Submitted:
30 June 2023
Posted:
04 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Drug administration
2.2. Animals
2.3. SNI model
2.4. Nociceptive tests
2.4.1. Hot Plate Test
2.4.2. Von Frey filaments
2.5. Evaluation of locomotor side effects
2.5.1. Rotarod test
2.5.2. Hole board test
2.6. DPPH Radical Scavenging Assay
2.7. Preparation of tissue and cell lysates
2.8. Western blotting
2.9. BV2 Cell culture
2.10. CCK-8 test
2.11. Statistical analysis
3. Results
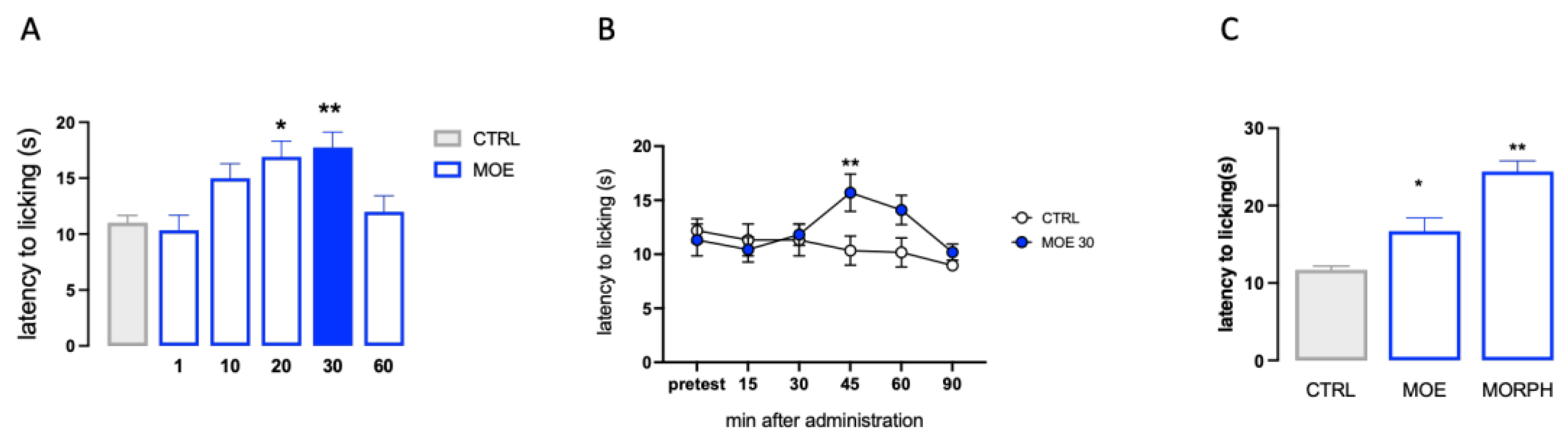
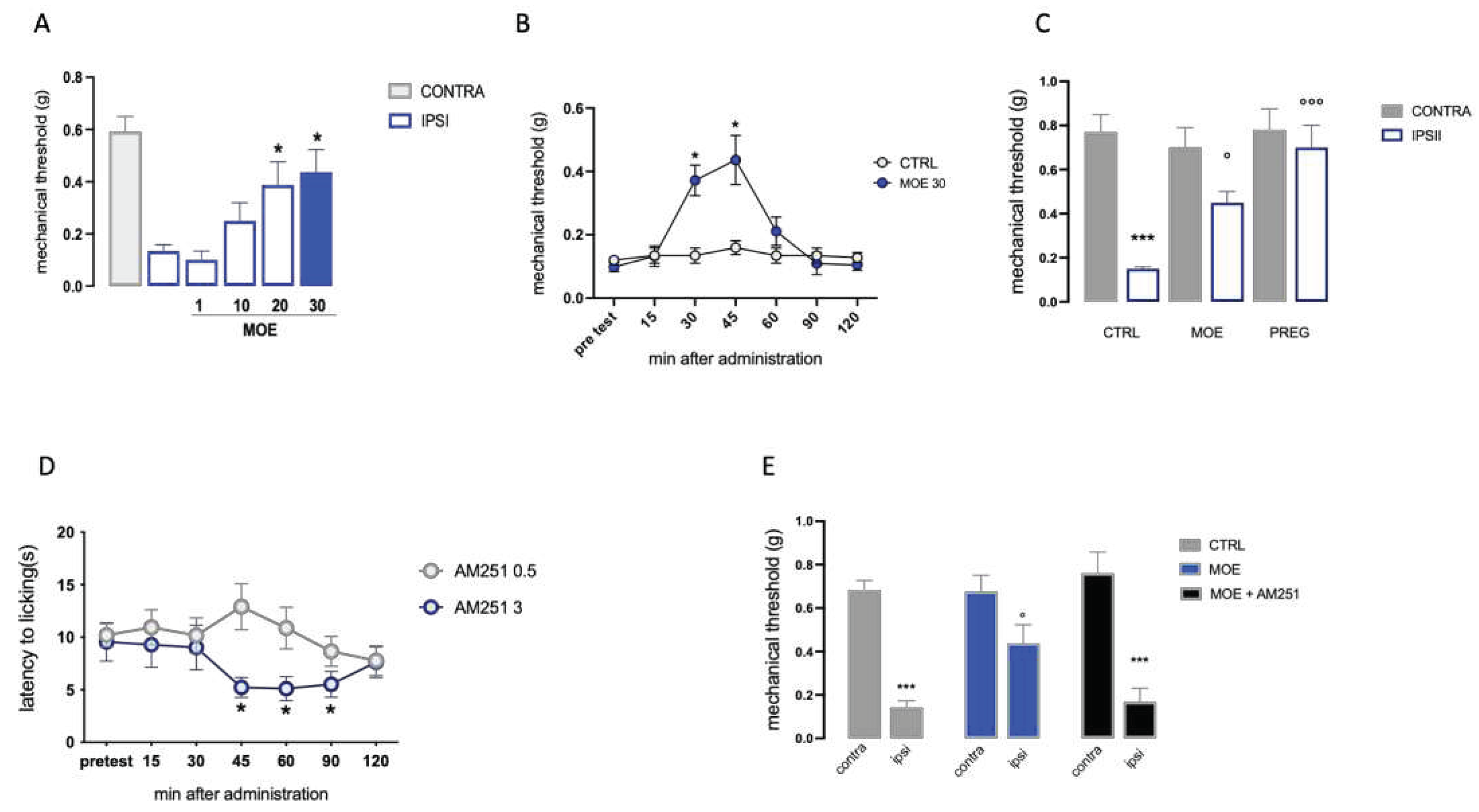
3.1. Effect of MOE on pain threshold
3.1.1. Antinociceptive activity of MOE
3.1.2. Antiallodynic effect of MOE
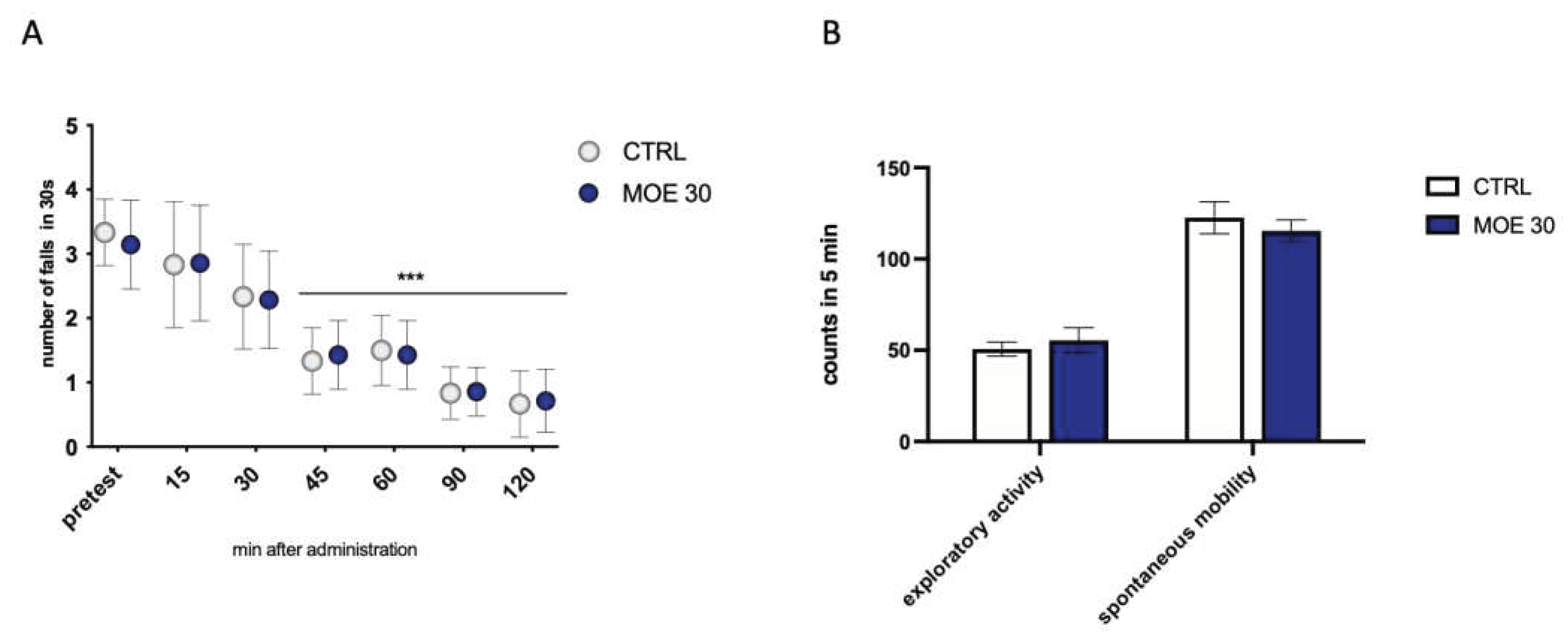
3.2. Evaluation of side effects on motility and exploratory ability of MOE
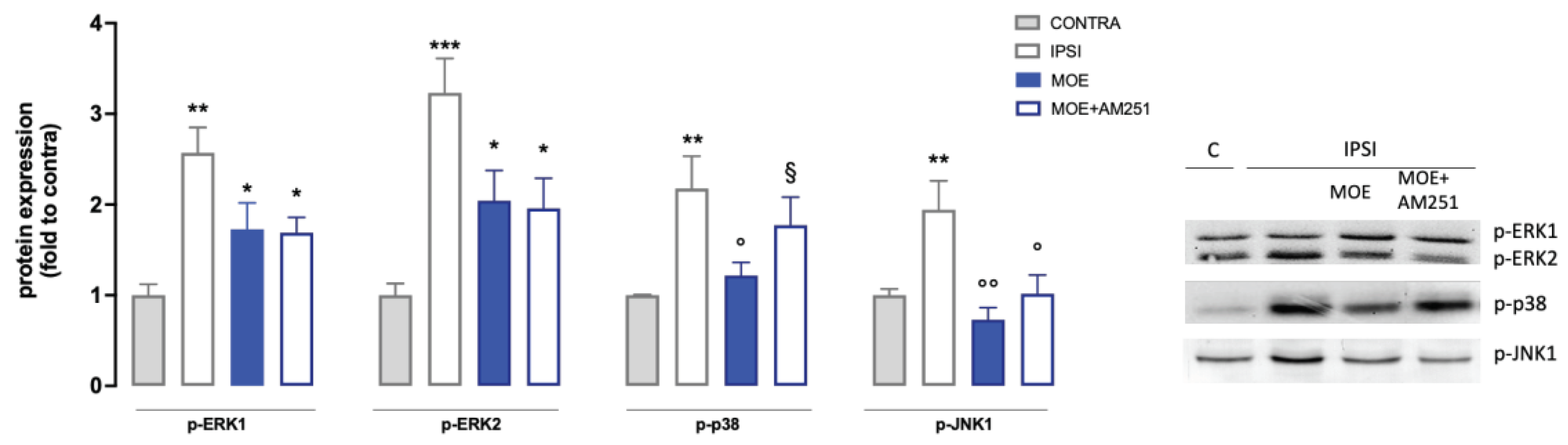
3.3. Effect of MOE on the phosphorylation of MAPK
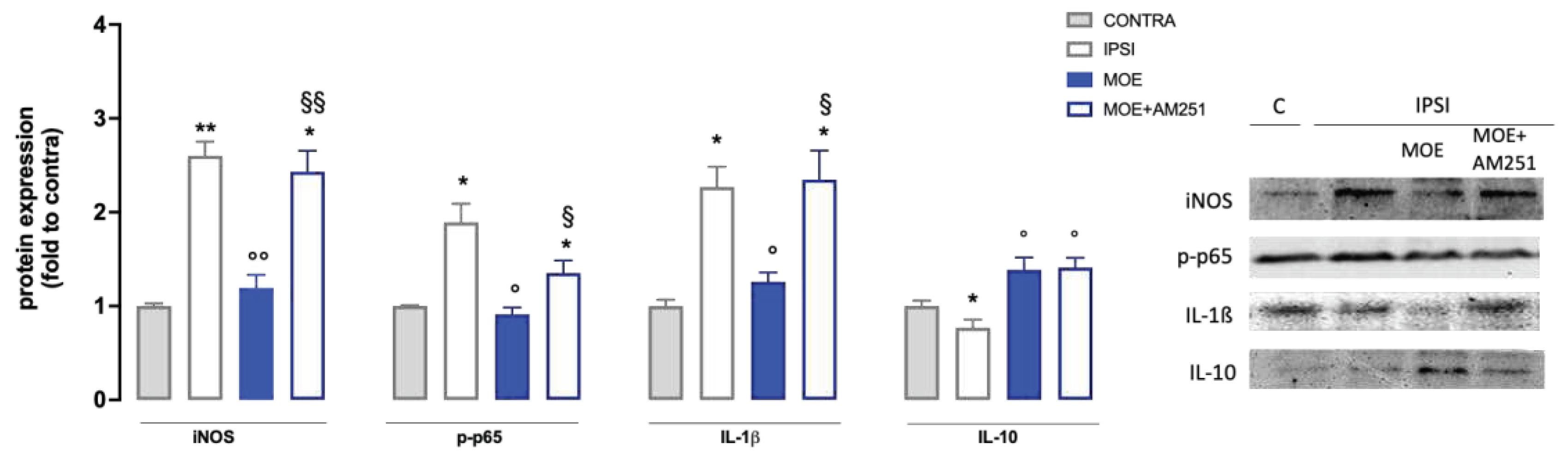
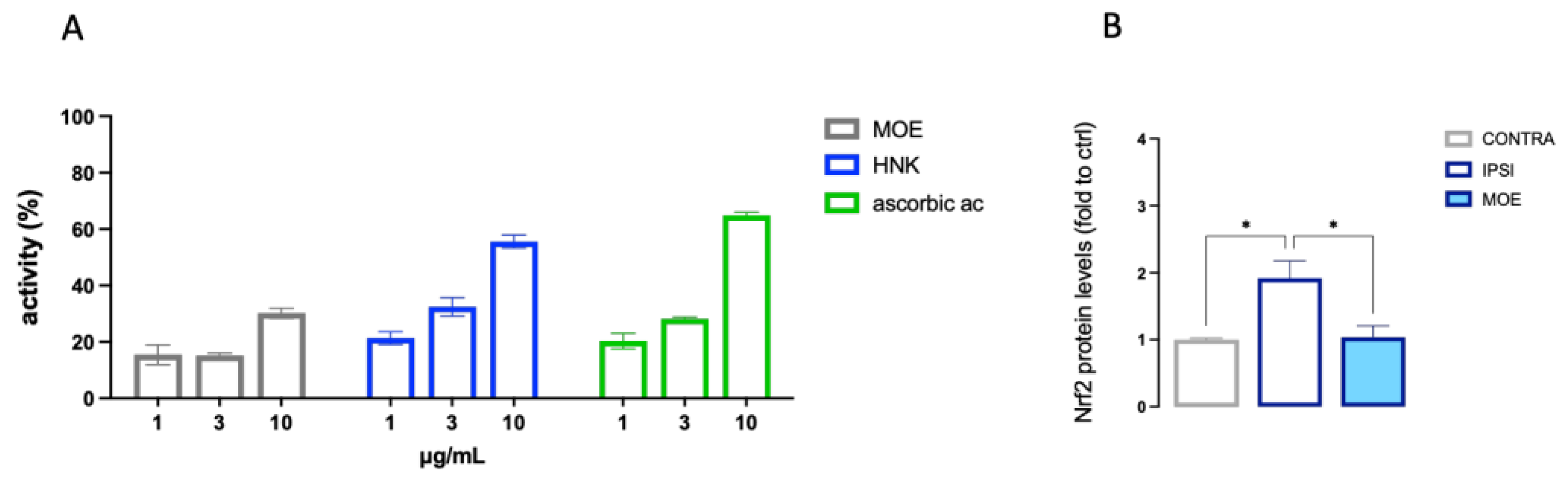
3.4. Effect of MOE on inflammatory markers and oxidative stress in spinal cord samples from SNI mice.
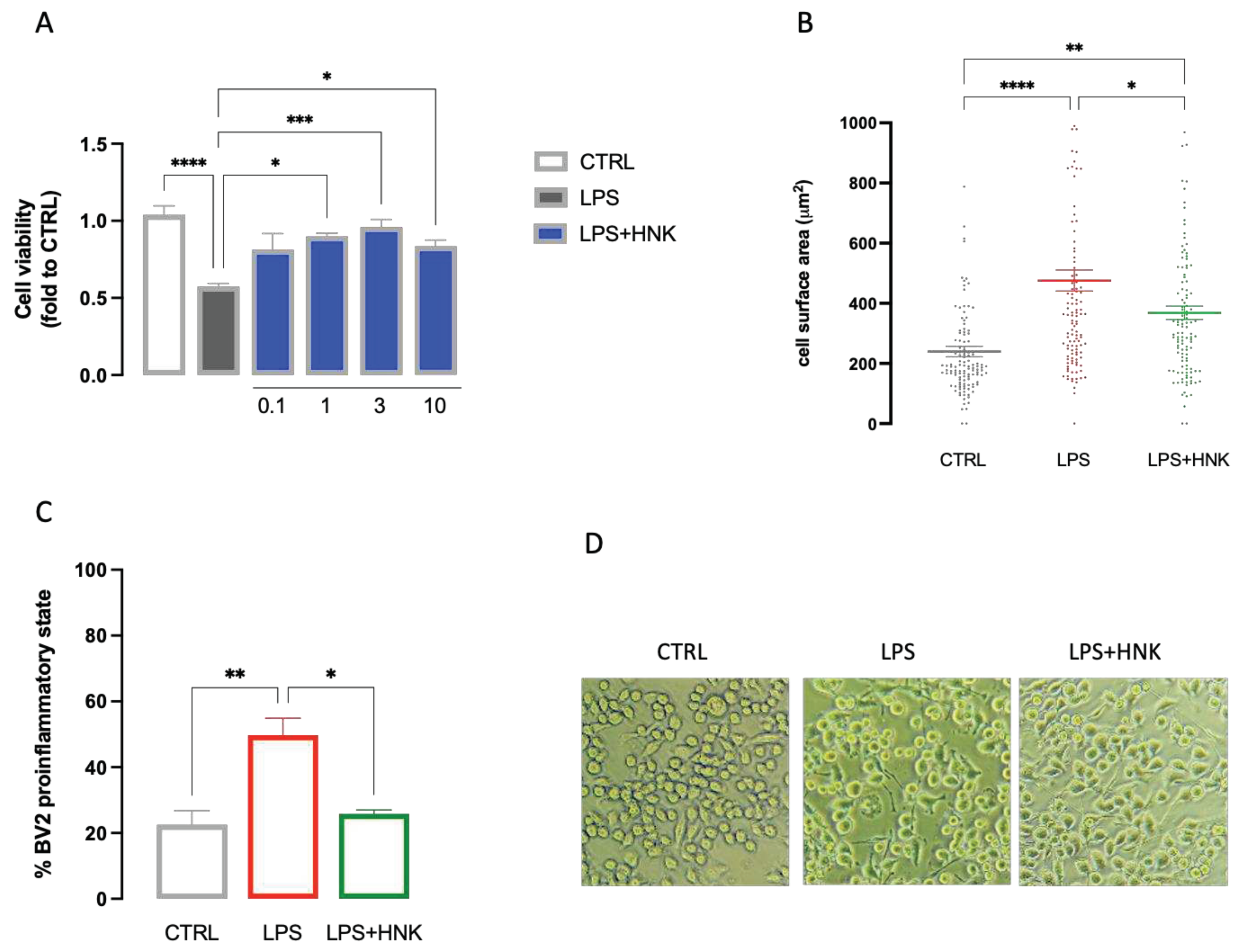
3.5. Effect of HNK on LPS-stimulated microglia cells
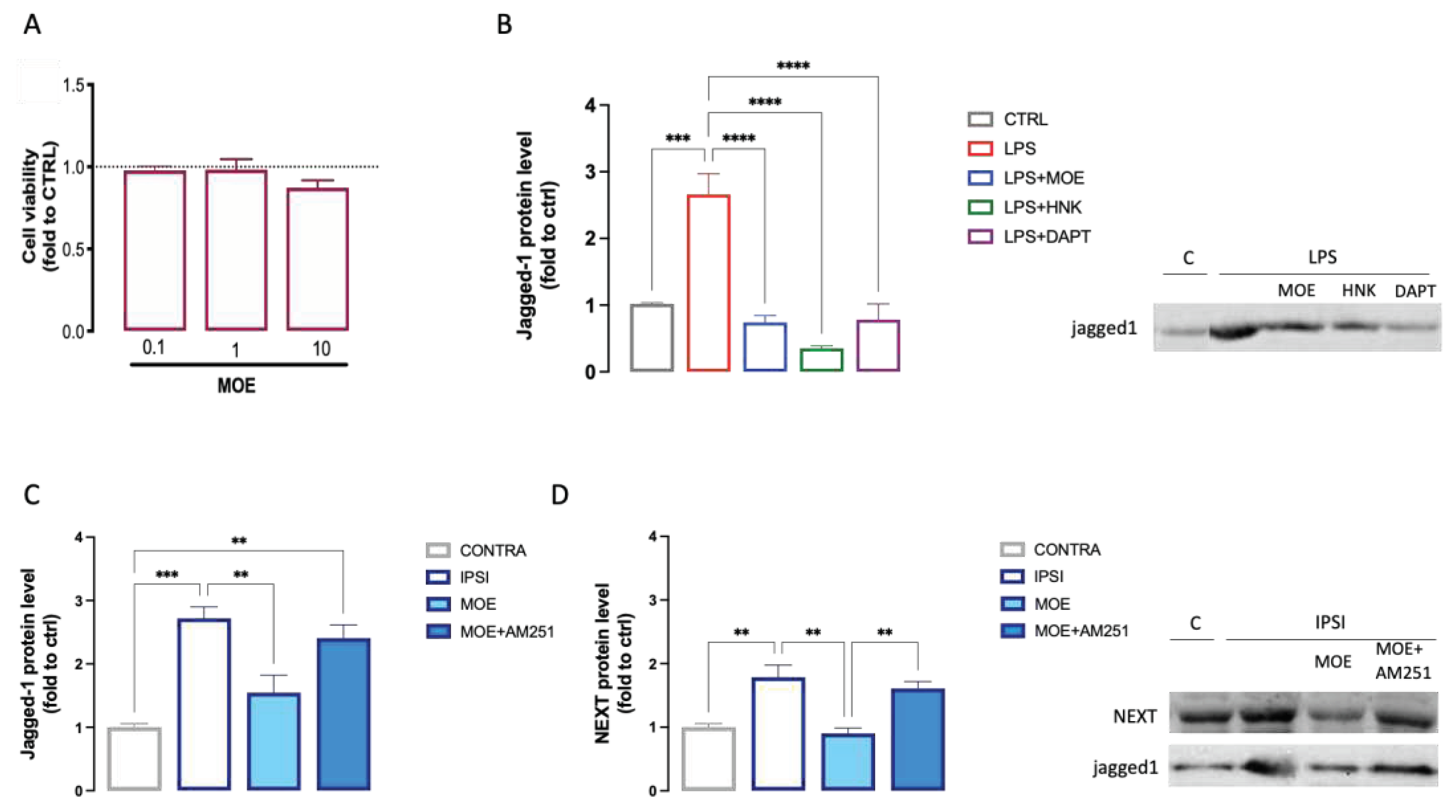
3.6. Evaluation of the effect of MOE and HNK on the Notch signaling pathway
3.7. Antioxidant activity of MOE and HNK
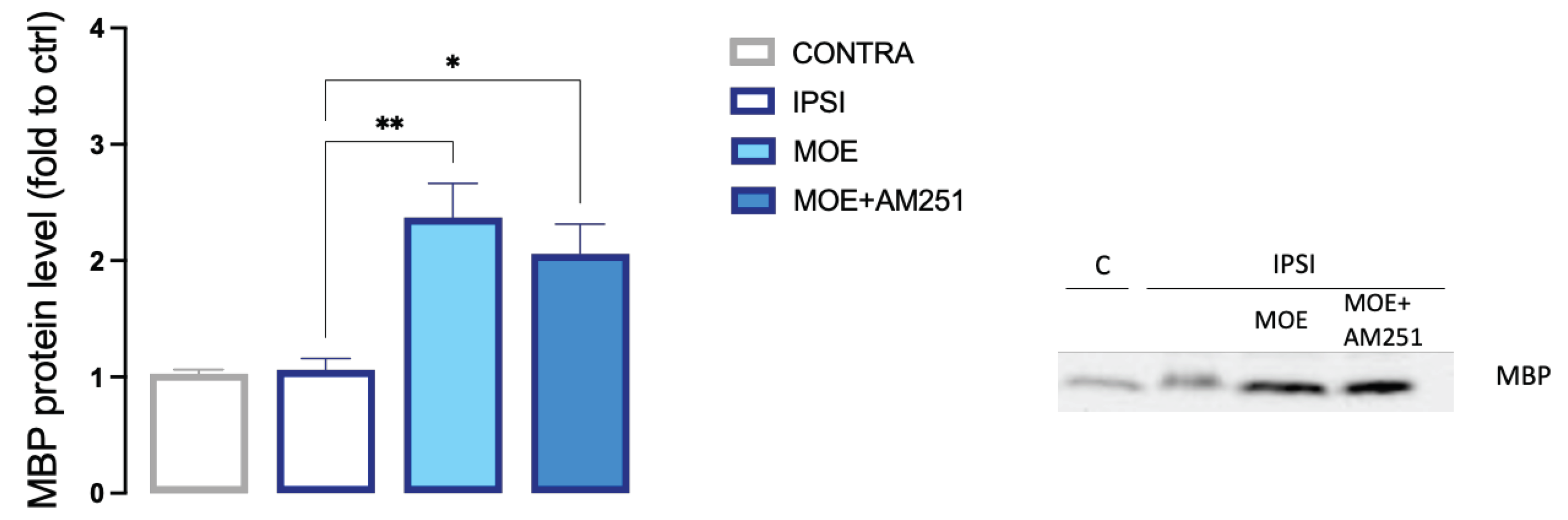
3.8. Effect of MOE on spinal expression of MBP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: Frommechanisms to Treatment. Physiol Rev 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Naranjo, C.; Del Reguero, L.; Moratalla, G.; Hercberg, M.; Valenzuela, M.; Failde, I. Anxiety, Depression and Sleep Disorders in Patients with Diabetic Neuropathic Pain: A Systematic Review. Expert Rev Neurother 2019, 19, 1201–1209. [Google Scholar] [CrossRef]
- Chen, Z.; Muscoli, C.; Doyle, T.; Bryant, L.; Cuzzocrea, S.; Mollace, V.; Mastroianni, R.; Masini, E.; Salvemini, D. NMDA-Receptor Activation and Nitroxidative Regulation of the Glutamatergic Pathway during Nociceptive Processing. Pain 2010, 149, 100–106. [Google Scholar] [CrossRef]
- Low, P.A.; Nickander, K.K.; Tritschler, H.J. The Roles of Oxidative Stress and Antioxidant Treatment in Experimental Diabetic Neuropathy. Diabetes 1997, 46, S38–S42. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural Antioxidant Control of Neuropathic Pain-Exploring the Role of Mitochondrial SIRT3 Pathway. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Boadas-Vaello, P.; Vela, J.M.; Verdu, E. New Pharmacological Approaches Using Polyphenols on the Physiopathology of Neuropathic Pain. Curr Drug Targets 2017, 18, 160–173. [Google Scholar] [CrossRef]
- Shen, C.-L.; Castro, L.; Fang, C.-Y.; Castro, M.; Sherali, S.; White, S.; Wang, R.; Neugebauer, V. Bioactive Compounds for Neuropathic Pain: An Update on Preclinical Studies and Future Perspectives. J Nutr Biochem 2022, 104, 108979. [Google Scholar] [CrossRef]
- Kim, J.H.; Kismali, G.; Gupta, S.C. Natural Products for the Prevention and Treatment of Chronic Inflammatory Diseases: Integrating Traditional Medicine into Modern Chronic Diseases Care. Evid Based Complement Alternat Med 2018, 2018, 9837863. [Google Scholar] [CrossRef]
- Ilari, S.; Proietti, S.; Russo, P.; Malafoglia, V.; Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Palma, E.; Tomino, C.; Fini, M.; et al. A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies. Antioxidants 2022, 11, 2361. [Google Scholar] [CrossRef]
- Poivre, M.; Duez, P. Biological Activity and Toxicity of the Chinese Herb Magnolia Officinalis Rehder & E. Wilson (Houpo) and Its Constituents. J Zhejiang Univ Sci B 18, 194–214. [CrossRef] [PubMed]
- Lee, Y.-J.; Lee, Y.M.; Lee, C.-K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic Applications of Compounds in the Magnolia Family. Pharmacol Ther 2011, 130, 157–176. [Google Scholar] [CrossRef]
- Luo, H.; Wu, H.; Yu, X.; Zhang, X.; Lu, Y.; Fan, J.; Tang, L.; Wang, Z. A Review of the Phytochemistry and Pharmacological Activities of Magnoliae Officinalis Cortex. J Ethnopharmacol 2019, 236, 412–442. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-L.; Man, K.-M.; Huang, P.-H.; Chen, W.-C.; Chen, D.-C.; Cheng, Y.-W.; Liu, P.-L.; Chou, M.-C.; Chen, Y.-H. Honokiol and Magnolol as Multifunctional Antioxidative Molecules for Dermatologic Disorders. Molecules 2010, 15, 6452–6465. [Google Scholar] [CrossRef]
- Lo, Y.C.; Teng, C.M.; Chen, C.F.; Chen, C.C.; Hong, C.Y. Magnolol and Honokiol Isolated from Magnolia Officinalis Protect Rat Heart Mitochondria against Lipid Peroxidation. Biochem Pharmacol 1994, 47, 549–553. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Leinders, M.; Üçeyler, N. Inflammation in the Pathophysiology of Neuropathic Pain. Pain 2018, 159, 595–602. [Google Scholar] [CrossRef]
- Rickert, U.; Cossais, F.; Heimke, M.; Arnold, P.; Preuße-Prange, A.; Wilms, H.; Lucius, R. Anti-Inflammatory Properties of Honokiol in Activated Primary Microglia and Astrocytes. J Neuroimmunol 2018, 323, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Rempel, V.; Fuchs, A.; Hinz, S.; Karcz, T.; Lehr, M.; Koetter, U.; Müller, C.E. Magnolia Extract, Magnolol, and Metabolites: Activation of Cannabinoid CB 2 Receptors and Blockade of the Related GPR55. ACS Med Chem Lett 2013, 4, 41–45. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis Sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Lilley, E.; Stanford, S.C.; Kendall, D.E.; Alexander, S.P.H.; Cirino, G.; Docherty, J.R.; George, C.H.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. ARRIVE 2.0 and the British Journal of Pharmacology: Updated Guidance for 2020. Br J Pharmacol 2020, 177, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N. How to Calculate Sample Size in Animal Studies? J Pharmacol Pharmacother 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Galeotti, N. Intranasal Delivery of an Antisense Oligonucleotide to the RNA-Binding Protein HuR Relieves Nerve Injury-Induced Neuropathic Pain. Pain 2021, 162, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Les, F.; Lopez, V.; Galeotti, N. Lavender (Lavandula Angustifolia Mill.) Essential Oil Alleviates Neuropathic Pain in Mice with Spared Nerve Injury. Front Pharmacol 2019, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Governa, P.; Biagi, M.; Pellati, F.; Galeotti, N. Zingiber Officinale Roscoe Rhizome Extract Alleviates Neuropathic Pain by Inhibiting Neuroinflammation in Mice. Phytomedicine 2020, 78, 153307. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Bartolini, A.; Ghelardini, C. Blockade of Intracellular Calcium Release Induces an Antidepressant-like Effect in the Mouse Forced Swimming Test. Neuropharmacology 2006, 50, 309–316. [Google Scholar] [CrossRef]
- Galeotti, N.; Ghelardini, C.; Zoppi, M.; Del Bene, E.; Raimondi, L.; Beneforti, E.; Bartolini, A. Hypofunctionality of Gi Proteins as Aetiopathogenic Mechanism for Migraine and Cluster Headache. Cephalalgia 2001, 21, 38–45. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Biagi, M.; Pellati, F.; Galeotti, N. Zingiber Officinale Roscoe Rhizome Extract Alleviates Neuropathic Pain by Inhibiting Neuroinflammation in Mice. Phytomedicine 2020, 78, 153307. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Benatti, C.; Governa, P.; Isoldi, G.; Pellati, F.; Alboni, S.; Tascedda, F.; Montopoli, M.; Galeotti, N.; Manetti, F.; et al. Non-Psychotropic Cannabis Sativa L. Phytocomplex Modulates Microglial Inflammatory Response through CB2 Receptors-, Endocannabinoids-, and NF-ΚB-Mediated Signaling. Phytotherapy Research 2022, 36, 2246–2263. [Google Scholar] [CrossRef]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental Design and Analysis and Their Reporting II: Updated and Simplified Guidance for Authors and Peer Reviewers. Br J Pharmacol 2018, 175. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Wei, Z.; Chigurupati, S.; Arumugam, T. V.; Jo, D.-G.; Li, H.; Chan, S.L. Notch Activation Enhances the Microglia-Mediated Inflammatory Response Associated With Focal Cerebral Ischemia. Stroke 2011, 42, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Li, L.; Liu, X.-H.; Gu, N.; Dong, H.-L.; Xiong, L. The Spinal Notch Signaling Pathway Plays a Pivotal Role in the Development of Neuropathic Pain. Mol Brain 2012, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, Z.; Xing, Z.; Zhu, H.; Zhou, W.; Xie, S.; Zhang, Z.; Li, M.-H. The Notch Signaling Pathway Regulates Differentiation of NG2 Cells into Oligodendrocytes in Demyelinating Diseases. Cell Mol Neurobiol 2022, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Galeotti, N. Posttranscriptional Regulation of Gene Expression Participates in the Myelin Restoration in Mouse Models of Multiple Sclerosis: Antisense Modulation of HuR and HuD ELAV RNA Binding Protein. Mol Neurobiol 2023, 60, 2661–2677. [Google Scholar] [CrossRef]
- Doth, A.H.; Hansson, P.T.; Jensen, M.P.; Taylor, R.S. The Burden of Neuropathic Pain: A Systematic Review and Meta-Analysis of Health Utilities. Pain 2010, 149, 338–344. [Google Scholar] [CrossRef]
- Singh, H.; Bhushan, S.; Arora, R.; Singh Buttar, H.; Arora, S.; Singh, B. Alternative Treatment Strategies for Neuropathic Pain: Role of Indian Medicinal Plants and Compounds of Plant Origin-A Review. Biomedicine & Pharmacotherapy 2017, 92, 634–650. [Google Scholar] [CrossRef]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med 2018, 84, 1151–1164. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural Antioxidant Control of Neuropathic Pain-Exploring the Role of Mitochondrial SIRT3 Pathway. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Chen, Z.; Muscoli, C.; Doyle, T.; Bryant, L.; Cuzzocrea, S.; Mollace, V.; Mastroianni, R.; Masini, E.; Salvemini, D. NMDA-Receptor Activation and Nitroxidative Regulation of the Glutamatergic Pathway during Nociceptive Processing. Pain 2010, 149, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Edelmayer, R.M.; Brederson, J.D.; Jarvis, M.F.; Bitner, R.S. Biochemical and Pharmacological Assessment of MAP-Kinase Signaling along Pain Pathways in Experimental Rodent Models: A Potential Tool for the Discovery of Novel Antinociceptive Therapeutics. Biochem Pharmacol 2014, 87, 390–398. [Google Scholar] [CrossRef]
- Ji, R.-R.; Gereau, R.W.; Malcangio, M.; Strichartz, G.R. MAP Kinase and Pain. Brain Res Rev 2009, 60, 135–148. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Roelofs, N.; Muñoz, E.; Fiebich, B.L. Alleviation of Microglial Activation Induced by P38 MAPK/MK2/PGE2 Axis by Capsaicin: Potential Involvement of Other than TRPV1 Mechanism/s. Sci Rep 2017, 7, 116. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.-Z.; Park, J.-Y.; Lind, A.-L.; Ma, Q.; Ji, R.-R. JNK-Induced MCP-1 Production in Spinal Cord Astrocytes Contributes to Central Sensitization and Neuropathic Pain. J Neurosci 2009, 29, 4096–4108. [Google Scholar] [CrossRef]
- Hervera, A.; Negrete, R.; Leánez, S.; Martín-Campos, J.M.; Pol, O. The Spinal Cord Expression of Neuronal and Inducible Nitric Oxide Synthases and Their Contribution in the Maintenance of Neuropathic Pain in Mice. PLoS One 2010, 5, e14321. [Google Scholar] [CrossRef]
- Qu, W.; Cheng, Y.; Peng, W.; Wu, Y.; Rui, T.; Luo, C.; Zhang, J. Targeting INOS Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation. Mol Neurobiol 2022, 59, 3124–3139. [Google Scholar] [CrossRef]
- Béchade, C.; Colasse, S.; Diana, M.A.; Rouault, M.; Bessis, A. NOS2 Expression Is Restricted to Neurons in the Healthy Brain but Is Triggered in Microglia upon Inflammation. Glia 2014, 62, 956–963. [Google Scholar] [CrossRef]
- Wang, L.; Yin, C.; Liu, T.; Abdul, M.; Zhou, Y.; Cao, J.-L.; Lu, C. Pellino1 Regulates Neuropathic Pain as Well as Microglial Activation through the Regulation of MAPK/NF-ΚB Signaling in the Spinal Cord. J Neuroinflammation 2020, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu Rev Immunol 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Iwamoto, S.; Yoshinaga, R.; Tozaki-Saitoh, H.; Nishiyama, A.; Mak, T.W.; Tamura, T.; Tsuda, M.; Inoue, K. Transcription Factor IRF5 Drives P2X4R+-Reactive Microglia Gating Neuropathic Pain. Nat Commun 2014, 5, 3771. [Google Scholar] [CrossRef]
- Ramachandran, C.; Wilk, B.; Melnick, S.J.; Eliaz, I. Synergistic Antioxidant and Anti-Inflammatory Effects between Modified Citrus Pectin and Honokiol. Evid Based Complement Alternat Med 2017, 2017, 8379843. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Shivakumar, M.; Joshi, V.; Subbanna, S. Endocannabinoid System in Neurodegenerative Disorders. J Neurochem 2017, 142, 624–648. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Baños, J.E.; Cabañero, D. The Endocannabinoid System and Neuropathic Pain. Pain 2016, 157 Suppl 1, S23–S32. [Google Scholar] [CrossRef]
- Ponnurangam, S.; Mammen, J.M. V; Ramalingam, S.; He, Z.; Zhang, Y.; Umar, S.; Subramaniam, D.; Anant, S. Honokiol in Combination with Radiation Targets Notch Signaling to Inhibit Colon Cancer Stem Cells. Mol Cancer Ther 2012, 11, 963–972. [Google Scholar] [CrossRef]
- Kaushik, G.; Venugopal, A.; Ramamoorthy, P.; Standing, D.; Subramaniam, D.; Umar, S.; Jensen, R.A.; Anant, S.; Mammen, J.M. V Honokiol Inhibits Melanoma Stem Cells by Targeting Notch Signaling. Mol Carcinog 2015, 54, 1710–1721. [Google Scholar] [CrossRef]
- Xie, K.; Qiao, F.; Sun, Y.; Wang, G.; Hou, L. Notch Signaling Activation Is Critical to the Development of Neuropathic Pain. BMC Anesthesiol 2015, 15, 41. [Google Scholar] [CrossRef]
- Xie, K.; Jia, Y.; Hu, Y.; Sun, Y.; Hou, L.; Wang, G. Activation of Notch Signaling Mediates the Induction and Maintenance of Mechanical Allodynia in a Rat Model of Neuropathic Pain. Mol Med Rep 2015, 12, 639–644. [Google Scholar] [CrossRef]
- Jin, G.-L.; Hong, L.-M.; Liu, H.-P.; Yue, R.-C.; Shen, Z.-C.; Yang, J.; Xu, Y.; Huang, H.-H.; Li, Y.; Xiong, B.-J.; et al. Koumine Modulates Spinal Microglial M1 Polarization and the Inflammatory Response through the Notch-RBP-Jκ Signaling Pathway, Ameliorating Diabetic Neuropathic Pain in Rats. Phytomedicine 2021, 90, 153640. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Qin, W.; Yang, A.-X.; Yan, F.-F.; Chen, Y.; Ma, J.-X. Propofol Alleviates Neuropathic Pain in Chronic Constriction Injury Rat Models via the MicroRNA-140-3p/Jagged-1 Peptide/Notch Signaling Pathway. Synapse 2021, 75, e22219. [Google Scholar] [CrossRef] [PubMed]
- Pierfelice, T.; Alberi, L.; Gaiano, N. Notch in the Vertebrate Nervous System: An Old Dog with New Tricks. Neuron 2011, 69, 840–855. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Luo, T.; Mei, Y.; Liu, H.; Dong, J.; Fang, Y.; Peng, J.; Guo, Y. Simvastatin Alters M1/M2 Polarization of Murine BV2 Microglia via Notch Signaling. J Neuroimmunol 2018, 316, 56–64. [Google Scholar] [CrossRef]
- Cao, Q.; Lu, J.; Kaur, C.; Sivakumar, V.; Li, F.; Cheah, P.S.; Dheen, S.T.; Ling, E.-A. Expression of Notch-1 Receptor and Its Ligands Jagged-1 and Delta-1 in Amoeboid Microglia in Postnatal Rat Brain and Murine BV-2 Cells. Glia 2008, 56, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




