Submitted:
02 July 2023
Posted:
03 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Endpoints
2.3. Variables Evaluated
2.4. Statistical Analysis
3. Results
3.1. Continence and satisfaction outcomes according to incontinence severity baseline
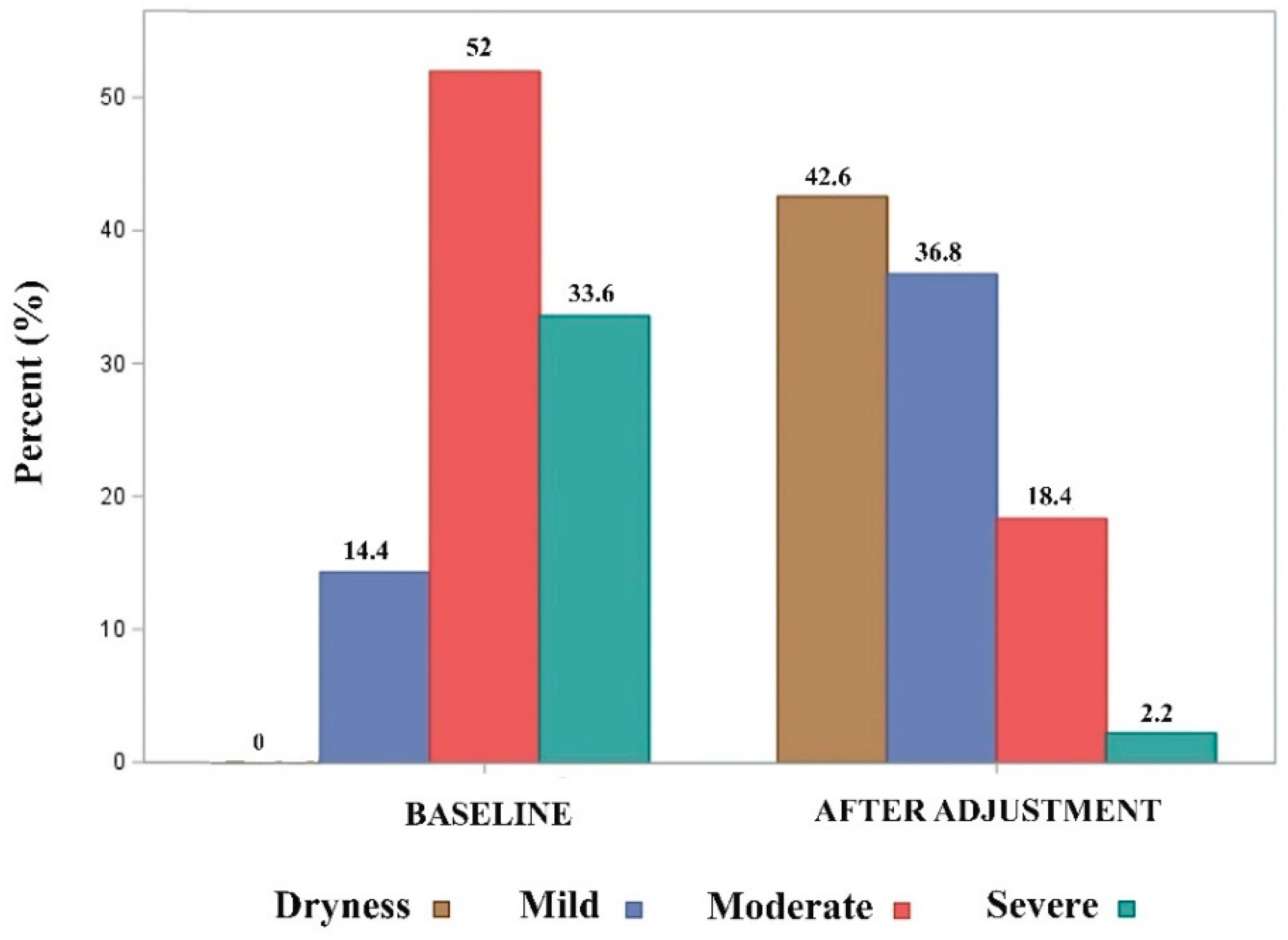
3.2. Comparison of effectiveness between baseline and after adjustment data
3.3. Postoperative and late complications
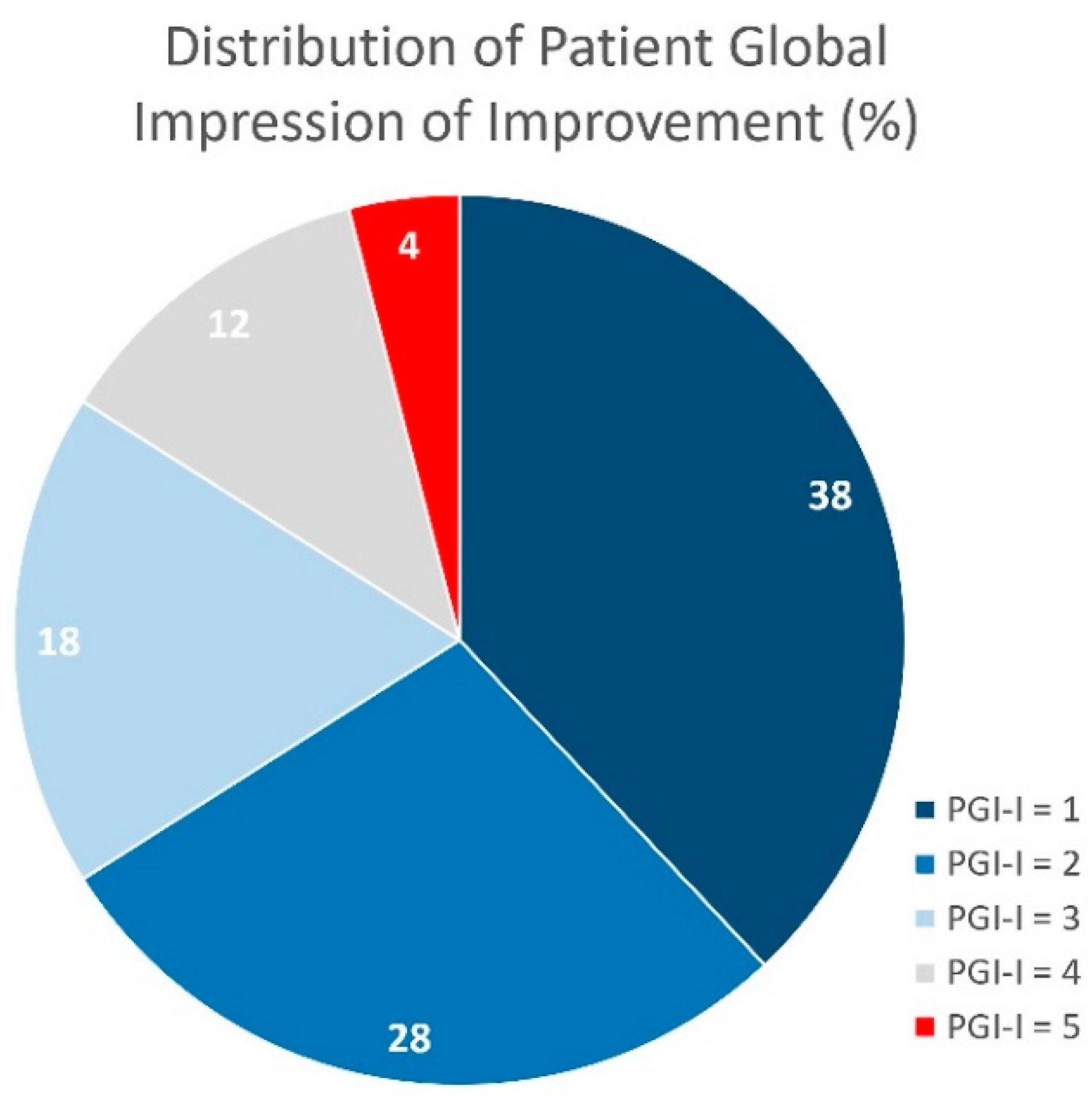
3.4. Self-perceived satisfaction
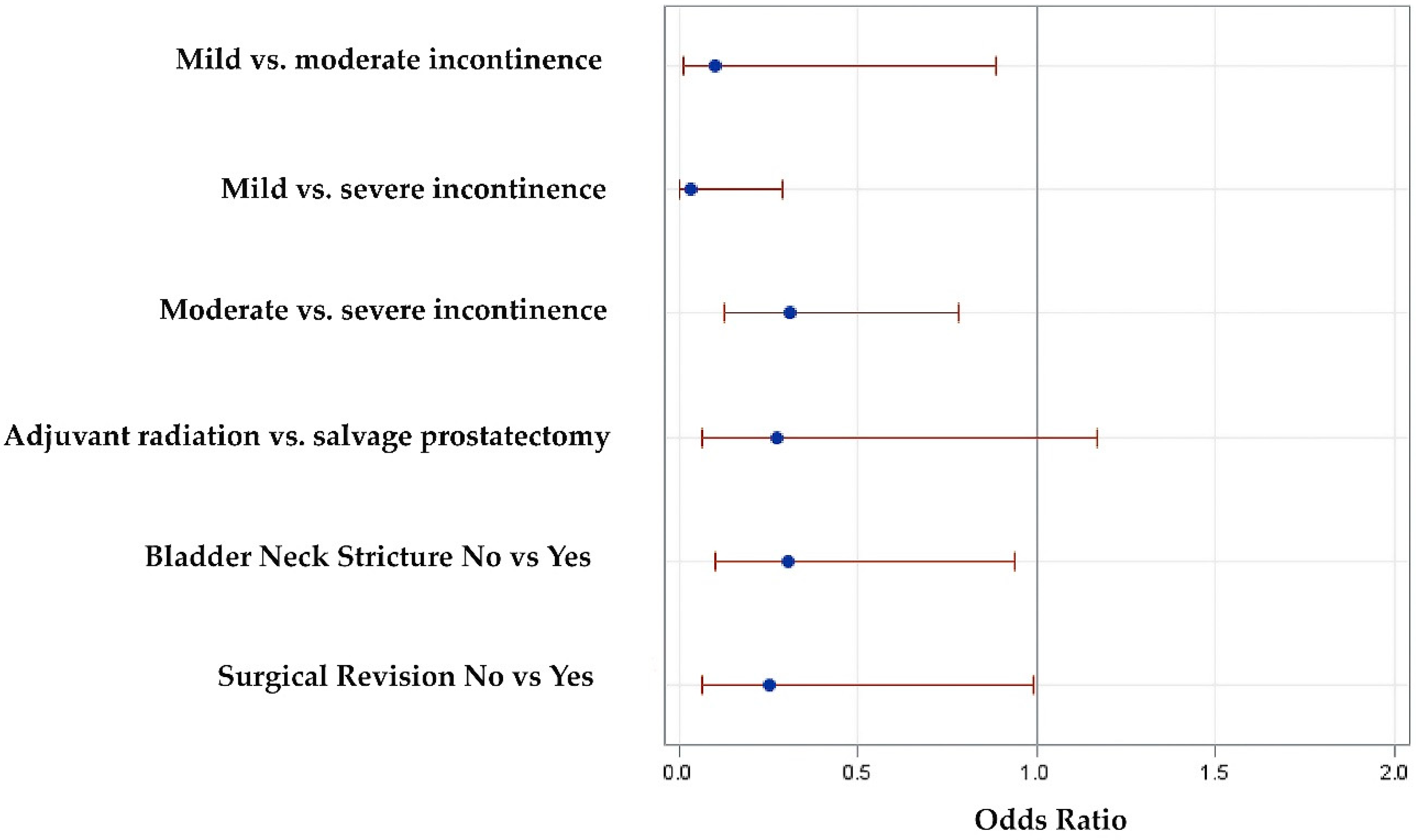
3.5. Logistic regression analysis for factors predictive of dryness
3.6. Nomogram to predict dryness after ATOMS adjustment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Oromendia, C.; Halpern, J.A.; Ballman, K.V. National trends in management of localized prostate cancer: A population based analysis 2004-2013. Prostate 2018, 78, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Ganswindt, U.; Stenzl, A.; Bamberg, M.; Belka, C. Adjuvant radiotherapy for patients with locally advanced prostate cancer - a new standard? Eur. Urol. 2008, 54, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Ghia, A.J.; Shrieve, D.C.; Tward, J.D. Adjuvant Radiotherapy Use and Patterns of Care Analysis for Margin-positive Prostate Adenocarcinoma with Extracapsular Extension: Postprostatectomy Adjuvant Radiotherapy: A SEER Analysis. Urology 2010, 76, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Huelster, H.L.; Laviana, A.A.; Joyce, D.D.; Huang, L.-C.; Zhao, Z.; Koyama, T.; Hoffman, K.E.; Conwill, R.; Goodman, M.; Hamilton, A.S.; et al. Radiotherapy after radical prostatectomy: Effect of timing of postprostatectomy radiation on functional outcomes. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 930.e23–930.e32. [Google Scholar] [CrossRef]
- Schick, U.; Latorzeff, I.; Sargos, P. Postoperative radiotherapy in prostate cancer: Dose and volumes. Cancer Radiother. 2021, 25, 674–678. [Google Scholar] [CrossRef]
- Grise, P.; Thurman, S. Urinary Incontinence following Treatment of Localized Prostate Cancer. Cancer Control. 2001, 8, 532–539. [Google Scholar] [CrossRef]
- Van Cangh, P.J.; Richard, F.; Lorge, F.; Castille, Y.; Moxhon, A.; Opsomer, R.; De Visscher, L.; Wese, F.X.; Scaillet, P. Adjuvant radiation therapy does not cause urinary incontinence after radical prostatectomy: results of a prospective randomized study. J. Urol. 1998, 159, 164–166. [Google Scholar] [CrossRef]
- Pontes, J.E.; Montie, J.; Klein, E.; Huben, R. Salvage surgery for radiation failure in prostate cancer. Cancer 1993, 71, 976–980. [Google Scholar] [CrossRef]
- Liu, M.; Pickles, T.; Berthelet, E.; Agranovich, A.; Kwan, W.; Tyldesley, S.; McKenzie, M.; Keyes, M.; Morris, J.; Pai, H.; et al. Urinary incontinence in prostate cancer patients treated with external beam radiotherapy. Radiother. Oncol. 2005, 74, 197–201. [Google Scholar] [CrossRef]
- Mamane, J.; Sanchez, S.; Lellouch, A.G.; Gaillard, V.; Poussot, B.; Tricard, T.; Saussine, C.; Brierre, T.; Game, X.; Beraud, F.; et al. Impact of radiation therapy on artificial urinary sphincter implantation in male patients: A multicenter study. Neurourol. Urodynamics 2021, 41, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Torrey, R.; Rajeshuni, N.; Ruel, N.; Muldrew, S.; Chan, K. Radiation History Affects Continence Outcomes After AdVance Transobturator Sling Placement in Patients With Post-prostatectomy Incontinence. Urology 2013, 82, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, T.; Guillaumier, S.; Solomon, E.; Jenks, J.; Pakzad, M.; Hamid, R.; Ockrim, J.; Shah, J. Radiotherapy is associated with reduced continence outcomes following implantation of the artificial urinary sphincter in men with post-radical prostatectomy incontinence. Urol. Ann. 2017, 9, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, D.; Hong, J.H.; Kim, C.-S.; Ahn, H.; Choo, M.-S. Factors contributing to treatment outcomes of post-prostatectomy incontinence surgery for the selection of the proper surgical procedure for individual patients: A single-center experience. Neurourol. Urodynamics 2018, 37, 1978–1987. [Google Scholar] [CrossRef]
- Abrams, P.; Constable, L.D.; Cooper, D.; MacLennan, G.; Drake, M.J.; Harding, C.; Mundy, A.; McCormack, K.; McDonald, A.; Norrie, J.; et al. Outcomes of a Noninferiority Randomised Controlled Trial of Surgery for Men with Urodynamic Stress Incontinence After Prostate Surgery (MASTER). Eur. Urol. 2021, 79, 812–823. [Google Scholar] [CrossRef]
- Dorado, J.F.; Angulo, J.C. Refined Nomogram Incorporating Standing Cough Test Improves Prediction of Adjustable Trans-Obturator Male System (ATOMS) Success to Treat Post-Prostatectomy Male Stress Incontinence. J. Pers. Med. 2022, 12, 94. [Google Scholar] [CrossRef]
- Téllez, C.; Szczesniewski, J.; Virseda-Chamorro, M.; Arance, I.; Angulo, J.C. Update on Adjustable Trans-Obturator Male System (ATOMS) for Male Incontinence after Prostate Cancer Surgery. Curr. Oncol. 2023, 30, 4153–4165. [Google Scholar] [CrossRef]
- Seweryn, J.; Bauer, W.; Ponholzer, A.; Schramek, P. Initial Experience and Results With a New Adjustable Transobturator Male System for the Treatment of Stress Urinary Incontinence. J. Urol. 2012, 187, 956–961. [Google Scholar] [CrossRef]
- Angulo, J.C.; Arance, I.; Esquinas, C.; Dorado, J.F.; Marcelino, J.P.; Martins, F.E. Outcome Measures of Adjustable Transobturator Male System with Pre-attached Scrotal Port for Male Stress Urinary Incontinence After Radical Prostatectomy: A Prospective Study. Adv. Ther. 2017, 34, 1173–1183. [Google Scholar] [CrossRef]
- International Surgical Outcomes Study (ISOS) group; Abbott, T. E.F.; E Greaves, K.; Patel, A.; Pearse, R.M.; Beattie, S.; Clavien, P.-A.; Demartines, N.; A Fleisher, L.; Grocott, M.; et al. Prospective observational cohort study on grading the severity of postoperative complications in global surgery research. Br. J. Surg. 2019, 106, e73–e80. [Google Scholar] [CrossRef]
- Bjelic-Radisic, V.; Ulrich, D.; Hinterholzer, S.; Reinstadler, E.; Geiss, I.; Aigmueller, T.; Tamussino, K.; Greimel, E.; Trutnovsky, G.; Austrian Urogynecology Working Group. Psychometric properties and validation of two global impression questionnaires (PGI-S, PGI-I) for stress incontinence in a German-speaking female population. Neurourol. Urodyn. 2018, 37, 1365–1371. [Google Scholar] [CrossRef]
- Daugherty, M.; Chelluri, R.; Bratslavsky, G.; Byler, T. Are we underestimating the rates of incontinence after prostate cancer treatment? Results from NHANES. Int. Urol. Nephrol. 2017, 49, 1715–1721. [Google Scholar] [CrossRef]
- Suardi, N.; Gallina, A.; Lista, G.; Gandaglia, G.; Abdollah, F.; Capitanio, U.; Dell’oglio, P.; Nini, A.; Salonia, A.; Montorsi, F.; et al. Impact of Adjuvant Radiation Therapy on Urinary Continence Recovery After Radical Prostatectomy. Eur. Urol. 2013, 65, 546–551. [Google Scholar] [CrossRef]
- Munoz, F.; Sanguineti, G.; Bresolin, A.; Cante, D.; Vavassori, V.; Waskiewicz, J.M.; Girelli, G.; Avuzzi, B.; Garibaldi, E.; Faiella, A.; et al. Predictors of Patient-Reported Incontinence at Adjuvant/Salvage Radiotherapy after Prostatectomy: Impact of Time between Surgery and Radiotherapy. Cancers 2021, 13, 3243. [Google Scholar] [CrossRef]
- Bresolin, A.; Garibaldi, E.; Faiella, A.; Cante, D.; Vavassori, V.; Waskiewicz, J.M.; Girelli, G.; Avuzzi, B.; Villa, E.; Magli, A.; et al. Predictors of 2-Year Incidence of Patient-Reported Urinary Incontinence After Post-prostatectomy Radiotherapy: Evidence of Dose and Fractionation Effects. Front. Oncol. 2020, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.V.; Ferro, M.; Jereczek-Fossa, B.A.; Renne, G.; Crisan, N.; Bottero, D.; Mazzarella, C.; Terracciano, D.; Autorino, R.; De Cobelli, O. Salvage Radical Prostatectomy after External Beam Radiation Therapy: A Systematic Review of Current Approaches. Urol. Int. 2015, 94, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.M.; Soljanik, I.; Füllhase, C.; Buchner, A.; May, F.; Stief, C.G.; Gozzi, C. Results of the AdVance Transobturator Male Sling After Radical Prostatectomy and Adjuvant Radiotherapy. Urology 2011, 77, 474–479. [Google Scholar] [CrossRef]
- Wright, H.C.; McGeagh, K.; Richter, L.A.; Hwang, J.J.; Venkatesan, K.; Pysher, A.; Koch, G.E.; Kowalczyk, K.; Bandi, G.; Marchalik, D. Transobturator sling for post-prostatectomy incontinence: radiation's effect on efficacy/satisfaction. Can J Urol. 2017, 24, 8998–9002. [Google Scholar]
- Del Favero, L.; Tasso, G.; Deruyver, Y.; Tutolo, M.; Beels, E.; Schillebeeckx, C.; De Ridder, D.; Van der Aa, F. Long-term Functional Outcomes and Patient Satisfaction After AdVance and AdVanceXP Male Sling Surgery. Eur. Urol. Focus 2022, 8, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Meisterhofer, K.; Herzog, S.; Strini, K.A.; Sebastianelli, L.; Bauer, R.; Dalpiaz, O. Male Slings for Postprostatectomy Incontinence: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2019, 6, 575–592. [Google Scholar] [CrossRef]
- Friedl, A.; Mühlstädt, S.; Zachoval, R.; Giammò, A.; Kivaranovic, D.; Rom, M.; Fornara, P.; Brössner, C. Long-term outcome of the adjustable transobturator male system (ATOMS): results of a European multicentre study. BJU Int. 2016, 119, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.C.; Cruz, F.; Esquinas, C.; Arance, I.; Manso, M.; Rodríguez, A.; Pereira, J.; Ojea, A.; Carballo, M.; Rabassa, M.; et al. Treatment of male stress urinary incontinence with the adjustable transobturator male system: Outcomes of a multi-center Iberian study. Neurourol. Urodynamics 2018, 37, 1458–1466. [Google Scholar] [CrossRef]
- Angulo, J.C.; Virseda-Chamorro, M.; Arance, I.; Ruiz, S.; Ojea, A.; Carballo, M.; Rodríguez, A.; Pereira, J.; Teyrouz, A.; Rebassa, M.; et al. Long-term outcome of adjustable transobturator male system for stress urinary incontinence in the Iberian multicentre study. Neurourol. Urodynamics 2020, 39, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Giammò, A.; Ammirati, E.; Tullio, A.; Morgia, G.; Sandri, S.; Introini, C.; Canepa, G.; Timossi, L.; Rossi, C.; Mozzi, C.; et al. Implant of ATOMS® system for the treatment of postoperative male stress urinary incontinence: an Italian multicentric study. Minerva Urol. Nephrol. 2020, 72, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Redmond, E.J.; Nadeau, G.; Tu, L.-M.; Doiron, R.C.; Steele, S.S.; Herschorn, S.; Locke, J.A.; Maciejewski, C.C.; Dwyer, N.T.; Campeau, L.; et al. Multicentered Assessment of Clinical Outcomes and Factors Associated With Failure of the Adjustable TransObturator Male System (ATOMS). Urology 2020, 148, 280–286. [Google Scholar] [CrossRef]
- Esquinas, C.; Angulo, J.C. Effectiveness of Adjustable Transobturator Male System (ATOMS) to Treat Male Stress Incontinence: A Systematic Review and Meta-Analysis. Adv. Ther. 2018, 36, 426–441. [Google Scholar] [CrossRef]
- Mühlstädt, S.; Angulo, J.C.; Mohammed, N.; Schumann, A.; Fornara, P. Complications of the urinary incontinence system ATOMS: description of risk factors and how to prevent these pitfalls. World J. Urol. 2019, 38, 1795–1803. [Google Scholar] [CrossRef]
- Angulo, J.C.; Arance, I.; Ojea, A.; Carballo, M.; Rodríguez, A.; Pereira, J.; Rebassa, M.; Teyrouz, A.; Escribano, G.; Teba, F.; et al. Patient satisfaction with adjustable transobturator male system in the Iberian multicenter study. World J. Urol. 2019, 37, 2189–2197. [Google Scholar] [CrossRef]
- Angulo, J.C.; Schönburg, S.; Giammò, A.; Queissert, F.; Gonsior, A.; González-Enguita, C.; Martins, F.E.; Rourke, K.; Cruz, F. Artificial urinary sphincter or a second adjustable transobturator male system offer equivalent outcomes in patients whom required revision on the initial ATOMS device: An international multi-institutional experience. Neurourol. Urodynamics 2021, 40, 897–909. [Google Scholar] [CrossRef]
- Angulo, J.C.; Esquinas, C.; Arance, I.; Rodríguez, A.; Pereira, J.; Rabassa, M.; Teyrouz, A.; Teba, F.; Celada, G.; Marcelino, J.P.; et al. Adjustable Transobturator Male System after Failed Surgical Devices for Male Stress Urinary Incontinence: A Feasibility Study. Urol. Int. 2018, 101, 106–113. [Google Scholar] [CrossRef]
- Queissert, F.; Huesch, T.; Kretschmer, A.; Kirschner-Hermanns, R.; Pottek, T.; Olianas, R.; Friedl, A.; Homberg, R.; Pfitzenmaier, J.; Naumann, C.M.; et al. Is the Standard Artificial Urinary Sphincter AMS 800 Still a Treatment Option for the Irradiated Male Patient Presenting with a Devastated Bladder Outlet? J. Clin. Med. 2023, 12, 4002. [Google Scholar] [CrossRef] [PubMed]
- Ullate, A.; Arance, I.; Virseda-Chamorro, M.; Ruiz, S.; Szczesniewski, J.; Téllez, C.; Queissert, F.; Dorado, J.F.; Angulo, J.C. ATOMS (Adjustable Trans-Obturator Male System) in Patients with Post-Prostatectomy Incontinence and Previously Treated Urethral Stricture or Bladder Neck Contracture. J. Clin. Med. 2022, 11, 4882. [Google Scholar] [CrossRef] [PubMed]
- Manunta, A.; Guillé, F.; Patard, J.J.; Lobel, B. Artificial sphincter insertion after radiotherapy: is it worthwhile? BJU Int. 2000, 85, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Ravier, E.; Fassi-Fehri, H.; Crouzet, S.; Gelet, A.; Abid, N.; Martin, X. Complications after artificial urinary sphincter implantation in patients with or without prior radiotherapy. BJU Int. 2014, 115, 300–307. [Google Scholar] [CrossRef]
- Mann, R.A.; Kasabwala, K.; Buckley, J.C.; Smith, T.G.; Westney, O.L.; Amend, G.M.; Breyer, B.N.; Erickson, B.A.; Alsikafi, N.F.; Broghammer, A.J.; et al. The “Fragile” Urethra as a Predictor of Early Artificial Urinary Sphincter Erosion. Urology 2022, 169, 233–236. [Google Scholar] [CrossRef]
- Gomha, M.A.; Boone, T.B. Artificial urinary sphincter for post-prostatectomy incontinence in men who had prior radiotherapy: a risk and outcome analysis. J. Urol. 2002, 167, 591–596. [Google Scholar] [CrossRef]
- Jhavar, S.; Swanson, G.; Deb, N.; Littlejohn, L.; Pruszynski, J.; Machen, G.; Milburn, P.; Bird, E. Durability of Artificial Urinary Sphincter With Prior Radiation Therapy. Clin. Genitourin. Cancer 2017, 15, e175–e180. [Google Scholar] [CrossRef]
- Tutolo, M.; Cornu, J.; Bauer, R.M.; Ahyai, S.; Bozzini, G.; Heesakkers, J.; Drake, M.J.; Tikkinen, K.A.; Launonen, E.; Larré, S.; et al. Efficacy and safety of artificial urinary sphincter (AUS): Results of a large multi-institutional cohort of patients with mid-term follow-up. Neurourol. Urodynamics 2018, 38, 710–718. [Google Scholar] [CrossRef]
- Kaufman, M.R.; Milam, D.F.; Johnsen, N.V.; Cleves, M.A.; Broghammer, J.A.; Brant, W.O.; Jones, L.A.; Brady, J.D.; Gross, M.S.; Henry, G.D. Prior Radiation Therapy Decreases Time to Idiopathic Erosion of Artificial Urinary Sphincter: A Multi-Institutional Analysis. J. Urol. 2018, 199, 1037–1041. [Google Scholar] [CrossRef]
- Gallet, P.; Phulpin, B.; Merlin, J.-L.; Leroux, A.; Bravetti, P.; Mecellem, H.; Tran, N.; Dolivet, G. Long-Term Alterations of Cytokines and Growth Factors Expression in Irradiated Tissues and Relation with Histological Severity Scoring. PLOS ONE 2011, 6, e29399. [Google Scholar] [CrossRef]
- Sterling, J.; Rahman, S.N.; Varghese, A.; Angulo, J.C.; Nikolavsky, D. Complications after Prostate Cancer Treatment: Pathophysiology and Repair of Post-Radiation Urethral Stricture Disease. J. Clin. Med. 2023, 12, 3950. [Google Scholar] [CrossRef]
- Giammò, A.; Ammirati, E. Long-Term Survival Rate of ATOMS Implant for Male Stress Urinary Incontinence and Management of Late Complications. J. Clin. Med. 2023, 12, 2296. [Google Scholar] [CrossRef]
- Schönburg, S.; Bauer, W.; Mohammed, N.; Brössner, C.; Fornara, P. De novo OAB After ATOMS: An Underestimated Problem or a Rare Side Effect? Front. Surg. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Cozzarini, C.; Rancati, T.; Palorini, F.; Avuzzi, B.; Garibaldi, E.; Balestrini, D.; Cante, D.; Munoz, F.; Franco, P.; Girelli, G.; et al. Patient-reported urinary incontinence after radiotherapy for prostate cancer: Quantifying the dose–effect. Radiother. Oncol. 2017, 125, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Mühlstädt, S.; Friedl, A.; Mohammed, N.; Schumann, A.; Weigand, K.; Kawan, F.; Göllert, C.; Kahlert, C.; Theil, G.; Fischer, K.; et al. Five-year experience with the adjustable transobturator male system for the treatment of male stress urinary incontinence: a single-center evaluation. World J. Urol. 2016, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
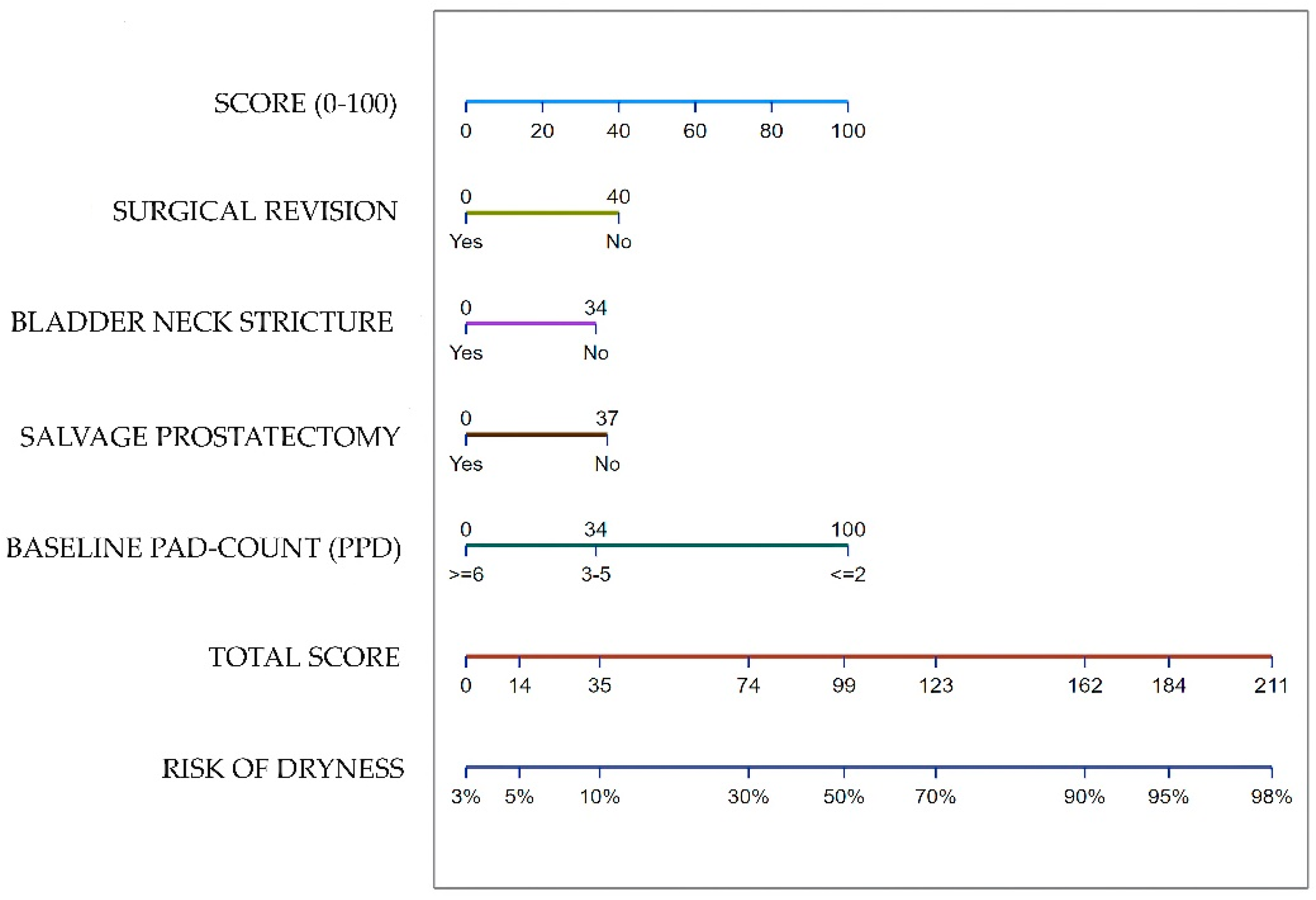
| Variable | Total Series (n = 223) |
|---|---|
| Preoperative data | |
| Age, years, median (IQR) | 71 (11) |
| Time since prostatectomy to ATOMS, months, median (IQR) | 52 (58) |
| Previous incontinence surgery, n (%) | 27 (12.1) |
| Salvage prostatectomy, n (%) | 12 (5.4) |
| Previous TUR-P, n (%) | 8 (3.6) |
| Previous brachytherapy, n (%) | 2 (.9) |
| Androgen deprivation therapy, n (%) | 46 (20.6) |
| Bladder neck stricture, n (%) | 63 (28.2) |
| OAB symptoms, n (%) | 27 (12.1) |
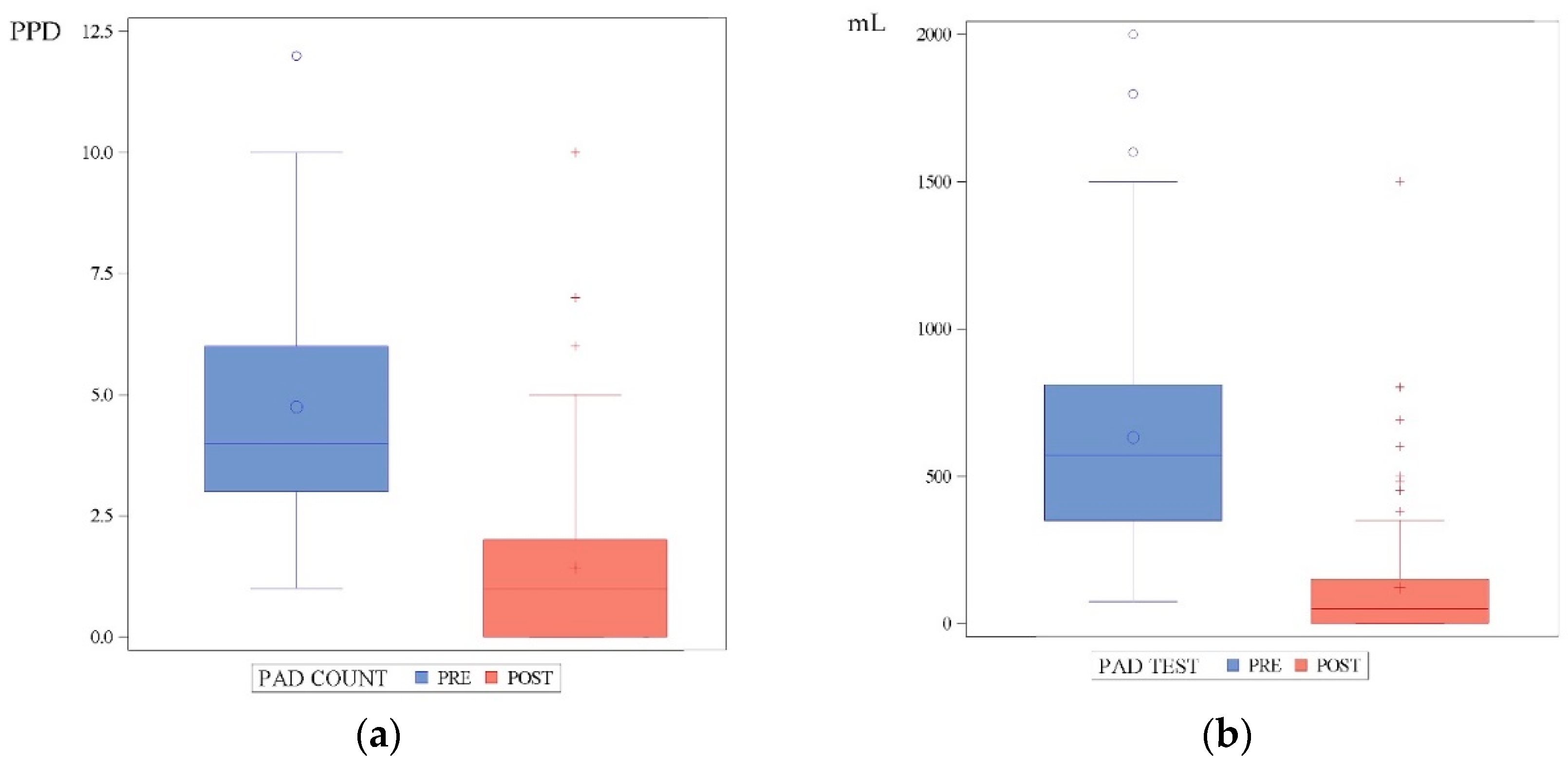
| 24-h pad-count, PPD, median (IQR) | 4 (3) |
| 24-h pad-test, mL, median (IQR) (1) | 570 (460) |
| Operative data | |
| Operative time, min, median (IQR) | 62 (24) |
| Perioperative complication, n (%) | 2 (.9) |
| VAS for pain (0 – 10), median (IQR) (2) | 2 (2) |
| Postoperative data | |
| Postoperative complications (3), any grade, n (%) | 36 (16.1) |
| Grade I (3), n (%) | 20 (9) |
| Grade II (3), n (%) | 11 (4.9) |
| Grade III (3), n (%) | 5 (2.2) |
| Surgical revision, n (%) | 29 (13) |
| Device explant, n (%) | 26 (11.7) |
| De novo OAB symptoms, n (%) | 11 (4.9) |
| Total filling volume, mL, median (IQR) | 16 (11.5) |
| Number of fillings, median (IQR) | 1 (3) |
| Patients with pad-test ≤ 20 mL, n (%) | 95 (42.6) |
| 24-h pad-count, PPD, median (IQR) | 1 (2) |
| 24-h pad-test, mL, median (IQR) (4) | 50 (150) |
| PGI-I = 1 (very much better), n (%) (4) | 48 (38.4) |
| PGI-I = 2 (much better), n (%) (4) | 35 (28) |
| PGI-I = 3 (slightly better), n (%) (4) | 22 (17.6) |
| PGI-I = 4 (same), n (%) (4) | 15 (12) |
| PGI-I = 5 (worse), n (%) (4) | 5 (5) |
| Degree of Incontinence | N (%) | Dryness (*) N (%) | Satisfaction (#) N (%) | Mean change in PPD | Mean change in pad-test (@) | Mean number of adjustments |
|---|---|---|---|---|---|---|
| Total series | 223 (100) | 95 (42.6) | 105 (84) | 3 ± 2.5 | 450 ± 400 | 3 ± 3 |
| Mild (1-2 PPD) | 32 (14.3) | 21 (65.6) | 10 (90.9) | 1.5 ± 1 | 200 ± 130 | 2.5 ± 3.5 |
| Moderate (3-5 PPD) | 116 (52) | 56 (48.3) | 52 (85.3) | 3 ± 2 | 350 ± 200 | 3 ± 3 |
| Severe (≥6 PPD) | 75 (33.7) | 18 (24) | 43 (81.1) | 5 ± 2 | 700 ± 270 | 4 ± 2 |
| P-value | <.0001 | .67 | <.0001 | <.0001 | <.0001 |
| Univariate Analysis | OR | 95% CI Upper limit | 95% CI Lower limit | P value |
| Patient age <70 vs. ≥70 years | .59 | .34 | 1.01 | .06 |
| Adjuvant radiotherapy vs. salvage prostatectomy | .35 | .1 | 1.2 | .08 |
| Primary incontinence surgery | .34 | .13 | .89 | .02 |
| OAB symptoms | .37 | .14 | .96 | .04 |
| Bladder neck stricture | .35 | .18 | .67 | .001 |
| Surgical revision | .32 | .13 | .77 | .008 |
| Baseline incontinence, Mild vs. moderate | .54 | .23 | 1.25 | .0003 |
| Mild vs. severe | .21 | .09 | .50 | |
| Moderate vs. severe | .40 | .22 | .73 | |
| Multivariate Analysis | OR | 95% CI Upper limit | 95% CI Lower limit | P value |
| Surgical revision | .25 | .06 | .99 | .018 |
| Adjuvant radiotherapy vs. salvage prostatectomy | .28 | .07 | 1.17 | .07 |
| Bladder neck stricture | .3 | .1 | .94 | .05 |
| Baseline incontinence, Mild vs. moderate | .1 | .01 | .89 | <.0001 |
| Mild vs. severe | .03 | 0 | .29 | |
| Moderate vs. severe | .31 | .13 | .78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





