption and delayed re-initiation” (n=54, 70.1%),
“interruption and bridging with heparin” (n=13, 16.9%), and
“uninterrupted”(n=10, 13.0%). Less delay was observed in Group 1A for both peri-procedural interruption and reinitiation. Mean interruption was 21.4±5.2 hours before the procedure for Group 1A and 27.0±27.1 hours for Group 1B (p=0.032); mean re-initiation delay after the procedure was 7.4± 5.9 for Group 1A compared to 27.7± 16.8 hours for Group 1B (p<0.001).
Differing peri-procedural NOAC regimens and clinical outcomes
Procedural and pre-discharge characteristics are presented in
Table 3. No differences were found in terms of procedural efficacy including successful leadless pacemaker implantation (100 % in both groups; p=1.000). Major intra- and post-procedural complications up to 30 days after discharge were low and did not differ between the two groups (1A 2.6% vs 1B 3.8%, p=0.685). The occurrence of minor groin site hematoma was also similar between the two groups (1A: 5.2% vs 1B: 3.9%, p=0.743). Median length of hospital stay was significantly lower in Group 1A compared to 1B (1A: 3.0 [IQR 2.0-4.8] vs 1B: 4.0 [IQR 3.0-12.5] days, p=0.019).
Discussion
In this retrospective study, patients undergoing leadless pacemaker implantation in two Swiss tertiary institutions were included. Almost 1 in every 2 patients in this cohort were under chronic oral anticoagulation therapy with DOAC. A standardized peri-procedural DOAC therapy regimen (interruption of 1 dose prior to the intervention with re-initiation 6-24 hours after the procedure) had comparable peri-procedural clinical outcomes compared to other different peri-procedural DOAC management approaches.
The growing importance of DOAC during TPS procedure
In the experience reported herein, which extends from September 2015 to the end of 2021, more than two-thirds of anticoagulated patients (68.1%) undergoing device implantation, were under DOAC. This number is considerably higher compared with previously reported other single-center cohorts (7,8). San Antonio and colleagues (8) reported that only around 20 % of anticoagulated patients were under a DOAC regime. One possible explanation for this difference is the time line of patient inclusion. The two above-cited series (7,8) included patients between the year 2014-2018 compared to 2015-2021 in our cohort. There is a clear shift from vitamin K antagonists to DOACs over time which is even more predominant in the last two years (
Figure 3). This trend can be explained by the growing amount of evidence outlining the safety and efficacy of DOACs in high-risk patient subgroups (16), including patients with mild-to-moderate renal impairment (11,17,18) as well as patients with stable coronary artery disease (8,16). In fact, no difference was found in the proportion of patients presenting renal impairment or structural heart disease between patients treated with DOAC and those treated with VKA. Furthermore, the preferential use of DOACs in patients suffering from atrial fibrillation is also underscored in the most recent European guidelines on the management of atrial fibrillation (16).
Peri-procedural management of NOAC during TPS procedure: which is the best approach?
Peri-procedural management of anticoagulation is a relevant issue in patients undergoing leadless pacemaker implantation, especially in regard to the number of frail patients in need for such an intervention. According to the EHRA practical recommendations for DOAC management (10,11), leadless pacemaker implantation could be considered an intervention with low bleeding risk, since clinically important bleeding is usually infrequent and controllable. As such, the recommendations propose skipping 1 DOAC dose prior to the procedure and re-initiation on the same day (generally between 6-8 hours after the procedure). Although it may be difficult to extend these recommendations to leadless pacemaker implantation given the limited outcome data available, there is however substantial evidence for a shorter anticoagulation interruption timing in patients undergoing conventional transvenous device implantation and box change (10,11). Indeed, both Bruise CONTROL trials I-II (12,19) demonstrated that careful intraoperative hemostasis of the device pocket allows effective control of bleeding in patients with either uninterrupted VKA or DOAC. However, during implantation of standard CIED tranvenous systems the main source of bleeding is the subclavicular subcutaneous pouch. Given the fact that leadless pacemaker implantation procedure implicates a completely different implantation technique compared to transvenous pacemakers, the extension of the results of the Bruise CONTROL 2 trial (19) for the peri-procedural management of DOAC during TPS is rather inappropriate.
San Antonio and colleagues (8), followed a similar management protocol as the one reported herein for the standardized approach consisting in discontinuing DOAC 12-24 hours before the procedure and reinitiating the medication 6-24 hours after. In the series reported by Kiani et al (7), two different peri-procedural DOAC management strategies were pursued: the more commonly practiced approach (n=36 patients) consisted in interrupting dabigatran or apixaban for at least two consecutive doses (rivaroxaban was withheld for at least one dose) before the procedure. The second approach, performed in only 5 patients, consisted in withholding apixaban/ dabigatran for one or less consecutive pre-procedural dose or no dose interruption for rivaroxaban. The incidences of major and minor complications in this series were overall low indicating an appropriate anticoagulation management; however, the low number of patients limits the clincal impact of these data.
In our experience, for the patient cohort treated with a standardized peri-procedural approach, 3 major complications occurred and did not differ compared to other approaches. The standardized approach was most often the approach of choice for periprocedural management of DOAC in patients with a planned TPS procedure, presenting better overall clinical status in whom the intervention could be performed on the same day or the day after admission. Administration of DOAC within 6-24 hours after the procedure and early mobilization are known measures that control thromboembolic risk. Conversely, patients treated with other periprocedural DOAC regimens (Group 1B) were usually patients with prolonged hospitalization, limited mobility, in overall poorer clinical status, and who required a pacemaker urgently. In these patients, DOAC management was heterogenous, based on patient-specific risk profile and procedural urgency.
Study Limitations
This is a retrospective two-center study, gathering patients chronically treated with DOAC therapy undergoing implantation of a leadless pacing system. The lack of a clearly defined control group to compare the standardized approach represents a limitation. Moreover, the patient cohort remains small and the rate of major complications low. The scope of the present study was to provide some preliminary, hypothesis-generating findings for the optimization of peri-procedural DOAC management during TPS, given the limited data in this field.
Conclusions
A standardized peri-procedural management of DOAC during TPS implantation consisting in skipping a single DOAC dose before the procedure and reinitiating the therapy within 6-24 hours, does not increase bleeding and thromboembolic events. This approach was prescribed more frequently in patients presenting a good overall clinical status, who were not pacemaker-dependent, and who underwent an elective procedure. Prospectively designed studies would be useful to further optimise the management of DOAC during TPS.
Author Contributions
The authors contributed in the following way: Conceptualization: FDR and AB; Methodology: FDR and AB; Software: FDR; Validation and analysis: FDR, AB, AS; Investigation: AD, AB, AS; AD, MLC, EP; Data Curation: FDR, AB, AS, AD, MLC; Writing Original Draft Preparation: FDR, AB; Writing Review & Editing: AB, AS, AA, GC, MLC, AD, EP, TO; Supervision: FDR, AB.; Project Administration and Funding Acquisition: not applicable.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Canton Zurich (KEK-ZH-NR: 2020-00811, 15 July 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
AMS received educational grants through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, BMS/Pfizer, and Medtronic; and speaker fees from Boston Scientific and BMS/Pfizer; AA is a consultant with Boston Scientific, Cairdac, EP Solution, Medtronic, Microport CRM, Philips and XSpline; received speaker fees from Boston Scientific, Medtronic, Microport CRM, and Philips; participates in clinical trials sponsored by Boston Scientific, EP Solution, Medtronic, and Zoll Medical; and has intellectual properties assigned to Boston Scientific, Biosense Webster, and Microport CRM; TO received a research grant from Medtronic; AB has received consultant and / or speaker fees from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cook Medical, Daiichi Sankyo, Medtronic, Pfizer, and Spectranetics/Philipps. Other authors: no conflicts of interest.
References
- Regoli, F.D. Reducing CIED-Related Morbidity: "LESS Is More". J. Clin. Med. 2022, 11, 4782. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Al-Samadi, F.; Clementy, N.; Garweg, C.; Martinez-Sande, J.L.; Piccini, J.P.; Iacopino, S.; Lloyd, M.; Prat, X.V.; Jacobsen, M.D.; et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Hear. Rhythm. 2018, 15, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Exner, D.V.; Cantillon, D.J.; Doshi, R.; Bunch, T.J.; Tomassoni, G.F.; Friedman, P.A.; Estes, N.M.; Ip, J.; Niazi, I.; et al. Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker. New Engl. J. Med. 2015, 373, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Boveda, S.; Higuera, L.; Longacre, C.; Wolff, C.; Wherry, K.; Stromberg, K.; El-Chami, M.F. Two-year outcomes of leadless vs. transvenous single-chamber ventricular pacemaker in high-risk subgroups. Eur. 2023, 25, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Cunnane, R.; Steffel, J.; El-Chami, M.F.; Reynolds, D.; Roberts, P.R.; Soejima, K.; Steinwender, C.; Garweg, C.; Chinitz, L.; et al. Development and validation of a risk score for predicting pericardial effusion in patients undergoing leadless pacemaker implantation: experience with the Micra transcatheter pacemaker. Eur. 2022, 24, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Valiton, V.; Graf, D.; Pruvot, E.; Carroz, P.; Fromer, M.; Bisch, L.; Tran, V.N.; Cook, S.; Scharf, C.; Burri, H. Leadless pacing using the transcatheter pacing system (Micra TPS) in the real world: initial Swiss experience from the Romandie region. Europace 2019, 21, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Black, G.B.; Rao, B.; Thakkar, N.; Massad, C.; Patel, A.V.; Merchant, F.M.; Hoskins, M.H.; Lurgio, D.B.; Patel, A.M.; et al. Outcomes of Micra leadless pacemaker implantation with uninterrupted anticoagulation. J. Cardiovasc. Electrophysiol. 2019, 30, 1313–1318. [Google Scholar] [CrossRef]
- Antonio, R.S.; Chipa-Ccasani, F.; Apolo, J.; Linhart, M.; Trotta, O.; Pujol-López, M.; Niebla, M.; Alarcón, F.; Trucco, E.; Arbelo, E.; et al. Management of anticoagulation in patients undergoing leadless pacemaker implantation. Hear. Rhythm. 2019, 16, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Garweg, C.; Iacopino, S.; Al-Samadi, F.; Martinez-Sande, J.L.; Tondo, C.; Johansen, J.B.; Prat, X.V.; Piccini, J.P.; Cha, Y.M.; et al. Leadless pacemaker implant, anticoagulation status, and outcomes: Results from the Micra Transcatheter Pacing System Post-Approval Registry. Hear. Rhythm. 2022, 19, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Sticherling, C.; Marin, F.; Birnie, D.; Boriani, G.; Calkins, H.; Dan, G.-A.; Gulizia, M.; Halvorsen, S.; Hindricks, G.; Kuck, K.-H.; et al. Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS). Eur. 2015, 17, 1197–1214. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Birnie, D.H.; Healey, J.S.; Wells, G.A.; Verma, A.; Tang, A.S.; Krahn, A.D.; Simpson, C.S.; Ayala-Paredes, F.; Coutu, B.; Leiria, T.L.; et al. Pacemaker or Defibrillator Surgery without Interruption of Anticoagulation. N. Engl. J. Med. 2013, 368, 2084–2093. [Google Scholar] [CrossRef]
- Regoli, F.; Araco, M.; Moccetti, T.; Caputo, M.L.; Conte, G.; Auricchio, A.; Moccetti, M. Potential Clinical Utility and Feasibility of Combined Left Atrial Appendage Closure and Positioning of Miniaturized Pacemaker Through a Single Right Femoral Vein Access. Am. J. Cardiol. 2017, 120, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ritter, P.; Duray, G.Z.; Steinwender, C.; Soejima, K.; Omar, R.; Mont, L.; Boersma, L.V.; Knops, R.E.; Chinitz, L.; Zhang, S.; et al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur. Hear. J. 2015, 36, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Roberto, M.; Grazioli-Gauthier, L.; Cioffi, G.; Pasotti, E.; Caputo, M.L.; Conte, G.; Breitenstein, A.; Moccetti, T. Feasibility and clinical efficacy of double suture-mediated closure device technique for hemostasis during positioning of miniaturized wireless pacemaker. J. Interv. Card. Electrophysiol. 2022, 64, 129–135. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; E Dilaveris, P.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Hear. J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Fox, K.A.; Piccini, J.P.; Wojdyla, D.; Becker, R.C.; Halperin, J.L.; Nessel, C.C.; Paolini, J.F.; Hankey, G.J.; Mahaffey, K.W.; Patel, M.R.; et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur. Hear. J. 2011, 32, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Healey, J.S.; A Wells, G.; Ayala-Paredes, F.; Coutu, B.; Sumner, G.L.; Becker, G.; Verma, A.; Philippon, F.; Kalfon, E.; et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur. Hear. J. 2018, 39, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).