Submitted:
03 July 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodologies
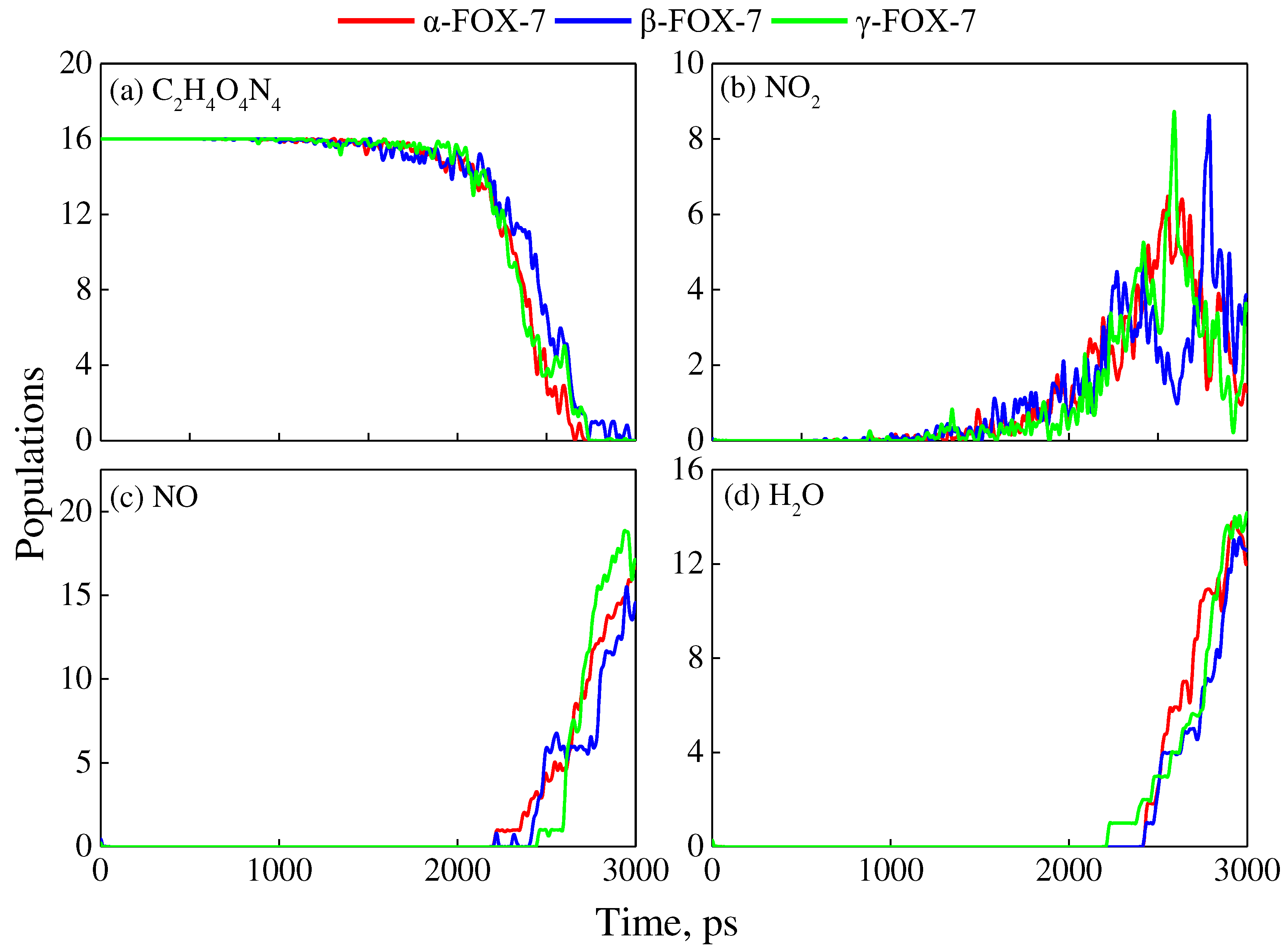
3. Resulds and Discussion
4. Conculsions
Notes
Acknowledgment
References
- Bu, R.; Li, H.; Zhang, C. Polymorphic Transition in Traditional Energetic Materials: Influencing Factors and Effects on Structure, Property, and Performance. Crystal Growth & Design 2020, 20, 3561–3576. [Google Scholar]
- Bemm, U.; Ostmark, H. 1,1-Diamino-2,2-Dinitroethylene: A Novel Energetic Material with Infinite Layers in Two Dimensions. Acta Cryst. 1998, C54, 1997–1999. [Google Scholar] [CrossRef]
- Evers, J.; Klapotke, T.M.; Mayer, P.; Oehlinger, G.; Welch, J. α- and β-Fox-7, Polymorphs of a High Energy Density Material, Studied by X-Ray Single Crystal and Powder Investigations in the Temperature Range from 200 to 423 K. Inorg. Chem. 2006, 45, 4996–5007. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.-J.; Evers, J.; Göbel, M.; Klapötke, T.M.; Mayer, P.; Oehlinger, G.; Welch, J.M. γ-Fox-7: Structure of a High Energy Density Material Immediately Prior to Decomposition. Propellants, Explos. Pyrotech. 2007, 32, 478–495. [Google Scholar] [CrossRef]
- Chemagina, I.V.; Filin, V.P.; Loboiko, B.G.; Kazakova, M.B.; Shaktorin, Y.A.; Lagutina, V.M.; Taibinov, N.P.; Garmasheva, N.V.; Alekseev, A.V. Investigation of Diaminodinitroethylene (Dadne) Thermal Decomposition. In ZABABAKHIN SCIENTIFIC TALKS - 2005: International Conference on High Energy Density Physics 2005; Vol. 849, pp 174-178.
- Hu, A.; Larade, B.; Abou-Rachid, H.; Lussier, L.-S.; Guo, H. A First Principles Density Functional Study of Crystalline FOX-7 Chemical Decomposition Process under External Pressure. Propellants Explos. Pyrotech. 2006, 31, 355–360. [Google Scholar] [CrossRef]
- Latypov, N.V.; Bergman, J.; Langlet, A.; Wellmar, U.; Bemm, U. Synthesis and Reactions of 1,1-Diamino-2,2-Dinitroethylene. Tetrahedron 2010, 30. [Google Scholar] [CrossRef]
- Bu, R.; Xie, W.; Zhang, C. Heat-Induced Polymorphic Transformation Facilitating the Low Impact-Sensitivity of 2,2-Dinitroethylene-1,1-Diamine (FOX-7). J. Phys. Chem. C 2019, 123, 16014–16022. [Google Scholar] [CrossRef]
- Guangrui, L.; Rui-Jun, G.; Hongzhen, L.; Chaoyang, Z. Polymorphism of energetic materials: a comprehensive study of molecular conformers, crystal packing and the dominance of their energetics in governing the most stable polymorph. Crystal Growth & Design 2018, 18, 4174–4186. [Google Scholar]
- Pravica, M.; Galley, M.; Park, C.; Ruiz, H.; Wojno, J. A High Pressure, High Temperature Study of 1,1-Diamino-2,2-Dinitro Ethylene. High Pressure Research 2011, 31, 80–85. [Google Scholar] [CrossRef]
- Klerk, W.P.C. d.; Popescu, C.; Heijden, A.E.D.M.v.d. Study on the Decomposition Kinetics of FOX-7 and HNF. J. Therm. Anal. Cal. 2003, 72, 955–966. [Google Scholar] [CrossRef]
- GindulytėLou, A.; Huang, M.; Karle, J. Proposed Mechanism of 1,1-Diamino-Dinitroethylene Decomposition: A Density Functional Theory Study. J. Phys. Chem. A 1999, 103, 11045–11051. [Google Scholar] [CrossRef]
- Booth, R.S.; Butler, L.J. Thermal Decomposition Pathways for 1,1-Diamino-2,2-Dinitroethene (FOX-7). J Chem Phys 2014, 141, 134315. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Sun, H. Thermal Decomposition of FOX-7 Studied by Ab Initio Molecular Dynamics Simulations. Theor Chem Acc 2014, 133. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Z.; Bernstein, E.R. Initial Decomposition Mechanism for the Energy Release from Electronically Excited Energetic Materials: FOX-7 (1,1-Diamino-2,2-Dinitroethene, C2H4N4O4). J Chem Phys 2014, 140, 074708. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xu, J.; Zhao, J. First-Principles Studies on the Thermal Decomposition Behavior of FOX-7. High Pressure Research 2010, 30, 301–309. [Google Scholar] [CrossRef]
- Kimmel, A.V.; Sushko, P.V.; Shluger, A.L.; Kuklja, M.M. Effect of Charged and Excited States on the Decomposition of 1,1-Diamino-2,2-Dinitroethylene Molecules. J Chem Phys 2007, 126, 234711. [Google Scholar] [CrossRef]
- Jiang, H.; Jiao, Q.; Zhang, C. Early Events When Heating 1,1-Diamino-2,2-Dinitroethylene: Self-Consistent Charge Density-Functional Tight-Binding Molecular Dynamics Simulations. J. Phys. Chem. C 2018, 122, 15125–15132. [Google Scholar] [CrossRef]
- Kiselev, V.G.; Gritsan, N.P. Unexpected Primary Reactions for Thermolysis of 1,1-Diamino-2,2-Dinitroethylene (FOX-7) Revealed by Ab Initio Calculations. J Phys Chem A 2014, 118, 8002–8008. [Google Scholar] [CrossRef]
- Ostmark, H.; Langlet, A.; Bergman, H.; Wellmar, U.; Bemm, U. FOX-7──A New Explosive with Low Sensitivity and High Performance. Proceedings of the 11th International Detonation Symposium, Snowmass, CO, USA; Office of Naval Research: Arlington, VA, USA, 1998. [Google Scholar]
- Ostmark, H.; Bergman, H.; Bemm, U.; Goede, P.; Holmgren, E.; Johansson, M.; Langlet, A.; Latypov, N.V.; Pettersson, A.; Pettersson, M.-L.; Wingborg, N.; Vorde, C.; Stenmark, H.; Karlsson, L.; Hihkio, M. 2,2-Dinitro-ethene-1,1-diamine (FOX-7)──Properties, Analysis and Scale-up. 32th International Annual Conference of ICT, 2002. [Google Scholar]
- Wu, Q.; Zhu, W.; Xiao, H. First-Principles Study of the Three Polymorphs of Crystalline 1,1-Diamino-2,2-Dinitrotheylene. Bull. Korean Chem. Soc. 2013, 34, 2281–2285. [Google Scholar] [CrossRef]
- Liu, G.; Tian, B.; Wei, S.-H.; Zhang, C. Polymorph-Dependent Initial Thermal Decay Mechanism of Energetic Materials: A Case of 1,3,5,7-Tetranitro-1,3,5,7-Tetrazocane. J. Phys. Chem. C 2021, 125, 10057–10067. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Y.; Gou, R.; Zhang, C. Difference in the Thermal Stability of Polymorphic Organic Crystals: A Comparative Study of the Early Events of the Thermal Decay of 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-Hexaazaisowurtzitane (Cl-20) Polymorphs under the Volume Constraint Condition. J. Phys. Chem. C 2019, 123, 16565–16576. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Xue, X. Pressure and Polymorph Dependent Thermal Decomposition Mechanism of Molecular Materials: A Case of 1, 3, 5,-Trinitro-1, 3, 5,-Triazine. J. Phys. Chem. A 2022, 126, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Elstner, M. The SCC-DFTB Method and Its Application to Biological Systems. Theor Chem Acc 2005, 116, 316–325. [Google Scholar] [CrossRef]
- Porezag, D.; Frauenheim, T.; Köhler, S.; Seifert, G.; Kaschner, R. Köhler, S.G.; Kaschner, R. Construction of Tight-Binding-Like Potentials on the Basis of Density-Functional Theory: Application to Carbon. Physical review B 1995, 51, 12947–12957. [Google Scholar] [CrossRef]
- Aradi, B.; Hourahine, B.; Frauenheim, T. Dftb+, a Sparse Matrix-Based Implementation of the DFTB Method. J Phys Chem A 2007, 111, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Cheng, X.L.; Zhang, H. Reactive Molecular Dynamics Simulation of Solid Nitromethane Impact on (010) Surfaces Induced and Nonimpact Thermal Decomposition. J Phys Chem A 2012, 116, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
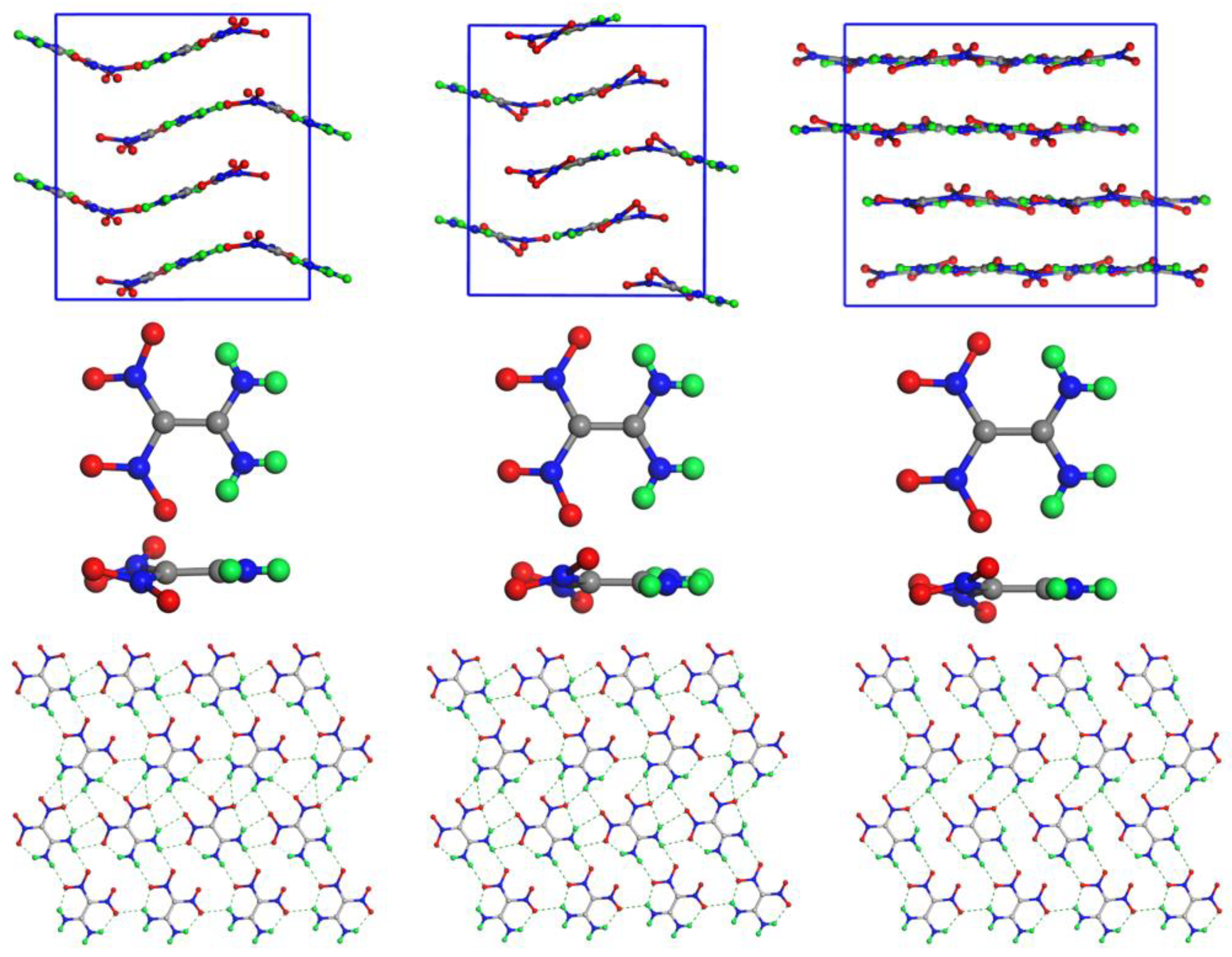





| Polymorphs | Time range (ps) | Frequency | Reaction |
|---|---|---|---|
 -FOX-7 -FOX-7 |
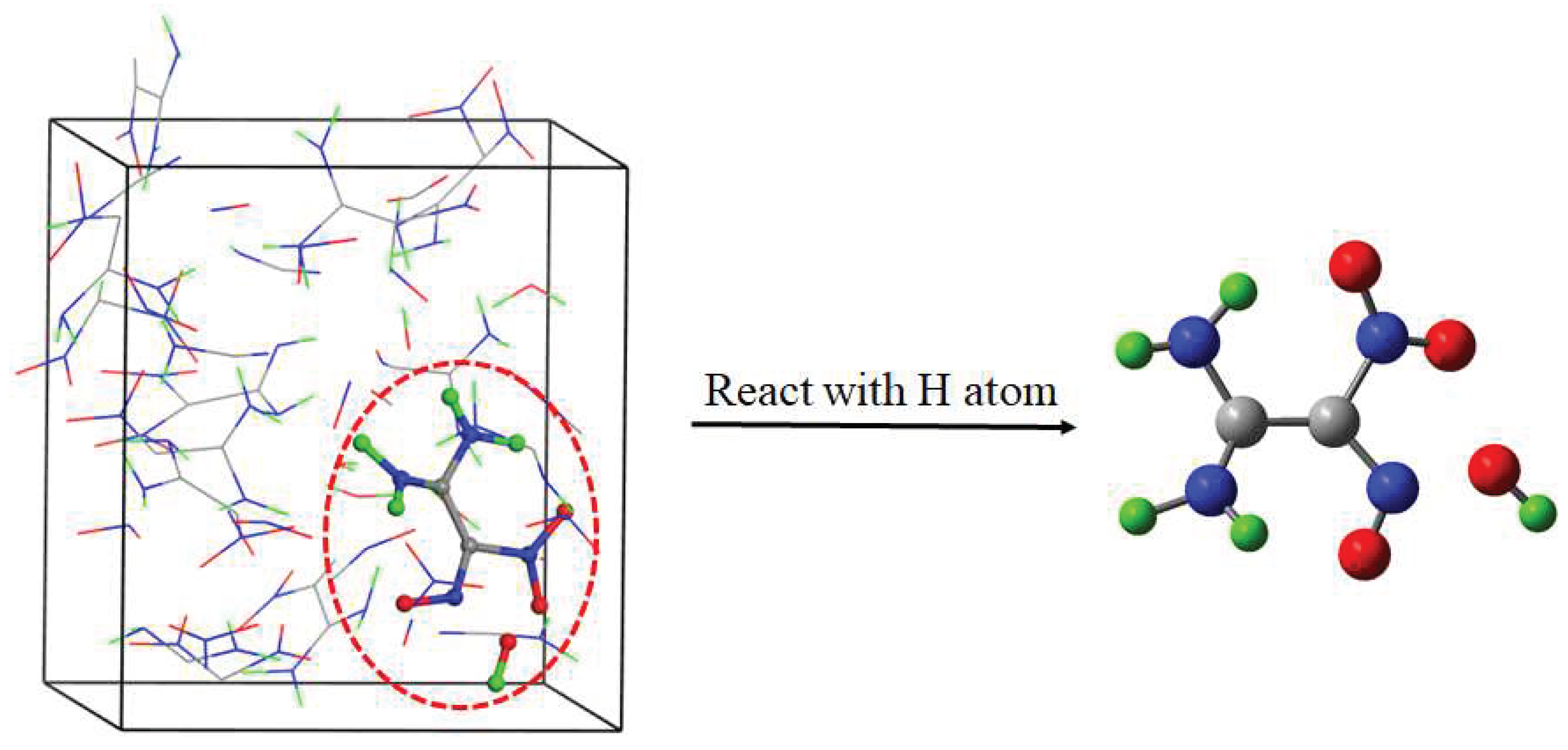
1.760-10.396 | 6 | C2H4O4N4 → C2H4O2N3 + NO2 (R1) |
| 3.502-14.734 | 9 | C2H4O4N4 + P → C2H3O4N4 + H[P] (R2) | |
| 7.684 | 1 | C2H4O4N4 + P → C2H4O3N4 + O[P] (R3) | |
 -FOX-7 -FOX-7 |
10.496-10.574 | 2 | C2H4O4N4 → C2H4O2N3 + NO2 |
| 5.548 | 1 | Intramolecular hydrogen transfer (R4) | |
| 2.590-14.042 | 9 | C2H4O4N4 + P → C2H3O4N4 + H[P] | |
| 2.714-10.354 | 3 | C2H4O4N4 + P → C2H4O3N4 + O[P] | |
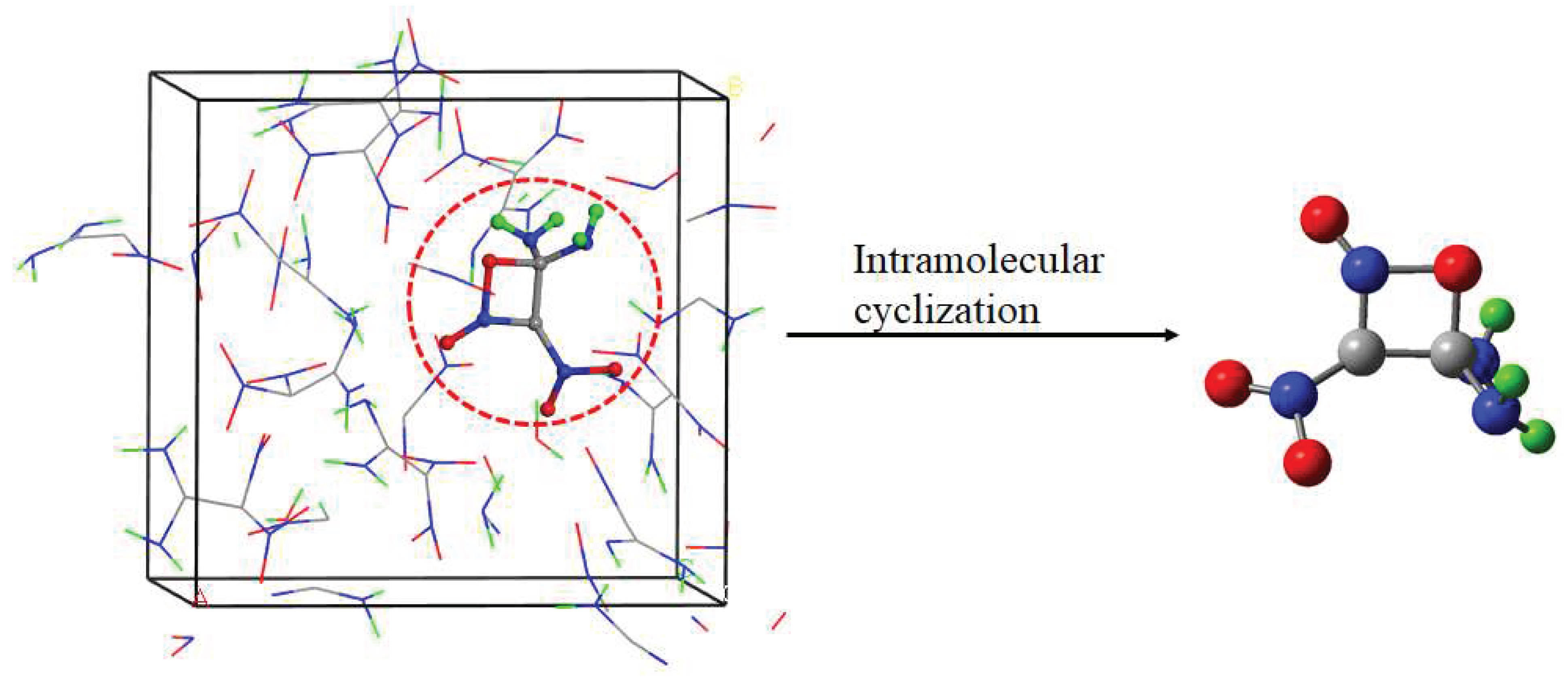
| 7.32 | 1 | Intramolecular cyclization (R5) | |
 -FOX-7 -FOX-7 |
0.792-3.672 | 2 | C2H4O4N4 → C2H4O2N3 + NO2 |
| 1.162-11.928 | 9 | C2H4O4N4 + P → C2H3O4N4 + H[P] | |
| 3.720-6.254 | 3 | C2H4O4N4 + P → C2H4O3N4 + O[P] | |
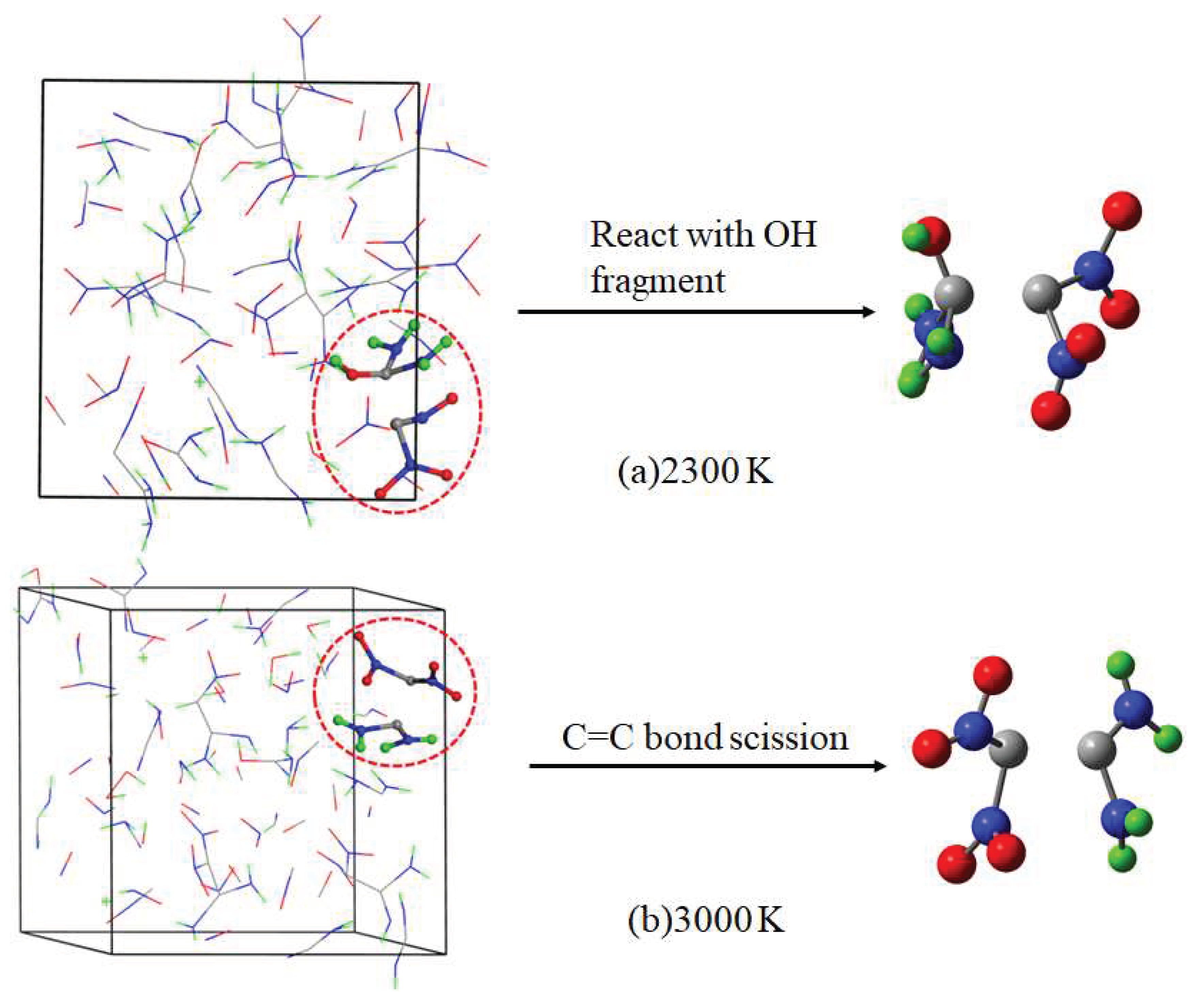
| 0.812 | 1 | C2H4O4N4 + O → CO4N2 + CH4ON2 (R6) | |
| 5.146 | 1 | C2H4O4N4 + OH →CO4N2 + CH5ON2 (R7) |
| Polymorphs | Time range (ps) | Frequency | Reaction |
|---|---|---|---|
 -FOX-7 -FOX-7 |
0.118-0.540 | 5 | C2H4O4N4 → C2H4O2N3 + NO2 |
| 0.080 | 1 | Intramolecular hydrogen transfer | |
| 0.078 | 1 | 2C2H4O4N4 → C2H3O4N4 + C2H5O4N4 (R8) | |
| 0.298-3.810 | 9 | C2H4O4N4 + P → C2H3O4N4 + H[P] | |
 -FOX-7 -FOX-7 |
0.062-0.882 | 10 | C2H4O4N4 → C2H4O2N3 + NO2 |
| 0.214-1.148 | 6 | C2H4O4N4 + P → C2H3O4N4 + H[P] | |
 -FOX-7 -FOX-7 |
0.092-0.562 | 8 | C2H4O4N4 → C2H4O2N3 + NO2 |
| 0.166-1.462 | 7 | C2H4O4N4 + P → C2H3O4N4 + H[P] | |
| 1.354 | 1 | C2H4O4N4 → CO4N2 + CH4N2 (R9) |
| Polymorphs | Time range (ps) | Frequency | Reaction |
|---|---|---|---|
 -FOX-7 -FOX-7 |
20.550-23.028 | 3 | C2H4O4N4 → C2H4O2N3 + NO2 |
| 22.244-27.046 | 11 | C2H4O4N4 + P → C2H3O4N4 + H[P] | |
| 24.054-24.404 | 2 | C2H4O4N4 + P → C2H4O3N4 + O[P] | |
 -FOX-7 -FOX-7 |
21.742-27.254 | 7 | C2H4O4N4 → C2H4O2N3 + NO2 |
| 22.872-26.460 | 7 | C2H4O4N4 + P → C2H3O4N4 + H[P] | |
| 24.694-25.788 | 2 | C2H4O4N4 + P → C2H4O3N4 + O[P] | |
 -FOX-7 -FOX-7 |
23.704-26.068 | 4 | C2H4O4N4 → C2H4O2N3 + NO2 |
| 22.020-26.358 | 10 | C2H4O4N4 + P → C2H3O4N4 + H[P] | |
| 20.594-21.900 | 2 | Intramolecular hydrogen transfer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




