1. Introduction
Bruxism is a repetitive activity of the masticatory musculature, characterized by grinding, clenching of the teeth and/or sustained jaw thrust without tooth contact. In addition, bruxism can occur during sleep (sleep bruxism) and/or during wakefulness (awake bruxism)[
1].
Sleep Bruxism (SB) also occurs concomitantly or secondarily to other sleep disorders, such as Obstructive Sleep Apnea (OSA) [
2,
3,
4,
5,
6,
7,
8,
9]. OSA consists of recurrent episodes of partial or total upper airway obstruction, accompanied by sleep fragmentation caused by arousals and commonly accompanied by snoring [
10], in addition to other complications (hypertension, arrhythmias, cardiovascular disease, etc.) [
11,
12]. This increases the difficulty in achieving a high diagnostic yield with electromyography (EMG) devices, and the criteria for neurophysiological analysis of these recordings vary among different studies [
13,
14,
15,
16,
17,
18,
19]. Differentiating between masticatory muscle activity (MMA), rhythmic masticatory muscle activity (RMMA), sleep-related oromotor activity (OMA) and recognized bruxing activity is a challenge during the polysomnography (PSG) analysis itself. These types of activity are not always properly recognized by the algorithms programmed into the automatic analysis mode of EMG devices and not all EMG devices offer manual analysis mode. Moreover, the criteria used for the manual mode of these recordings are not uniform [
13,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. PSG studies in sleep laboratories include, electroencephalography (EEG), electrooculogram (EOG), electrocardiogram (EKG), EMG recordings (of the masticatory muscles and tibial muscles), and the thoracoabdominal movements recordings. Also include oronasal flow and oxygen saturation, allowing a definitive evaluation of SB and the detection of other disorders such as OSA, parasomnias, or restless legs syndrome [
31,
32].
However, the cost of PSG is high and requires very sophisticated instruments and highly specialized personnel, making its application unfeasible in dental clinics. Therefore, in recent years, portable ambulatory instruments have been developed that provide information similar to that provided by PSG but are more affordable and easier to handle. Their validity is still under discussion and requires further research, but it can be very useful as a clinical approach to the SB evaluation [
21].
EMG-EKG is a three-channel Holter-type device designed to detect the surface EMG signal of the two masseter muscles and the heart rate (HR) by EKG. This EKG capability is what differentiates this device from other portable devices and what would support its efficacy. The reliability of EMG-EKG has been proven with very good diagnostic yield [
33,
34,
35]. However, the studies have not been developed in an OSA population. There are many patients with undiagnosed OSA. Knowing that it is a disease that in many cases is concomitant with SB, we considered the need for assess the reliability of the ambulatory EMG for this type of population.
2. Materials and Methods
Twenty-three participants underwent one night of PSG study with simultaneous recording with the EMG-EKG device Bruxoff
® (OT Bioelettronica, Italy). Procedures will be conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement and checklist(36). The study protocol has been approved by the Ethics Committee of the Hospital Clínico San Carlos in Madrid, (C.P. - C.I. 14/380-E). Written informed consent will be obtained from all participants and all procedures were conductedaccording to the Declaration of Helsinki. The variables referring to the number of episodes and the SB index measured with both tools and analyzed in their manual and automatic modes were compared. Masticatory muscle activity was scored according to published criteria [
25,
32]. The sample was segmented by severity of OSA according to AASM criteria [
25,
36].
2.1. Sample Selection:
The participants of the study are adult patients attended by the Sleep Unit (Clinical Neurophysiology Department) of San Carlos University Hospital (Madrid, Spain) who underwent an earlier screening according to the suspicion of SB and OSA, by self-referred tests and physical examination. Exclusion criteria were major neurological disorders, psychiatric disorders, other sleep disorders, psychoactive medication, or edentulism. The clinical examination (tooth wear, masticatory muscle myalgia, TMJ arthralgia, hard tissue, soft tissue, and masseter and/or temporal hypertrophy) was performed according to DC/TMD and AAOP criteria and conducted by a dentist with ability in orofacial pain [
37,
38]. Finally, a PSG diagnosis was performed by an experienced neurophysiologist trained in SB. As a result, from both selections a sample of 23 subjects with an average age of 46,10 ± 10,98 was achieved, including 16 men and 7 women. A concordance between EMG-EKG device and the PSG (Gold Standard) design was used with six participants without OSA and seventeen with OSA. The sample of OSA patients were segmented by degree of severity in three groups (Mild OSA = 7, Moderate OSA = 3, Severe OSA = 7).
2.2. PSG recordings
The full-night monitoring recordings in the Sleep Laboratory (minimum of 8 hours in bed) were performed using a Deltamed Coherence 5.0 system. PSG recordings were made according to the AASM recommendations [
25], including: six EEG derivations; right and left EOG; submental, masseter and leg EMG; nasal cannula/pressure and oronasal thermal flow; thoracic and abdominal respiratory effort bands; snoring; body position sensor; pulse oximetry; audio and video recordings. Impedance values were checked and adjusted (< 5 Ω), and standard calibrations were performed. All PSG recordings were manually reviewed according to international criteria [
25]. In the SB and OSA group the diagnosis was confirmed by PSG performed by a sleep expert, following blinding masking with respect to the clinical examination.
2.2.1. PSG Sleep Bruxism Analysis
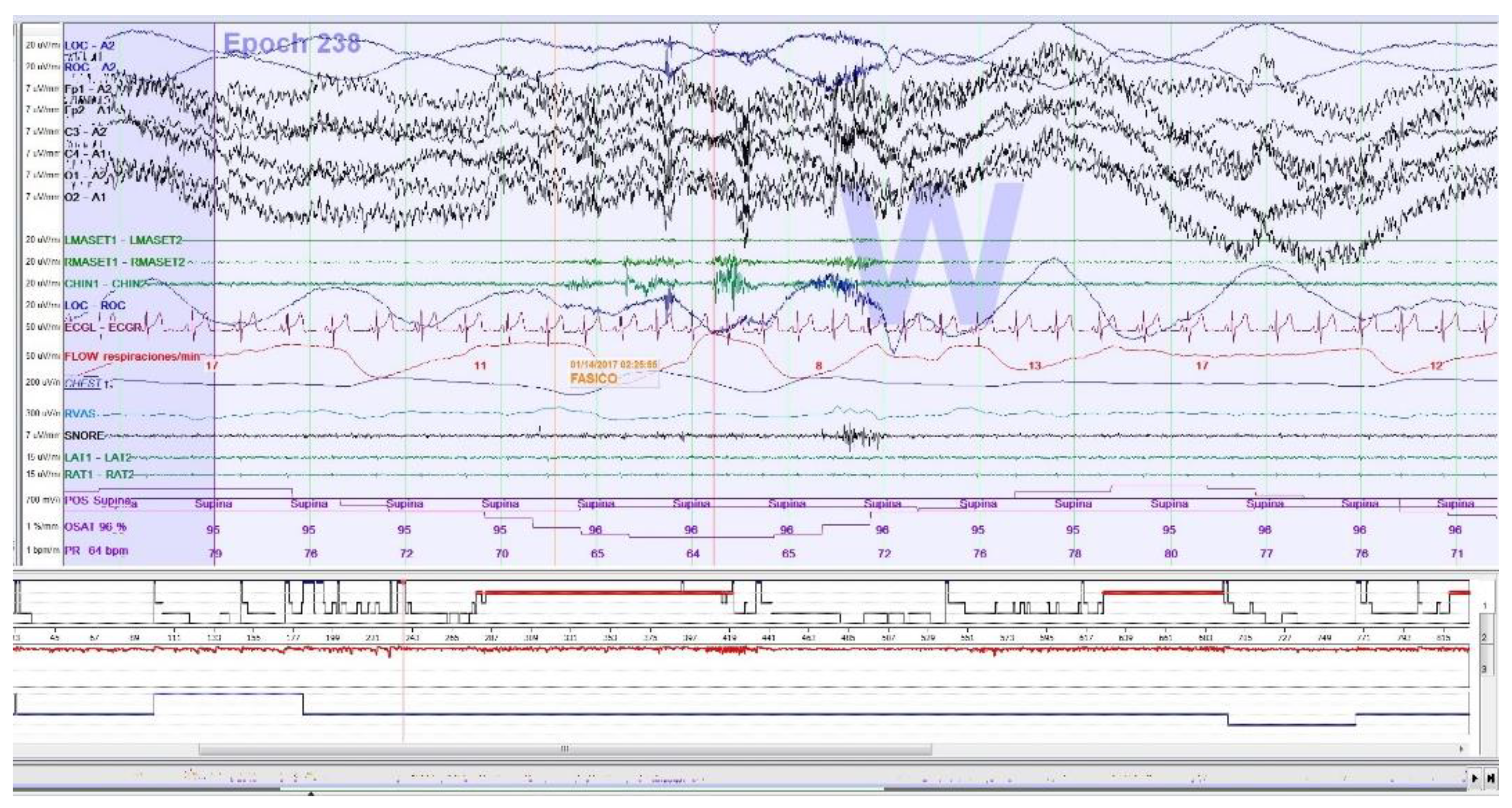
SB events are estimated through rhythmic (RMMA;
Figure 1), and non-rhythmic (AMM) masticatory muscle activity recorded with EMG on the masseter muscles (surface electrodes). Published criteria for SB episodes in PSG were followed [
25]. For the calculation of dichotomous variables, the presence of > 2 RMMA-MMA/BS episodes/h was considered. For the calculation of quantitative variables, the type of SB event is decided: phasic event (three or more EMG burst, at least 0.25 seconds and up to 2.0 seconds), tonic event (at least one EMG burst > 2.0 seconds), and mixed event (both types) (27,32,40–42). Increased muscle tone following an apnea episode, which is part of the American Academy of Sleep Medicine (AASM) definition of arousal [
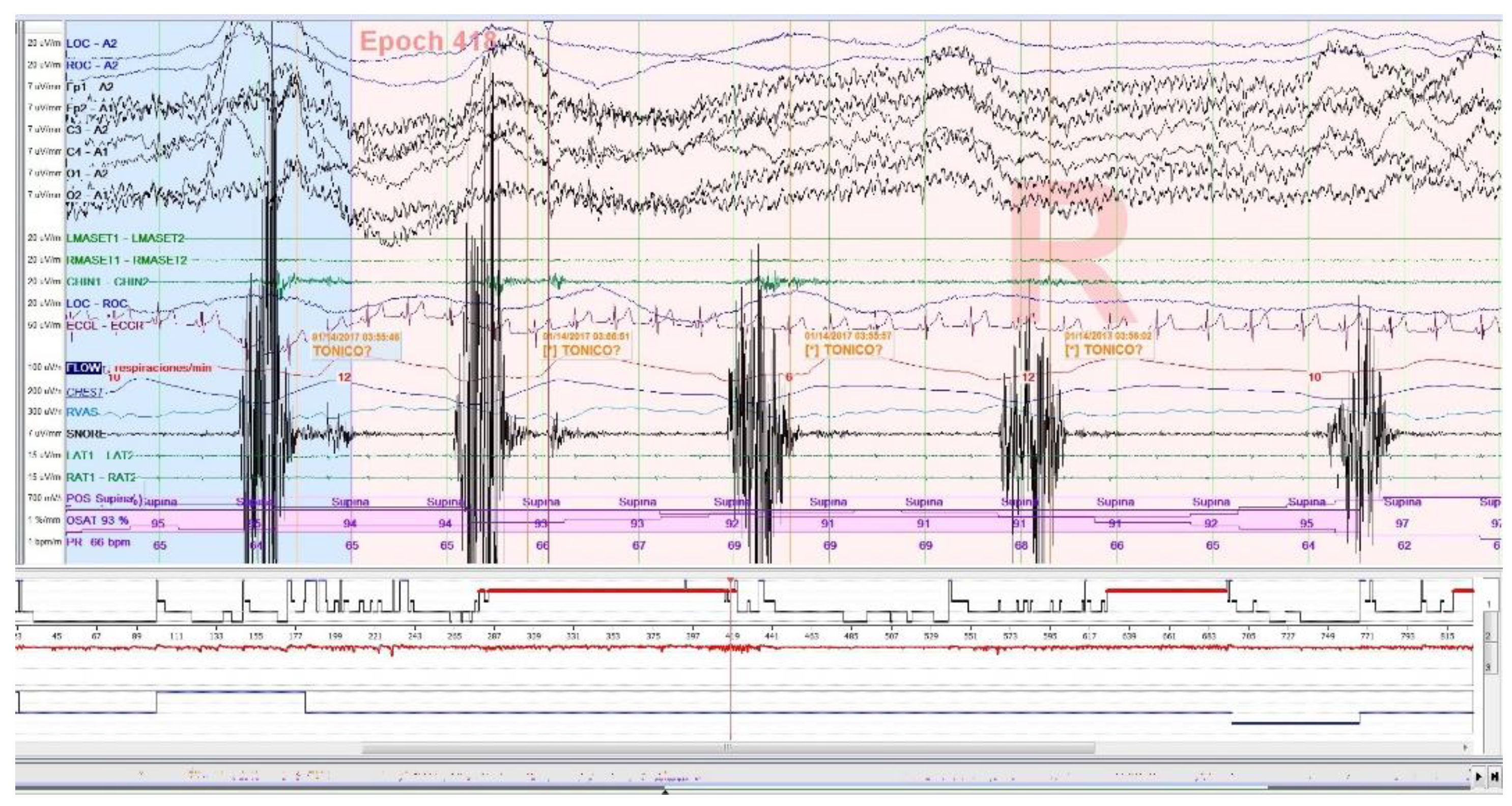
25], as well as sleep related oromotor activity (OMA;
Figure 2) different from RMMA-MMA/BS were excluded to avoid possible confounding bias. All isolated SB events, independent of respiratory events, were accepted according to EMG criteria, regardless of whether accompanied by arousals.
2.3. Bruxoff Sleep Bruxism Analysis
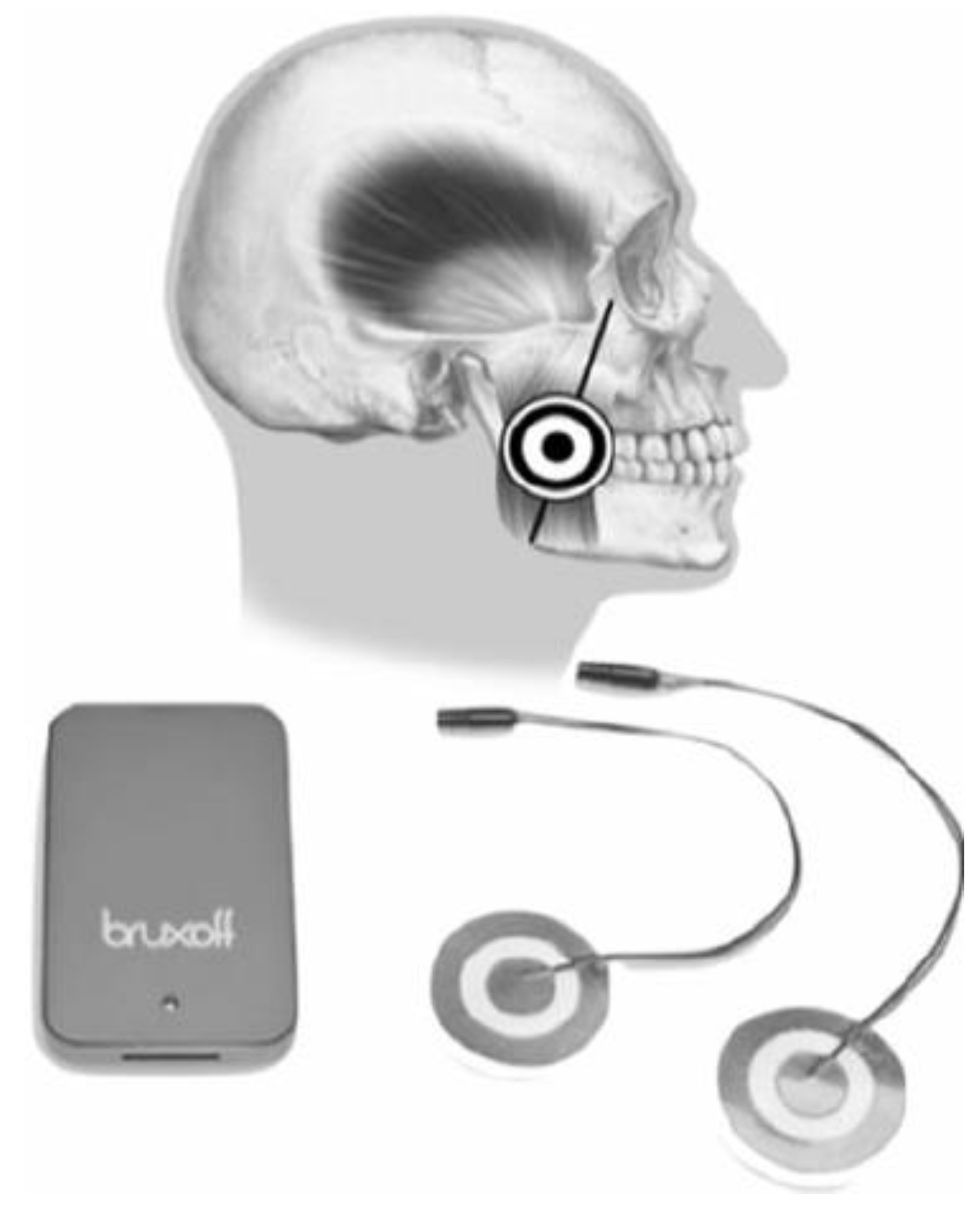
Bruxmeter is the software system of the EMG-EKG device (Bruxoff
®;
Figure 3 and Figure S1).
Interpretation is performed both manually, with the investigator analyzing the raw data, and automatically, with the device’s software analyzing the data to generate a diagnosis, which when analyzed automatically reaches a sensitivity of 91.6% [
20].
The MicroSD card provides the data for the diagnostic variables: bruxing event, number of bruxing events per hour of sleep (SB index), and number of bruxing events per night.
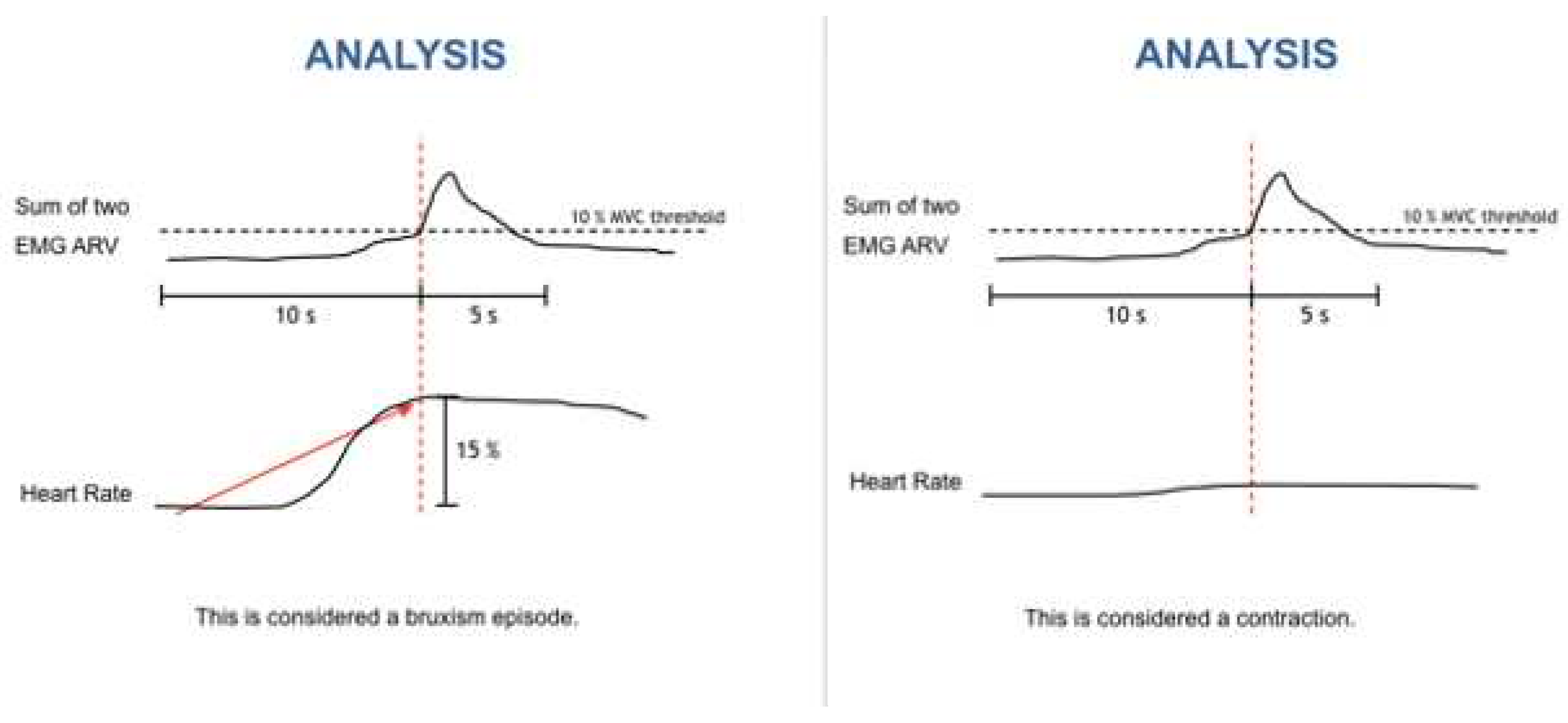
The bruxism event criteria depend on whether the analysis is performed in manual or automatic mode. Manual mode: EMG signal with peaks > 0.25s and an average amplitude of 10% of the patient’s maximum voluntary contraction (MVC), being preceded 1s earlier by an increase in heart rate (HR) of 15%. Automatic mode: EMG signal with an amplitude of at least 10% of the patient’s MCV, preceded by an increase in HR of 20%, 1-5s before (
Figure 4).
2.4. Statistical analysis
Continuous variables as means ± SD, ANOVA and corresponding non-parametric tests were applied when necessary. Correlations and the Bland–Altman plot were used to quantify the agreement between both methods. The concordance was calculated through the ICC. The variables used were Apnea Hypopnea Index (AHI), SB Index, number of apnea events, number of hypopnea events, and number of SB events. In addition, the sample was segmented according to the degree of severity of OSA and according to the types of SB episodes. All calculations were performed with the SPSS v17.0 statistical package (SPSS Inc., Chicago, IL) and Epidat 4.2. Values equal to or less than 0.05 were considered statistically significant.
3. Results
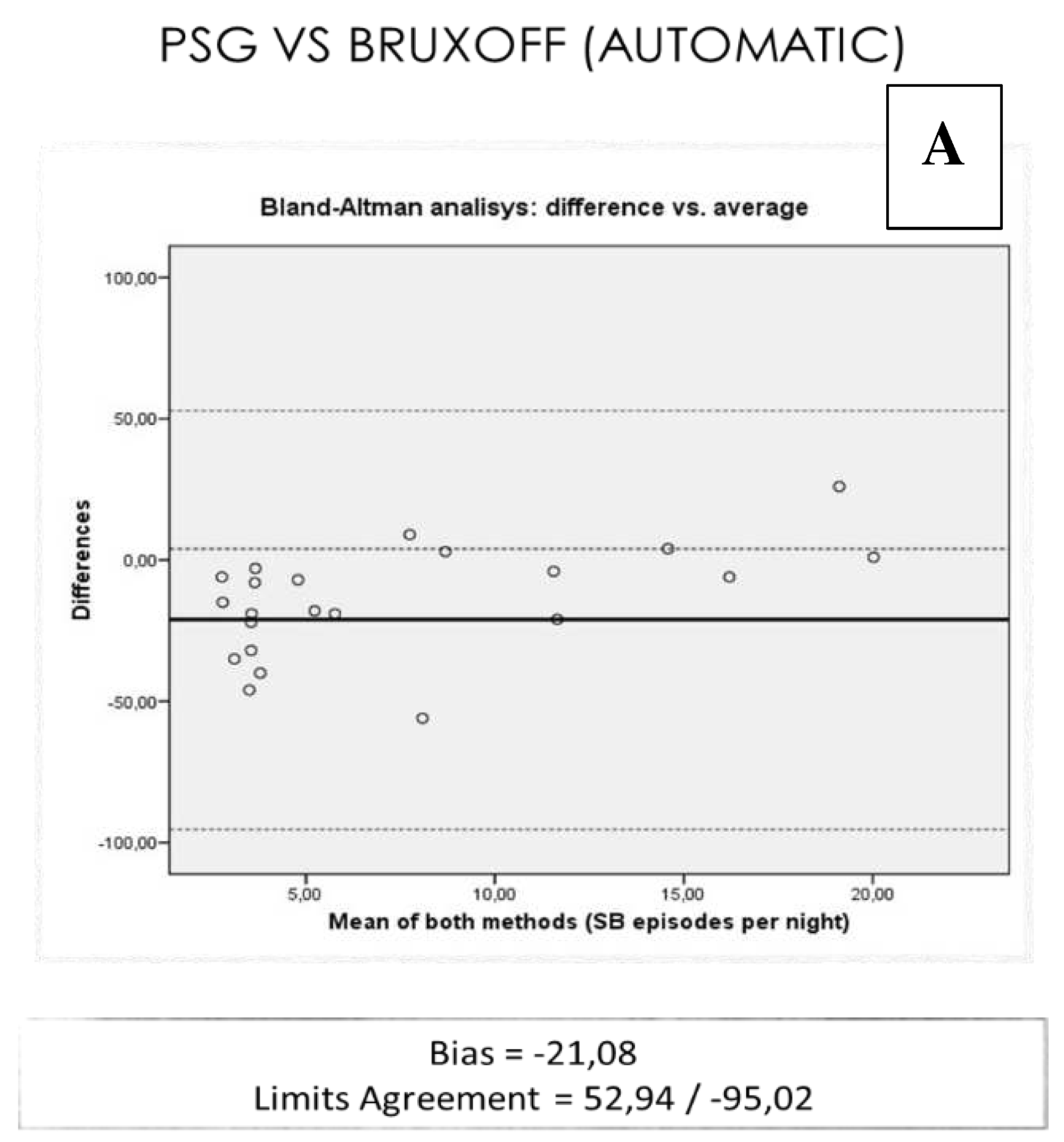
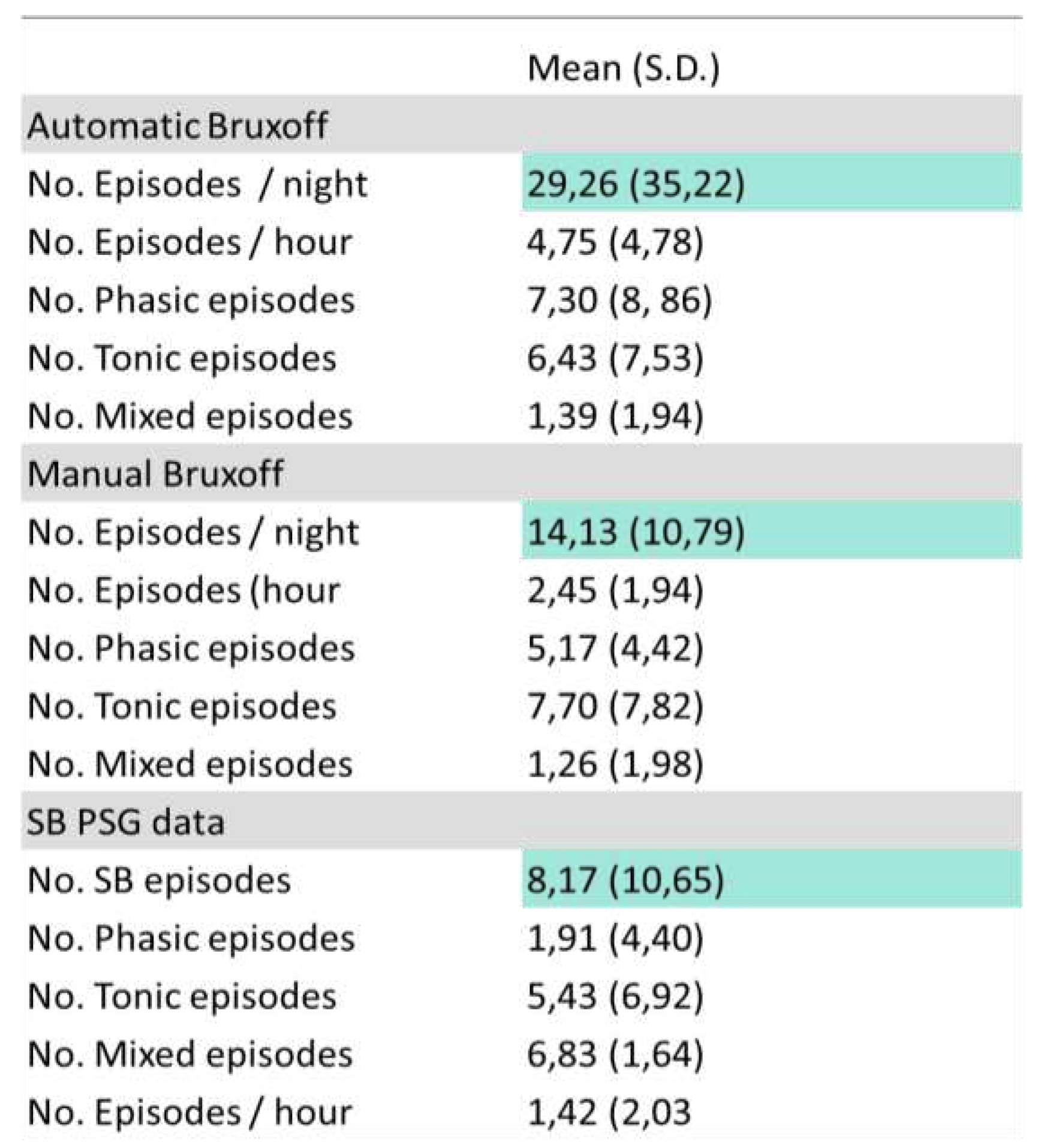
The total events of SB per night in the PSG study were on average (8.17), lower than the one obtained with EMG-EKG device manual analysis (14.13) and automatic (29.26) [r= -0,091 ICC (0-0,25)] (
Table 1).
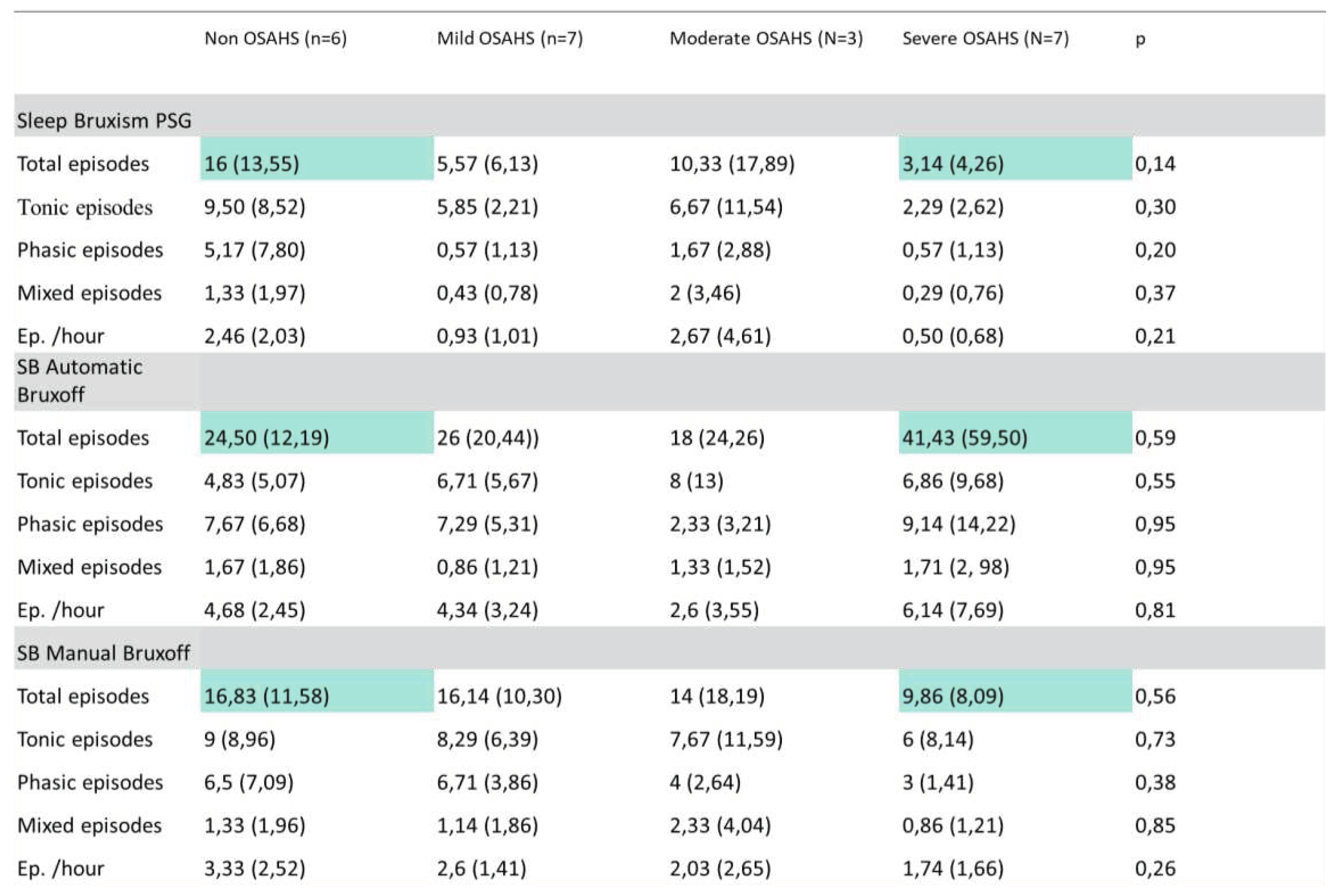
Both the SB PSG and Manual EMG-EKG device episodes decrease from non-OSA (PSG = 16 ± 13,55, EMG-EKG = 16,83 ±11,58) to severe OSA (PSG = 3,14 ± 4,26, EMG-EKG = 9,86 ± 8,09). However, in the case of automatic EMG-EKG the number of SB episodes in severe OSA doubles (41,23 ± 12,50) with respect to non OSA (24,50 ± 12,19); (
Table 2).
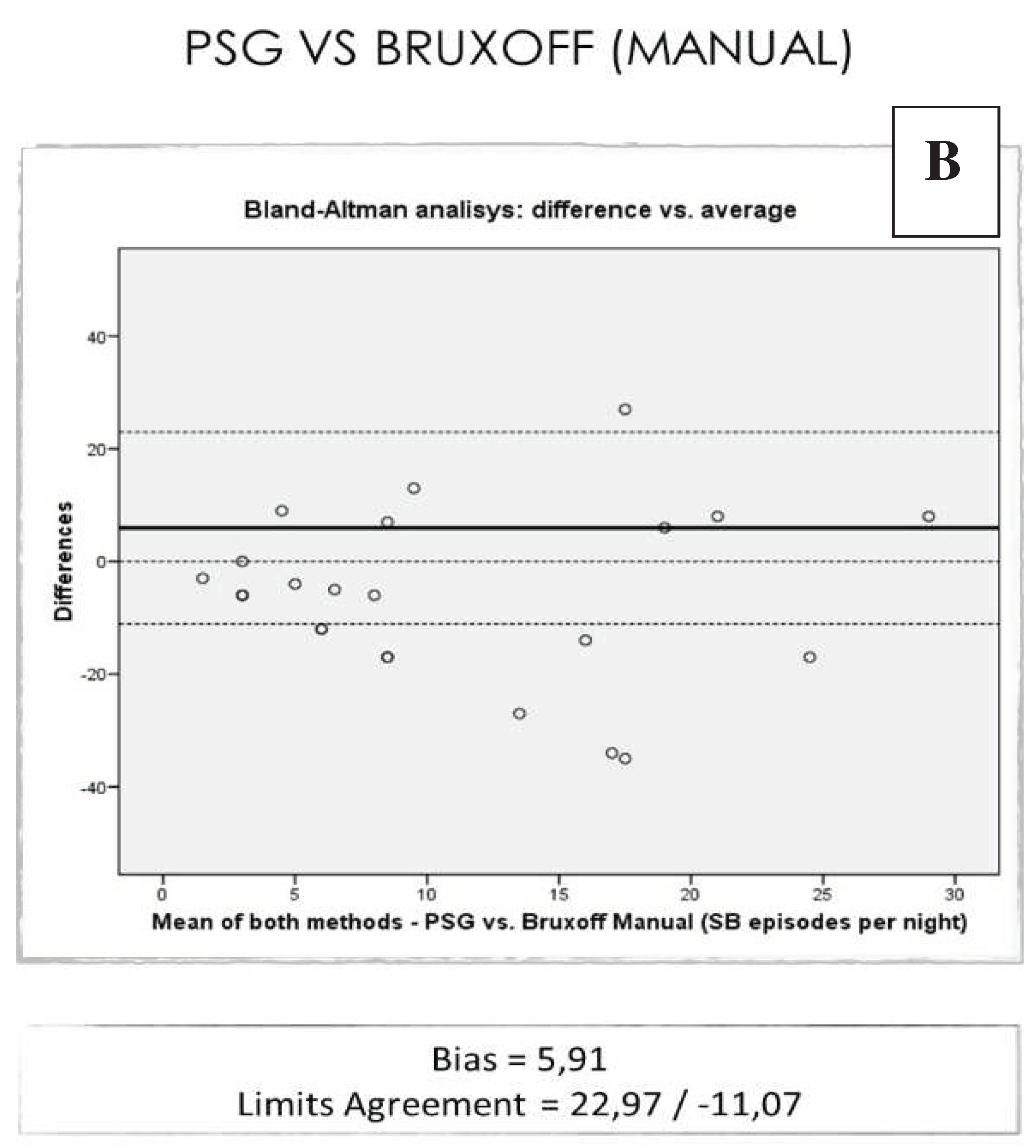
On average: the EMG-EKG device Automatic analysis measures 21.08 units more than PSG. The results with the manual EMG-EKG device analysis improved but were not good (
Figure 5).
Although have not obtained statistical significance, it would be useful to reproduce studies with a similar design and increasing the sample size to confirm these results. This would provide data to improve the automatic analysis algorithms of portable devices for the SB evaluation.
4. Discussion
Some authors describe the possibility that there is a subtype of patients with subclinical or mild OSA and that such EMG activity could play a protective role against OSA [
19]. We must bear in mind that OSA and SB share structures that play a fundamental role with protective functions during sleep. Also, there are inter-individual differences. Therefore, it is essential to clarify the PSG criteria for the evaluation of SB and its comorbidities, in order to design quality studies and avoid biases in that evaluation [
25,
32,
42]. Likewise, the refinement of ambulatory devices depends on the correlation and concordance obtained with the gold standard (PSG). Improving the ability to avoid bias with automatic analysis of portable EMG devices is essential to avoid overestimation of the disease. Overestimating SB would mean overestimating its association with other sleep disorders. In addition, it is essential to complement the instrumental diagnosis of SB with the clinical examination and the patient’s self-referred tests, as this would allow to assess the sequelae of SB [
1]. The clinical consequence of SB is the true indicator of the need for treatment [
43]. In any way, definitive EMG ambulatory evaluation of SB should be increasingly implemented in the clinical setting and not only in research, as it is the only reliable and objective measure that bruxing activity is present and active. Similarly, EMG is a useful tool for proper follow-up as a measure of the efficacy of certain therapeutic approach strategies. The use of EMG on a daily and reliable basis would mean being able to implement this tool in the same way that, for example, a periodontal chart is performed for the staging of periodontal disease and its evolution.
The combined use of respiratory polygraphy with EMG also allows a complete screening of both entities (SB-OSA), being also used for the follow-up of patients who are users of a mandibular advancement device. It would be interesting to use portable respiratory polygraphs that include EMG in masseters, like the one used by Winck [
22]. The EMG-only devices have not sufficient diagnostic yield for SB in populations which OSA has not been previously discarded. Including masseter and temporalis muscles EMG montage in sleep units as routine would be useful to improve the knowledge about SB-OSA relationship.
The bruxing activity is considered as a continuum, so instruments that allow the recording of several nights in a less costly way, such as EMG, should be refined. Therefore, the determination of new correlations and updated cut-off points is important [
42].
Once the performance of portable EMG has been improved, it could be used for concordance studies against other types of novel tools that are emerging due to the obvious evolution of technology, big data, and artificial intelligence [
44]. Such studies would allow them to be performed longitudinally and more fluently than with PSG in a sleep lab.
In the case of studies on dental materials used in oral rehabilitations in bruxism patients, there are large biases due to not objectively measuring SB. With the improvement of EMG, increasing its use by clinicians and researchers in the different fields of dentistry, a large part of these biases could be avoided.
The results show that manual analysis of SB events is more reliable than automatic analysis in our sample. Training in this type of analysis and calibration among professionals, as is done for example with DC/TMD exploration for temporomandibular disorders, would provide great advantages for professionals who handle this type of patient [
37]. It would be advisable to perform a basic OSA screening of all patients with suspected SB. In the case of EMG-EKG device, manual analysis of bruxing events in these patients shows greater reliability than automatic analysis.
Likewise, there are already EMG devices that are used for the management of SB through biofeedback [
45]. Further research along these lines could provide new non-invasive, reversible, and inexpensive management methods for the patient.
Limitations
The patient attending the sleep unit may suffer from “laboratory” effects on the first night, but it was not feasible for us to perform more than one night of PSG recording. The groups are not balanced due to the low sample size and there is a predominantly OSA population.
5. Conclusions
There is no concordance between the results obtained in the PSG neurophysiologic analysis and those obtained by means of the EMG-EKG device automatic and manual analysis for the diagnosis of SB in a population mostly with OSA.
There is EMG activity in OSA patients which could act as a confounding factor in the analysis of SB episodes with portable devices.
It would be recommended to clarify the analysis scores to differentiate the muscle activity consecutive to the apnea episode from the masticatory muscle activity that meets the criteria for SB, especially for the programming of portable devices algorithms.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: EMG-EKG Device.
Author Contributions
Conceptualization, R.C-V, F.J.M.O, I.A.G; A.A.D.G.; Methodology, R.C-V, A.A.D.G, F.J.M.O.; Formal analysis, R.C-V., F.J.M.O., A.A.D.G.; Investigation, R.C-V., F.J.M.O.; Writing—Original Draft, R.C-V.; Supervision, I.A.G., F.J.M.O., A.A.D.G.; Resources F.J.M.O., I.A.G., E.A.S.R; Writing-Review and editing, F.J.M.O., I.A.G., E.A.S.R.; Project administration, F.J.M.O.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Hospital Clínico San Carlos in Madrid, (C.P.—C.I. 14/380-E).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
the data presented in this study are available on request from the corresponding authors. The data are not publicly available due to ethical restrictions. Acknowledgments: We thank the patients of the study for making this possible.
Conflicts of Interest
The authors declare no conflict of interest
References
- Lobbezoo F, Ahlberg J, Raphael KG, Wetselaar P, Glaros AG, Kato T, et al. International consensus on the assessment of bruxism: Report of a work in progress. J Oral Rehabil 2018, 45, 837–844. [CrossRef]
- Carra MC, Bruni O, Huynh N. Topical review: sleep bruxism, headaches, and sleep-disordered breathing in children and adolescents. J Orofac Pain. 2021, 26, 267–276.
- Lavigne G, Manzini C, Huynh N. Sleep bruxism. In: Elsevier Saunders, editor. Principles and Practice of Sleep Medicine. 5th ed. St. Louis; 2011. p. 1129–39.
- Glaros AG. Incidence of diurnal and nocturnal bruxism. J Prosthet Dent. 1981, 45, 545–549. [CrossRef] [PubMed]
- Allen JD, Rivera-Morales WC, Zwemer JD. The Occurrence of Temporomandibular Disorder Symptoms in Healthy Young Adults With and Without Evidence of Bruxism. CRANIO®. 1990, 8, 312–318.
- Reding GR, Rubright WC, Zimmerman SO. Incidence of bruxism. J Dent Rest. 1866, 45, 1198–1204. [CrossRef]
- Laberge L, Tremblay RE, Vitaro F, Montplaisir J, PhD C. Development of Parasomnias From Childhood to Early Adolescence. Pediatrics 2000, 106, 67–74. [CrossRef] [PubMed]
- Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of Bruxism in Adults: A Systematic Review of the Literature. J Orofac Pain. 2013, 27, 99–110. [CrossRef] [PubMed]
- Kato T, Mikami A, Sugita H, Muraki H, Okura M, Ohi M, et al. Negative association between self-reported jaw symptoms and apnea–hypopnea index in patients with symptoms of obstructive sleep apnea syndrome: a pilot study. Sleep and Breathing 2013, 17, 373–379. [CrossRef]
- Roehrs T, Carskadon MA, Dement WC, Roth T. Day- time sleepiness and alertness. In: Saunders, editor. Principles and Practice of Sleep Medicine. Philadelphia; 2000. p. 43–52.
- Bassiri AG, Guilleminault C. Clinical features and evaluation of obstructive sleep apnea–hypopnea syndrome. In: Saunders WB, editor. Principles and Practices of Sleep Medicine. Philadelphia; 2000. p. 869–78.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2002, 165, 1217–1239. [CrossRef]
- INOKO Y, SHIMIZU K, MORITA O, KOHNO M. Relationship between masseter muscle activity and sleep-disordered breathing. Sleep Biol Rhythms. 2004, 2, 67–68. [CrossRef]
- Saito M, Yamaguchi T, Mikami S, Watanabe K, Gotouda A, Okada K, et al. Temporal association between sleep apnea-hypopnea and sleep bruxism events. J Sleep Res. 2014, 23, 196–203. [CrossRef] [PubMed]
- Saito M, Yamaguchi T, Mikami S, Watanabe K, Gotouda A, Okada K, et al. Weak association between sleep bruxism and obstructive sleep apnea. A sleep laboratory study. Sleep and Breathing 2016, 20, 703–709. [CrossRef]
- Hosoya H, Kitaura H, Hashimoto T, Ito M, Kinbara M, Deguchi T, et al. Relationship between sleep bruxism and sleep respiratory events in patients with obstructive sleep apnea syndrome. Sleep and Breathing 2014, 18, 837–844. [CrossRef]
- da Costa Lopes AJ, Cunha TCA, Monteiro MCM, Serra-Negra JM, Cabral LC, Júnior PCS. Is there an association between sleep bruxism and obstructive sleep apnea syndrome? A systematic review. Sleep and Breathing 2020, 24, 913–921. [CrossRef] [PubMed]
- Manfredini D, Guarda-Nardini L, Marchese-Ragona R, Lobbezoo F. Theories on possible temporal relationships between sleep bruxism and obstructive sleep apnea events. An expert opinion. Sleep and Breathing 2015, 19, 1459–1465. [CrossRef] [PubMed]
- Tan M, Yap A, Chua A, Wong J, Parot M, Tan K. Prevalence of Sleep Bruxism and Its Association with Obstructive Sleep Apnea in Adult Patients: A Retrospective Polysomnographic Investigation. J Oral Facial Pain Headache 2019, 33, 269–277. [CrossRef]
- Castroflorio T, Deregibus A, Bargellini A, Debernardi C, Manfredini D. Detection of sleep bruxism: comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J Oral Rehabil. 2014, 41, 163–169. [CrossRef]
- Manfredini D, Ahlberg J, Castroflorio T, Poggio CE, Guarda-Nardini L, Lobbezoo F. Diagnostic accuracy of portable instrumental devices to measure sleep bruxism: a systematic literature review of polysomnographic studies. J Oral Rehabil. 2014, 41, 836–842. [CrossRef]
- Winck M, Drummond M, Viana P, Pinho JC, Winck JC. Sleep bruxism associated with obstructive sleep apnoea syndrome – A pilot study using a new portable device. Revista Portuguesa de Pneumologia (English Edition). 2017 Jan;23(1):22–6.
- Sjöholm TT, Lowe AA, Miyamoto K, Fleetham JA, Ryan CF. Sleep bruxism in patients with sleep-disordered breathing. Arch Oral Biol 2000, 45, 889–896. [CrossRef]
- Lavigne G, Kato T, Herrero Babiloni A, Huynh N, Dal Fabbro C, Svensson P, et al. Research routes on improved sleep bruxism metrics: Toward a standardised approach. J Sleep Res. 2021 Oct 6;30(5). [CrossRef]
- The AASM Manual for the Scoring of Sleep and Associated Events. Vol. 2.6. 2020.
- Okeson JP, Phillips BA, Berry DT, Cook YR, Cabelka JF. Nocturnal bruxing events in subjects with sleep-disordered breathing and control subjects. J Craniomandib Disord. 1991;5(4)():258–64. [PubMed]
- Kato T, Blanchet PJ, Montplaisir JY, Lavigne GJ. Sleep bruxism and other disorders with orofacial activity during sleep. In: Butter-worth Heinemann, editor. Sleep and Movement Disorders. Philadelphia; 2003. p. 273–85.
- Lavigne GJ, Manzini C, Kato T. Sleep bruxism. Principles and practice of sleep medicine. 4th ed. Elsevier Saunder, editor. Philadelphia; 2005. 946–959 p.
- Miyawaki S, Lavigne GJ, Pierre M, Guitard F, Montplaisir JY, Kato T. Association between sleep bruxism, swallowing-related laryngeal movement, and sleep positions. Sleep. 2003 Jun 15;26(4)(PMID: 12841373):461–5. [CrossRef]
- Macaluso GM, Guerra P, Di Giovanni G, Boselli M, Parrino L, Terzano MG. Sleep Bruxism is a Disorder Related to Periodic Arousals During Sleep. J Dent Res. 1998 Apr 8;77(4):565–73. [CrossRef]
- Rundo JV, Downey R. Polysomnography. In 2019. p. 381–92.
- Lavigne GJ, Rompre PH, Montplaisir JY. Sleep Bruxism: Validity of Clinical Research Diagnostic Criteria in a Controlled Polysomnographic Study. J Dent Res. 1996 Jan 8;75(1):546–52. [CrossRef]
- Yanez-Regonesi F, Eisa E, Judge S, Carlson C, Okeson J, Moreno-Hay I. Diagnostic accuracy of a portable device (Bruxoff®) to measure sleep bruxism. J Oral Rehabil. 2023 Apr 25;50(4):258–66. [CrossRef]
- Castroflorio T, Deregibus A, Bargellini A, Debernardi C, Manfredini D. Detection of sleep bruxism: comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J Oral Rehabil. 2014 Mar;41(3):163–9. [CrossRef]
- Deregibus A, Castroflorio T, Bargellini A, Debernardi C. Reliability of a portable device for the detection of sleep bruxism. Clin Oral Investig. 2014 Nov 28;18(8):2037–43. [CrossRef]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008 Apr;61(4):344–9. [CrossRef]
- American Academy of Sleep Medicine. Sleep related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89.
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache. 2014 Jan;28(1):6–27. [CrossRef]
- De Leeuw R, Klasser GD. Orofacial Pain, Guidelines for assessment, diagnosis, and management. 5a ed. The American Academy of Orofacial Pain, editor. 2008.
- Lavigne GJ, Rompré PH, Poirier G, Huard H, Kato T, Montplaisir JY. Rhythmic Masticatory Muscle Activity during Sleep in Humans. J Dent Res. 2001 Feb 8;80(2):443–8. [CrossRef]
- Ward SLD. Principles and practice of sleep medicine, third edition. Edited by Meir H. Kryger, Thomas Roth, and William C. Dement. Philadelphia, W. B. Saunders Co., 2000, 1,336 pp. Pediatr Pulmonol. 2001 May;31(5):398–398.
- Kato T, Thie NM, Huynh N, Miyawaki S, Lavigne GJ. Topical review: sleep bruxism and the role of peripheral sensory influences. J Orofac Pain. 2003;Summer 17 (3)():191–213. [PubMed]
- Manfredini D, Ahlberg J, Wetselaar P, Svensson P, Lobbezoo F. The bruxism construct: From cut-off points to a continuum spectrum. J Oral Rehabil. 2019 Nov 2;46(11):991–7. [CrossRef]
- Raphael KG, Santiago V, Lobbezoo F. Is bruxism a disorder or a behaviour? Rethinking the international consensus on defining and grading of bruxism. J Oral Rehabil. 2016 Oct;43(10):791–8. [CrossRef]
- Ommerborn MA, Walentek N, Bergmann N, Franken M, Gotter A, Schäfer R. Validation of a new diagnostic method for quantification of sleep bruxism activity. Clin Oral Investig. 2022 Jun 23;26(6):4351–9. [CrossRef]
- Stuginski-Barbosa J, Porporatti AL, Costa YM, Svensson P, Conti PCR. Diagnostic validity of the use of a portable single-channel electromyography device for sleep bruxism. Sleep and Breathing. 2016 ;20(2):695–702. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).