1. Introduction
Anaerobic digestion is characterized by being a reaction where biogas is produced from biodegradable matter under anaerobic conditions. It has been widely used as a method to provide energy, especially in Asian countries, such as: China, India and African countries [
1]. As a beneficial method for the environment and as the best option for economic development and improvement of the quality of life in developing countries, anaerobic digestion is a suitable method to generate renewable energy [
2]. European countries and the United States have developed the technology for the anaerobic treatment of wastewater and the production of biogas [
3], this is possible with the use of modern reactors that improve the treatment of a variety of wastes. with different characteristics [
4]. This technology is capable of handling various organic residues, such as: cattle manure, biomass agricultural residues (crop residues) [
5], residues from dairy industries, municipal residues (vegetable waste) [
6], treatment of primary sludge and wastewater [
7].
In Ecuador, non-conventional renewable energy includes wind, solar energy and sources of biodegradable matter (MICSE, 2015). Biodegradable matter such as livestock excreta, food plant residues or crop residues could receive a treatment that converts them into value-added products. The best management practice as mentioned is anaerobic digestion [
8]. In the Balao canton (Guayas), cocoa shells have been used in co-digestion with cow manure on a laboratory scale to obtain biogas with an average methane yield of 174 (wet) and 193 (dry) L kg-1 SV [
9]. In the Bolívar Province, Guaranda city; Anaerobic co-digestion was used to produce biogas from cabbage waste and cattle manure. The study determined that the biogas yield was 389.47 cm3N g-1 initial SV with a methane composition of 61% [
10].
In order to obtain a higher yield of biogas and methane, and knowing that animal manure is high in nitrogen and low in carbon, anaerobic digestion has been hampered by the optimal C/N ratio required to start the process [
11]. For this reason, animal manure requires a carbon source, being lignocellulosic material a suitable candidate to compensate for the carbon deficiency of manure [
12]. [
7], studied the production of biogas and subsequent generation of power and heat from the co-digestion of food waste and primary sludge.
For this reason, the objective of this research is to carry out the anaerobic co-digestion of rumen residues with wheat straw in a potential biochemical test of methane and to define the change and effect of the C/N ratio that will occur during co-digestion, in addition to the synergistic effect and biodegradability of organic matter together with its kinetic contrast of biogas production.
2. Materials and methods
2.1. Origin of substrates and inoculum
All experimental procedures were performed following the guidelines of [
13,
14]. This study was performed in the Stated Bolivar University, Guaranda - Ecuador. The main substrate (ruminal residue) was collected from the Municipal Slaughtering Center of the city of Guaranda, located at Latitude: 1°35’52.062"S Longitude: 78°59’48.28"O at an altitude of 2765 masl. This residue represents the matter in the process of digestion that was interrupted when the cattle were slaughtered. Daily slaughter generates a large amount of this residue, making it a potential source of energy. Such samples were collected in polyethylene bags obtaining significant samples. Subsequently, they were stored in the laboratory at 6 C for 72 h before being added to the biodigesters.
The inoculum used in all the tests comes from the urban wastewater treatment station (WWTP) in the city of San Miguel de Ibarra (Ecuador). It is extracted from the primary sludge of the anaerobic digester that worked in mesophilic conditions (temperature between 35 and 37ºC approximately.
2.2. Characterization of raw materials and biogas
The materials were characterized by proximal analysis and elemental analysis. The total solids (ST) of the substrates, volatile solids percentage (VS) with respect to total solids and ashes were determined by the methodology proposed by the standards UNE-EN 18134-1: 2016, UNE-EN 18134-2: 2017, UNE-EN ISO 18134-3, 2016, UNE-EN ISO 18123: 2016 and [
15]
Similarly, for the proximal analysis of the inoculum, whose composition was mostly liquid, a more proper methodology of wastewater proposed by the American Public Health Association (APHA) [
16] sections 2540A-2540G was used, determining the TS, VS and ashes.
The elementary analysis from which the percentages of N, C, O, H, S and C/N ratio of the substrates and the inoculum are obtained were determined through the VARIOUS MACRO CUBE elemental analyzer, following the guidelines proposed by the standard UNE ISO16948 15104. The pH was determined at room temperature using a HACH HQ 40D digital multimeter meter potentiometer.
The biogas production was calculated from the pressure exerted by the biogas inside the biodigester. The pressure was measured daily by the manometer (Delta OHM HD 2124.2) equipped with a sensor (Delta TP 704 with a capacity of 100 bar). After the daily pressure measurement, the biogas accumulated in the upper space of the biodigester was completely released; this caused the pressure exerted by the biodigester to be reduced to a pressure close to atmospheric pressure. After releasing the biogas, the pressure in the head space of the biodigester was again measured as an initial condition for the next day measurement. The biogas components (CH4, H2S, CO2 and O2) were determined with the Geotech BIOGAS GA-5000 analyzer. The biogas estimate was evaluated daily from each biodigester by daily extraction of all the generated biogas.
2.3. Experimental methodology
The experiments were carried out following BMP protocols [
14]. Glass bottles of 311 mL with an effective volume of 186 mL, hermetically sealed with stoppers and control gas opening valves, were used as experimental units. The tests were performed using a concentration of substrate of 18 g VS/L and Inoculum/Substrate ratios of 2:1, 1:1, 1:2 based on VS; these are considered typical values for the BMP test [
8]. The experiments were conducted within the mesophilic temperature range, at 38°C±1. The experimental units were shaken manually twice a day during all the experiment. The treatments were evaluated by triplicates and 9 control bottles without substrate were also included to correct the methane production from the inoculum. The experiments were carried out for 45 days. The biogas production was measured daily.
The experimental biogas yield was calculated from the pressure difference generated each day in the digesters [
17]. Starting from the ideal gas equation, the volume of biogas generated at 38°C was estimated by the equation:
where, ΔP: Absolute pressure difference (KPa), V
f: free volume, C: Molar volume of a gas at standard pressure and temperature (22.41L/mol), R: ideal gas constant (8.314 L kPa/K/mol) and T
w: working temperature (mesophilic 38°C) 311.15K.
Calculation of theoretical performance, biodegradability and synergistic effect index.
Theoretical maximum methane yield (TMY, mL/g-VS) was calculated from the elemental composition and ash contents of the substrates, using the Buswell’s formula [
18] and Chen’s formula [
19], as shown in Eqs. (2) and (3), respectively.
Biodegradability (BD) was calculated from the highest cumulative methane yield (experimental methane yield, EMY) and TMY, as shown in Eq. (3):
The synergistic effect index (SEI) for the anaerobic co-digestion proposed in this study was calculated as shown in Eq. (4).
In Eq. (4), EMYco is the EMY of a co-digestion. EMY1 and EMY2 are the EMYs of the mono-digestions of R and WS, respectively. X1 and X2 are the VS fractions of R and WS in the co-digestion, respectively.
2.4. Kinetic modeling
The modified Gompertz model Eq. (5), which has been widely applied in simulating and predicting anaerobic digestion performance [
20,
21,
22], was used in this study.
In Eq. (5), B represents the simulated cumulative methane yield (mL/g-VS); B0 refers to the simulated maximum cumulative methane yield (mL/g-VS); Vmax means the maximum methane production rate (mL/g-VS/day); e is equal to 2.718; tlag stands for the lag phase time (day); t is the digestion time (day).
Transfer function model Eq. (6) allows predicting the maximum methane production based only on accumulated methane production over time and analyzes the anaerobic digestion process as a system receiving inputs and generating outputs [
15,
23]
The Logistic function Eq. (7) fits the global shape of the biogas production kinetics: it has an initial exponential increase and a final stabilization at a maximum production level. This model assumes that the rate of gas production is proportional to the amount of gas already produced, the maximum production rate and the maximum biogas production capacity. This model has been used for anaerobic fermentation, and to estimate the methane production in landfill leachate [
24]. In this case, a modified version of the logistic function was used [
25].
where B (mL g
VS-1) is the biogas produced at time t (d), B
o (mL g
VS-1) is the maximum biogas production, V
max (mL g
VS-1 d
-1) the maximum biogas production rate and t
lag (d) the lag time.
3. Results and discussion
3.1. Characterization of substrates and inoculums
Table 1 shows that the volatile solids of the ruminal residue and wheat straw is respectively, 70.74% and 74.79%; this indicates that both substrates have a moderate content of volatile solids, equivalent to a normal content of organic matter available for anaerobic digestion. The low percentages of ash for both substrates (12.81% and 8.45% respectively), justify the low volatile solids content of these substrates and a high amount of minerals. Likewise, the analyzed inoculum has 58.49% SV and 55.59% ash, confirming a high content of available organic matter, this is justified because there are viable and non-viable microorganisms, as well as remains of organic matter that is accompanied by the medium analyzed.
The C/N ratio of the substrates is 100.99 and 28.93 for the rumen residue and wheat straw, respectively, these values could generate a wide range of C/N ratios available in anaerobic co-digestion of both substrates, compared to the monodigestion of each substrate alone. On the one hand, the rumen residue contributes a large amount of degradable carbon to the medium, due to its high C/N ratio. While wheat straw supplies nitrogen to the system, this is due to its low C/N ratio. The high C/N ratio of the ruminal residue, as well as its high organic matter content, are properties that generate advantages when mixed with wheat straw, a substrate with a lot of available nitrogen. In short, the co-digestion of both substrates could improve the balance of nutrients in the digester and increase the amount of degradable organic matter, which leads to an increase in methane production. An appropriate ratio of C/N can accelerate the rate of substrate metabolism in fermentation. Some studies have shown that a C/N ratio of about 25:1 results in the highest methane synthesis output [
26,
27]
3.2. Cumulative production of biogas and methane
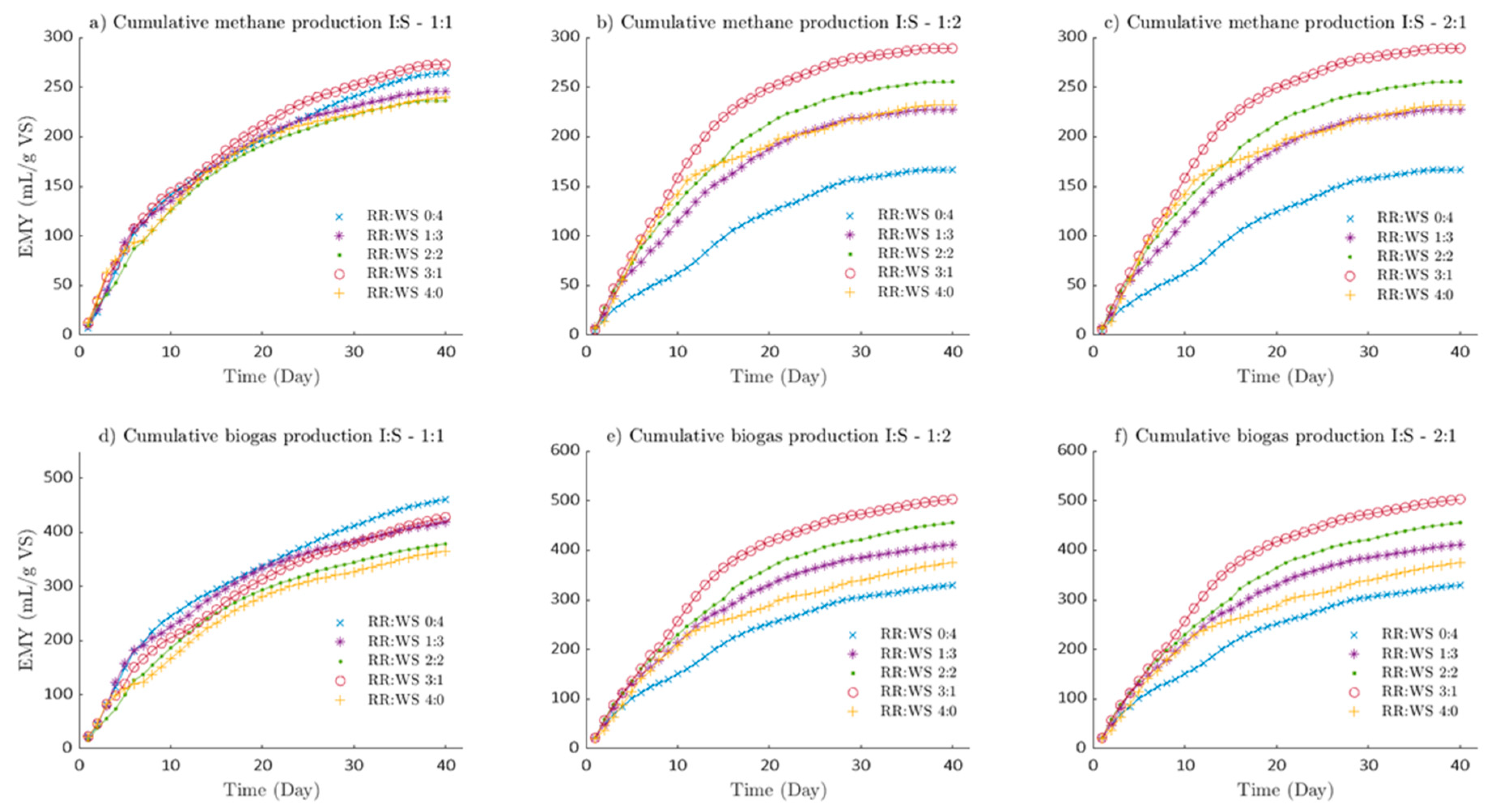
In
Figure 1 shows the similarity in the generation of biogas at 40 days of digestion (
Figure 1 a,b) when the I:S rate is 1:2 and 2:1. It can be noted that the order in which the substrate and co-substrate rate generate biogas is RR:WS (3:1, 2:2, 1:3, 4:0 and 0:4) for both proportions. There is an inversely proportional relationship of substrate and co-substrate (RR:WS), the higher RR and lower WS, the greater the biogas generated, which implies that RR is easily biodegraded. It is well known that the rumen has high cellulose-degrading efficiency and the bacteria involved in this may also be involved in degradation in the AD process. Methanogens active in the rumen may also contribute to methanogenesis in the biogas production process [
28]. Indeed, it can be observed that biodegradation starts immediately. When the amount of inoculum used is 2 times the amount of substrate (I:S - 2:1), it can be observed that during the co-digestion of RR and WS, the biogas generated improves as the fraction of RR added to the digester increases up to 50% with respect to WS,
Figure 1c. At a 2:2 rate of RR to WS, the biogas yield was 493.247mL/g-VS, which was higher than RR (421.726mL/g-VS) and WS (477.754mL/g-VS) in monodigestion. Something similar occurs with the experiment I:S – 1:1,
Figure 1a. At a 3:1 rate of RR to WS, the biogas yield was 427.787mL/g-VS, slightly lower than WS (460.891mL/g-VS) and higher than RR (365.335mL/g-VS) in monodigestion. When the inoculum to substrate rate was 1:2,
Figure 1b, the highest biogas production occurred. At a 3:1 rate of RR to WS, the biogas yield was 502.903mL/g-VS, higher than RR (375.441mL/g-VS) and WS (329.639mL/g-VS) in monodigestion. As the amount of inoculum decreases in the digesters with respect to the substrate, the cumulative biogas yield (CBY) also decreases for the RR:WS treatments at rates 0:4 to 2:2. However, at 3:1 and 4:0 rates of RR to WS, the amount of accumulated biogas increases from 421.726mL/g-VS to 502.903mL/g-VS, the same occurs with the 4:0 rate. Indicating that adding a low percentage of inoculum and a high percentage of RR favors biogas production.
Something similar occurs with the methane generated (CMY), the order in which the substrate and co-substrate rate generate methane is RR:WS (3:1, 2:2, 4:0, 1:3 and 0:4). A production maximum can be noted for different fractions of inoculum. A maximum of 306.205mLCH4/g-VS (RE:TR-2:2), 272.836mLCH4/g-VS and 289.078mLCH4/g-VS were identified at an I:S rate of 2:1, 1:1 and 1:2, respectively. An optimal range of methane generated could be identified, corresponding to fractions of ruminal residue from 50 to 75% at any inoculum concentration. [
27,
29] reported that the gastrointestinal tract of animals provides an ideal ecosystem for anaerobic microorganisms to thrive and for there to be a great diversity of methanogens that favor methane production.
3.3. Effect of pH, C/N rate, biodegradability and synergic effect index on accumulated methane yield.
Table 2 shows the average pH variation that occurred during co-digestion at 40 days. It can be observed that the initial pH rises as the amount of RR increases in the digesters regardless of the amount of inoculum present, indicating the favorable buffering capacity of RR; a slight drop in pH occurs at the end of the experiment, however, negligible enough to consider it less than the optimal level. For the three experimental cases, the pH in the digesters is maintained within the optimum range of 6.8 to 7.8 [
30]. [
31] reported the pH of the reactor influences both the AD process and the efficiency of the digestion process. Methanogens perform their roles more effectively between pH ranges of 6.5–8.2, with an optimum pH of 7.0. Although it has been previously reported that the optimum pH ranges for obtaining the highest biogas and methane yield in AD are 6.5–7.5, the pH range is generally wide for biogas production plants, and the optimal value of pH may vary with the type of substrate and digestion process [
32]. According to which there could have been no inhibition due to the accumulation of VGA that causes such a decrease. The reason that the pH is similar in the three experiments is because the organic loading rate (OLR = 18g-VS/L) is the same and does not cause a significant drop in pH.
Table 2 also indicates that the optimal initial pH to maximize the CMY is 7.34, a lower amount of inoculum caused the pH to rise, leading the digester to a medium that tends to alkalinity (7.76); this affected the CMY. Indeed, the pH variation can be due to some influential digestion parameters, such as VFA, bicarbonate concentration, alkalinity of the system, and also by the fraction of CO2 produced during the process [
31]. The initial and final pH levels belonging to the experiment I:S – 2:1 is adequate to increase the CMY.
There is, a range of C/N rates for which the CMY is the maximum.
Table 2 presents the variation and effect of the C/N rate that occurred during the co-digestion of RR and WS in different fractions, on the CMY. It can be seen that the RME reaches a maximum of 306.21, 272.84 and 289.08mL/g-SV when C/N is 23.26, 38.15 and 38.15 respectively. Adding more inoculum (I:S – 2:1) favors CMY as expected and C/N rates lower than 23.26 provide a higher methane concentration at 40 days of digestion; this could be due to the low C/N rate of the innocuous (7.46) which generates an additional medium with an optimal nutrient balance and therefore a higher methane concentration. When the amount of inoculum drops to 50% (I:S - 1:1) or lower concentrations (I:S - 1:2),
Table 2 reveals that a C/N rate of (16.65 - 38.15) is optimal for the co-digestion of RR and WS, similar results were reported by [
23] with a C/N ratio of (26–27.5). The tendency is, therefore, to decrease the amount of inoculum and maintain the C/N rate at 38.15 to maximize the CMY. The AD process is more stable when the C/N ratio oscillates between 20 and 30, however, for AcoD the existing carbon of the easily degradable part must be taken into account, and the carbon that is not specifically affected by microorganisms [
33].
With the help of the empirical formulas of the substrates, the theoretical methane yield (TMY) in monodigestion and co-digestion is estimated. Regardless of the amount of inoculum used, the TMY is the same for each case. A high synergistic effect index (SEI) corresponds to a high biodegradability percentage. The SEI and BD for the co-digestion of RE and TR are detailed in
Table 2 with a range of SEI from -8.08% to 33.86%. The highest percentages of SEI occur in the co-digestion of RR to WS at a ratio of 3:1 (33.86%), 2:2 (16.48%) and 3:1 (11.10%) for I:S ratios of 1:2, 2:1 and 1:1, respectively. This is due to the adjustment of the C/N ratios during the AcoD and the synergistic effect of (24.20-33.86). These values are higher than those reported by [
23], whose SEI was (14.34 - 29.70). The negative values indicate that at a ratio of RR to WS of 1:3 and 2:2, one of the substrates has a higher percentage of lignin (it is not easily degraded) and as a consequence they present very low BD values. From
Table 2 it can be seen that the BD of WS ranges from 39.05% to 64.25%, of RR ranges from 53.59% to 57.88% in monodigestion and at different fractions of inoculum; a higher load of inoculum increases the BD but not the SEI. Higher SEI percentages occurred at lower inoculum concentrations (I:S – 1:2) and higher concentrations of RR with respect to WS (RR:WS 3:1); furthermore, the BD in this experiment was slightly less than I:S – 2:1 and had similar cumulative methane concentrations. Mixtures of RR and WS in ratios of 1:3-3:1 could increase the variety of nutritional components and subsequently promote the growth of various microorganisms involved in methane production, [
19] reported a relative abundance of 78% of
Methanospirillum as the most dominant archaea in the co-digestion process, the remainder belonging to other archaeal species. The negative SEI percentages in the I:S-1:1 experiment indicate that equal amounts of inoculum and substrates do not favor SEI; the substrates could be generating methane at the expense of the organic matter present in the inoculum itself and not from the substrate as such.
3.4. Evaluation of the different kinetic models
A nonlinear least-square regression analysis was performed using the software MATLAB to fit the nonlinear equations (Gompertz, transfer and logistic) to the average specific cumulative methane production curves with respect to time generated from the triplicate BMP assays. A summary of the kinetic parameters obtained from three models is shown in
Table 3. The experimental data of methane production was compared with the equivalent parameters Bo (Eq. 5, Eq. 6 and Eq. 7) obtained from Gompertz equation (GE), Transfer equation (TE) and Logistic function (LF), respectively. It can be observed that the difference between experimental and adjusted data decreased as I:S rate decreased, particularly in TE model; the higher differences were found for all treatment observing that TE overestimated 27.53% the experimental value while GE and LF models did it in 6.13%. These results could be explained on the basis that smaller amount of inoculum added to the reactor required higher adaptation time of the methanogenic bacteria, reflected in the higher lag value obtained of most treatments in the I:S 1:2 experiment.
For the evaluation of the models, two statistics have been used (
Table 3); a) the coefficient of determination of the adjustment R
2 and b) the root of the mean of the squares of the errors (RMSE). In the table, it is observed that the highest values of R
2 were recorded of GM and LF in the IS 1:2 experiment, their highest R
2 values being between 0.996 and 0.997. Similar results were obtained by [
15,
34]. On the other hand, the remaining experiments present values of R
2 lower than 0.996 for the same fit. According to the
Table 3, TM cannot be useful to estimate the parameters of the experiments, since estimated parameters are much higher than those estimated by GM and LF. We can also note that the fraction of inoculum in each experiment influences the final estimate of the parameters, for example, as the fraction of inoculum increases in the reactor, a greater generation of methane occurs and less latency time than in most of cases are zero or close to zero. This is evidenced in the latency periods of the I:S experiments (2:1, 1:1 and 1:2) for the case of adjustment with GM and LF, the short periods of the lag phase of these experiments indicates the high bioavailability of organic compounds within the substrates [
25], Therefore, the fitting results were consistent with the experimental results.
Vmax follows a parabolic pattern for the three settings, particularly GM in the three inoculum and substrate concentrations, starting with 16.687 mLCH4/gVSd for the I:S 2:1 - RR:WS 0:4 experiment and as the amount of inoculum in the experiment decreases reactor the values drop to 6.708 mLCH4/gVSd for the I:S 1:2 -RR:WS 0:4 experiment, after which it increase to 16.683 mLCH4/gVSd for I:S 1:2 - RR:WS 3:1. It is notorious that there is no significant difference with respect to the inoculum concentration to obtain a high methane generation rate; however, there is a difference with respect to the concentration of substrate and co-substrate. In view of the fact that a lower concentration of inoculum is required in the reactors for the experiments to reflect the degradation of the substrate and co-substrate, we can suggest the experiment I:S 1:2 - RR:WS 3:1 as the most representative.
In general, there is a deviation of the CMY between the measured and the estimated less than 9.29%. The low deviations between the measured and estimated cumulative methane indicate that these models accurately predict behavior in the reactors; [
15,
35], reported a deviation of 10% in their research. This is because RR and WS have a low protein and fat content and a higher carbohydrate content. Carbohydrate conversion can be very fast (within a few days) but protein and fat conversion could take several weeks [
21].
4. Conclusions
In this study, methane production from anaerobic codigestion of RR with WS was investigated. The results suggested that anaerobic co-digestion of RR and WS at a ratio of 3:1 in I:S concentrations of 1:1 and 1:2 could be a viable alternative to produce methane in the future, because the co-digestion process not only could promote methane production, but also reduce the amount of agricultural waste and waste from slaughter plants. Specifically, the co-digestion of RR and WS in a 3:1 ratio (I:S 1:2 - RR:WS) showed an SEI of 33.86% and produced 289.08 mL/g-VS of methane. An SEI of 11.10% was observed for the codigestion of RR and WS in a 3:1 ratio (I:S 1:1 - RR:WS), and a similar methane yield of 272.84 mL/g-VS was achieved, in both experiments and with smaller amounts of inoculum we obtained similar productions, this could be due to the diversity and microbial richness of the codigests.
Experiments reveal that a C/N ratio of (16.65 - 38.15) is optimal for codigestion of RR and WS. Therefore, the tendency is to decrease the amount of inoculum and maintain the C/N ratio at 38.15 to maximize the CMA. It also indicates that the optimal initial pH to maximize the CMY is 7.34, a smaller amount of inoculum caused the pH to rise, leading the digester to a medium that tends towards alkalinity (7.76); this affected the CMY. The highest R2 values were recorded for GM and LF in the IS 1:2 experiment, their highest R2 values being between 0.996 and 0.997. On the other hand, the remaining experiments present R2 values less than 0.996 for the same setting.
References
- T. Bond, M.R. Templeton, History and future of domestic biogas plants in the developing world, Energy for Sustainable Development. 15 (2011) 347–354. [CrossRef]
- J.H. Ebner, R.A. Labatut, J.S. Lodge, A.A. Williamson, T.A. Trabold, Anaerobic co-digestion of commercial food waste and dairy manure: Characterizing biochemical parameters and synergistic effects, Waste Management. 52 (2016) 286–294. [CrossRef]
- J.B. Holm-Nielsen, T. Al Seadi, P. Oleskowicz-Popiel, The future of anaerobic digestion and biogas utilization, Bioresource Technology. 100 (2009) 5478–5484. [CrossRef]
- K. Boe, Online monitoring and control of the biogas process, 2006. Available online: https://doi.org/nei-dk-4757.pdf.
- S.R. Paudel, S.P. Banjara, O.K. Choi, K.Y. Park, Y.M. Kim, J.W. Lee, Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges, Bioresource Technology. 245 (2017) 1194–1205. [CrossRef]
- T.G. Poulsen, L. Adelard, Improving biogas quality and methane yield via co-digestion of agricultural and urban biomass wastes, Waste Management. 54 (2016) 118–125. [CrossRef]
- M.C. Aguilar, Y.D. Wang, T. Roskilly, P.B. Pathare, R.O. Lamidi, Biogas from anaerobic co-digestion of food waste and primary sludge for cogeneration of power and heat, Energy Procedia. 142 (2017) 70–76. [CrossRef]
- S.A. Neshat, M. Mohammadi, G.D. Najafpour, P. Lahijani, Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production, Renewable and Sustainable Energy Reviews. 79 (2017) 308–322. [CrossRef]
- N. Acosta, J. De Vrieze, V. Sandoval, D. Sinche, I. Wierinck, K. Rabaey, Cocoa residues as viable biomass for renewable energy production through anaerobic digestion, Bioresource Technology. 265 (2018) 568–572. [CrossRef]
- J. Gaibor-Chávez, Z. Niño-Ruiz, B. Velázquez-Martí, A. Lucio-Quintana, Viability of Biogas Production and Determination of Bacterial Kinetics in Anaerobic Co-digestion of Cabbage Waste and Livestock Manure, Waste and Biomass Valorization. 0 (2018) 1–9. [CrossRef]
- F. Tufaner, Y. Avşar, Effects of co-substrate on biogas production from cattle manure: a review, International Journal of Environmental Science and Technology. 13 (2016) 2303–2312. [CrossRef]
- F. Valenti, Y. Zhong, M. Sun, S.M.C. Porto, A. Toscano, B.E. Dale, F. Sibilla, W. Liao, Anaerobic co-digestion of multiple agricultural residues to enhance biogas production in southern Italy, Waste Management. 78 (2018) 151–157. [CrossRef]
- C. Holliger, M. Alves, D. Andrade, I. Angelidaki, S. Astals, U. Baier, C. Bougrier, P. Buffière, M. Carballa, V. De Wilde, F. Ebertseder, B. Fernández, E. Ficara, I. Fotidis, J.C. Frigon, H.F. De Laclos, D.S.M. Ghasimi, G. Hack, M. Hartel, J. Heerenklage, I.S. Horvath, P. Jenicek, K. Koch, J. Krautwald, J. Lizasoain, J. Liu, L. Mosberger, M. Nistor, H. Oechsner, J.V. Oliveira, M. Paterson, A. Pauss, S. Pommier, I. Porqueddu, F. Raposo, T. Ribeiro, F.R. Pfund, S. Strömberg, M. Torrijos, M. Van Eekert, J. Van Lier, H. Wedwitschka, I. Wierinck, Towards a standardization of biomethane potential tests, Water Science and Technology. 74 (2016) 2515–2522. [CrossRef]
- I. Angelidaki, M. Alves, D. Bolzonella, L. Borzacconi, J.L. Campos, A.J. Guwy, S. Kalyuzhnyi, P. Jenicek, J.B. Van Lier, Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays, Water Science and Technology. 59 (2009) 927–934. [CrossRef]
- W.O. Meneses-Quelal, B. Velázquez-Martí, J. Gaibor-Chávez, Z. Niño-Ruiz, Biochemical potential of methane (BMP) of camelid waste and the Andean region agricultural crops, Renewable Energy. 168 (2021) 406–415. [CrossRef]
- APHA (American Public Health Association), Standard Methods for the Examination of Water and Wastewater, Standard Methods for the Examination of Water and Wastewater. (2012) 1496.
- D. Valero, J.A. Montes, J.L. Rico, C. Rico, Influence of headspace pressure on methane production in Biochemical Methane Potential (BMP) tests, Waste Management. 48 (2016) 193–198. [CrossRef]
- M. Buswell, H.F. Muellepi, Mechanis of Methane Fermentation, Industrial and Engineering Chemistry. 44 (1952) 550–552. [CrossRef]
- W. Li, M.A.H. Siddhu, F.R. Amin, Y. He, R. Zhang, G. Liu, C. Chen, Methane production through anaerobic co-digestion of sheep dung and waste paper, Energy Conversion and Management. 156 (2018) 279–287. [CrossRef]
- Y. Zhao, F. Sun, J. Yu, Y. Cai, X. Luo, Z. Cui, Y. Hu, X. Wang, Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation, Bioresource Technology. (2018). [CrossRef]
- G.K. Kafle, L. Chen, Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models, Waste Management. 48 (2016) 492–502. [CrossRef]
- W. Li, H. Khalid, Z. Zhu, R. Zhang, G. Liu, C. Chen, E. Thorin, Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin, Applied Energy. 226 (2018) 1219–1228. [CrossRef]
- Z. Zahan, M.Z. Othman, T.H. Muster, Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comparative batch study for C/N optimisation, Waste Management. 71 (2018) 663–674. [CrossRef]
- X.L. S. Pommier, D. Chenu, M. Quintard, A Logistic Model for the Prediction of the Influence of Water on the Solid Waste Methanization in Landfill, Biotechnology and Bioengineering. 97 (2007) 473–481. [CrossRef]
- A. Ware, N. Power, Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions, Renewable Energy. 104 (2017) 50–59. [CrossRef]
- F. Almomani, Prediction of biogas production from chemically treated co-digested agricultural waste using arti fi cial neural network, Fuel. 280 (2020) 118573. [CrossRef]
- Y. Li, Z. Meng, Y. Xu, Q. Shi, Y. Ma, M. Aung, Y. Cheng, Interactions between Anaerobic Fungi and Methanogens in the Rumen and Their Biotechnological Potential in Biogas Production from Lignocellulosic Materials, (2021).
- L. Sun, P.B. Pope, V.G.H. Eijsink, A. Schnürer, Characterization of microbial community structure during continuous anaerobic digestion of straw and cow manure, Microbial Biotechnology. 8 (2015) 815–827. [CrossRef]
- S. Karekar, R. Stefanini, Homo-Acetogens : Their Metabolism and Competitive Relationship with Hydrogenotrophic Methanogens, (2022) 1–22.
- K. Hagos, J. Zong, D. Li, C. Liu, X. Lu, Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives, Renewable and Sustainable Energy Reviews. 76 (2017) 1485–1496. [CrossRef]
- I. Sumantri, P. Kusnadi, I.G.R. Handoyo, A.C. Kumoro, Biodigestion of Mixed Substrates of Cow Manure-Delignified Spent Coffee Ground ( DSCG ) using Microorganism Enhancer for Biogas Production and Its Kinetic Study, (2022) 96–109. [CrossRef]
- M.J. Hahn, L.A. Figueroa, Pilot scale application of anaerobic baf fl ed reactor for biologically enhanced primary treatment of raw municipal wastewater, Water Research. (2015) 1–9. [CrossRef]
- M.N.I. Siddique, Z.A. Wahid, Achievements and perspectives of anaerobic co-digestion: A review, Journal of Cleaner Production. 194 (2018) 359–371. [CrossRef]
- B. Deepanraj, V. Sivasubramanian, S. Jayaraj, Kinetic study on the effect of temperature on biogas production using a lab scale batch reactor, Ecotoxicology and Environmental Safety. 121 (2015) 100–104. [CrossRef]
- F. Raposo, R. Borja, M.A. Martín, A. Martín, M.A. de la Rubia, B. Rincón, Influence of inoculum-substrate ratio on the anaerobic digestion of sunflower oil cake in batch mode: Process stability and kinetic evaluation, Chemical Engineering Journal. 149 (2009) 70–77. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).