Submitted:
02 July 2023
Posted:
03 July 2023
You are already at the latest version
Abstract
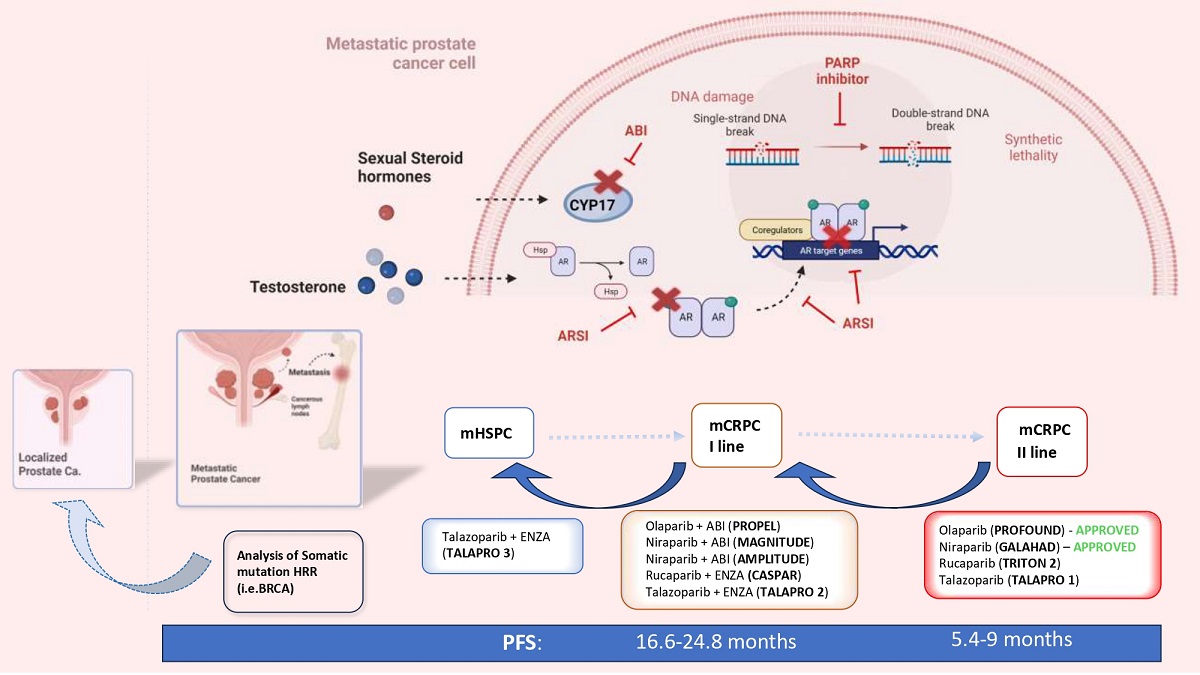
Keywords:
1. Introduction: the concept of anticipation in the systemic therapy of prostate cancer
2. The rational and the genetic profile that sustain PARP inhibitors in PC
3. Clinical trials and actual recommendations for PARP inhibitors in second line mCRPC
4. The prognostic role of HRR PV in non-metastatic and mHSPC as indicators of anticipation tailored treatment
5. PARP-AR crosstalk and current clinical trials with PARPi in anticipated first line mCRPC
5.1. PROPEL trial: abiraterone + Olaparib
5.2. MAGNITUDE: abiraterone + Niraparib
5.3. AMPLITUDE trial: Abiraterone + niraparib
5.4. CASPAR trial: enzalutamide + rucaparib
5.5. TALAPRO-2: enzalutamide + talazoparib
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poon DM, Chan K, Lee SH, Chan TW, Sze H, Lee EK, Lam D, Chan MF. Abirateroneacetate in metastatic castration-resistant prostate cancer - the unanticipated real-world clinical experience. BMC Urol. 2016 Mar 22;16:12-18. [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.-S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2016, 71, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A,Alcaraz A, Alekseev B, Iguchi T, Shore ND, Rosbrook B, Sugg J, Baron B, Chen L,Stenzl A. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive ProstateCancer. J Clin Oncol. 2019 Nov 10;37(32):2974-2986. [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.d.S.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, KopyltsovE, Park CH, Alekseev B, Montesa-Pino Á, Ye D, Parnis F, Cruz F, Tammela TLJ,Suzuki H, Utriainen T, Fu C, Uemura M, Méndez-Vidal MJ, Maughan BL, Joensuu H,Thiele S, Li R, Kuss I, Tombal B; ARASENS Trial Investigators. Darolutamide andSurvival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142. [CrossRef]
- Sciarra, A.; Frisenda, M.; Bevilacqua, G.; Gentilucci, A.; Cattarino, S.; Mariotti, G.; Del Giudice, F.; Di Pierro, G.B.; Viscuso, P.; Casale, P.; et al. How the Analysis of the Pathogenetic Variants of DDR Genes Will Change the Management of Prostate Cancer Patients. Int. J. Mol. Sci. 2022, 24, 674. [Google Scholar] [CrossRef]
- Sciarra A, Fiori C, Del Giudice F, DI Pierro G, Bevilacqua G, Gentilucci A,Cattarino S, Mariotti G, Salciccia S. DDR genes analysis and PARP-inhibitors therapy as tailored management in metastatic prostate cancer: achieved answers, open questions and future perspectives. Minerva Urol Nephrol. 2022 Dec;74(6):649-652. [CrossRef]
- Giglia-Mari G, Zotter A, Vermeulen W. DNA damage response. Cold Spring Harb Perspect Biol. 2011;3(1):a000745. [CrossRef]
- Caldecott, K.W. Mammalian single-strand break repair: Mechanisms and links with chromatin. DNA Repair 2007, 6, 443–453. [Google Scholar] [CrossRef]
- Rouleau, M.; Patel, A.; Hendzel, M.J.; Kaufmann, S.H.; Poirier, G.G. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 2010, 10, 293–301. [Google Scholar] [CrossRef]
- Liu, C.; Vyas, A.; Kassab, M.A.; Singh, A.K.; Yu, X. The role of poly ADP-ribosylation in the first wave of DNA damage response. Nucleic Acids Res. 2017, 45, 8129–8141. [Google Scholar] [CrossRef]
- Ceccaldi, R.; Liu, J.C.; Amunugama, R.; Hajdu, I.; Primack, B.; Petalcorin, M.I.R.; O’connor, K.W.; Konstantinopoulos, P.A.; Elledge, S.J.; Boulton, S.J.; et al. Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature 2015, 518, 258–262. [Google Scholar] [CrossRef]
- Hegan, D.C.; Lu, Y.; Stachelek, G.C.; Crosby, M.E.; Bindra, R.S.; Glazer, P.M. Inhibition of poly(ADP-ribose) polymerase down-regulates BRCA1 and RAD51 in a pathway mediated by E2F4 and p130. Proc. Natl. Acad. Sci. 2010, 107, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Domchek SM, Aghajanian C, Shapira-Frommer R. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199-203. [CrossRef]
- Castro E, Goh C, Olmos D. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748-1757. [CrossRef]
- Carter HB, Helfand B, Mamawala M. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur Urol. 2019;75(5):743-749. [CrossRef]
- Castro E, Goh C, Leongamornlert D. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur Urol. 2015;68(2):186-193. [CrossRef]
- Lang, S.H.; Swift, S.L.; White, H.; Misso, K.; Kleijnen, J.; Quek, R.G. A systematic review of the prevalence of DNA damage response gene mutations in prostate cancer. Int. J. Oncol. 2019, 55, 597–616. [Google Scholar] [CrossRef] [PubMed]
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes Nature. 2020;578(7793):82-93. [CrossRef]
- Eeles, R.; Goh, C.; Castro, E.; Bancroft, E.; Guy, M.; Al Olama, A.A.; Easton, D.; Kote-Jarai, Z. The genetic epidemiology of prostate cancer and its clinical implications. Nat. Rev. Urol. 2013, 11, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Kim IE Jr, Kim S, Srivastava A. Similar incidence of DNA damage response pathway alterations between clinically localized and metastatic prostate cancer. BMC Urol. 2019;19(1):33. [CrossRef]
- Marshall, C.H.; Fu, W.; Wang, H.; Baras, A.S.; Lotan, T.L.; Antonarakis, E.S. Prevalence of DNA repair gene mutations in localized prostate cancer according to clinical and pathologic features: association of Gleason score and tumor stage. Prostate Cancer Prostatic Dis. 2018, 22, 59–65. [Google Scholar] [CrossRef]
- Hussain, M.; Corcoran, C.; Sibilla, C.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Mateo, J.; Olmos, D.; Mehra, N.; et al. Tumor Genomic Testing for >4,000 Men with Metastatic Castration-resistant Prostate Cancer in the Phase III Trial PROfound (Olaparib). Clin. Cancer Res. 2022, 28, 1518–1530. [Google Scholar] [CrossRef]
- I Scher, H.; Sandhu, S.; Efstathiou, E.; Lara, P.N.; Yu, E.Y.; Saad, F.; Ståhl, O.; Olmos, D.; E Mason, G.; Espina, B.M.; et al. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2022, 23, 362–373. [Google Scholar] [CrossRef]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 21, 162–174. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Mehra, N.; Scagliotti, G.V.; Castro, E.; Dorff, T.; Stirling, A.; Stenzl, A.; Fleming, M.T.; Higano, C.S.; Saad, F.; et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021, 22, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Collaborators, T.I.A.E.; Fisher, C.; Foster, C.S.; Jameson, C.; Barbachanno, Y.; Bartlett, J.; Bancroft, E.; Doherty, R.; Kote-Jarai, Z.; et al. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br. J. Cancer 2008, 98, 502–507. [Google Scholar] [CrossRef]
- Thorne, H.; Willems, A.J.; Niedermayr, E.; Hoh, I.M.Y.; Li, J.; Clouston, D.; Mitchell, G.; Fox, S.; Hopper, J.L.; Bolton, D.; et al. Decreased Prostate Cancer-Specific Survival of Men with BRCA2 Mutations from Multiple Breast Cancer Families. Cancer Prev. Res. 2011, 4, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Page, E.C.; Bancroft, E.K.; Brook, M.N.; Assel, M.; Hassan Al Battat, M.; Thomas, S.; Taylor, N.; Chamberlain, A.; Pope, J.; Ni Raghallaigh, H.; et al. Interim Results from the IMPACT Study: Evidence for Prostate-specific Antigen Screening in BRCA2 Mutation Carriers. Eur. Urol. 2019, 76, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Rumble, R.B.; Loblaw, D.A.; Finelli, A.; Ehdaie, B.; Cooperberg, M.R.; Morgan, S.C.; Tyldesley, S.; Haluschak, J.J.; Tan, W.; et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2016, 34, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Chanza, N.M.; Bernard, B.; Barthelemy, P.; Accarain, A.; Paesmans, M.; Desmyter, L.; de Roodenbeke, D.T.; Gil, T.; Sideris, S.; Roumeguere, T.; et al. Prevalence and clinical impact of tumor BRCA1 and BRCA2 mutations in patients presenting with localized or metastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2021, 25, 199–207. [Google Scholar] [CrossRef]
- Antonarakis ES, Lu C, Luber B, Liang C, Wang H, Chen Y, Silberstein JL, Piana D, Lai Z, Chen Y, Isaacs WB, Luo J. Germline DNA-repair Gene Mutations and Outcomes in Men with Metastatic Castration-resistant Prostate Cancer Receiving First-line Abiraterone and Enzalutamide. Eur Urol. 2018 Aug;74(2):218-225. [CrossRef]
- Annala M, Struss WJ, Warner EW, Beja K, Vandekerkhove G, Wong A, Khalaf D, Seppälä IL, So A, Lo G, Aggarwal R, Small EJ, Nykter M, Gleave ME, Chi KN, Wyatt AW. Treatment Outcomes and Tumor Loss of Heterozygosity in Germline DNA Repair-deficient Prostate Cancer. Eur Urol. 2017 Jul;72(1):34-42. [CrossRef]
- Hussain M, Daignault-Newton S, Twardowski PW. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J Clin Oncol 2018, 36, 991–9. [CrossRef]
- Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, Piulats JM, Lorente D, Saez MI, Morales-Barrera R, Gonzalez-Billalabeitia E, Cendón Y, García-Carbonero I, Borrega P, Mendez Vidal MJ, Montesa A, Nombela P, Fernández-Parra E, Gonzalez Del Alba A, Villa-Guzmán JC, Ibáñez K, Rodriguez-Vida A, Magraner-Pardo L, Perez-Valderrama B, Vallespín E, Gallardo E, Vazquez S, Pritchard CC, Lapunzina P, Olmos D. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2019 Feb 20;37(6):490-503. [CrossRef]
- Rao A, Moka N, Hamstra DA, Ryan CJ. Co-inhibition of androgen receptor and PARP as a novel treatment paradigm in prostate cancer: where are we now? Cancer 2022;14;801:1-23. [CrossRef]
- Clarke NW, Armstrong AJ, Oya M, Shore N, Loredo E, Procopio G, Girotto G, for the Propel investigators. Abiraterone and Olaparib for metastatic castration resistant prostate cancer. NEJM Evidence,; 1 (9): 1-16. 3 June. [CrossRef]
- Carr, T.H.; Adelman, C.; Barnicle, A.; Kozarewa, I.; Luke, S.; Lai, Z.; Hollis, S.; Dougherty, B.; Harrington, E.A.; Kang, J.; et al. Homologous Recombination Repair Gene Mutation Characterization by Liquid Biopsy: A Phase II Trial of Olaparib and Abiraterone in Metastatic Castrate-Resistant Prostate Cancer. Cancers 2021, 13, 5830. [Google Scholar] [CrossRef]
- Saad, F.; Thiery-Vuillemin, A.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; et al. Patient-reported outcomes with olaparib plus abiraterone versus placebo plus abiraterone for metastatic castration-resistant prostate cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2022, 23, 1297–1307. [Google Scholar] [CrossRef]
- Clarke N. Final overall survival in PROpel: abiraterone and Olaparib versus abiraterone and placebo as first-line therapy for metastatic castration-resistant prostate cancer. ASCO GU 2023. Available online: https://meetings.asco.org/abstracts-presentations/217650.
- Chi, K.N.; Rathkopf, D.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; Gomes, A.J.P.d.S.; Roubaud, G.; et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2023, 41, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Rathkopf, D. A Phase 3 Randomized, Placebo-controlled, Double-blind Study of Niraparib in Combination With Abiraterone Acetate and Prednisone Versus Abiraterone Acetate and Prednisone for the Treatment of Participants With Deleterious Germline or Somatic Homologous Recombination Repair (HRR) Gene-Mutated Metastatic Castration-Sensitive Prostate Cancer (mCSPC).2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04497844.
- Rao A, Ryan CJ, VanderWeele DJ, Heller G, Lewis LD, Watt C, Chen RC, Grubb R, Hahn OM, Beltran H. CASPAR (Alliance A031902): A Randomized, Phase III Trial of Enzalutamide (ENZ) with Rucaparib (RUCA)/Placebo (PBO) as aNovel Therapy in First-Line Metastatic Castration-Resistant Prostate Cancer (MCRPC). J. Clin. Oncol. 2021, 39, TPS181.
- ClinicalTrials.gov. A Clinical Study Evaluating The Benefit of Adding Rucaparib to Enzalutamide for Men With Metastatic Prostate Cancer That Has Become Resistant To Testosterone-Deprivation Therapy. Available online: https://clinicaltrials.gov/ct2/show/NCT04455750.
- Agarwal, N.; Azad, A.; Shore, N.D.; Carles, J.; Fay, A.P.; Dunshee, C.; Karsh, L.I.; Paccagnella, M.L.; Di Santo, N.; Elmeliegy, M.; et al. Talazoparib plus enzalutamide in metastatic castration-resistant prostate cancer: TALAPRO-2 phase III study design. Futur. Oncol. 2022, 18, 425–436. [Google Scholar] [CrossRef] [PubMed]
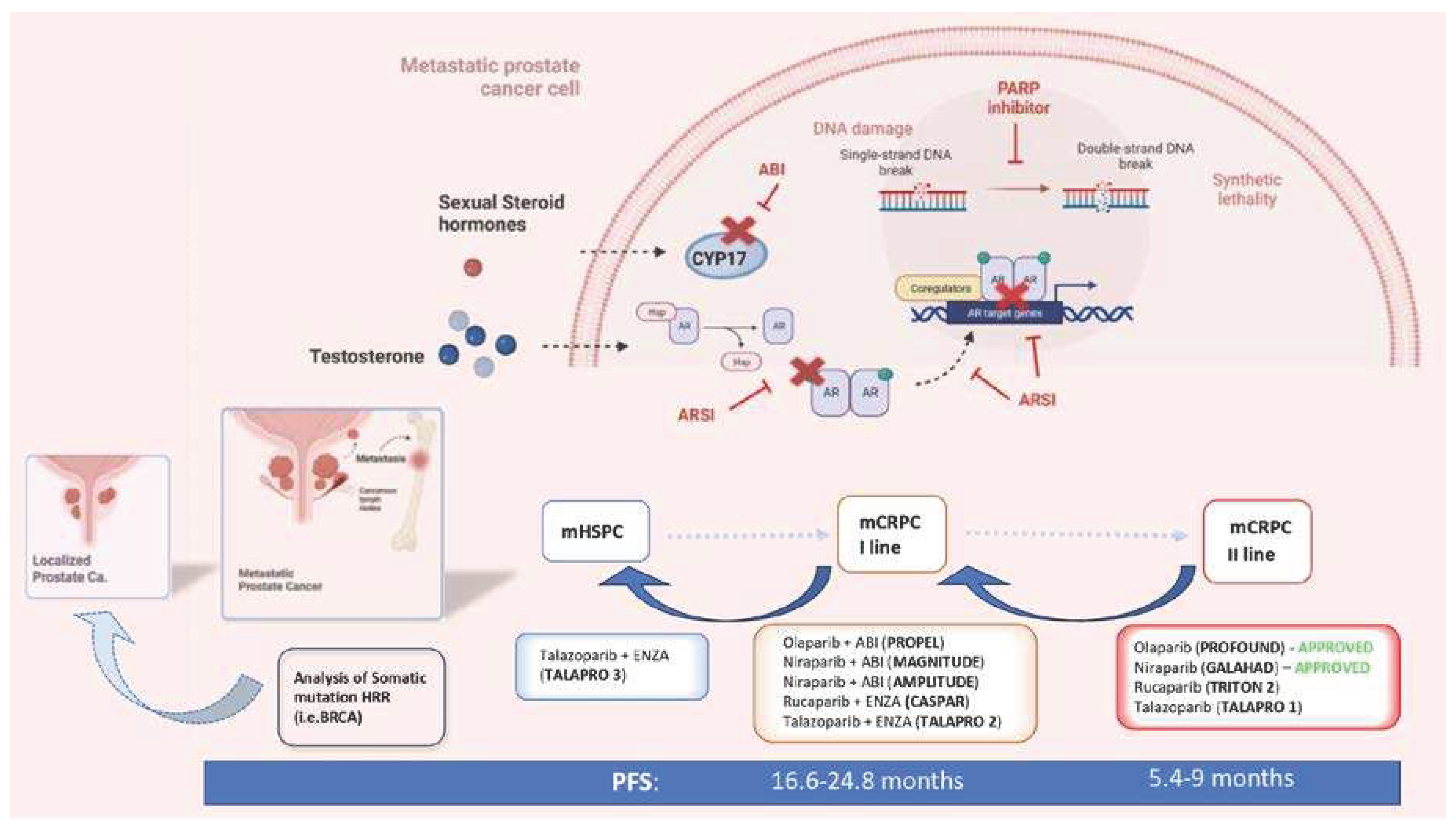
| Study | Design | Treatments | Patient Selection | Primary Endpoint | Main Results |
|---|---|---|---|---|---|
| PROPEL [44] | Phase 3, randomized, placebo-controlled, double-blinded |
Abiraterone + Olaparib (360) Abiraterone + Placebo (360) |
First line mCRPC Prior ARSI not allowed Prior docetaxel allowed for mHSPC HRR mutation not required (but analyzed as subgroup) |
Radiographic PFS | Radiological PFS 24.8 vs 16.6 months; HR 0.66; 95%CI 0.54-0.81; p<0.001, irrespectively to HRR status (HRR mutated subgroup: HR 0.50; 95%CI 0.34-0.73; HRR non-mutated subgroup: HR 0.76; 95%CI 0.60-0.97). Overall survival 42.1 vs 34.7 months; HR 0.81; 95%CI 0.67-1.00; p=0.0544, irrespectively to HRR mutation (HRR mutated subgroup: HR 0.66; 95%CI 0.45-0.95; HRR non-mutated subgroup: HR 0.89; 95%CI 0.70-1.14) |
| CASPAR [50] | Phase 3, randomized, placebo-controlled, double-blinded |
Enzalutamide + Rucaparib (496) Enzalutamide + Placebo (496) |
First line mCRPC Prior ARSI allowed For mHSPC and nmCRPC Prior docetaxel allowed for mHSPC HRR mutation not required (but analyzed as subgroups) |
Radiographic PFS and overall survival |
ongoing |
| MAGNITUDE [48] | Phase 3, randomized, placebo-controlled, double-blinded |
HRR-mutant cohort Abiraterone + Niraparib (200) Abiraterone + Placebo (200) -------------------------- HRR- no mutated cohort Abiraterone + Niraparib (300) Abiraterone + Placebo (300) |
First line mCRPC Prior ARSI not allowed Prior docetaxel allowed for mHSPC ------------------------------ First line mCRPC Prior ARSI not allowed Prior docetaxel allowed for mHSPC |
Radiographic PFS ----------------------- Radiographic PFS |
In HRR- mutant : PFS 16.5 vs 13.7 months; HR 0.73; 95%CI 0.56-0.96;p=0.022. In HRR- no mutant: HR 1.09; 95%CI 0.75-1.57;p=0.66 and the cohort was closed to further enrollment. |
| TALAPRO-2 [52] | Phase 3, randomized, placebo-controlled, double-blinded |
Enzalutamide + Talazoparib (509) Enzalutamide + Placebo (509) |
First line mCRPC Prior abiraterone allowed (no novel AR inhibitors) for mHSPC and nmCRPC Prior docetaxel allowed for mHSPC HRR mutation not required but analyzed as subgroups |
Radiographic PFS | ongoing |
| AMPLITUDE [49] | Phase 3, randomized, placebo-controlled, double-blinded |
Abiraterone+ niraparib (395) Abiraterone + Placebo (395) |
First line mCRPC with HRR pathogenetic variants Prior ARSI not allowed |
ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





