Submitted:
29 June 2023
Posted:
03 July 2023
You are already at the latest version
Abstract
Keywords:
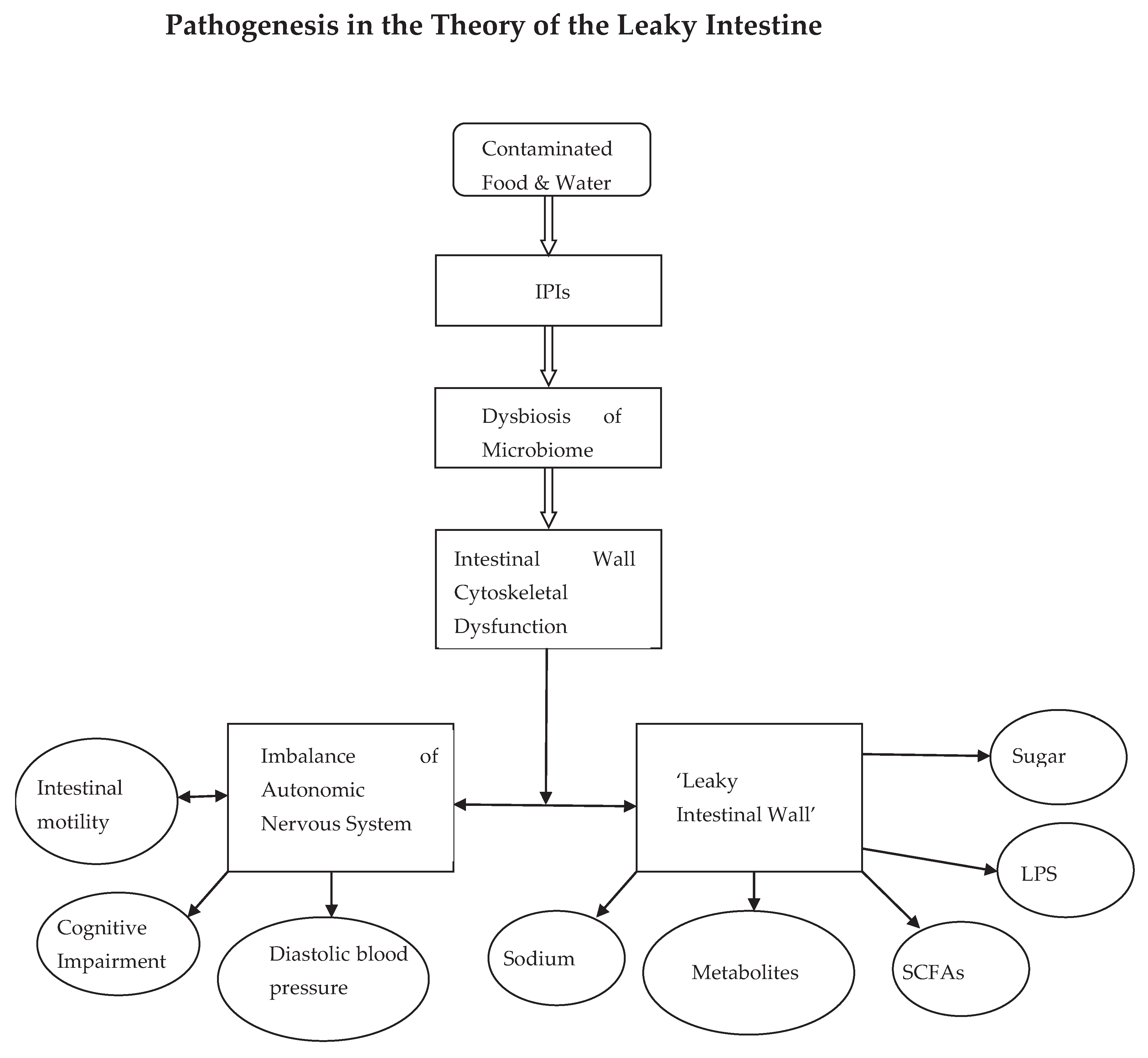
INTRODUCTION
METHODS
Laboratory Tests:
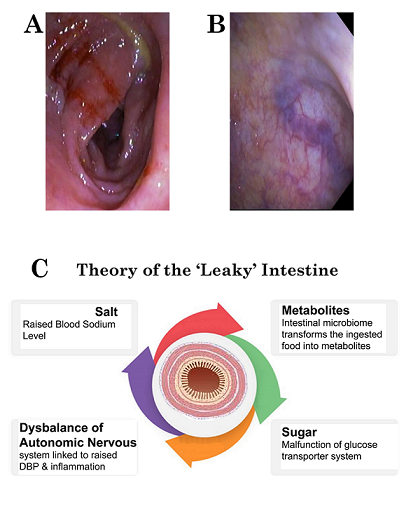
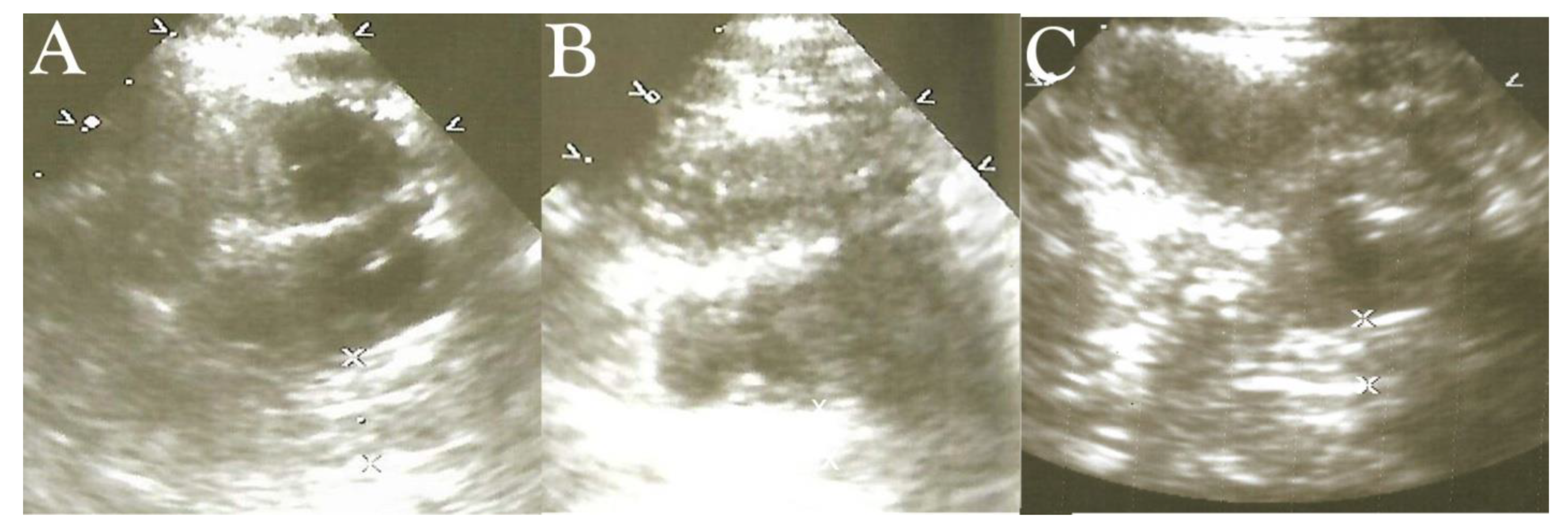
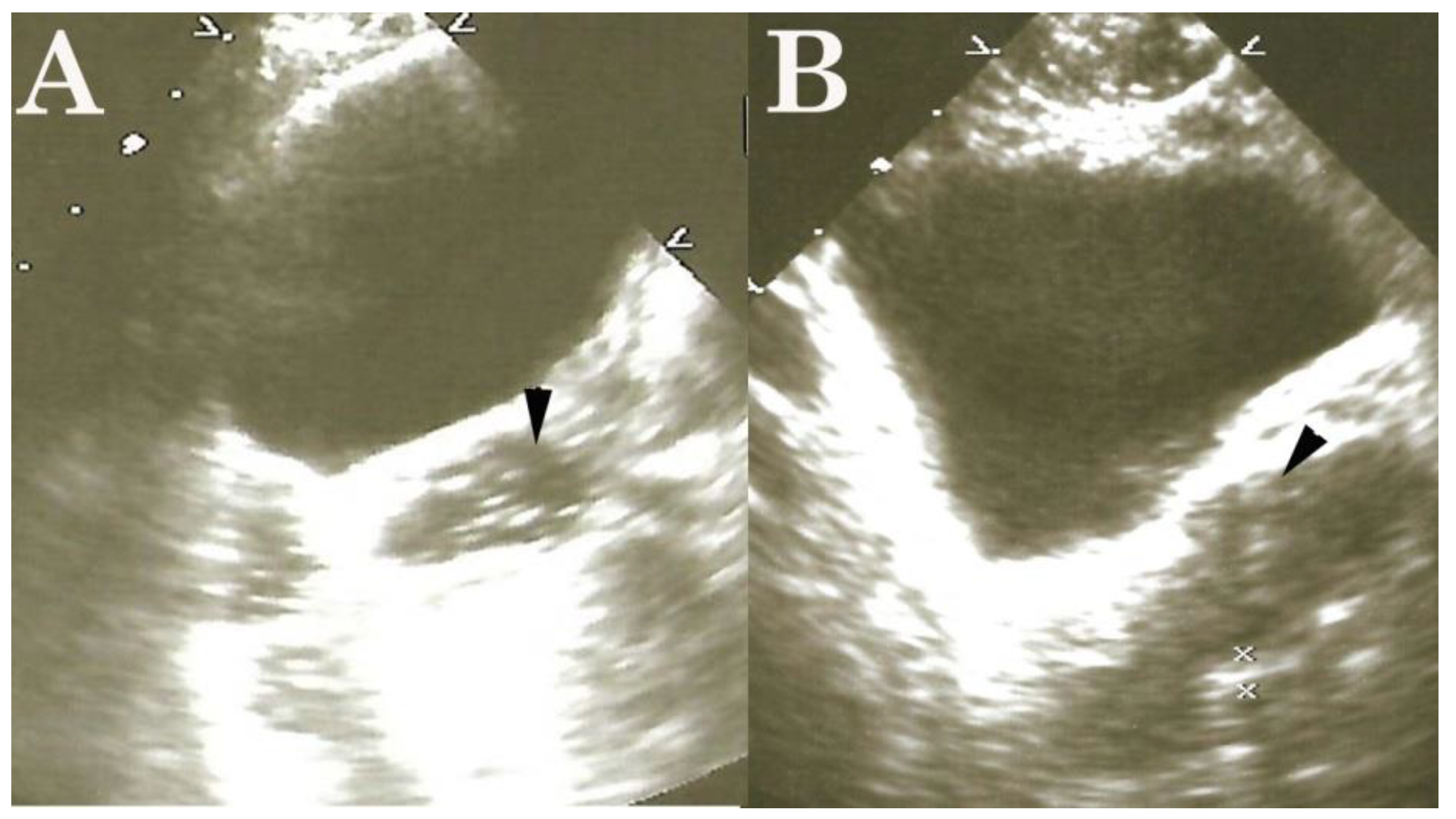
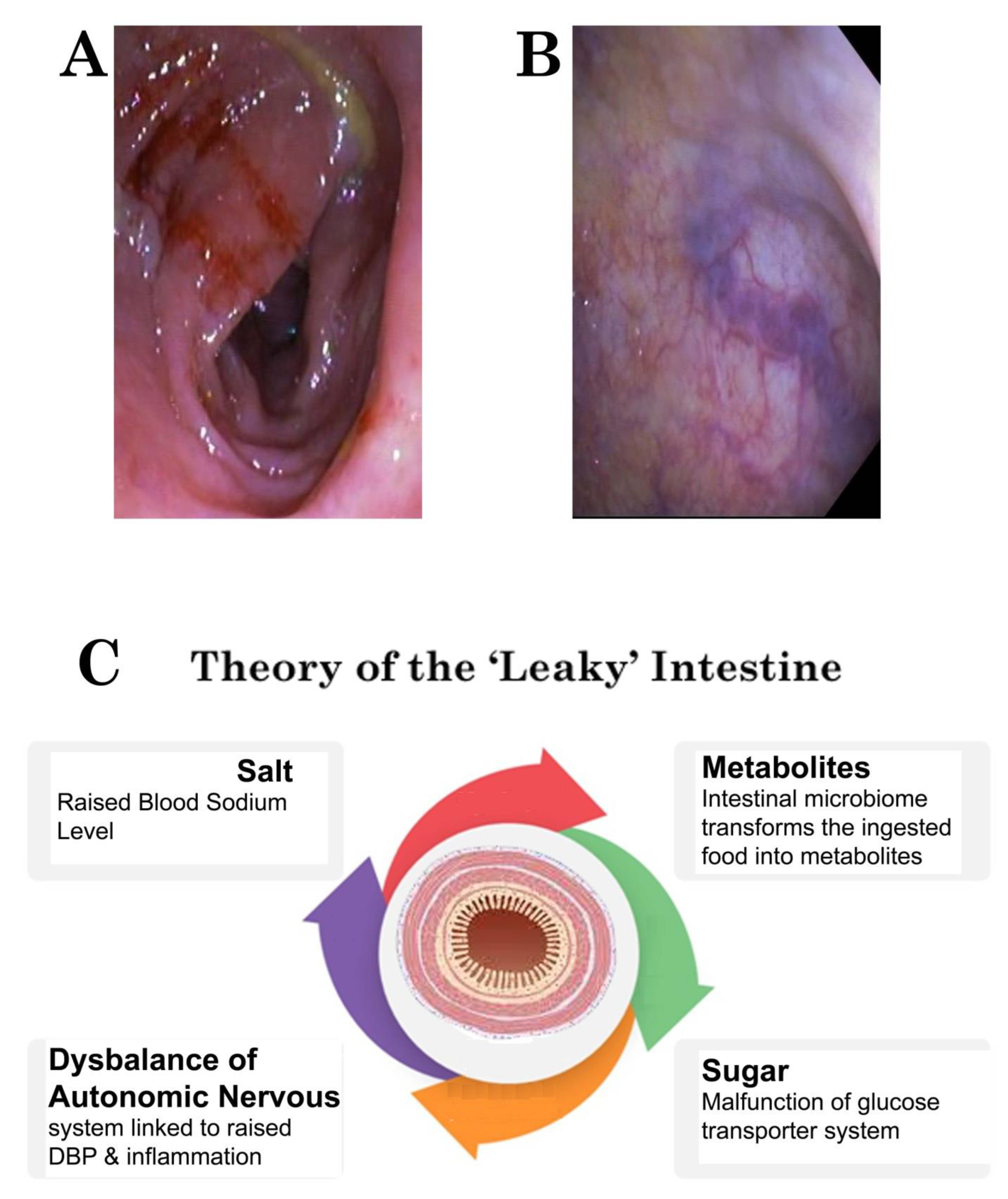
High-Frequency Ultrasound Duodenography and Colonography
Anti-Parasitic Treatment
Blood Pressure Classification
Statistical analysis
RESULTS
General Effects
DISCUSSION
Funding
Conflicts of interest
References
- WHO. Conquering, Suffering and Enriching Humanity Report of an Informal Consultation. 2000.
- Olubunmi, O. Parasites of Man and Animals: Concept publication limited. 2013.
- Vandenberg O, Van Laethem Y, Souayah H, Kutane WT, Van Gool T. Improvement of routine diagnosis of intestinal parasites with multiple sampling and SAF-fixative in the triple-feces-test. Gastroenterology Belg. 2006; 69: 361-366.
- 4. Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg A. Prevalence and Factors Associated with Intestinal Parasitic Infection among Children in an Urban Slum of Karachi. PLoS ONE 2008; 3(11): e3680. [CrossRef]
- Ahmed T, Khanum H, Uddin MS, Barua P, Arju T, Kabir M, Haque R. Entameoba Histolytica, Giardia Lamblia and Cryptosporidium spp. infection in children in an urban slum area of Bangladesh Bio res. Comm. 2016; 2: 175-181.
- World Health Organization. Prevention and control of intestinal parasitic infections: Report of a WHO Expert Committee [meeting held in Geneva from 3 to 7 March 1986]. World Health Organization; 1987.
- Abate A, Kibret B, Bekalu E, Abera S, Teklu T, Yalew A; et al. Cross-sectional study on the prevalence of intestinal parasites and associated risk factors in Teda Health Centre, Northwest Ethiopia. International Scholarly Research Notices. 2013;2013. [CrossRef]
- Ayelgn M, Worku L, Ferede G, Wondimeneh Y. A 5 year retrospective analysis of common intestinal parasites at Poly Health Center, Gondar, Northwest Ethiopia. BMC research notes. 2019;12(1):697. [CrossRef]
- Norhayati M, Fatmah M, Yusof S, Edariah A. Intestinal parasitic infections in man: A review. Medical Journal of Malaysia. 2003;58(2):296–305.
- Funso-Aina OI, Chineke HN, Adogu PO. A Review of Prevalence and Pattern of Intestinal Parasites in Nigeria (2006-2015). European Journal of Medical and Health Sciences. 2020;2(1). [CrossRef]
- Ogonaka IA, Nwoke BEB, Ukaga GN. Prevalence of Soil Transmitted Helminthes among Primary School Pupils in Owerri West Local Government Area in Imo State, Nigeria. Parasitol Public Health Soc Niger. 2011;27:92.
- Opara KN, Nwoke EA, Abanobico-Onwuliri COE, Iwuala C, Amadi AN. Intestinal parasites among children in day-care centres in Owerri metropolis Nigeria. Niger J Parasitol. 2010;31(1):45-49.
- Ahmed S, Spence JD. Sex differences in the intestinal microbiome: Interactions with risk factors for atherosclerosis and cardiovascular disease. Biol Sex Differ. 2021;12(1):35. [CrossRef]
- Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C; et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front Immunol. 2017;8:754. [CrossRef]
- Dan X, Mushi Z, Baili W, Han L, Enqi W, Huanhu Z; et al. Differential analysis of hypertension-associated intestinal microbiota. Int J Med Sci. 2019;16:872–881. [CrossRef]
- de la Cuesta-Zuluaga J, Mueller NT, Alvarez-Quintero R, Velasquez-Mejia EP, Sierra JA, Corrales-Agudelo V; et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients. 2018;11(1):51. [CrossRef]
- Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. [CrossRef]
- Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR; et al. Gut microbiota composition and blood pressure. Hypertension. 2019;73(5):998-1006. [CrossRef]
- Verhaar BJH, Collard D, Prodan A, Levels JHM, Zwinderman AH, Bäckhed F; et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: The HELIUS study. Eur Heart J. 2020;41(44):4259-4267. [CrossRef]
- Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C; et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017;7:381. [CrossRef]
- Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N; et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. [CrossRef]
- Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond). 2018;132:701–718. [CrossRef]
- Huart J, Leenders J, Taminiau B, Descy J, Saint-Remy A, Daube G; et al. Gut microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension. 2019;74:1005–1013. [CrossRef]
- Jackson MA, Verdi S, Maxan ME, Shin CM, Zierer J, Bowyer RCE, Martin T, Williams FMK, Menni C, Bell JT; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. 2018;9:2655. [CrossRef]
- Adeloye D, Owolabi EO, Ojji DB, Auta A, Dewan MT, Olanrewaju TO; et al. Prevalence, awareness, treatment, and control of hypertension in Nigeria in 1995 and 2020: A systematic analysis of current evidence. J Clin Hypertens. 2021;23:963–977. [CrossRef]
- Odili A, Chori B, Danladi B, Nwakile P, Okoye I, Abdullah U; et al. Prevalence, Awareness, Treatment and Control of Hypertension in Nigeria: Data from a Nationwide Survey 2017. Glob Heart. 2020. [CrossRef]
- Mohanty P, Patnaik L, Nayak G; et al. Gender difference in prevalence of hypertension among Indians across various age-groups: A report from multiple nationally representative samples. BMC Public Health. 2022;22:1524. [CrossRef]
- 28. Akinlua JT, Meakin R, Umar AM, Freemantle N. Current Prevalence Pattern of Hypertension in Nigeria: A Systematic Review. PLoS ONE 2015;10(10):e0140021. [CrossRef]
- Njemanze PC, Darlington CC, Onuchukwu JE, Ukeje NE, Amadi A, Mgbenu CU; et al. Intestinal parasitic infection-induced intestinal wall cytoskeleton dysfunction in diabetes mellitus. Niger J Gen Pract. 2022;20:29-35. [CrossRef]
- Sun, D. , Xiang H., Yan J., He L. Intestinal microbiota: A promising therapeutic target for hypertension. Front Cardiovasc Med. 2022 Nov 15;9:970036. PMID: 36457803; PMCID: PMC9705378. [CrossRef]
- Njemanze, P.C. , Njemanze J., Skelton A., Akudo A., Akagha O., Chukwu A.A ; et al. High-frequency ultrasound imaging of the duodenum and colon in patients with symptomatic giardiasis in comparison to amebiasis and healthy subjects. J Gastroenterol Hepatol 2008;23:e34-42. [CrossRef]
- Njemanze, P.C. , Njemanze J.T., Ofoegbu C.C., Darlington C.C., Nneke E., Onweni J.A.; et al. High-frequency ultrasound imaging of the intestine in normal subjects and patients with intestinal parasites. In: Ali Abdo Gamie S, Mahmoud Foda E, editors. Essentials of Abdominal Ultrasound. London: IntechOpen; 2019; pp. 91–105. [Google Scholar] [CrossRef]
- Njemanze, P.C. , Njemanze J.T., Ofoegbu C.C., Nneke E., Onweni I.A., Ejiogu U.V.; et al. Technical notes on high-frequency ultrasound duodenography and colonography imaging of giardial lesions. Niger J Gen Pract 2021;19:54-60.
- Zubcevic, J. , Richards E.M., Yang T., Kim S., Sumners C., Pepine C.J.; et al. Impaired Autonomic Nervous System-Microbiome Circuit in Hypertension. Circ Res. 2019 Jun 21;125(1):104-116. PMID: 31219753; PMCID: PMC6588177. [CrossRef]
- Straub, R. , Wiest R., Strauch U., Harle P., Schölmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut 2006;55(11):1640–649. [CrossRef]
- Jeong, J. , Kim K., Lim D., Kim K., Kim H., Lee S.; et al. Microvasculature remodeling in the mouse lower gut during inflammaging. Sci Rep 2017;7:39848. [CrossRef]
- Liu, A. , Wang X., Liang X., Wang W., Li C., Qian J., Zhang X. Human umbilical cord mesenchymal stem cells regulate immunoglobulin a secretion and remodel the diversification of intestinal microbiota to improve colitis. Front Cell Infect Microbiol. 2022 Sep 2;12:960208. [CrossRef]
- National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda (MD): National Heart, Lung, and Blood Institute (US); 2004 Aug. Classification of Blood Pressure. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9633/.
- Taneja, V. Sex hormones determine immune response. Front Immunol. 2018; 9:1931. [CrossRef]
- Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017 Sep;103:45-53. [CrossRef]
- Miró-Canturri A, Ayerbe-Algaba R, Vila-Domínguez A, Jiménez-Mejías ME, Pachón J, Smani Y. Repurposing of the Tamoxifen Metabolites to Combat Infections by Multidrug-Resistant Gram-Negative Bacilli. Antibiotics (Basel). 2021 Mar 22;10(3):336. [CrossRef]
- Quintanar-Quintanar ME, Jarillo-Luna A, Rivera-Aguilar V; et al. Immunosuppressive treatment inhibits the development of amebic liver abscesses in hamsters. Med Sci Monit. 2004;10(11):BR317-24.
- Koppula S, Kumar H. More than a pore: The therapeutic potential of stem cells for neurological disorders. Cell Tissue Res. 2019 Jan;375(1):11-24.
- Fujita T, Kishimoto K, Kato Y; et al. Sympathetic nervous system regulates adipocyte proliferation by modulating bone marrow mesenchymal stem cell niche through β2-adrenergic receptor. Nat Commun. 2015 Jun 22;6:8281. [CrossRef]
- Fasano, A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012 Feb;42(1):71-8. [CrossRef]
- Mu Q, Kirby J, Reilly CM, Luo XM. Leaky gut as a danger signal for autoimmune diseases. Front Immunol. 2017;8:598. [CrossRef]
- Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability - a new target for disease prevention and therapy. BMC Gastroenterol. 2014 Dec 19;14:189. [CrossRef]
- Hollander, D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999 Oct;1(5):410-6. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




