Submitted:
03 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
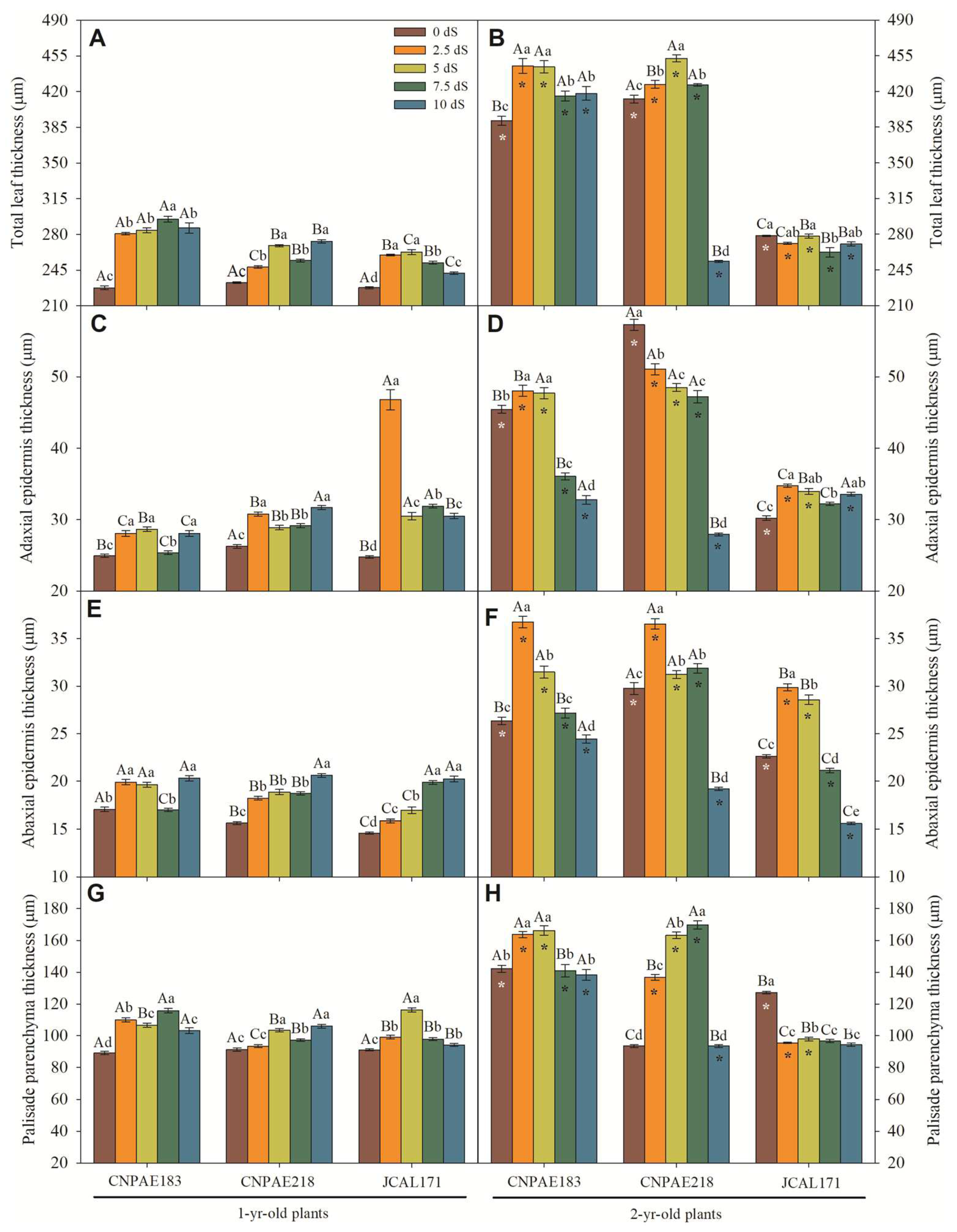
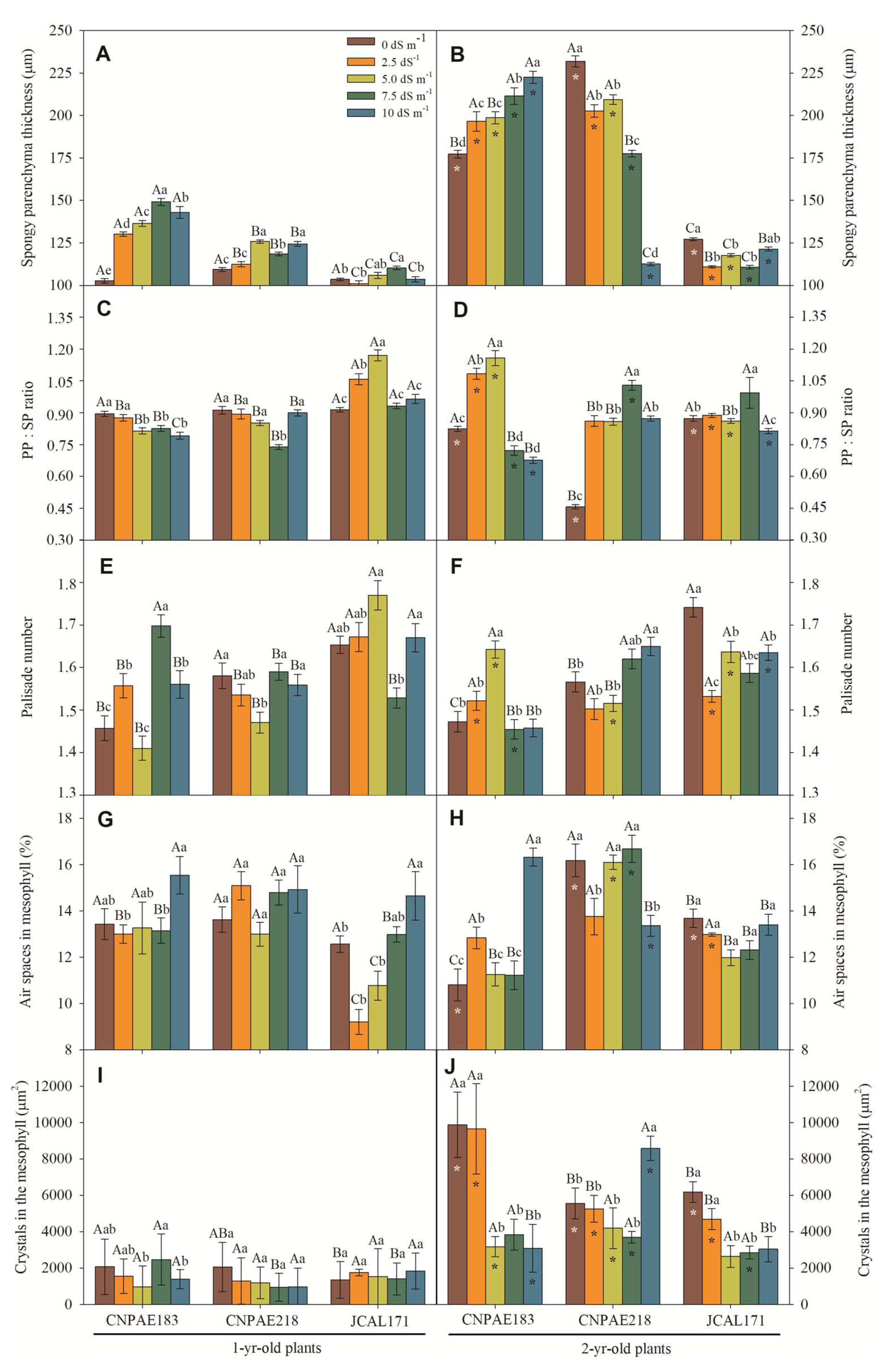
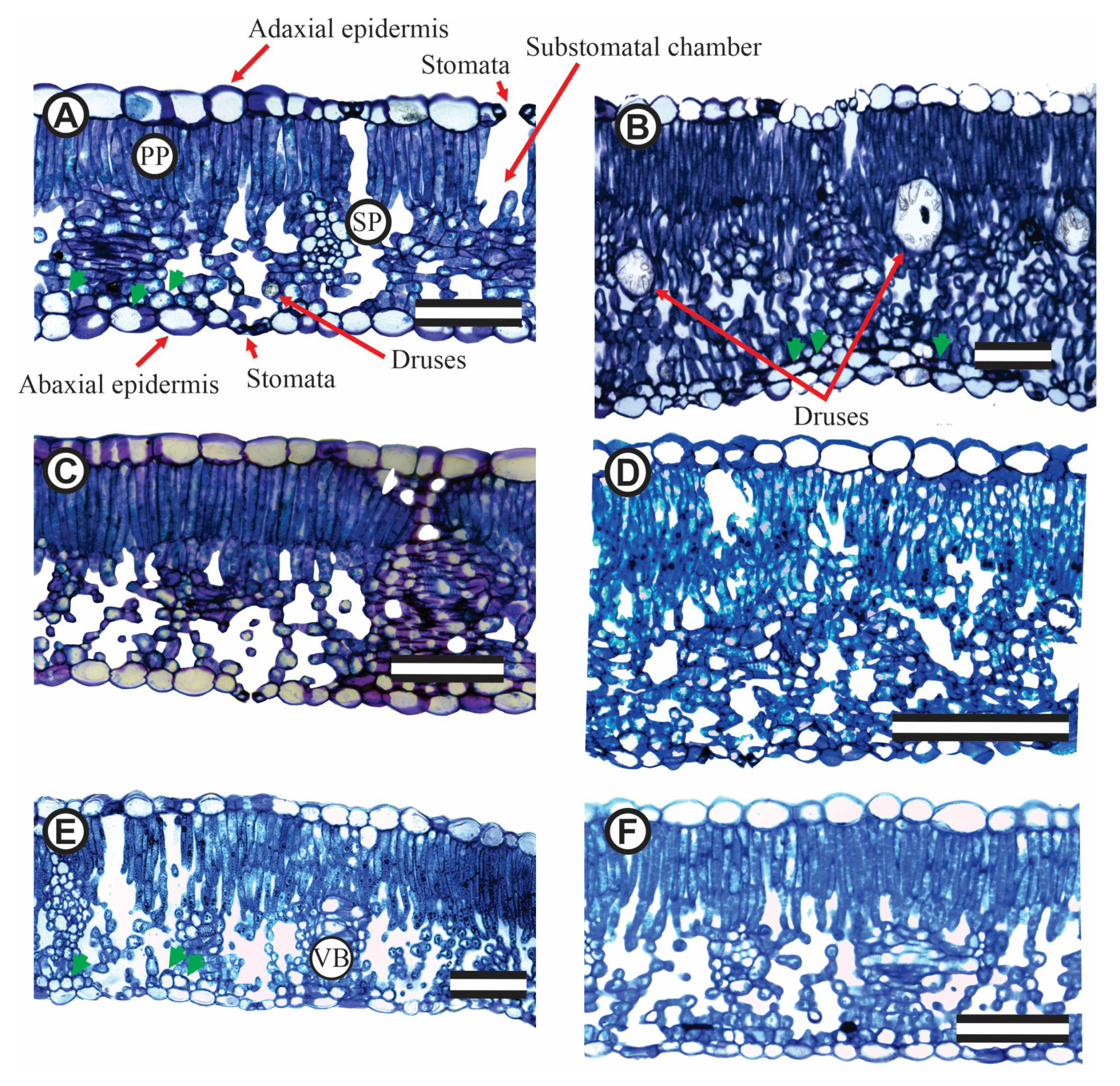
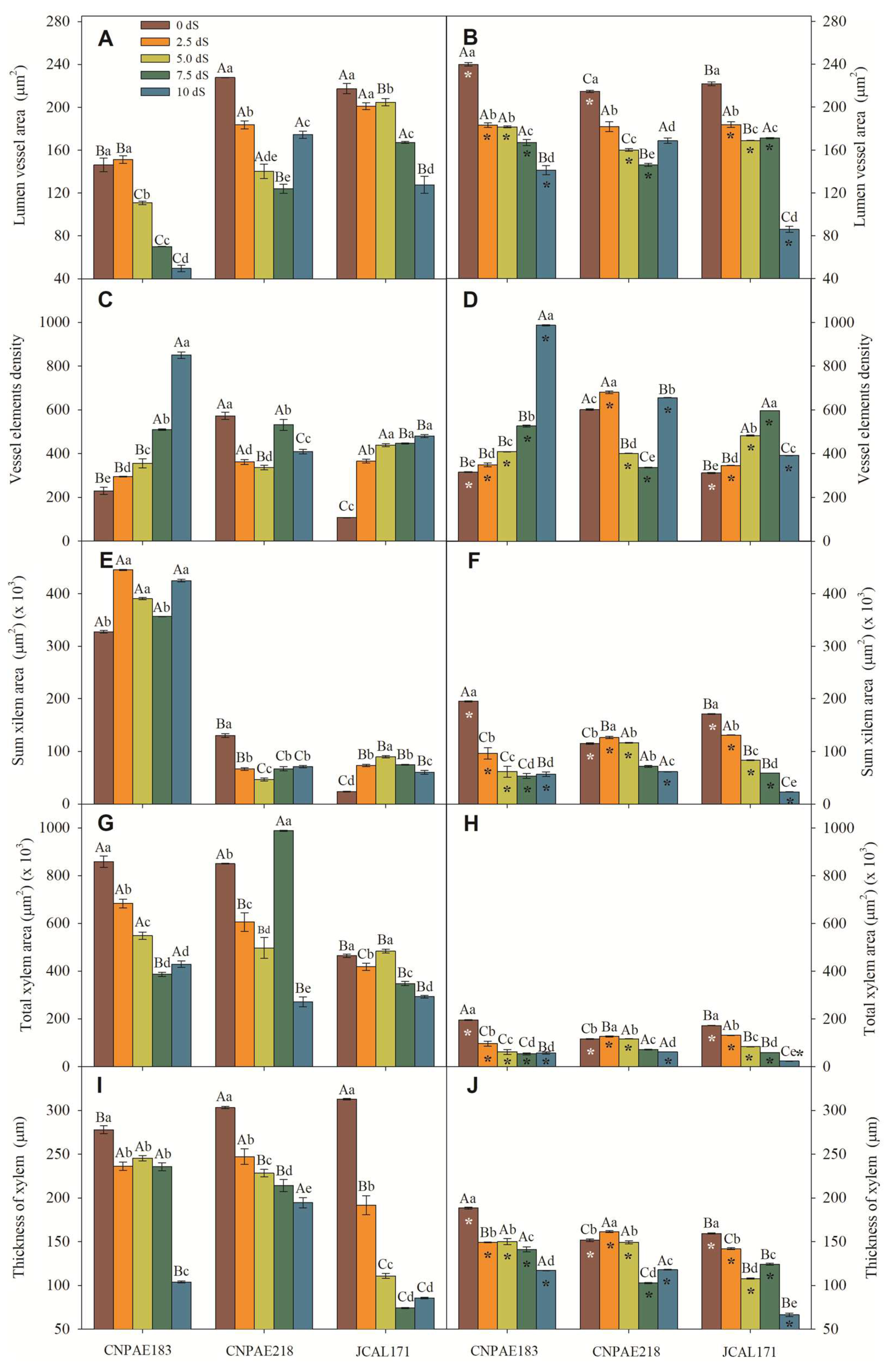
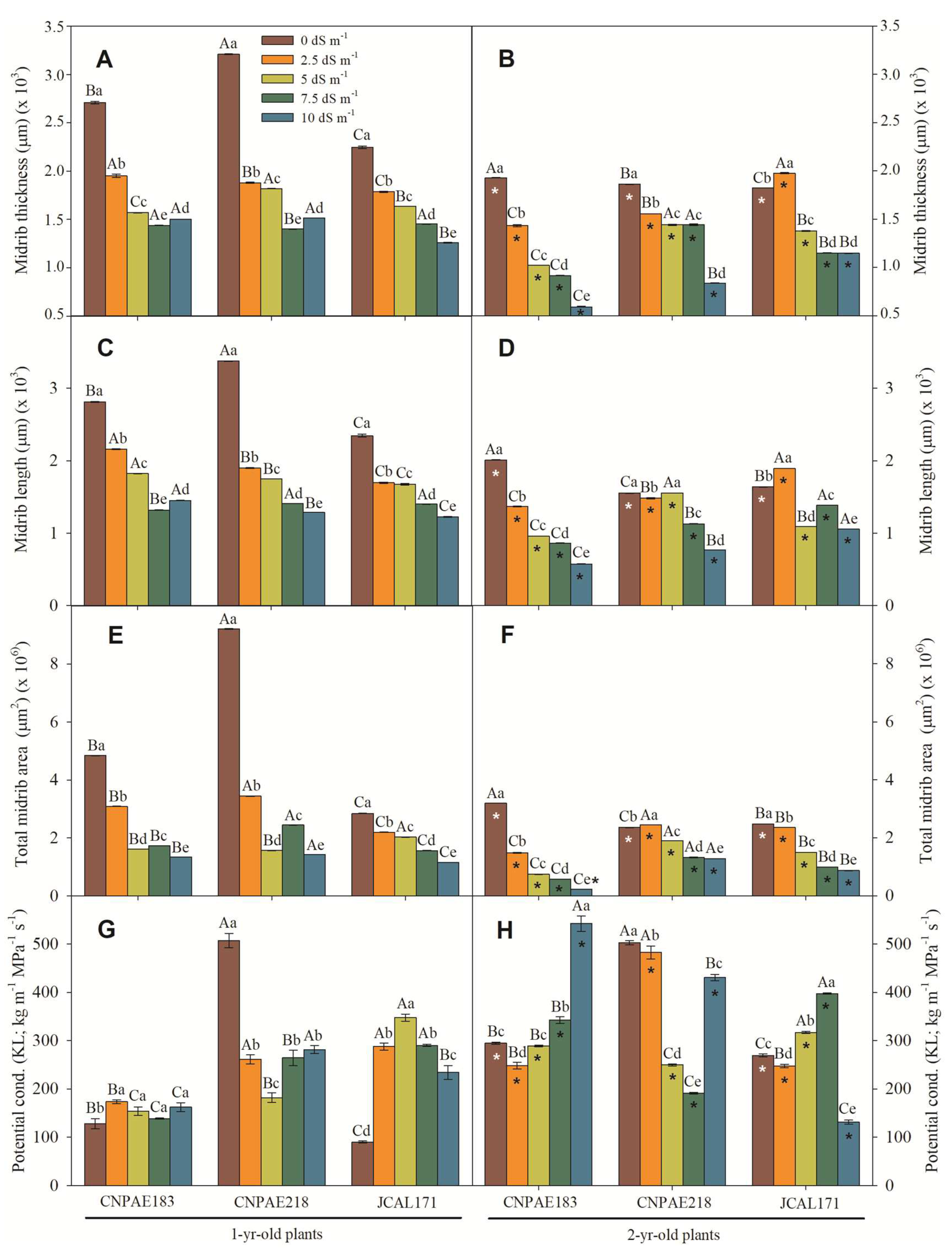
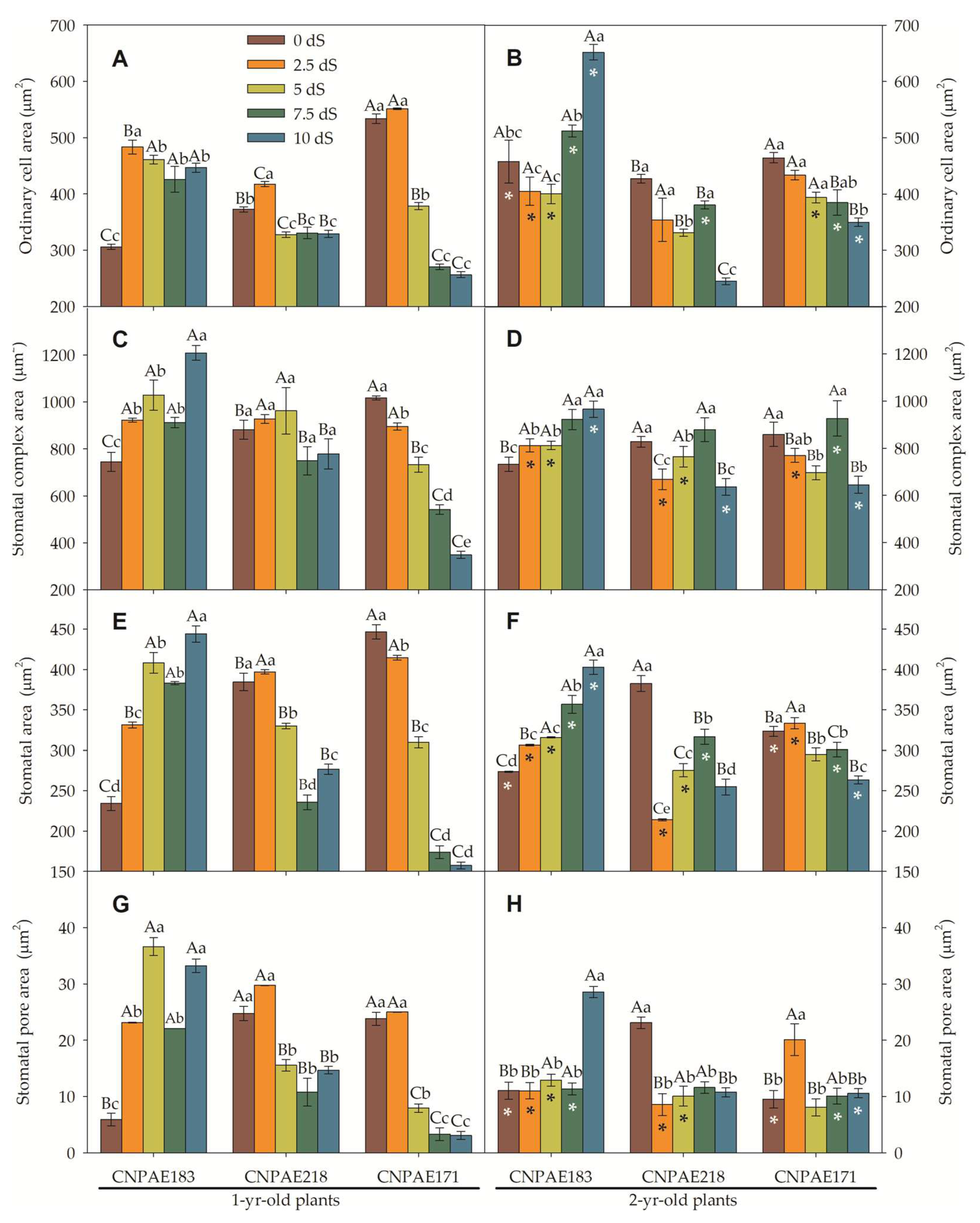
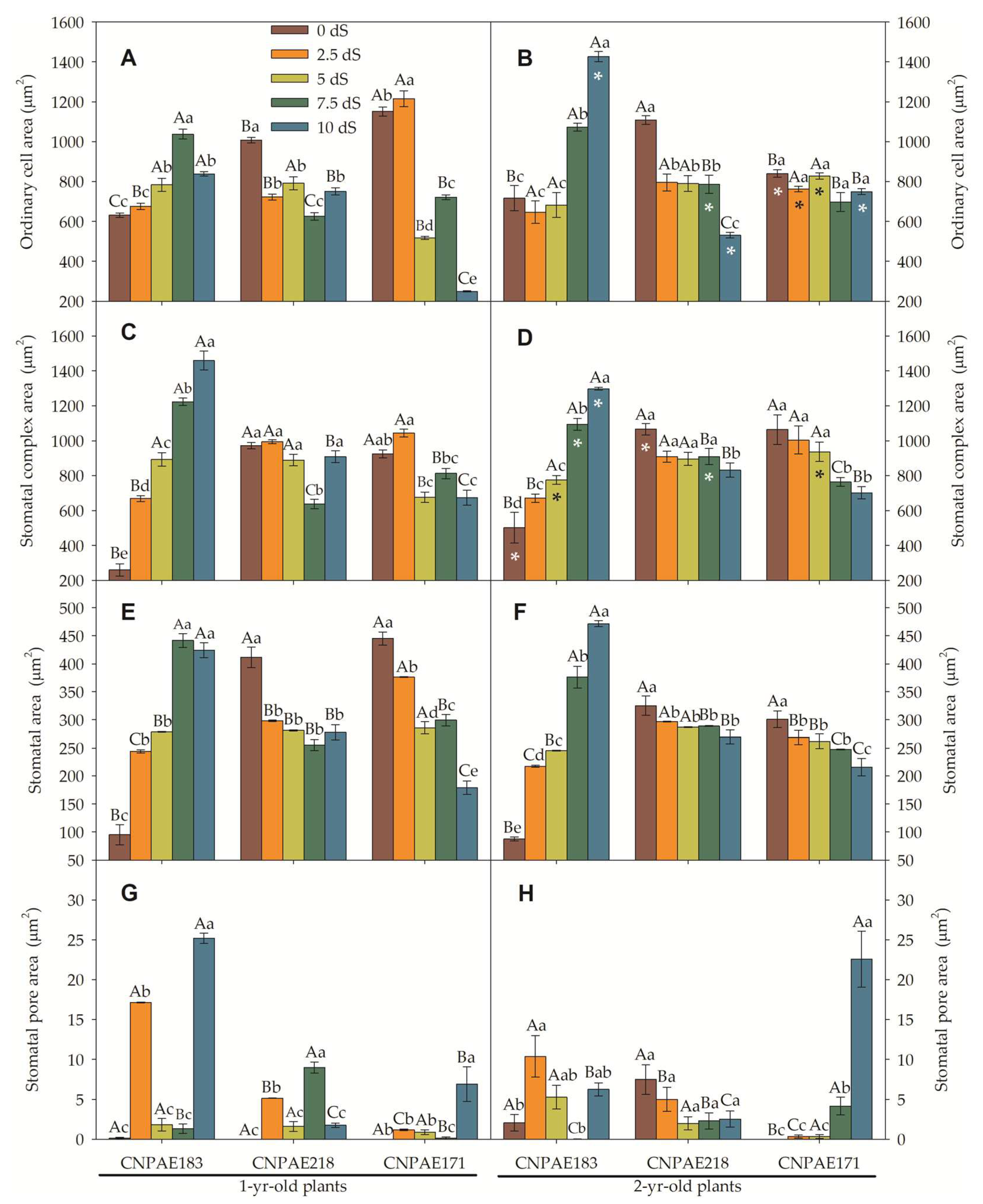
2.1. Cross section in leaves of J. curcas plants under salt stress
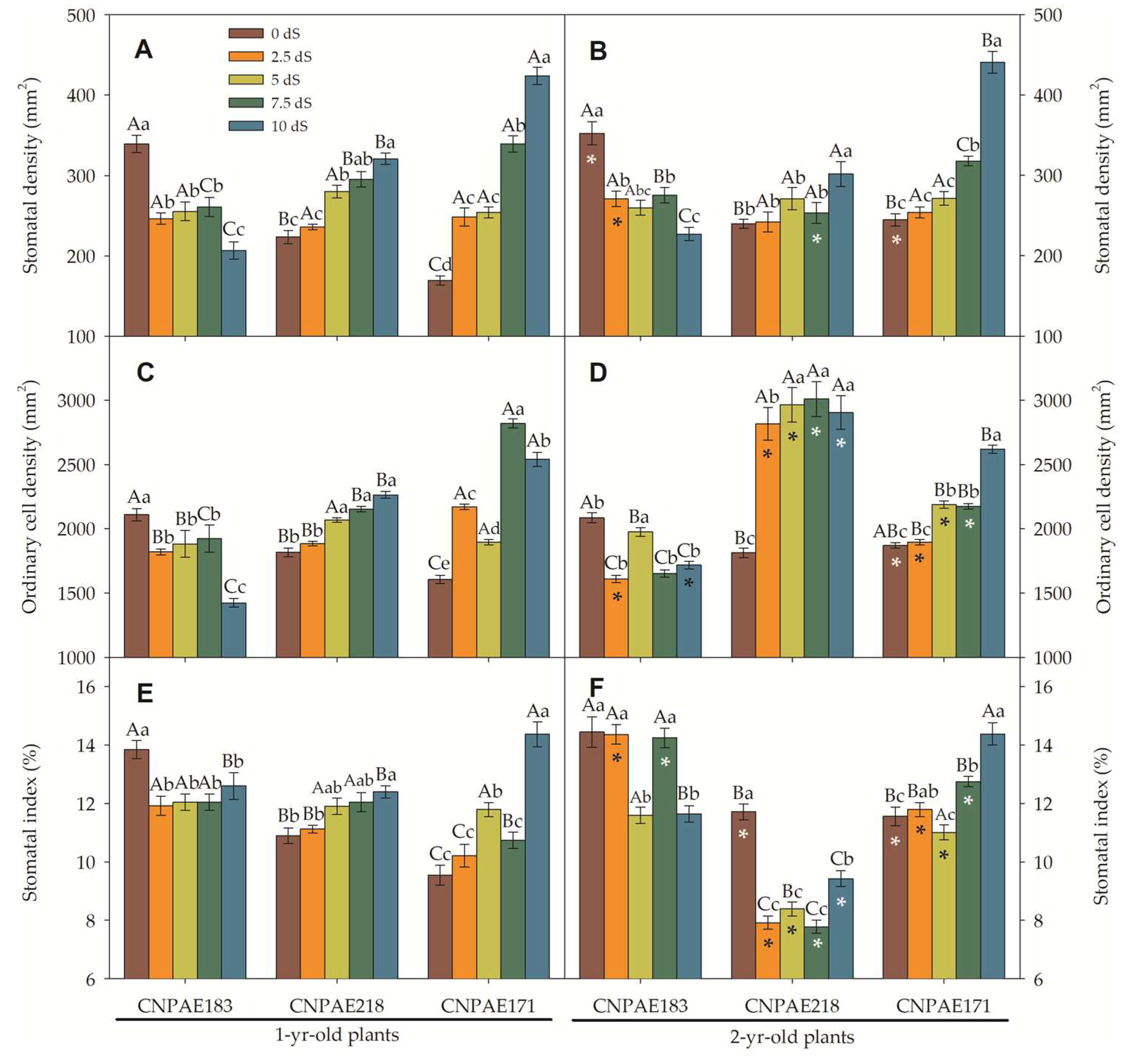
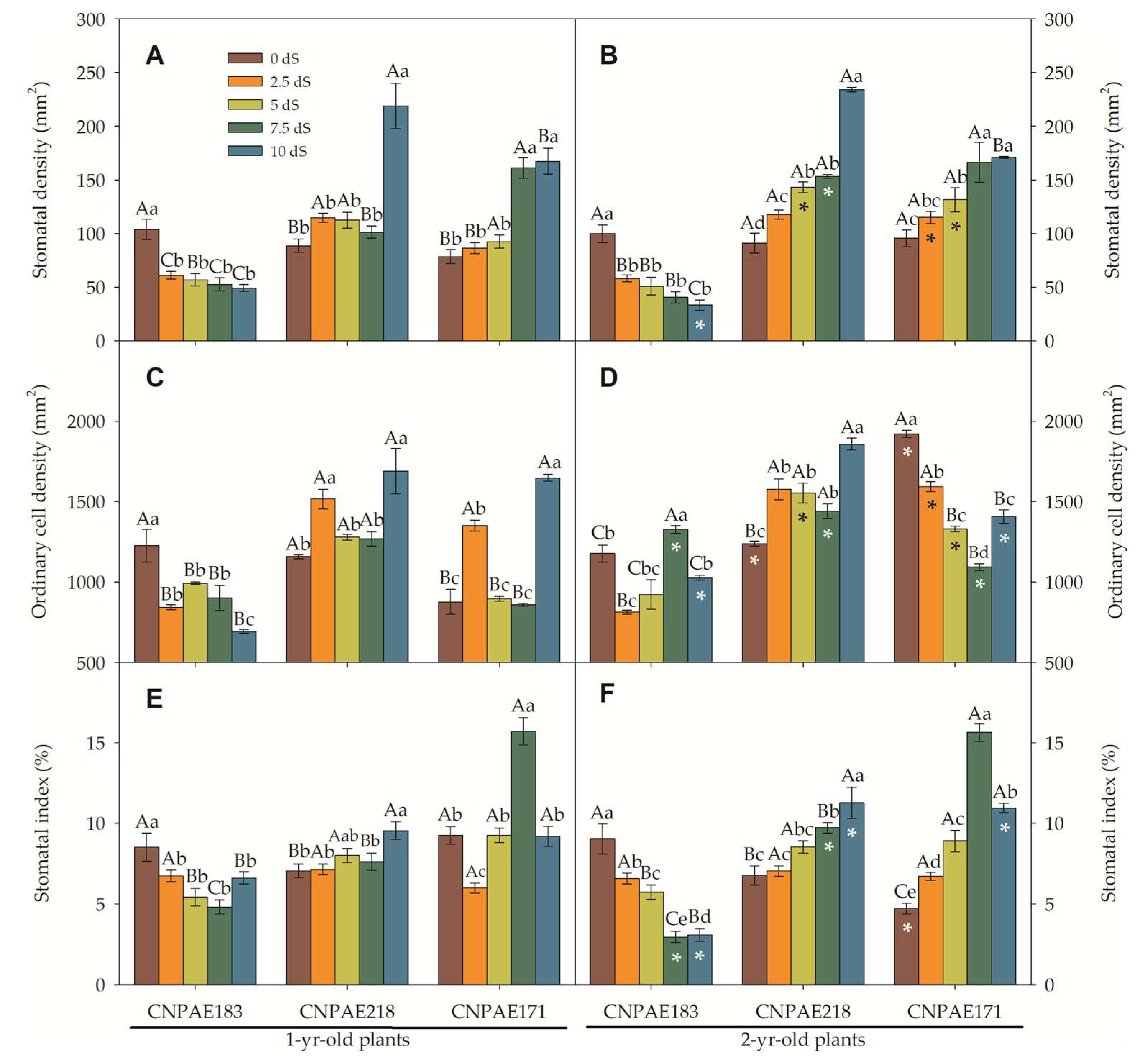
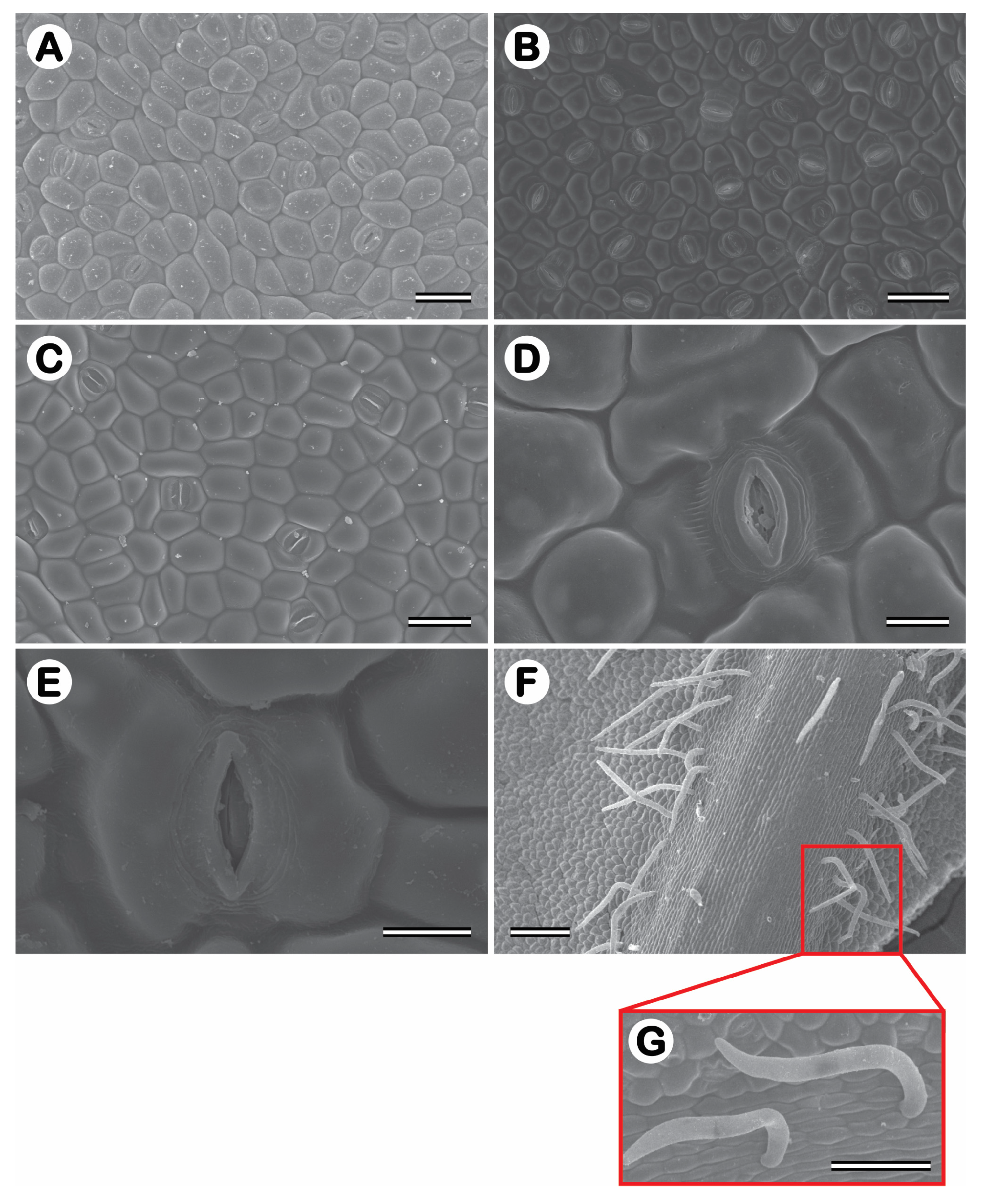
2.2. Scanning electron microscopy of the epidermis (SEM)
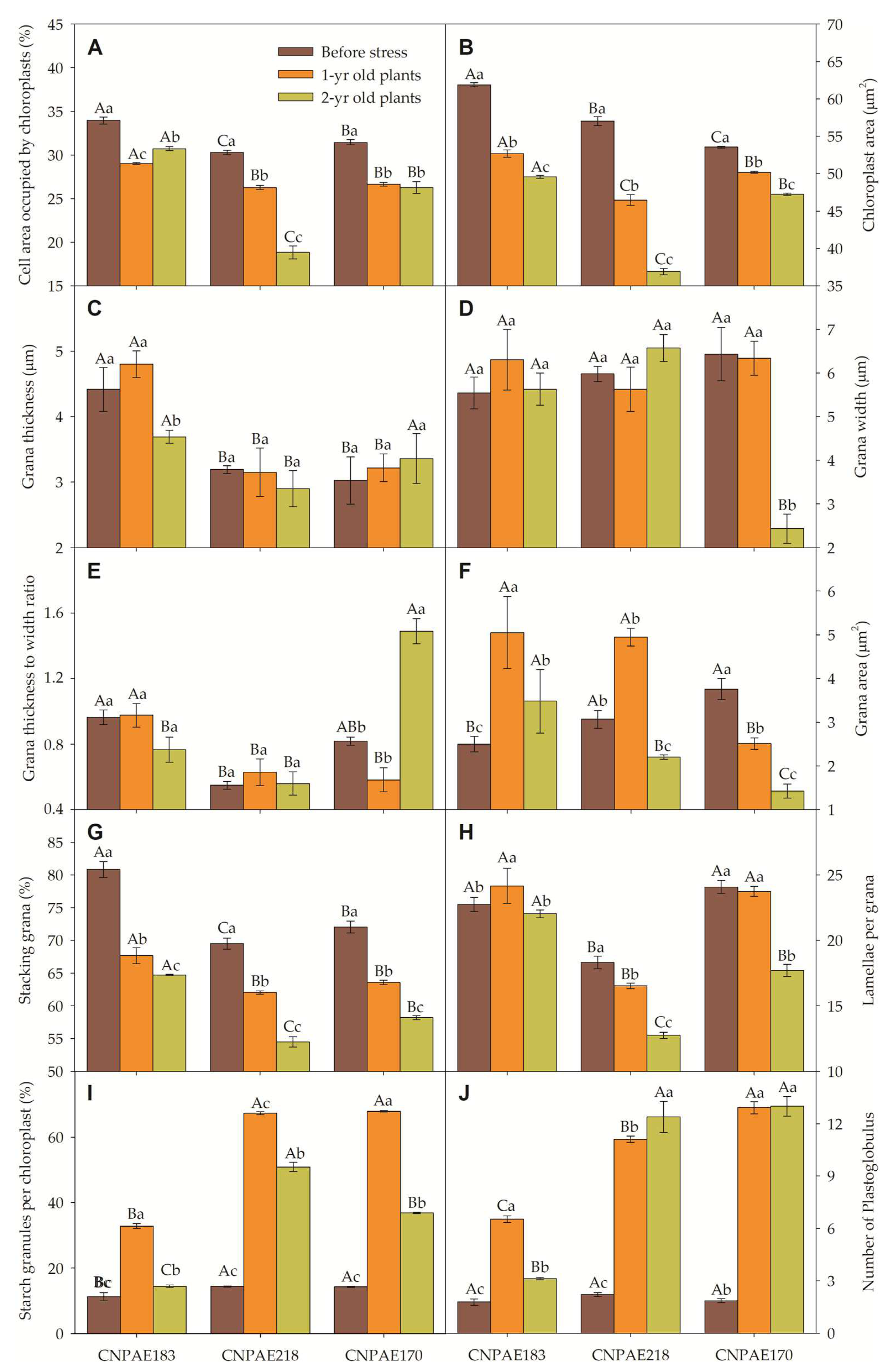
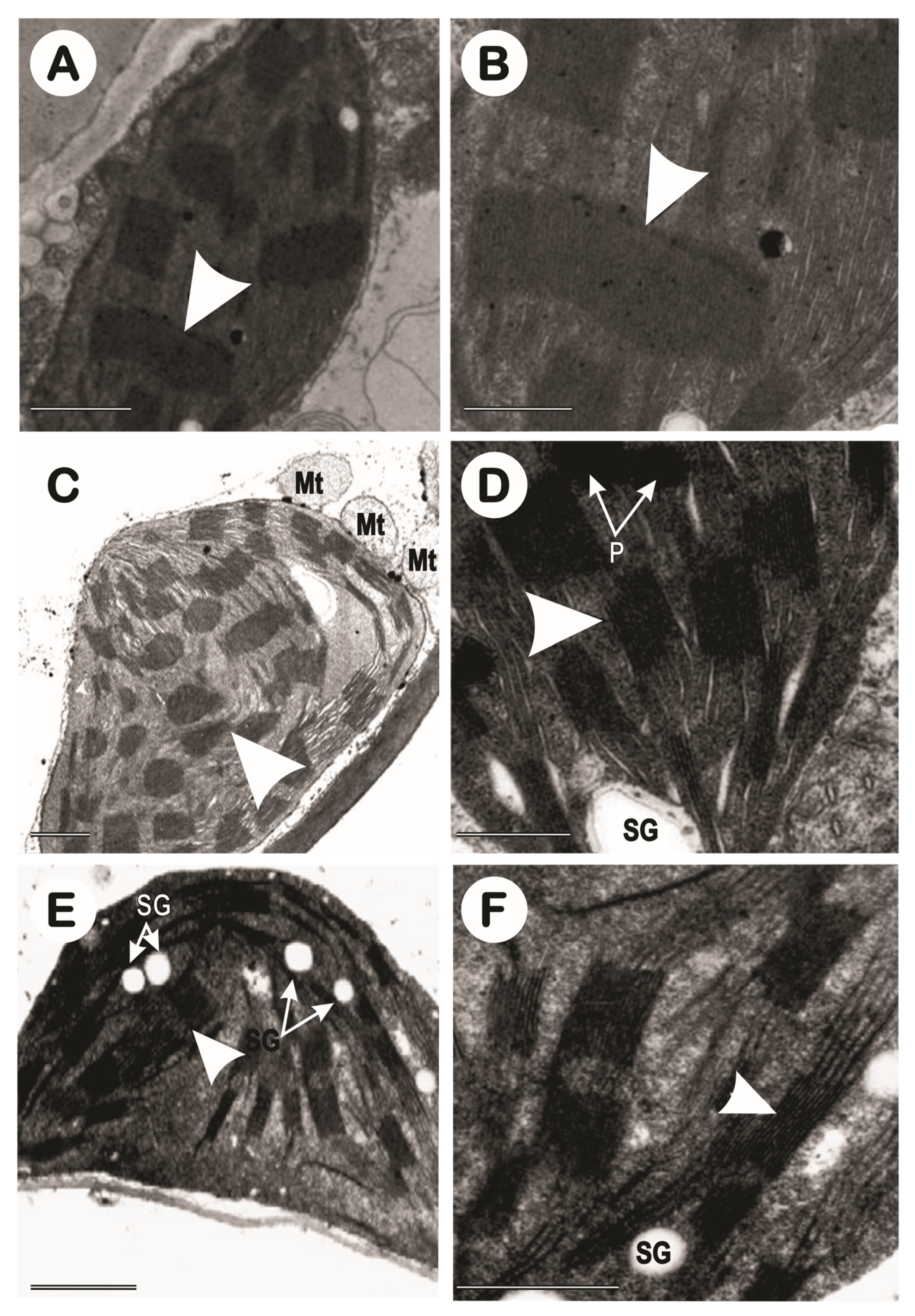
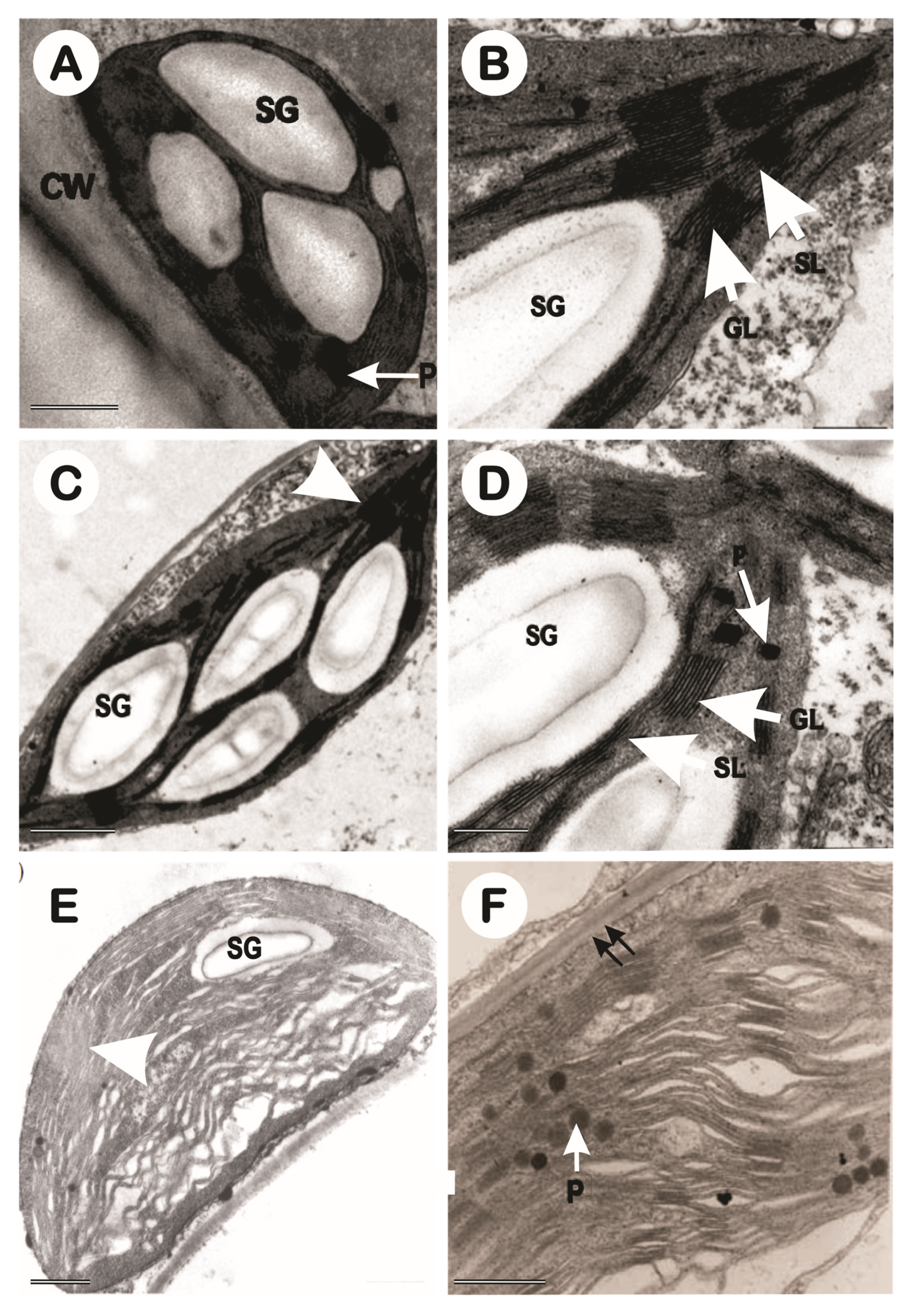
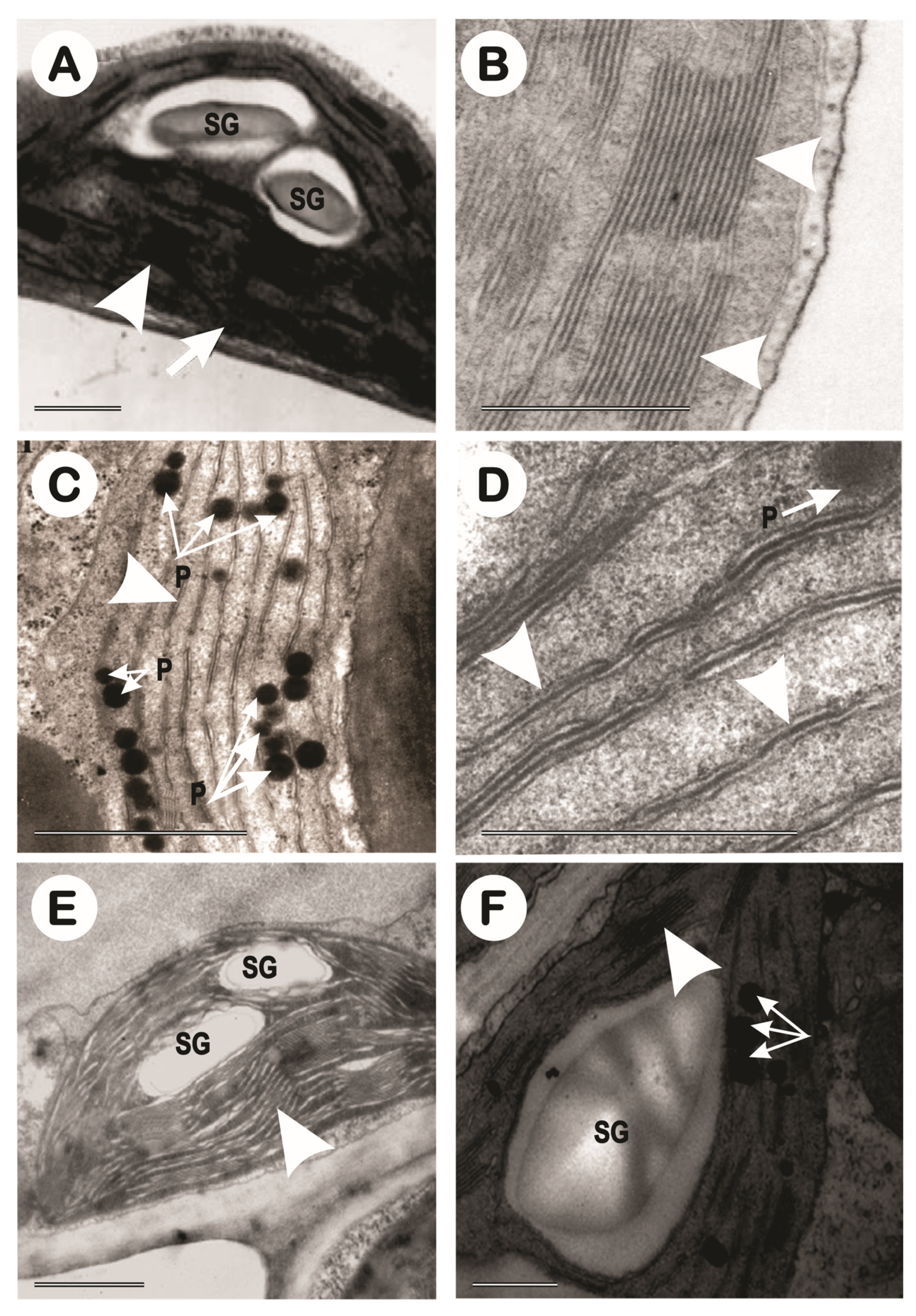
2.3. Transmission electron microscopy of chloroplasts (TEM)
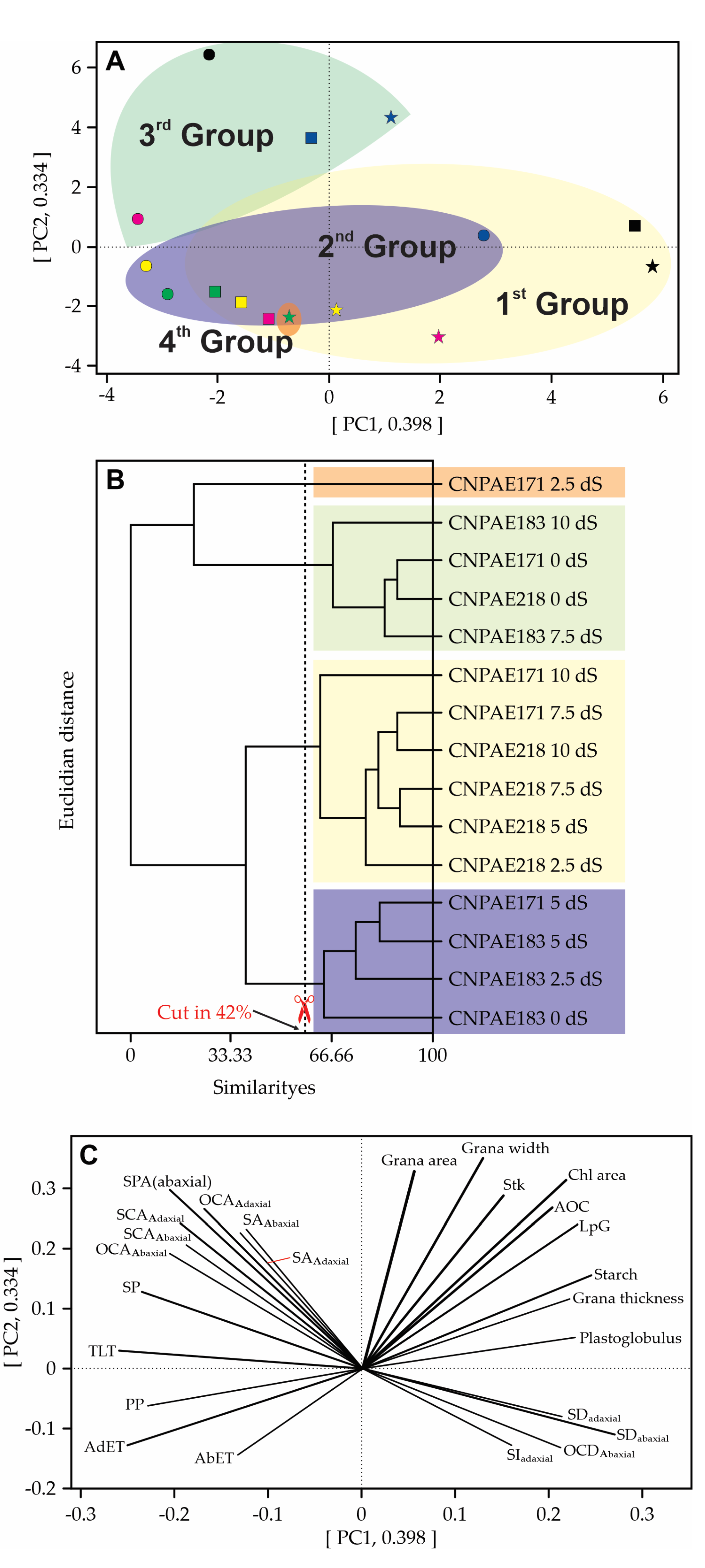
2.4. Principal component analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant material and environmental conditions
4.2. Optical anatomy
4.3. Scanning electron microscopy
4.4. Transmission electron microscopy
4.5. Experimental Design and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pompelli, M.F.; Ferreira, P.P.B.; Chaves, A.R.M.; Figueiredo, R.C.Q.Q.; Martins, A.O.; Jarma-Orozco, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol Bioch 2021, 168, 116–127. [Google Scholar] [CrossRef]
- Silva-Santos, L.; Corte-Real, N.; Dias-Pereira, J.; Figueiredo, R.C.B.Q.; Endres, L.; Pompelli, M.F. Salinity shock in Jatropha curcas leaves is more pronounced during recovery than during stress time. Braz J Develop 2019, 5, 11359–11369. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Melo, G.M.; da Cunha, P.C.; Pereira, J.A.F.; Willadino, L.; Ulisses, C. Anatomical changes in the leaves and roots of Jatropha curcas L. cultivated under saline stress. Rev Ciência Agr 2011, 42, 670–674. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P. Photosynthesis under stressful environments: an overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Arcoverde, G.B.; Rodrigues, B.M.; Pompelli, M.F.; Santos, M.G. Water relations and some aspects of leaf metabolism of Jatropha curcas young plants under two water deficit levels and recovery. Braz J Plant Physiol 2011, 23, 123–130. [Google Scholar] [CrossRef]
- Corte-Real, N.; Miranda, P.V.V.C.; Endres, L.; Souza, E.R.; Pompelli, M.F. Tolerance to salinity in Jatropha curcas are genotype-dependent. Braz J Develop 2019, 5, 22169–22199. [Google Scholar] [CrossRef]
- Gomes, M.F.C.; Binneck, E.; Ferreira-Neto, J.R.C.; Luz, G.A.; Benko-Iseppon, A.M.; Pompelli, M.F.E.; Kido, É.A. Transcriptome analysis uncovers the Jatropha curcas L. transportome after NaCl (150 mM) exposure. Physiol Plant 2021, in press. [Google Scholar]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodrígues-Páez, L.A. Salinity in Jatropha curcas: a review of physiological, biochemical, and molecular factors involved. Agriculture 2022, 12, 594. [Google Scholar] [CrossRef]
- Bezerra-Neto, E.; Coelho, J.B.M.; Jarma-Orozco, A.; Rodríguez-Páez, L.A.; Pompelli, M.F. Modulation of photosynthesis under salinity and the role of mineral nutrients in Jatropha curcas L. J Agron Crop Sci 2021, 208, 314–334. [Google Scholar] [CrossRef]
- Melcher, P.J.; Holbrook, N.M.; Burns, M.J.; Zwieniecki, M.A.; Cobb, A.R.; Brodribb, T.J.; Sack, L. Measurements of stem xylem hydraulic conductivity in the laboratory and field. Methods Ecol Evol 2012, 3, 685–694. [Google Scholar] [CrossRef]
- Oliveira, P.S.; Pereira, L.S.; Silva, D.C.; Souza Júnior, J.O.; Laviola, B.G.; Gomes, F.P. Hydraulic conductivity in stem of young plants of Jatropha curcas L. cultivated under irrigated or water deficit conditions. Ind Crop Prod 2018, 116, 15–23. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Gulías, J.; Figueroa, C.M.; Iñiguez, C.; Clemente-Moreno, M.J.; Nunes-Nesi, A.; Fernie, A.R.; Cavieres, L.A., Bravo; García-Plazaola, J.I.; et al. How do vascular plants perform photosynthesis in extremeenvironments? An integrative ecophysiological andbiochemical story. Plant J 2020, 101, 979–1000. [Google Scholar] [CrossRef]
- Gauhl, E.; Björkman, O. Simultaneous measurements on the effect of oxygen concentration on water vapor and carbon dioxide exchange in leaves. Planta 1969, 88, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, J.P.; Yuan, F.; Yang, Z.; Wang, B.S.; Chen, M. Transcriptome profiling of genes involved in photosynthesis in Elaeagnus angustifolia L. under salt stress. Photosynthetica 2018, 56, 998–1009. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sharmila, P.; Pardha-Saradhi, P. Proline alleviates salt-stress-induced enhancement in Ribulose-1,5-bisphosphate oxygenase activity. Biochem Biophys Res Commun 2000, 279, 512–515. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J 2016, 90, 856–867. [Google Scholar] [CrossRef]
- Muchate, M.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot Rev 2016, 82, 371–406. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Abdelaal, K.A.A. Response of strawberry plants grown in the hydroponic system to pretreatment with H2O2 before exposure to salinity stress. J Plant Production, Mansoura Univ 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- Bongi, G.; Loreto, F. Gas-exchange properties of saltstressed olive (Olea europea L.) leaves. Plant Physiol 1989, 90, 1408–1416. [Google Scholar] [CrossRef]
- Terletskaya, N.; Kurmanbayeva, M. Change in leaf anatomical parameters of seedlings of different wheat species under conditions of drought and salt stress. Pak J Bot 2017, 49, 857–865. [Google Scholar]
- Toscano, S.; Ferrante, A.; Tribulato, A.; Romano, D. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol Bioch 2018, 127, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Akcin, T.A.; Akcin, A.; Yalcin, E. Anatomical adaptations to salinity in Spergularia marina (Caryophyllaceae) from Turkey. Proc Nat Acad Sci, India Sect B Biol Sci 2014, 85, 625–634. [Google Scholar] [CrossRef]
- Trebst, A.; Tsujimoto, H.; Arnon, D. Separation of light and dark phases in the photosynthesis of isolated chloroplasts. Nature 1958, 182, 351–355. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Wolosiuk, R.A. Photosynthesis: the carbon reactions. In Plant Physiology, 5th ed.; Taiz, L., Zeiger, E., Eds.; Sinauer Associates, Inc.: Sunderland, USA, 2010; pp. 199–242. [Google Scholar]
- Atabayeva, S.; Nurmahanova, A.; Minocha, S.; Ahmetova, A.; Kenzhebayeva, S.; Aidosova, S.; Nurzhanova, A.; Zhardamalieva, A.; Asrandina, S.; Alybayeva, R.; et al. The effect of salinity on growth and anatomical attributes of barley seedling (Hordeum vulgare L.). Afr J Biotechnol 2013, 12, 2366–2377. [Google Scholar] [CrossRef]
- Hsie, B.S.; Mendes, K.R.; Antunes, W.C.; Endres, L.; Campos, M.L.O.; Souza, F.C.; Santos, N.D.; Singh, B.; Arruda, E.C.P.; Pompelli, M.F. Jatropha curcas L. (Euphorbiaceae) modulates stomatal traits in response to leaf-to-air vapor pressure deficit. Biomass Bioenerg 2015, 81, 273–281. [Google Scholar] [CrossRef]
- Lei, Z.Y.; Han, J.M.; Yi, X.P.; Zhang, W.F.; Zhang, Y.L. Coordinated variation between veins and stomata in cotton and its relationship with water-use efficiency under drought stress. Photosynthetica 2018, 56, 1326–1335. [Google Scholar] [CrossRef]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The influence of leaf size and shape on leaf thermal dynamics: does theory hold up under natural conditions? Plant Cell Environ 2016, 40, 237–248. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Mendes, K.R.; Ramos, M.V.; Santos, J.N.B.; Youssef, D.T.A.; Pereira, J.D.; Endres, L.; Jarma-Orozco, A.; Solano-Gomes, R.; Jarma-Arroyo, B.; et al. Mesophyll thickness and sclerophylly among Calotropis procera morphotypes reveal water-saved adaptation to environments. J Arid Land 2019, 11, 795–810. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Barrón, E.; Camarero, J.J.; Vilagrosa, A.; Gil-Pelegrín, E. Morphological and physiological divergences within Quercus ilex support the existence of different ecotypes depending on climatic dryness. Ann Bot 2014, 114, 301–313. [Google Scholar] [CrossRef]
- Gil-Pelegrín, E.; Saz, M.A.; Cuadrat, J.M.; Peguero-Pina, J.J.; Sancho-Knapik, D. Oaks Under Mediterranean-Type Climates: Functional Response to Summer Aridity. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Gil-Pelegrín, E., Peguero-Pina, J.J., Sancho-Knapik, D., Eds.; Springer: London, 2017; pp. 137–193. [Google Scholar]
- Fahn, A. Plant Anatomy, 2nd ed.; Butterworth Heinemann: Oxford, USA, 1990; p. 588. [Google Scholar]
- Evert, R.F. Esau's Plant Anatomy - meristems, cells, and tissues of tre plant body - their structures, function and development, 3rd ed.; John Wiley & Sons, Inc.: New Jersey, 2013; p. 624. [Google Scholar]
- Taratima, W.; Samattha, C.; Maneerattanarungroj, P.; Trunjaruen, A. Physiological and anatomical response of rice (Oryza sativa L.) ‘Hom Mali Daeng’ at different salinity stress levels. Acta Agrobot 2023, 76, 764. [Google Scholar] [CrossRef]
- Ola, H.; Elbar, A.; Farag, E.; Eisa, S.S.; Habib, S.A. Morpho-anatomical changes in salt stressed kallar grass (Leptochloa fusca L. Kunth). J Agric Biol Sci 2012, 8, 158–166. [Google Scholar]
- Barhoumi, Z.; Djebali, W.; Chaïbi, W.; Abdelly, C.; Smaoui, A. Salt impact on photosynthesis and leaf ultrastructure of Aeluropus littoralis. J Plant Res 2007, 120, 529–537. [Google Scholar] [CrossRef]
- Bastias, E.; Gonzales-Moro, M.B.; Gonzales-Murua, C. Zea mays L. amylacea from the Lluta Valley (Arica Chile) tolerates salinity stress when high levels of boron are available. Plant Soil 2005, 267, 73–84. [Google Scholar] [CrossRef]
- Clemente-Moreno, M.J.; Gago, J.; Díaz-Vivancos, P.; Bernal, A.; Miedes, E.; Bresta, P.; Liakopoulos, G.; Fernie, A.R.; Hernández, J.A.; Flexas, J. The apoplastic antioxidant system and altered cell wall dynamics influence mesophyll conductance and the rate of photosynthesis. Plant J 2019, 99, 1031–1046. [Google Scholar] [CrossRef]
- Taratima, W.; Ritmaha, T.; Jongrungklang, N.; Maneerattanarungroj, P.; Kunpratum, N. Effect of stress on the leaf anatomy of sugarcane cultivars with different drought tolerance (Saccharum officinarum, Poaceae). Rev Biol Trop 2020, 68, 1159–1170. [Google Scholar] [CrossRef]
- Cavalcante, P.G.S.; Santos, C.M.; Filho, H.C.L.W.; Avelino, J.R.L.; Endres, L. Morpho-physiological adaptation of Jatropha curcas L. to salinity stress. Aust J Crop Sci 2018, 12, 563–571. [Google Scholar] [CrossRef]
- Keates, S.E.; Tarlyn, N.M.; Loewus, F.A.; Franceschi, V.R. L-Acorbic acid and L-galactose are sources for oxalic acid and calcium oxalate in Pistia stratiotes. Phytochem 2000, 53, 433–440. [Google Scholar] [CrossRef]
- Nakata, P.; McConn, M. Isolated Medicago truncatula mutants with increased calcium oxalate crystal accumulation have decreased ascorbic acid levels. Plant Physiol Biochem 2007, 45, 216–220. [Google Scholar] [CrossRef]
- Masrahi, Y.; Al-Namazi, A.; Alammari, B.; Alturki, T. Adaptations facilitate the invasion of Cylindropuntia rosea (DC.) Backeb. (Cactaceae) in the highlands of southwestern Saudi Arabia. Plant Signal Behav 2022, 17, 2144593. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Ecological anatomy investigation related to some halophyte species from Moldovia. Rom J Biol - Plant Biol 2008, 53, 23–30. [Google Scholar]
- Hunsche, M.; Bürling, K.; Saied, A.S.; Schmitz-Eiberger, M.; Sohail, M.; Gebauer, J.; Noga, G.; Buerkert, A. Effects of NaCl on surface properties, chlorophyll fluorescence and light remission, and cellular compounds of Grewia tenax (Forssk.) Fiori and Tamarindus indica L. leaves. Plant Growth Regul 2010, 61, 253–263. [Google Scholar] [CrossRef]
- Tsuda, M.; Tyree, M.T. Plant hydraulic conductance measured by the high pressure flow meter in crop plants. J Exp Bot 2000, 51, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; McDonald, I.; Alarcón, A.; Fichtler, E.; Licona, J.-C.; Peña-Claros, M.; Sterck, F.; Villegas, Z.; Sass-Klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol 2010, 185, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C. Abscisic acid and stomatal closure: a hydraulic conductance conundrum? New Phytol 2013, 197, 6–8. [Google Scholar] [CrossRef]
- Meinzer, F.; Grantz, D. Coordination of stomatal, hydraulic, and canopy boundary layer properties: Do stomata balance conductances by measuring transpiration? Physiol Plant 1991, 83, 324–329. [Google Scholar] [CrossRef]
- North, G.B.; Brinton, E.K.; Browne, M.G.; Gillman, M.G.; Roddy, A.B.; Kho, T.L.; Wang, E.; Fung, V.A.; Brodersen, C.R. Hydraulic conductance, resistance, and resilience: how leaves of a tropical epiphyte respond to drought. Am J Bot 2019, 106, 943–957. [Google Scholar] [CrossRef]
- Ladjal, M.; Huc, R.; Ducrey, M. Drought effects on hydraulic conductivity and xylem vulnerability to embolism in diverse species and provenances of Mediterranean cedars. Tree Physiol 2005, 25, 1109–1117. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Li, Z. Computational fluid dynamics model and flow resistance characteristics of Jatropha curcas L xylem vessel. Sci Rep 2020, 10, 14728. [Google Scholar] [CrossRef]
- Sterck, F.J.; Zweifel, R.; Sass-Klaassen, U.; Chowdhury, Q. Persisting soil drought reduces leaf specific conductivity in Scots pine (Pinus sylvestris) and pubescent oak (Quercus pubescens). Tree Physiol 2008, 28, 529–536. [Google Scholar] [CrossRef]
- Linton, M.J.; Sperry, J.S.; D.G., W. Limits to water transport in Juniperus osteosperma and Pinus edulis: implications for drought tolerance and regulation of transpiration. Funct Ecol 1998, 12, 906–911. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pittermann, J. Drought experience and cavitation resistance in six shrubs from the Great Basin, Utah. Basic Appl Ecol 2000, 1, 31–41. [Google Scholar] [CrossRef]
- Santana, T.A.; Oliveira, P.S.; Silva, L.D.; Laviola, B.G.F.; de Almeida, A.-A.; Gomes, F.P. Water use efficiency and consumption in different Brazilian genotypes of Jatropha curcas L. subjected to soil water deficit. Biomass Bioenerg 2015, 75, 119–125. [Google Scholar] [CrossRef]
- Maherali, H.; E.H., D. Xylem conductivity and vulnerability to cavitation of ponderosa pine growing in contrasting climates. Tree Physiol 2000, 20, 859–868. [Google Scholar] [CrossRef]
- Franks, P.J.; Gibson, A.; Bachelard, E.P. Xylem permeability and embolism susceptibility in seedlings of Eucalyptus camaldulensis Dehnh. from two different climatic zones. Aust J Plant Physiol 1995, 22, 15–21. [Google Scholar] [CrossRef]
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S. How to quantify conduits in wood? Front Plant Sci 2013, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M. Dynamic measurements of root hydraulic conductance using a high pressure flowmeter in the laboratory and field. J of Exp Bot 1995, 46, 83–94. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol 1997, 17, 490. [Google Scholar] [CrossRef]
- Charrier, G.; Torres-Ruiz, J.M.; Badel, E.; Burlett, R.; Choat, B.; Cochard, H.; Delmas, C.E.L.; Domec, J.C.; Jansen, S.; King, A.; et al. Evidence for hydraulic vulnerability segmentation and lack of xylem refilling under tension. Plant Physiol 2016, 172, 1657–1668. [Google Scholar] [CrossRef]
- Neves, E.L.; Funch, L.S.; Viana, B.F. Comportamento fenológico de três espécies de Jatropha (Euphorbiaceae) da Caatinga, semi-árido do Brasil. Rev Bras Bot 2010, 33, 155–166. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Zaman-Allah, M.; Marimuthu, S.; Wani, S.P.; Kesava Rao, A.V.R. Root growth in Jatropha and its implications for drought adaptation. Biomass Bioenerg 2012, 39, 247–252. [Google Scholar] [CrossRef]
- Tavecchio, N.; Reinoso, H.; Ruffini Castiglione, M.; Spanò, C.; Pedranzani, H.E. Anatomical studies of two Jatropha species with importance for biodiesel production. J Agric Sci 2016, 8, 84–94. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Carins, M.R.; Jordan, G.J.; Brodribb, T.J. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ 2012, 35, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Drake, P.L.; Beerling, D.J. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant Cell Environ 2009, 32, 1737–1748. [Google Scholar] [CrossRef]
- Rahman, A.A.N.S.; Rahman, M.; Shimanto, M.H.; Kibria, M.G.; Islam, M.A. Stomatal size and density trade-off varies with leaf phenology and species shade tolerance in a South Asian moist tropical forest. Funct Plant Biol 2022, 49, 307–318. [Google Scholar] [CrossRef]
- Hameed, M.; Ashraf, M.; Naz, N.; Al-Qurainy, F. Anatomical adaptations of Cynodon dactylon (L.) Pers. from the salt range Pakistan to salinity stress. I. Root and stem anatomy. Pak J Bot 2010, 42, 279–289. [Google Scholar]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Guan, Z.J.; Sun, M.; Zhang, J.-J.; Cao, K.-F.; Hu, H. Evolutionary association of stomatal traits with leaf vein density in Paphiopedilum, Orchidaceae. PLoS ONE 2012, 7, e40080. [Google Scholar] [CrossRef] [PubMed]
- Dunford, S. Translocation in the Phloem. In Plant Physiology, 5th ed.; Taiz, L., Zeiger, E., Eds.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; pp. 271–303. [Google Scholar]
- DaMatta, F.M.; Cunha, R.L.; Antunes, W.C.; Martins, S.C.V.; Araujo, W.L.; Fernie, A.R.; Moraes, G.A.B.K. In field-grown coffee trees source-sink manipulation alters photosynthetic rates, independently of carbon metabolism, via alterations in stomatal function. New Phytol 2008, 178, 348–357. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front Plant Sci 2018, 9, 1889. [Google Scholar] [CrossRef]
- Hovenden, M.J.; Shimanski, L.J. Genotypic differences in growth and stomatal morphology of Southern Beech, Nothofagus cunninghamii, exposed to depleted CO2 concentration. Aust J Plant Physiol 2000, 27, 281–287. [Google Scholar] [CrossRef]
- Pandey, R.; Chacko, P.M.; Choudhary, M.L.; Prasad, K.V.; Pal, M. Higher than optimum temperature under CO2 enrichment influences stomata anatomical characters in rose (Rosa hybrida). Sci Hortic-Amsterdam 2007, 113, 74–81. [Google Scholar] [CrossRef]
- Zhai, L.; Yan, A.; Shao, K.; Wang, S.; Wang, Y.; Chen, Z.-H.; Xu, J. Large vascular bundle phloem area 4 enhances grain yield and quality in rice via source–sink–flow. Plant Physiol 2023, 191, 317–334. [Google Scholar] [CrossRef]
- Haibo, D.; Zhang, W.; Hua, B.; Zhu, Z.; Zhang, J.; Zhang, Z. Cucumber STACHYOSE SYNTHASE is regulated by its cis-antisense RNA asCsSTS to balance source–sink carbon partitioning. Plant Cell 2023, 35, 435–452. [Google Scholar] [CrossRef]
- Miehe, W.; Czempik, L.; Klebl, F.; Lohaus, G. Sugar concentrations and expression of SUTs suggest active phloem loading in tall trees of Fagus sylvatica and Quercus robur. Tree Physiol 2023, 43, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ganguly, D.R.; Shafik, S.H.; Danila, F.; Grof, C.P.L.; Sharwood, R.E.; Furbank, R.T. The role of SWEET4 proteins in the post-phloem sugar transport pathway of Setaria viridis sink tissues. J Exp Bot 2023, 74, 2968–2986. [Google Scholar] [CrossRef]
- Taylor, A.O.; Craig, A.S. Plants under climatic stress: II. low temperature, high light effects on chloroplast ultrastructure. Plant Physiol 1971, 47, 719–725. [Google Scholar] [CrossRef]
- Ackerson, R.C.; Hebert, E.R. Osmoregulation in cotton in response to water stress : I. alterations in photosynthesis, leaf conductance, translocation, and ultrastructure. Plant Physiol 1981, 67, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Capellades, M.; Lemeur, R.; Debergh, P. Effects of sucrose on starch accumulation and rate of photosynthesis in rosa cultured in vitro. Plant Cell Tiss Organ Cult 1991, 25, 21–26. [Google Scholar] [CrossRef]
- Achten, W.M.J.; Maes, W.H.; Reubens, B.; Mathijs, E.; Singh, V.P.; Verchot, L.; Muys, B. Biomass production and allocation in Jatropha curcas L. seedlings under different levels of drought stress. Biomass Bioenerg 2010, 34, 667–676. [Google Scholar] [CrossRef]
- Díaz-López, L.; Gimeno, V.; Simón, I.; Martínez, V.; Rodrígues-Ortega, W.M.; García-Sánchez, F. Jatropha curcas seedlings show a water conservation strategy under drought conditions based on decreasing leaf growth and stomatal conductance. Agr Water Manage 2012, 105, 48–56. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Barata-Luís, R.M.; Vitorino, H.S.; Gonçalves, E.R.; Rolim, E.V.; Santos, M.G.; Almeida-Cortez, J.S.; Endres, L. Photosynthesis, photoprotection and antioxidant activity of purging nut under drought deficit and recovery. Biomass Bioenerg 2010, 34, 1207–1215. [Google Scholar] [CrossRef]
- Silva, L.D.; Gomes, F.P.; Oliveira, P.S.; Almeida, F.R.; Pirovani, C.P.; Laviola, B.G.; Amaral, J.F.T. Plasticity of photosynthetic metabolism in Jatropha curcas genotypes under water stress. Gen Mol Res 2019, 18, 1–17. [Google Scholar] [CrossRef]
- Cabrales-Rodríguez, R.; Betancur-Hurtado, C.A.; Rodríguez-Páez, L.A. Cultivo del piñón (Jatropha curcas L.); manejo nutricional y usos en Córdoba, Colombia, Ed.; Universidad de Córdoba: Montería, Colombia, 2019; p. 88. [Google Scholar]
- Cabral, G.A.L.; Binneck, E.; Souza, M.C.P.; Silva, M.D.; Ferreira Neto, J.R.C.; Pompelli, M.F.; Endres, L.; Kido, E.A. First expressed TFome of physic nut (Jatropha curcas L.) after salt stimulus. Plant Mol Biol Rep 2020, 38, 189–208. [Google Scholar] [CrossRef]
- Campos, M.L.O.; Hsie, B.S.; Granja, J.A.A.; Correia, R.M.; Silva, S.R.S.; Almeida-Cortez, J.S.; Pompelli, M.F. Photosynthesis and antioxidant activity mechanisms in Jatropha curcas L. under salt stress. Braz J Plant Physiol 2012, 24, 55–67. [Google Scholar] [CrossRef]
- Dorta-Santos, M.A.; Barriola, I.; Wassner, D.F.; Ploschuk, E.L. Photosynthesis, fluorescence and mesophyll conductance responses to increasing salinity levels in Jatropha curcas at early vegetative stages. J Agron Crop Sci 2020, 206, 52–63. [Google Scholar] [CrossRef]
- Gadelha, C.G.; Miranda, R.S.; Alencar, N.L.M.; Costa, J.H.; Prisco, J.T.; Gomes-Filho, E. Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J Plant Physiol 2017, 212, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Reubens, B.; Achten, W.M.J.; Maes, W.H.; Danjon, F.; Aerts, R.; Poesen, J.; Muys, B. More than biofuel? Jatropha curcas root system symmetry and potential for soil erosion control. J Arid Environ 2011, 75, 201–205. [Google Scholar] [CrossRef]
- Fairless, D. The little shrub that could - maybe. Nature 2007, 449, 652–655. [Google Scholar] [CrossRef]
- Arockiasamy, S.; Kumpatla, J.; Hadole, S.; Yepuri, V.; Patil, M.; Shrivastava, V.; Rao, C.; Kancharla, N.; Jalali, S.; Varshney, A.; et al. Breeding and biotechnological efforts in Jatropha curcas L. for sustainable yields. Oil Crop Sci 2021, 6, 180–191. [Google Scholar] [CrossRef]
- Souza, M.C.P.; Silva, M.D.; Binneck, E.; Cabral, G.A.L.; Iseppon, A.M.B.; Pompelli, M.F.; Endres, L.; Kido, E.A. RNA-Seq transcriptome analysis of Jatropha curcas L. accessions after salt stimulus and unigene-derived microsatellite mining. Ind Crop Prod 2020, 147, 112168. [Google Scholar] [CrossRef]
- Mendes, K.R.; Batista-Silva, W.; Dias-Pereira, J.; Pereira, M.P.S.; Souza, E.V.; Serrão, J.E.; Granja, J.A.A.; Pereira, E.C.; Gallacher, D.J.; Mutti, P.R.; et al. Leaf plasticity across wet and dry seasons in Croton blanchetianus (Euphorbiaceae) at a tropical dry forest. Sci Rep 2022, 12, 954. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 1965, 27, 137–138. [Google Scholar]
- Pompelli, M.F.; Martins, S.C.; Celin, E.F.; Ventrella, M.C.; DaMatta, F.M. What is the influence of ordinary epidermal cells and stomata on the leaf plasticity of coffee plants grown under full-sun and shady conditions? Braz J Biol 2010, 70, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Huang, C.F. Stochastic flow network reliability with tolerable error rate. Qual Technol Quant Manag 2013, 10, 57–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




