1. Introduction
Polymers characterized by a low Tg (glass transition temperature) below about -20°C by an amorphous structure are predisposed for the manufacturing of PSA (pressure-sensitive adhesives) [
1]. Since their introduction half a century ago, pressure-sensitive acrylic adhesives have been successfully applied in many fields. They are used in self-adhesive tapes [
2,
3,
4,
5,
6,
7,
8,
9,
10], labels [
11,
12,
13,
14,
15,
16,
17], sign and marking films [
12], and protective films [
14,
15], assembly operations [
4,
11,
12,
18,
19,
20,
21,
22] as well as in dermal dosage systems for pharmaceutical applications [
2,
6,
7,
10,
11,
12,
17,
23,
24,
25,
26,
27], and biomedical electrodes [
28]. In the last sixty years or so, pressure-sensitive adhesive acrylics have made tremendous strides from what was virtually a black art to what is now a sophisticated science. So much so that both the few larger manufacturers of pressure-sensitive adhesive articles and their even larger suppliers now use very expensive equipment to study pressure-sensitive adhesive behavior: tack, adhesion, and cohesion [
16,
29,
30,
31]. In the case of protective films very important is shrinkage of pressure-sensitive adhesives after crosslinking process. Three properties which are useful in characterizing the nature of pressure-sensitive adhesives are tack initial adhesion, peel (adhesion) and shear (cohesion). The first measures the adhesive's ability to adhere quickly, the second its ability to resist removal by peeling, and the third its ability to hold in position when shearing forces are exerted. The first two are directly related to each other but are inversely related to the third. The pressure-sensitive adhesives are called, in the adhesives trade, pressure-sensitive adhesives (PSA). The performance of PSA such as tack, peel, and shear, are to a large degree determined by the polymerization method, crosslinking process and especially by the type and quantity of the crosslinking agents added to the acrylic PSA [
4,
7,
12,
17,
27,
32]. The common technologies for manufacturing pressure-sensitive adhesives include the following kind of acrylic pressure-sensitive adhesives (PSA). Solvent-based acrylic PSA-the adhesive ingredients are polymerized in solvent and cast onto the web. After coating, the solvents are dried off, leaving behind the functional adhesive [
11,
12,
19,
21,
31,
33]. Water-borne acrylic dispersions PSA, the adhesive ingredients are polymerized in water and applied to the web, then the water is dried off, leaving behind the functional adhesive. Solvent-free acrylic PSA in form of typical hot-melt, LVS (low viscosity systems) and photoreactive prepolymers. The adhesives based on photoreactive prepolymers are coated at room temperature and crosslinked by application of UV-technology using UV-lamps emitted radiation in UV-A and UV-C areas [
12,
13,
19,
21,
28,
31,
33,
34,
35,
36].
2. Materials and Methods
2.1. Materials
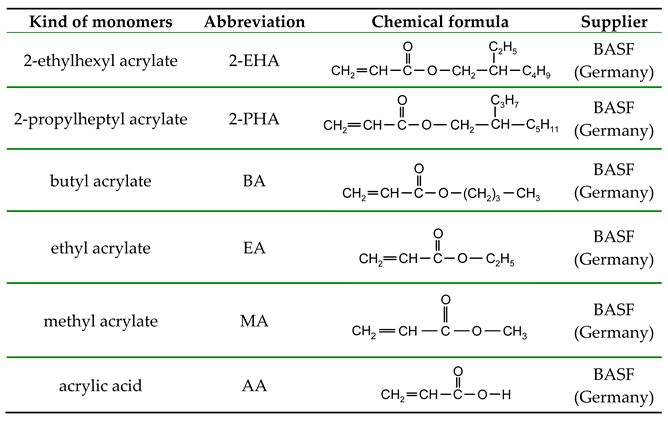
The following acrylate monomers, as shown in
Table 1, were used in the tests performed below.
2.2. Synthesis of investigated solvent-free photoreactive acrylic pressure-sensitive adhesives
The solvent-free polyacrylate-based photoreactive pressure-sensitive adhesives (PSA) were synthesized in a 0.25-liter glass reactor with water cooling using UV radiation as a source of free radicals resulting from photolytic degradation of the photoinitiator. The monomer mixture consisted of 35 wt.% 2-ethylhexyl acrylate (2-EHA), 25 wt.% 2-propylheptyl acrylate (2-PHA), 15 wt.% butyl acrylate (BA), 10 wt.% ethyl acrylate (EA), 10 wt.% methyl acrylate (MA) and 5 wt.% acrylic acid (AA). 0.1 wt.% Omnirad 127, based on the weight of the monomer mixture, was used as the radical photoinitiator.
The photoreactive acrylic PSA were synthesized using UV-initiated polymerization in stirred reactor under inert atmosphere (N
2) according to bulk radical polymerization method. Reactor of 0.25-liter capacity was equipped with stirrer, nitrogen inlet, temperature sensor and vent. UV radiation from a lamp mounted by the reactor was used to initiate the reaction. A UV lamp was placed by the reactor wall at a distance of 50 mm. Lamp with a power of 11W (Osram Dulux S BL UVA 11 W/78) equipped with a reflector, provided the radiation intensity at the level of 2.5 mW/cm
2 at a given distance (
Figure 1). The irradiation time was changed in the range from 1 min to 5 min. After exposure to UV radiation, oxygen is added to the pre-polymerization product to stabilize it.
2.3. Viscosity of synthesized solvent free photoreactive acrylic PSA
The viscosity of synthesized acrylic PSA was determined with a Rheomat RM 189 from Rheomatric Scientific, with spindle No 3 at room temperature (23°C).
2.4. Free monomers concentration in synthesized acrylic PSA
The residual of monomers was measured with gas chromatograph CP−3800 from Varian (
Figure 2). It was applied chromatographic column J&W DB−1 with the length of 30 mm and diameter of 0.25 mm. The 0.1 μl of gaseous phase sample for analysis from above PSA were injected into chromatographic column with syringe Hamilton 7001 with the volume from 0.01 to 1.0 μl.
2.5. Coat weight of transfer acrylic PSA and type of cover material
The coating weight of transfer of pressure-sensitive adhesives (thickness of the adhesive layer influence essentially pressure-sensitive adhesives (PSA) performance. The base weight of the adhesive layer covering the foil was maintained 100 g/m². Throughout the study the adhesives were coated on the 50 g/m² siliconized polyester film and after coating covered with the same 50 g/m² siliconized polyester film.
2.6. UV-initiated crosslinking
The coated layer of the photoreactive prepolymer is covered with a polyester adhesive film in order to exclude the so-called oxygen inhibition, negatively affecting the polymerization process. The "sandwich" obtained in this way is transferred from the coater to a device that allows the prepolymer layer to be illuminated on both sides with very low power UV-A lamps (
Figure 3).
Photoreactive acrylic prepolymer are modified with photoreactive crosslinking agent 1,6-hexanediol diacrylate (1,6-HDDA) in concentration between 0.3 and 0.7 wt.% are coated with coat weight 100 g/m² directly on 50 µ thick siliconized polyester film, covered with the same 50 µ thick siliconized polyester film and after that crosslinked from 5 to 10 min under Hg Philips lamps with UV dose of about 3 mW/cm² (
Figure 3). The UV-exposure can be measure using an integrating radiometer Dynachem TM Model 500, available from Dynachem Corporation.
2.7. Conditioning
Before testing the adhesive-coated strips were stored for 7 days at room temperature and 50% relative humidity. Three samples were tested, and the given value of tested property was the arithmetic mean of the achieved results.
2.8. Measurement of tack, peel adhesion, shear strength and shrinkage
The influence of residue acrylate monomers concentration, crosslinking agents 1,6-HDDA amount and UV-crosslinking time on pressure-sensitive adhesives properties, such as tack, peel adhesion, shear strength and shrinkage were determined by standard A.F.E.R.A. (Association des Fabricants Europeens de Rubans Auto-Adhesifs) standard. Exact details can be found in AFERA 4015 (loop tack), AFERA 4001 (peel adhesion) and AFERA 4012 (shear strength) measured at 20°C and 70°C.
Loop tack method measures the instantaneous adhesion of a loop of adhesive-coated material using no external pressure to secure contact (acc. AFERA 4015). According to another definition the quick stick tack value is the force required to separate at a specific rate a loop of material, which was brought into contact with a standard surface. A sample of PSA-coated material 1-inch (about 2,5 cm) wide and 7-inch (about 17,5 cm) long is bonded to a vertical of a clean steel test plate at least 10 lineal cm in firm contact. The vertical steel test plate is clamped in the jaws of a tensile testing machine. The scale reading in Newtons is recorded as the tape is peeled from the steel surface with a constant rate of 300 mm per minute. Loop tack has the advantage of allowing to use wood substrates from Rocholl (Germany).
Peel adhesion (acc. AFERA 4001) is the force required to remove a coated flexible pressure-sensitive adhesive sheet material from a test panel measured at 180° on wood surface as rate of removal. A sample of PSA-coated material 1-inch (about 2,5 cm) wide and 5-in (about 12,7 cm) long is bonded to a horizontal target substrate surface of a clean steel test plate at least 12,7 cm in firm contact. A 2 kg hard rubber roller is used to apply the strip. The free end of the coated strip is doubled back nearly touching itself so the angle of removal will be 180°. The free end is attached to the adhesion tester scale. The steel test plate is clamped in the jaws of a tensile testing machine, which is capable of moving the plate away from the scale at a constant rate of 300 mm per minute.
Shear strength is a measure of the cohesiveness or internal strength of an adhesive according to AFERA 4012, at 20°C and at 70°C. It is measured in minutes or hours required to pull a standard area of adhesive coated sheet material from a stainless-steel test panel under stress of a constant, standard load of 10 N. Each test is conducted on an adhesive-coated strip applied to a standard stainless-steel panel in a manner such that a 1-inch × 1-inch (2,5 cm × 2,5 cm) portion of the strip is in fixed contact with the panel with one end of the strip being free.
Shrinkage presents the percentage or millimetre change of dimensions of the PVC foil covered with PSA after PSA crosslinking and attached to the glass after keeping it 3 weeks at temperature of 60°C. Self-adhesive products with shrinkage greater than 0.3 % or 0.3 mm are not or only partially acceptable in the adhesive industry.
3. Results
3.1. Viscosity of synthesized solvent free photoreactive acrylic PSA
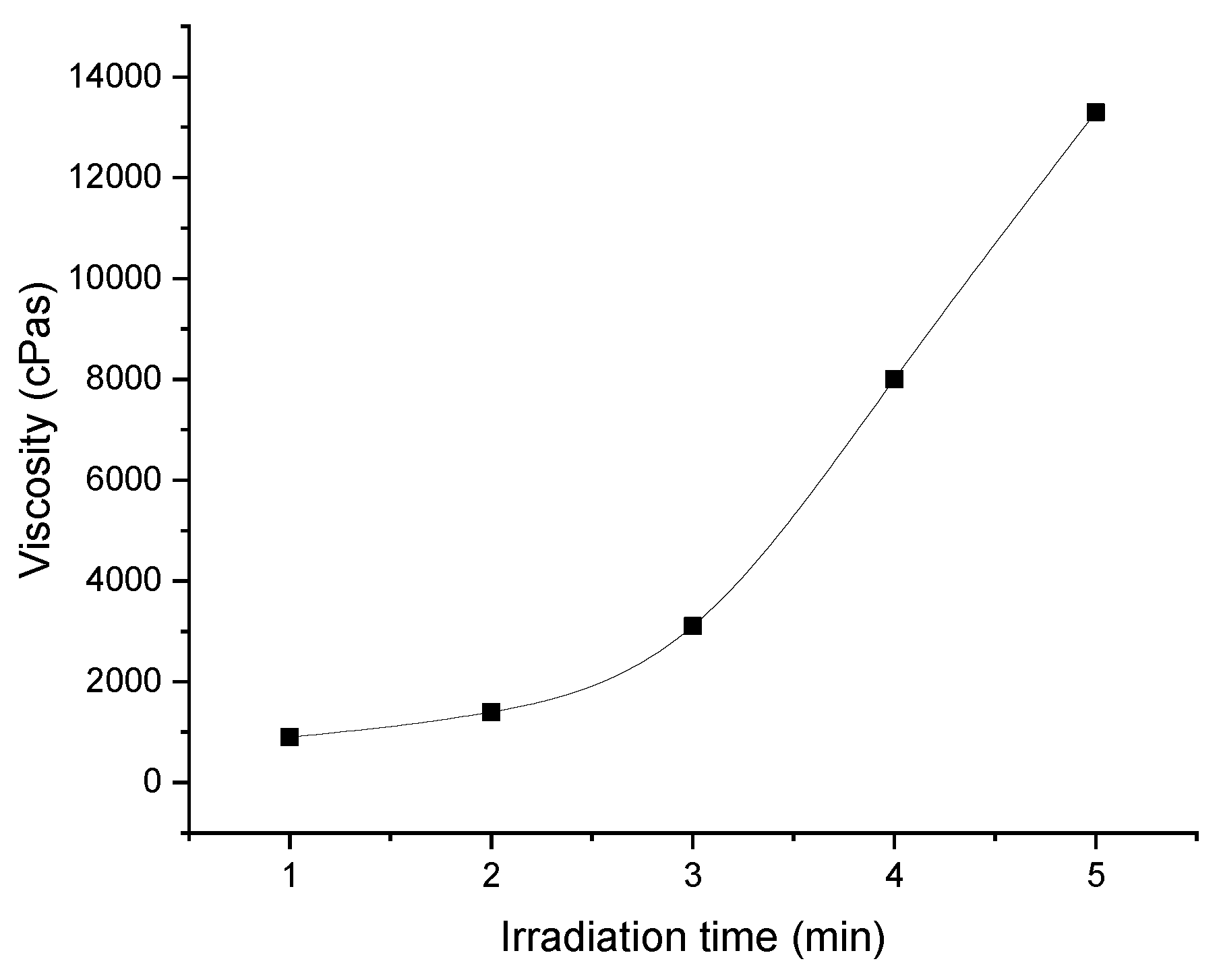
As shown in
Figure 4, the viscosity of the synthesized prepolymer increases with increasing exposure time of the photoreactive mixture under the UV lamp. Exposure time is one of the parameters that allow to adjust the viscosity of the prepolymer. Too low a viscosity leads to difficulties in coating, since the prepolymer spills too quickly during the coating process. Too high lightness, in turn, requires slower coating, which has a negative impact on the economics of production. In general, prepolymers with low viscosities of 1000 to 4000 mPa·s are used in the production of removable self-adhesive materials. Photoreactive prepolymers with viscosities of 8,000 to 16,000 mPa·s are used in the production of classic self-adhesive materials, such as transfer adhesive tapes.
3.4. Free monomers concentration in synthesized acrylic PSA
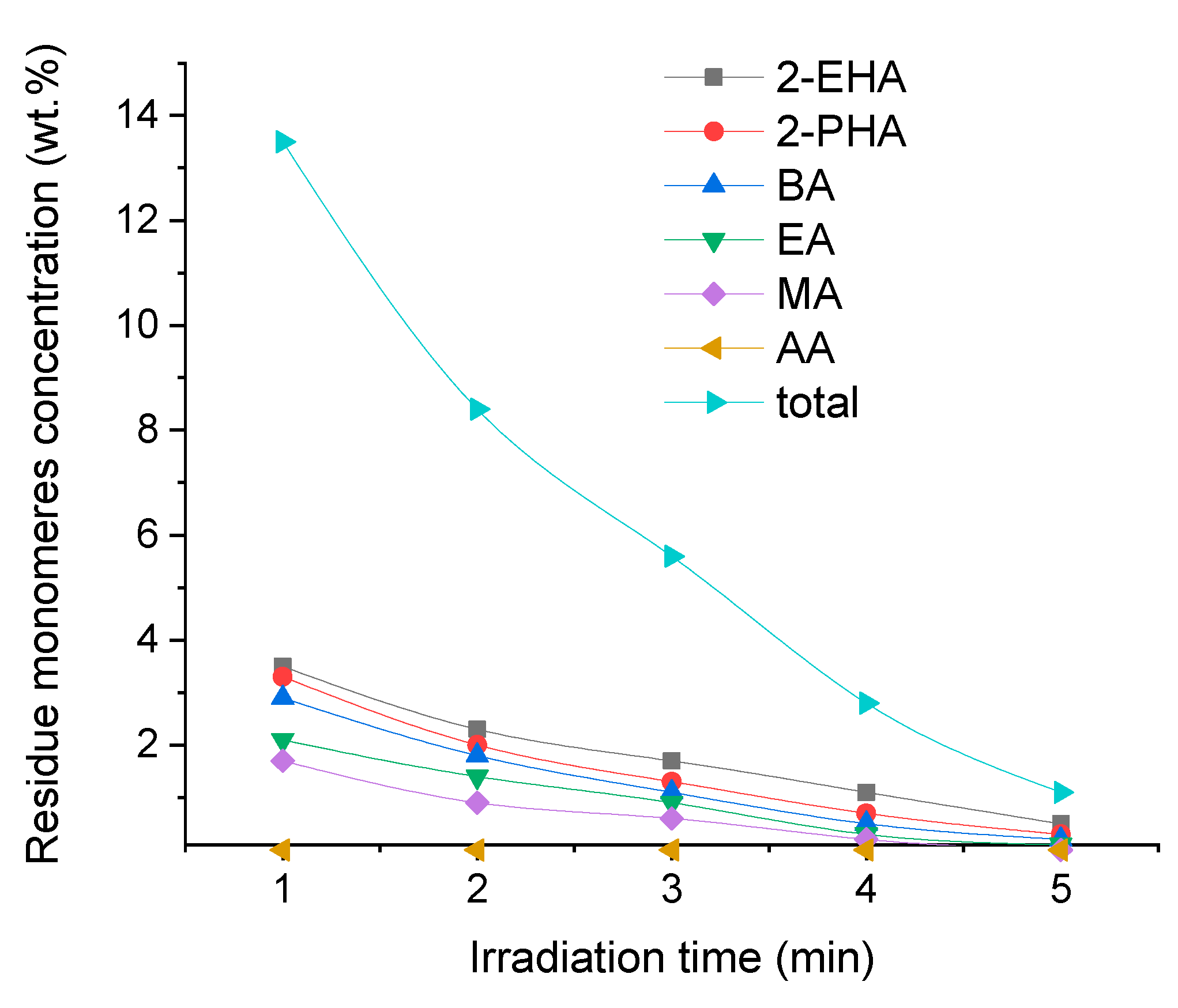
The concentration of all residue monomers in synthesized solvent-free prepolymer acrylic PSA was relatively high and it was 13.5 wt.% (
Figure 5) and exact concentration values of used acrylate monomers corresponded with their reactivity, understood as the ability to radically polymerization with other monomers. And so, as can be seen in
Figure 5, 2-ethylhexyl acrylate (2-EHA) and 2-propylheptyl acrylate (2-PHA) are characterized by the lowest reactivity. As the alkyl substituent decreases, the reactivity of acryl alkylates increases. Thus, methyl acrylate is more reactive than ethyl acrylate, which in turn is more reactive than butyl acrylate. The most reactive monomer is acrylic acid, the concentration of which as unreacted monomer is 0 wt.%. Acrylic acid with extremely high reactivity was not present in synthesized PSA.
3.5. UV-initiated crosslinking of photoreactive prepolymers
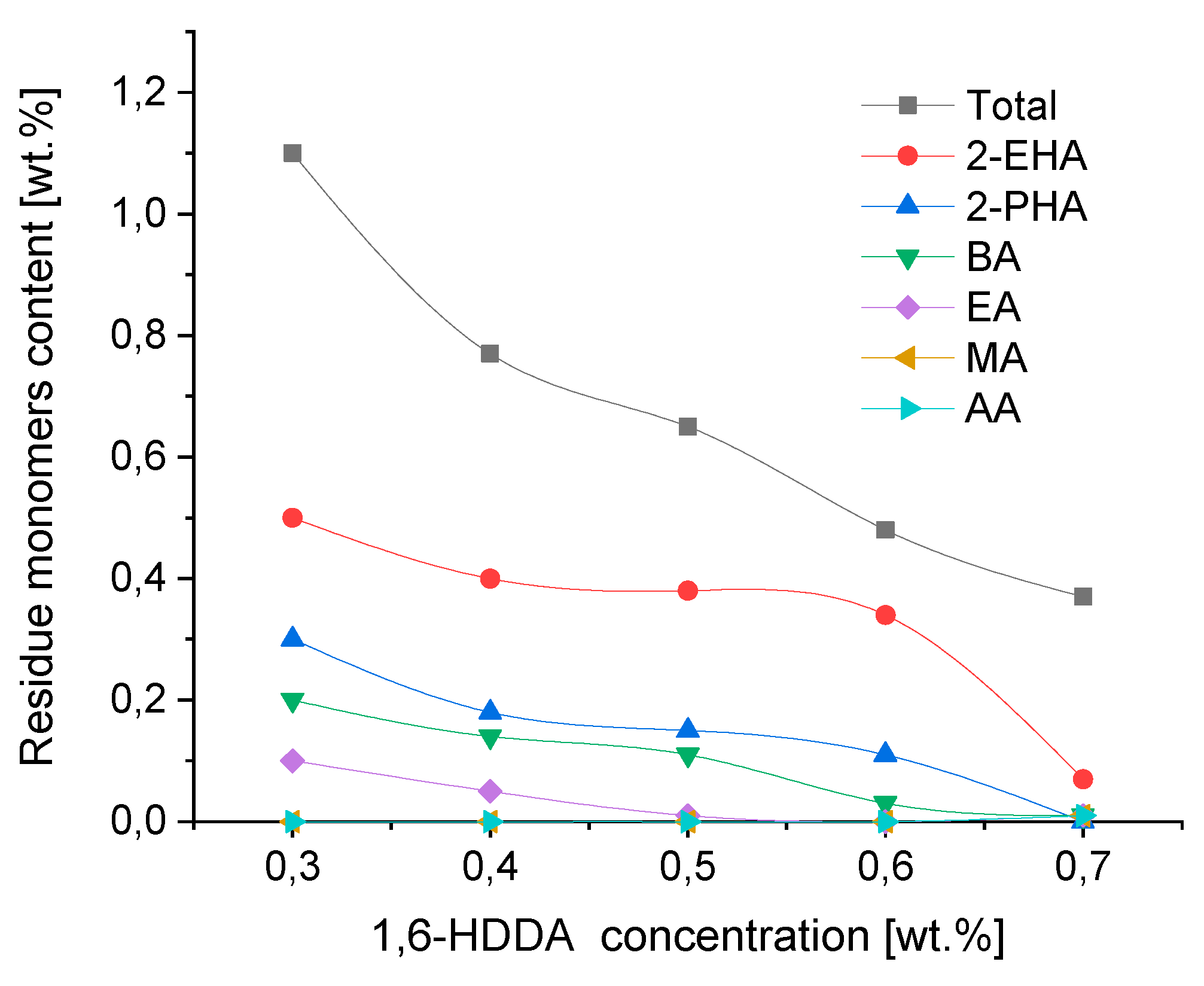
Figure 6 shows the effect of the concentration of the difunctional monomer 1,6-hexanediol diacrylate used as a photoreactive crosslinking compound in concentration between 0.3 and 0.7 wt.% on the residue of unreacted monomers in the photo-crosslinking process of acrylic adhesive films with a thickness of 100 g/m² (ca. 100 µ), in this particular case of photo-crosslinking transfer adhesive films 5 minutes under a UV lamp.
The use of photoreactive cross-linking compounds in the form of multifunctional acrylates, in this case 1,6-hexanediol diacrylate (1,6-HDDA), is intended to obtain the appropriate internal strength (cohesion) of self-adhesive layers cross-linked under a UV lamp. During the increase in the cohesion of the UV-crosslinked adhesive films, there is also an increase in the conversion of the acrylate monomers used, and thus a decrease in the content of unreacted monomers. As shown in
Figure 6, the increase in the concentration of the difunctional monomer 1,6-HDDA positively affects the reduction of residues monomers used in the polymerization process of the photoreactive prepolymer. A further increase in the concentration of 1,6-HDDA, due to the increased degree of cross-linking of the adhesive film, leads to a decrease in its flexibility, which in turn leads to the deterioration of its self-adhesive properties, such as tack and peel adhesion.
3.6. UV-initiated crosslinking according to crosslinking time
The duration of UV curing of the photoreactive prepolymer used for the production of transfer adhesive films is an important parameter influencing their final applicable properties. Too short cross-linking time under the UV lamp causes non-reacting of all, or almost all, of the monomers used for the production to produce of transfer adhesive films. Remains of unreacted monomers, apart from an unpleasant odor, may negatively affect the properties of adhesive adhesives, such as tack, peel adhesion or shear strength and shrinkage.
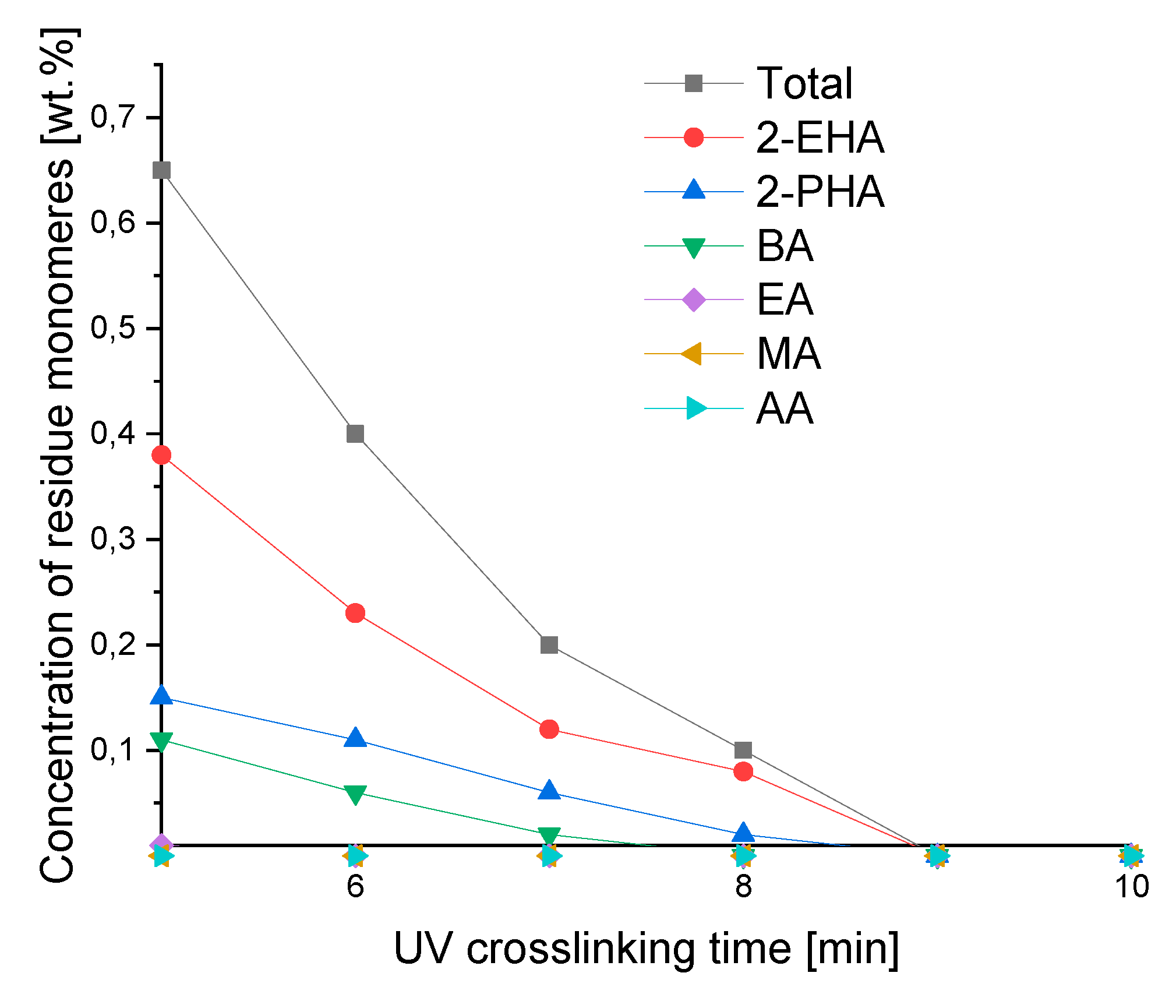
As shown in
Figure 7, the cross-linking time of adhesive films is important for reducing the concentration of classic monomers and, as in the case of more reactive monomers, for their complete conversion. The more reactive ones include acrylic acid (AA) and methyl acrylate (MA), where after 5 minutes of UV crosslinking time the adhesive layer does not contain them. After 6 minutes of exposure to the UV lamp, no ethyl acrylate (EA) is observed, and after 8 minutes of butyl acrylate (BA). After 9 minutes, unreacted 2-ethylhexyl acrylate (2-EHA) and 2-propylheptyl acrylate (2-PHA) disappear. The cross-linking time of 10 minutes under a UV lamp allows to obtain, free of unreacted monomers, a high-quality self-adhesive transfer tape.
3.7. Influence of residue monomers on tack, peel adhesion, shear strength and shrinkage
The goal of this part of publication was conducted the experiment was to find the influence of residue unreacted acrylic monomers in transfer self-adhesive tapes based on photoreactive acrylic PSA on their important properties, such as tack, peel adhesion, shear strength and shrinkage. The details of the tests and the obtained results of tack, peel adhesion, cohesion and shrinkage on a wooden substrate obtained from the German company Rocholl are presented in
Figure 8,
Figure 9,
Figure 10 and
Figure 11, tack (
Figure 8), peel adhesion (
Figure 9), shear strength (
Figure 10), respectively and shrinkage (
Figure 11).
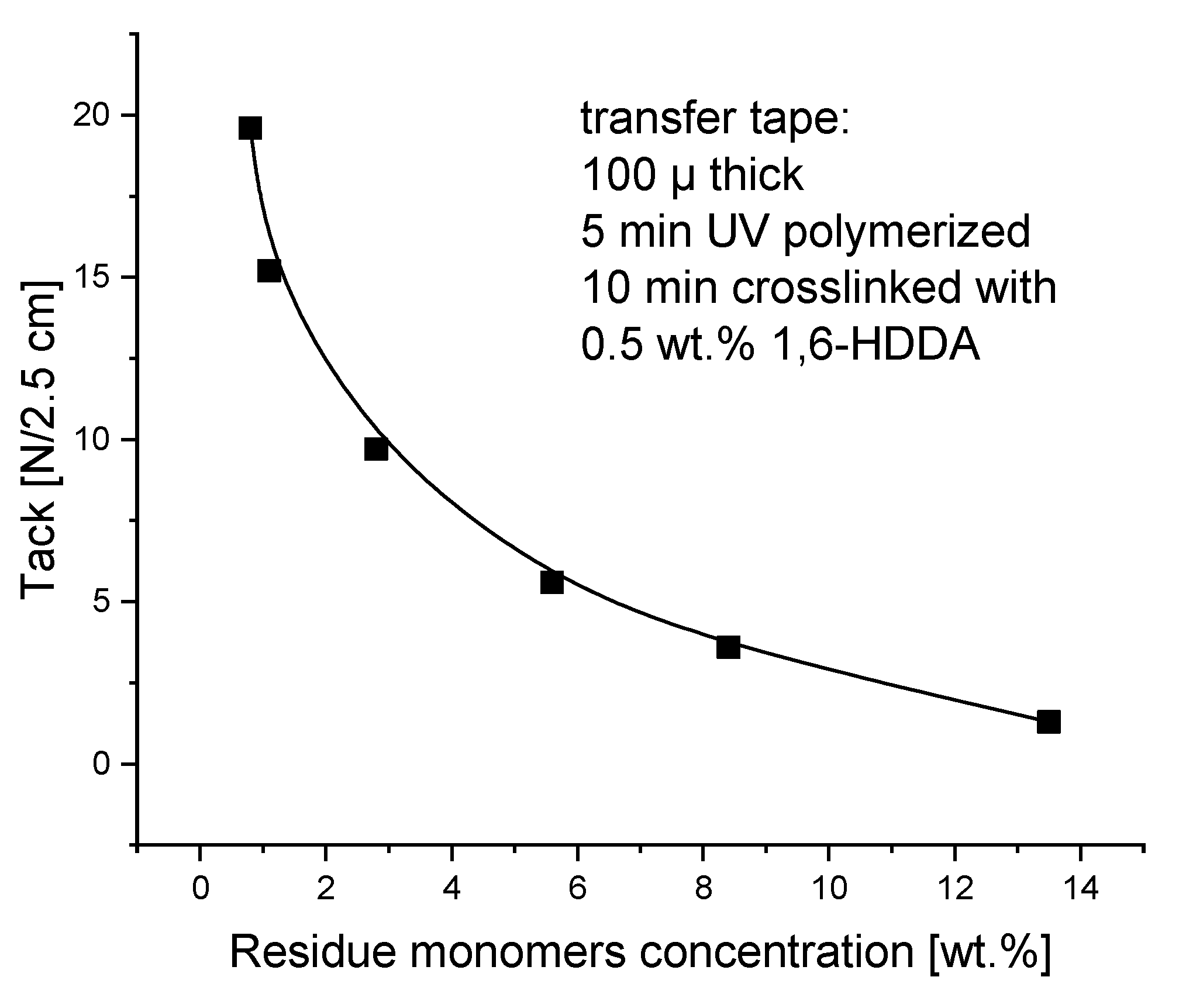
Predictably, the presence of free unreacted acrylate monomers adversely affects the transfer tack of adhesive tapes to the wood surface. Of course, this unfavorable tendency depends on the concentration of residue monomers and is intensified by the increase in the concentration of unreacted monomers. The more unreacted monomers there are, the smaller the tack, also called initial adhesion. The increased content of unreacted monomers migrates faster than the smaller content of unreacted monomers to the surface of the wood, which logically justifies the reduction of tack. As shown in
Figure 8, the highest tack value of 20 N was achieved with transfer adhesive tapes containing approximately 1 wt.% residue monomers. The content of residue monomers above 2-3 wt.% has a very negative effect on the tack and is unacceptable in the technology of joining wooden elements.
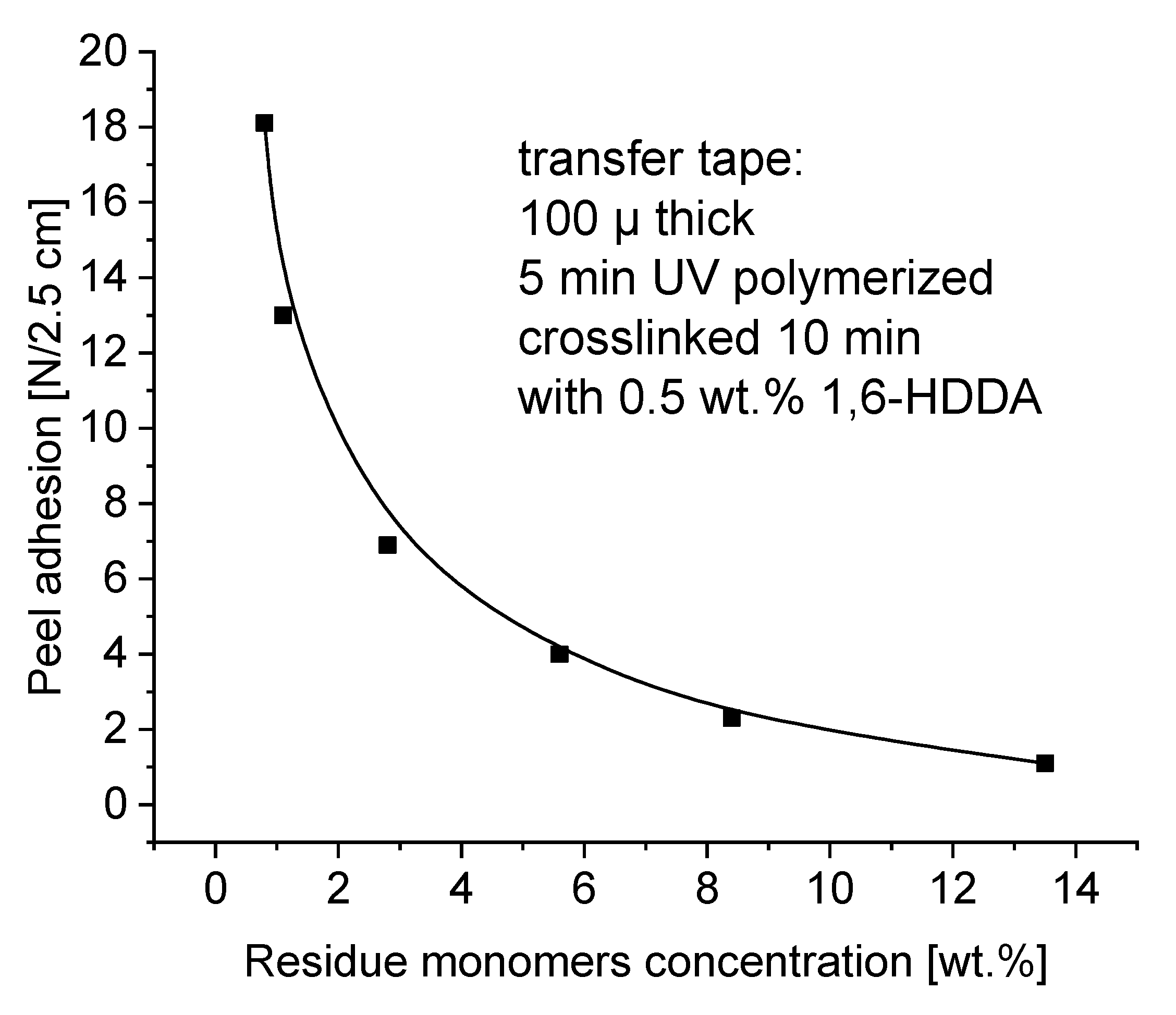
As in the case of tack, the presence of residue monomers in transfer adhesive tapes adversely affects adhesion, which is referred to as peel adhesion (
Figure 9). As in the case of tack, the presence of residue monomers in transfer adhesive tapes adversely affects adhesion, which is referred to as peel adhesion. Adhesion, measured as peel adhesion, depends on the contact time of the adhesive with the wooden substrate. In application conditions, the contact time of the transfer adhesive tape with the substrate to be glued very often does not exceed a few minutes. For this reason, the peel adhesion test was performed after 2 minutes.
As in tack testing, the measured values of peel adhesion decrease due to the presence of unreacted free residues. Although the measured values of adhesion are generally higher than the tack value, we also observe a negative impact of residue monomers on its value here. Remains of unreacted acrylate monomers migrate from the inside of the adhesive film, thus worsening the adhesion of the transfer adhesive tape to the wooden substrate. As in the case of tack, peel adhesion on wood substrate requires the use of adhesive tapes with less than 2 wt.% residue monomers (
Figure 9).
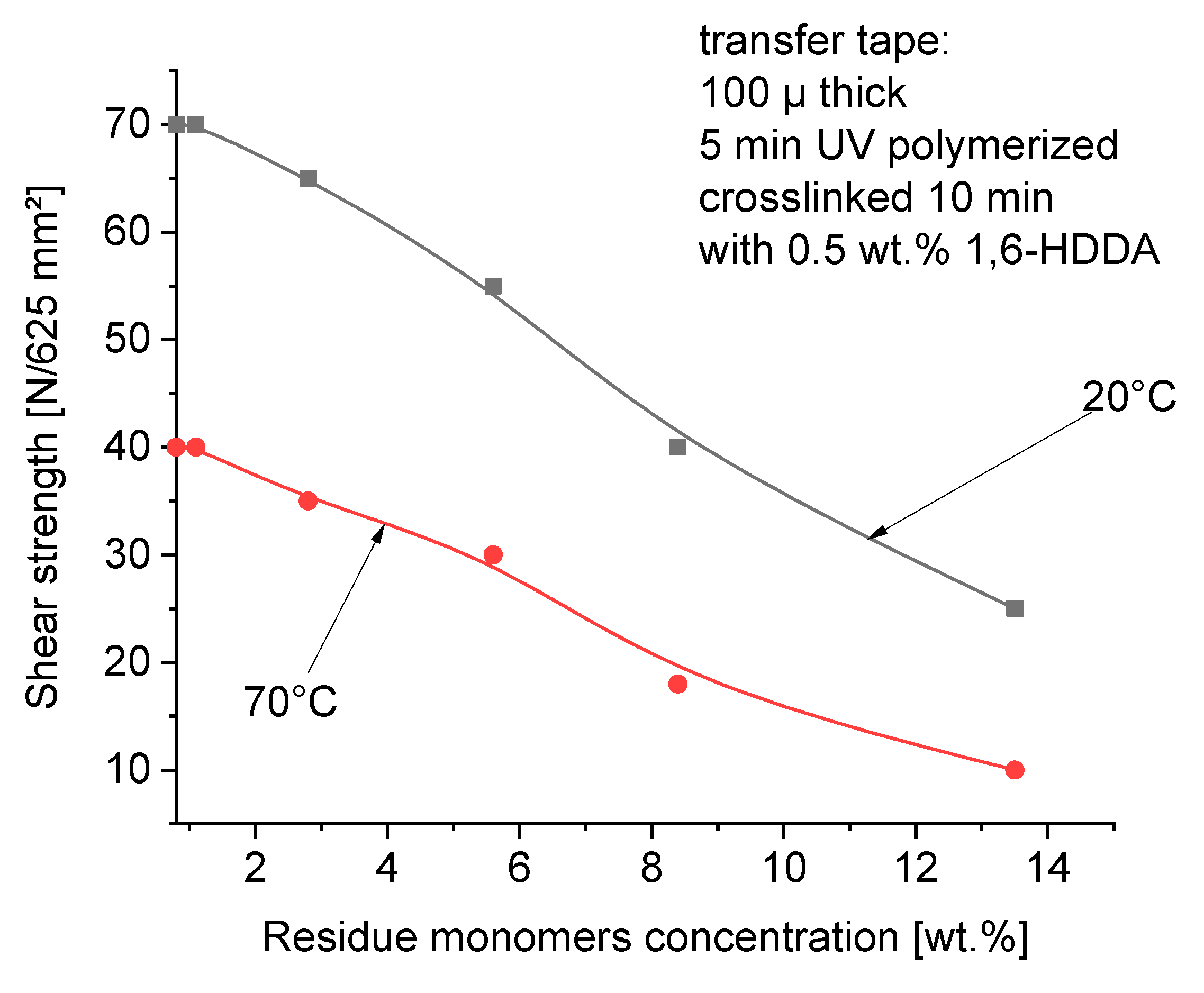
Typical shear strength resistance testing is performed with a controlled area of adhesive tape applied to a standard test surface, in this case wood surface. Because shear failure is the inability of the pressure-sensitive adhesive to resist contentious stress, any task that is a measure of stress relaxation within the adhesives gives meaningful data. A high shear resistant adhesive will maintain the stress, while a poor shear resistant adhesive will relieve the stress quite rapidly.
Figure 10 presents the shear strength of acrylic self-adhesive layers dependent on the free monomer’s concentration in the polymer layers.
The content of free unreacted acrylate monomers in self-adhesive tapes reduces the internal strength of the adhesive joint (cohesion), measured both at 20°C and 70°C. The cohesion values measured at 20°C are obviously higher than at 70°C. At 70°C, the migration of residue monomers from the adhesive layer of the adhesive tape to the surface of the joined wooden elements is higher than at room temperature, therefore the cohesion at 70°C is lower than at 20°C. Here, as in the case of tack and peel adhesion, the content of residue monomers in the range of 2-3 wt.% ensures even high strength of the bonded adhesive joint (
Figure 10).
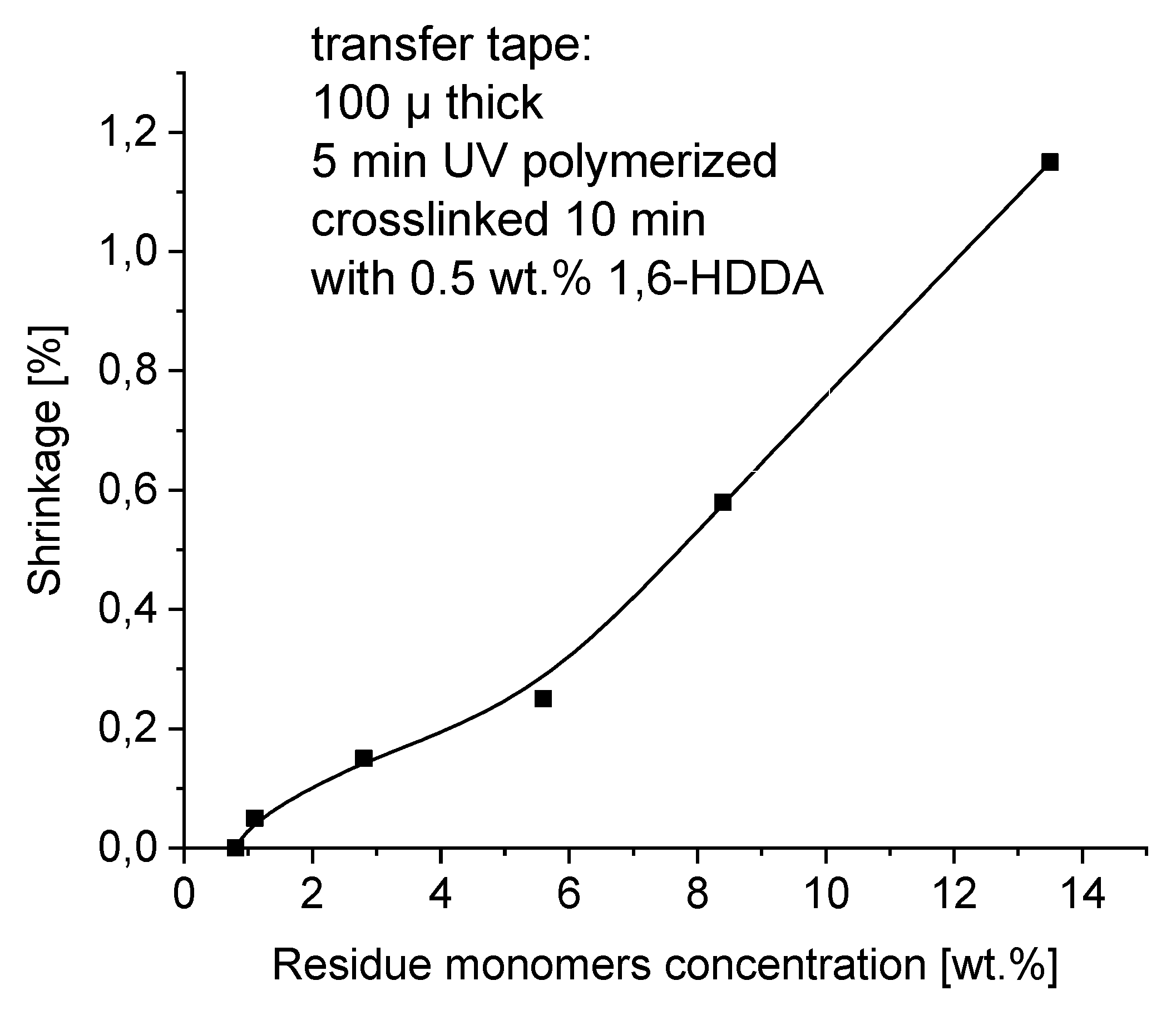
Shrinkage of transfer adhesive tapes is an extremely important parameter when bonding substrates with different coefficients of thermal expansion and different surface energies. It depends on many factors, in this particular case on the amount of double bonds in the non-crosslinked and then in the crosslinked polymer in the form of an adhesive tape. Shrinkage is also affected by unreacted monomer residues in the polymer after UV cross-linking.
Although acrylic polymers have been used successfully as pressure-sensitive adhesives in a variety of industries, a property inherent to all acrylic PSAs, which negatively impacts adhesion performance is shrinkage on different surfaces, for example wood, upon cross-linking. The shrinkage profiles of solvent-free acrylic PSA dependence on free monomers content are shown in
Figure 11. The best shrinkage results under 0.05 - 0.2 % were achieved for self-adhesive layers containing not more than about 2 - 3 wt.% of free monomers. The shrinkage of PSA greater than 0.3 % is completely unacceptable.
4. Conclusions
Summing up, from the performed evaluation of the experiments referring to tack, peel adhesion, shear strength and shrinkage of photoreactive solvent-free acrylic pressure-sensitive adhesives, by testing self-adhesive acrylic layers containing various concentration of residue unreacted free acrylate monomers discussed in this article, it can be concluded that the best results regarding investigated properties were obtained for carrier-free films characterized by coat weight of 100g/m² (100 µ) containing not more than 2-3 wt.% of residue monomers. When carrying out the synthesis of solvent-free photoreactive self-adhesive adhesives based on acrylates, it is not possible to obtain transfer (carrier-free) self-adhesive tapes free of unreacted monomers as the final product. The amount of unreacted monomer residues in the amount of about 13.5 wt.% corresponds to their reactivity, which is also dependent on other monomers present in the mixture. In general, the reactivity of acrylate monomers decreases with an increase in their alkyl part, and thus increases with a decrease in their alkyl part. This is confirmed by GC analyzes of mixtures of unreacted monomers, where 2-ethylhexyl acrylate, 2-propylheptyl acrylate and butyl acrylate predominate. As for other acrylates used in research, such as ethyl acrylate and methyl acrylate, their concentration is relatively low. The lack of acrylic acid in the mixture of unreacted monomers indicates its high reactivity, and thus complete conversion. The relatively high concentration of unreacted monomers in the self-adhesive photoreactive prepolymer results from the method of conducting bulk polymerization without the participation of a solvent. A few minutes are enough to obtain a photoreactive prepolymer with a specific viscosity that allows it to be coated. During this time, a prepolymer with a viscosity of about 2,000 to 14,000 mPas is formed, which, due to the very short polymerization time, contains a relatively large concentration of unreacted monomers, often exceeding 10% by weight. The classic acrylic polymer pressure-sensitive adhesives synthesized in a solvent within 2-6 h contains up to 2-4 wt. unreacted monomers. The content of residue acrylic monomers can be reduced by increasing the time of irradiation of the monomer mixture with UV radiation, but only up to a certain point, when the increase in the viscosity of the obtained prepolymers made it possible to coat it and obtain self-adhesive tapes. Residual monomers are also reduced during cross-linking of the prepolymer to the polymer with a multifunctional, in this particular case difunctional, 1,6-hexanediol diacrylate (1,6-HDDA). Increasing the concentration of the photoreactive cross-linking compound increases the conversion of the polymers used in the polymerization, and thus reduces the content of unreacted acrylate monomers. An additional possibility of reducing the concentration of residue monomers gives us an increase in the cross-linking time under the UV lamp. After 6 minutes of crosslinking of the photoreactive prepolymer containing the photoreactive crosslinker, transfer adhesive tapes containing unreacted acrylate monomers with a maximum concentration of about 0.4 wt% are obtained. Further cross-linking within 7-8 minutes allows for the reduction of the residue monomers to 01-0.2 wt.%, And after 10 minutes, the transfer adhesive tapes are obtained with the concentration of unreacted monomers below 0.1 wt.%. When examining the influence of residue monomers within 1-3 wt.% On tack (
Figure 8), peel adhesion (
Figure 9), shear strength at 20°C and 70°C (
Figure 10) and on shrinkage (
Figure 11), it can be noticed that that the limitation of residue monomers to about 1 wt.% does not have any significant effect on the application properties of transfer adhesive tapes used in the furniture industry. From the experimental results by application of prepared carrier-free PSA tapes, the conclusion can be inferred that the use acrylic PSA with higher than 2-3 wt.% free monomers negatively influence all evaluated properties of solvent-based acrylic PSA, especially the cohesion tested at 20°C and 70°C. The combination of synthesis of acrylic PSA containing less than 1 wt.% of free monomers, allows excellent tack, peel adhesion, shear strength and shrinkage performance to be reached.
Author Contributions
Conceptualization, Z.C.; methodology, Z.C., M.B.; software, M.B.; validation, Z.C., M.B., T.K.; formal analysis, Z.C.; investigation, M.B.; resources, T.K.; data curation, Z.C.; writing—original draft preparation, Z.C.; writing—review and editing, Z.C., M.B., T.K.; visualization, M.B.; supervision, Z.C.; project administration, Z.C.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this cannot be shared at this time as this data forms part of an ongoing study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elsmore, M.T.; Atkinson, R.L.; Irvine, D.J.; Howdle, S.M.; De Focatiis, D.S.A. Sustainable terpene triblock copolymers with tuneable properties for pressure sensitive adhesive applications, Polymer Testing 2022, 109, 107530, 1-12. [CrossRef]
- Taghizadeh, S.M.; Ghasemi, D. Synthesis and optimization of a four-component acrylic-based copolymer as pressure sensitive adhesive, Iranian Polymer Journal 2010, 19, 343–352 http://journal.ippi.ac.ir.
- Diethert, A.; Ecker, K.; Peykova, Y.; Willenbacher, N.; Müller-Buschbaum, P. Tailoring the near-surface composition profiles of pressure-sensitive adhesive films and the resulting mechanical properties. ACS applied materials & interfaces 2011, 3, 2012–2021. [Google Scholar]
- Lee, J.-H.; Shim, G.-S.; Park, J.-W.; Kim, H.-J.; Kim, Y. Adhesion performance and recovery of acrylic pressure-sensitive adhesives thermally crosslinked with styrene–isoprene–styrene elastomer blends for flexible display applications. " Journal of Industrial and Engineering Chemistry 2019, 78, 461–467. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shim, G.-S.; Kim, H.-J.; Kim, Y. Adhesion Performance and Recovery of Acrylic PSA with Acrylic Elastomer (AE) Blends via Thermal Crosslinking for Application in Flexible Displays. Polymers 2019, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Karnal, P.; Jha, A.; Wen, H.; Gryska, S.; Barrios, C.; Frechette, J. Contribution of Surface Energy to pH-Dependent Underwater Adhesion of an Acrylic Pressure-Sensitive Adhesive. Langmuir 2019, 35, 5151–5161. [Google Scholar] [CrossRef]
- Márquez, I.; Paredes, N.; Alarcia, F.; Velasco, J.I. Adhesive Performance of Acrylic Pressure-Sensitive Adhesives from Different Preparation Processes. Polymers 2021, 13, 2627. [Google Scholar] [CrossRef]
- Márquez, I.; Paredes, N.; Alarcia, F.; Velasco, JI. Influence of Acrylonitrile Content on the Adhesive Properties of Water-Based Acrylic Pressure-Sensitive Adhesives. " Polymers 2022, 14, 909. [Google Scholar] [CrossRef]
- Baraghoosh, M.; Zohuri, G.H.; Behzadpour, M.; Gholami, M.; Hosseinpour, P.; Arabi, S.M. . Effect of Different Tackifiers on Emulsion-Based Pressure-Sensitive Adhesive (PSA). Progress in Color, Colorants and Coatings 2022, 15, 295–303. [Google Scholar]
- Casas-Soto, C.R.; Conejo-Dávila, A.S.; Osuna, V.; Chávez-Flores, D.; Espinoza-Hicks, J.C.; Flores-Gallardo, S.G.; Vega-Rios, A. Dibutyl itaconate and lauryl methacrylate copolymers by emulsion polymerization for development of sustainable pressure-sensitive adhesives. Polymers 2022, 14, 632. [Google Scholar] [CrossRef]
- Goulding, T.M. Pressure-sensitive adhesives. Handbook of adhesive technology 1 ( 1994.
- Khanjani, J. Pressure-Sensitive Adhesive Joints, In Adhesives and Adhesive Joints in Industry Applications. Edited by Anna Rudawska, 2019, pp. 148. [CrossRef]
- Back, J.-H.; Kwon, Y.; Roldao, J.C.; Yu, Y.; Kim, H.-J.; Gierschner, J.; Lee, W.; Kwon, M.S. Synthesis of solvent-free acrylic pressure-sensitive adhesives via visible-light-driven photocatalytic radical polymerization without additives. Green Chemistry 2020, 22, 8289–8297. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martín-Martínez, J.M. Viscoelastic and Adhesion Properties of New Poly(Ether-Urethane) Pressure-Sensitive Adhesives. Front. Mech. Eng. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, D.Y.; Yoon, S.H.; Kim, S.H.; Han, M.S.; Jeon, S.; Kim, Y.; Lim, Y.K.; Hwang, D.-H.; Jung, S.-H.; Lim, B. Adhesion Improvement of Solvent-Free Pressure-Sensitive Adhesives by Semi-IPN Using Polyurethanes and Acrylic Polymers. Polymers 2022, 14, 3963. [Google Scholar] [CrossRef]
- Pandey, V.; Fleury, A.; Villey, R.; Creton, C.; Ciccotti, M. Linking peel and tack performances of pressure sensitive adhesives. Soft Matter, 2020, 16, 3267–3275. [Google Scholar] [CrossRef]
- Seok; W.C.; Leem, J.T.; Song, H.J. Acrylic pressure-sensitive adhesives based on ethylene glycol acrylate for flexible display application: Highly elastic and recoverable properties, Polymer Testing 2022, 108, 1–12.
- https://doi.org/10.1016/j.polymertesting.2022.107491. [CrossRef]
- Czech, Z.; Witczak, M.; Kowalska, J. The influence of residue monomers on selected properties of acrylic pressure-sensitive adhesives. Drewno 2012, 188, 59–70. [Google Scholar]
- Barrios, C.A. Pressure Sensitive Adhesive Tape: A Versatile Material Platform for Optical Sensors. Sensors 2020, 20, 5303. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Gziut, K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2020, 13, 5661. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Kraśkiewicz, A. The Effect of Type-I Photoinitiators on the Kinetics of the UV-Induced Cotelomerization Process of Acrylate Monomers and Properties of Obtained Pressure-Sensitive Adhesives. " Materials 2021, 14, 4563. [Google Scholar] [CrossRef]
- Rudawska, A.; Wahab, M.A.. Mechanical Properties of Adhesive Joints Made with Pressure-Sensitive Adhesives. Strojniški vestnik - Journal of Mechanical Engineering 2021, 67, 380–388. [CrossRef]
- Sun, S.; Li, M.; Liu, A.; A review on mechanical properties of pressure sensitive adhesives, International Journal of Adhesion and Adhesives 2013, 41, 98–106. [CrossRef]
- Sancho-Querol, S.; Yáñez-Pacios, A.J.; Martín-Martínez, J.M. New binary blends of ethylene-co-n-butyl acrylate (EBA) copolymer and low molecular weight rosin ester resin with potential as pressure sensitive adhesives. Materials 2018, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- Fuensanta, M.; Khoshnood, A.; Rodríguez-Llansola, F.; Martín-Martínez, J.M. New Waterborne Polyurethane-Urea Synthesized with Ether-Carbonate Copolymer and Amino-Alcohol Chain Extenders with Tailored Pressure-Sensitive Adhesion Properties. Materials 2020, 13, 627. [Google Scholar] [CrossRef]
- Takahashi, K.; Yanai, F.; Inaba, K.; Kishimoto, K.; Kozone, Y.; Sugizaki, T. Sticking Effect of a Tackifier on the Fibrillation of Acrylic Pressure-Sensitive Adhesives. Langmuir 2021, 37, 11457–11464. [Google Scholar] [CrossRef]
- Bakar, R.A.; Li, Y.; Hewitson, O.P.; Roth, P.J.; Keddie, J.L. Azide Photochemistry in Acrylic Copolymers for Ultraviolet Cross-Linkable Pressure-Sensitive Adhesives: Optimization, Debonding-on-Demand, and Chemical Modification. ACS Applied Materials & Interfaces 2022, 14, 30216–30227. [Google Scholar] [CrossRef]
- Back, J.-H.; Kwon, Y.; Kim, H.-J.; Yu, Y.; Lee, W.; Kwon, M.S. Visible-Light-Curable Solvent-Free Acrylic Pressure-Sensitive Adhesives via Photoredox-Mediated Radical Polymerization. Molecules 2021, 26, 385. [Google Scholar] [CrossRef]
- Antosik, A.K.; Bartkowiak, M.; Czech, Z. Pressure-sensitive adhesives based on acrylics. Wydawnictwo Uczelniane Zachodniopomorskiego Uniwersytetu Technologicznego w Szczecinie. Szczecin, 2022, 1-67. https://hdl.handle.net/20.500.12539/1548.
- Benedek, I.; Feldstein, M. M.. Technology of Pressure-Sensitive Adhesives and Products. Taylor & Francis Group, LLC, USA, 2009, pp. 568. [CrossRef]
- Creton, C. Pressure-Sensitive Adhesives: An Introductory Course. MRS Bulletin 2003, 28, 434–439. [Google Scholar] [CrossRef]
- Czech, Z.; Wesołowska, M. Development of solvent-free acrylic pressure-sensitive adhesives, European Polymer Journal 2007, 43, 3604–3612. [CrossRef]
- Ortega-Iguña, M.; Chludzinski, M.; Sánchez-Amaya, J.M. Comparative Mechanical Study of Pressure Sensitive Adhesives over Aluminium Substrates for Industrial Applications. Polymers 2022, 14, 4783. [Google Scholar] [CrossRef]
- Czech, Z.; Wesołowska, M. Development of solvent-free acrylic pressure-sensitive adhesives. European Polymer Journal 2007, 43, 3604–3612. [Google Scholar] [CrossRef]
- Shim, G.-S.; Kim, H.-J.; Bartkowiak, M. Curing behavior and impact of crosslinking agent variation in stepwise UV/UV cured acrylic pressure-sensitive adhesives, Journal of Materials Research and Technology 2021, 15, 1622–1629. [CrossRef]
- Zhu, M.; Cao, Z.; Haijun, Z.; Yijun, X., Li, G.; Nongyue, W.; Yingchun, L.; Lianqi, H..; Xiongwei, Q. Preparation of environmentally friendly acrylic pressure-sensitive adhesives by bulk photopolymerization and their performance. RSC Advances, 2020, 10, 10277–10284. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).