1. Introduction
The porcelain tile is a material that offers high compact resistance, frost resistance, and has good durability with low porosity [
1,
2,
3]. According to ISO 10545-3, its water absorption should be less than 0.5%. Porcelain tiles have been used for nearly 40 years as an excellent building material for walls, floors, pavements and paved areas, such as squares, in towns and cities. There are four types of porcelain tile available on the market, combining glazed and unglazed as well as polished and unpolished finishings. Aside from the technical performance, esthetic features are also critical for end-user decision making. The esthetic qualities of unglazed porcelain tiles are heavily influenced by the body color. The glaze layer covers the body color in the case of glazed porcelain tiles but the body color is still important, albeit to a lesser extent than in the case of unglazed products.
The porcelain tile is a silicate ceramic that is made up of many different types of clay minerals, feldspars and quartz [
4]. It can also be classified as a triaxial ceramic due to these components [
5]. With the rapid development of the porcelain industry, clay minerals, which are the most important component used in the porcelain tile preparation, will soon be depleted. The major oxide components are SiO
2 and Al
2O
3, followed by Na
2O, K
2O, CaO and MgO. Fe
2O
3 and TiO
2 are common impurities and during firing the presence of a high content of these components will produce a dark color [
6] in an oxidizing atmosphere. As a result, special consideration should be given to the Fe
2O
3 and TiO
2 contents. To compensate, small amounts of ZrSiO
4 have traditionally been used to improve the whiteness of unglazed porcelain tiles [
7].
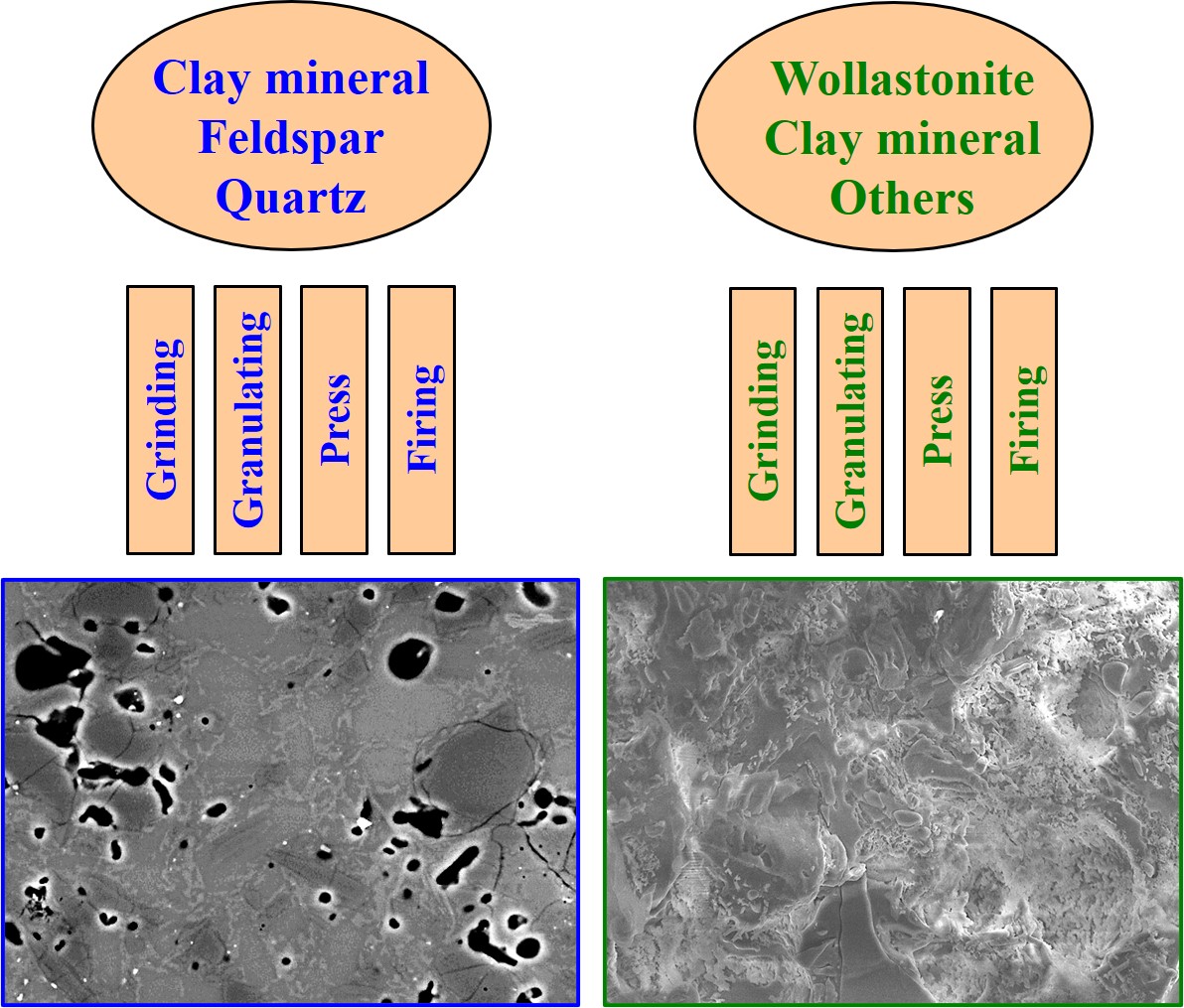
The manufacturing process used to produce porcelain tiles is well-established in the market and is similar to that of other types of ceramic tiles. Porcelain tiles differ from other classes in the following ways: raw material composition, which is more stable in terms of thermal deformations; finer particle size distribution after milling (median size 5–10 µm with 3–6% above 45 µm); higher pressing pressure (35–45 MPa); and higher firing temperature (1180–1220 °C) and time (40–60 min, cold-to-cold firing). In addition to these distinctions, the operational window is narrow in order to maintain competitive quality, productivity and global costs. Although the above are all conventional production parameters of porcelain tiles, the specific production parameters of porcelain tiles are not always exactly the same. To ensure that water absorption under 0.5% can be achieved, different sets of process parameters and types of unit operations can be applied.
The final microstructure of porcelain tiles is composed of a glassy matrix (55–65%) with closed pores (4–6%), dispersed crystalline particles, such as quartz, zirconite, unmolten feldspar, and an in situ crystalized phase, such as mullite (to balance the proportions). The mullite-based porcelain tile (MPT) dominates the market and the anorthite-based porcelain (APT), is the most promising candidate to coexist with or replace MPT. Although APT has been conducted successfully for many ceramic applications [
8], including porcelain tiles [
9] at a laboratory scale, it has not yet been industrial manufacturing ever since as well as MPT. As a result, a comprehensive comparison of MPT and APT is reported herein with regard to the raw materials, processing, phase evolution and mechanical behavior, based on the porcelain tile firing behavior and other process requirements.
2. Raw Materials
2.1. Mullite-Based Porcelain Tile
A typical MPT starting composition is 40–50% clay, 35–45% feldspar and 10–15% quartz sand, as reported by Andreola and co-workers [
10]. In particular, the formula contains 50% kaolinitic clay, 40% feldspar and 10% quartz sand has been studied in-depth by Romero and co-workers, with a focus on, but not limited to, the kinetic, microstructural and mechanical behavior of porcelain tiles [
11,
12,
13,
14,
15,
16,
17]. In this formula there are three main oxides: K
2O, Al
2O
3 and SiO
2, mainly introduced by feldspar, clay and quartz, respectively, while other oxides are added as low-content impurities of these three raw minerals. Due to the three main components, compositions are mostly members of the K
2O-Al
2O
3-SiO
2 system.
In general, kaolin, china clay [
18], illite clay [
19], pyrophyllite [
20] and flint clay are used as clay minerals to provide plasticity and dry mechanical strength to the green body. Clay minerals, including calcined kaolin (containing Al
2O
3), and flux components are used to develop the mullite and glassy phase. Microcline [
21], albite [
22], pegmatite [
23], perlite, nepheline syenite [
24], spodumene [
25], diopside [
26] and tremolite are used as feldspar minerals. Feldspar minerals produce a liquid phase at low temperature (the theoretical melting points for microcline and albite are around 1250°C and 1100°C, respectively) to promote densification of the porcelain tile. The use of these raw materials leads to a major liquid phase belonging to the Na
2O-K
2O-Al
2O
3-SiO
2 system. Talc and calcite can be used as minor fractions, up to 5%, to form additional eutectic phase with feldspar and reducing the firing temperature [
27]. Sands are the most commonly used quartz minerals but quartz is also present in industrial raw materials such as clays and feldspars. These improve the thermal and dimensional stability due to a high melting point, which partially offsets firing shrinkage. High representative oxide content, low impurity oxides and low loss on ignition (LOI) are the main criteria used to evaluate whether a raw mineral material is of high quality. Clay minerals with high Al
2O
3 content, feldspar minerals with high K
2O and Na
2O content and near zero LOI and quartz minerals with almost 100% SiO
2 are all ideal minerals for MPT preparation. High-grade minerals will provide MPT materials with low water absorption, high density, lower firing shrinkage and enhanced mechanical properties.
With the rapid depletion of natural mineral resources, alternative compositions for porcelain tile preparation urgently need to be developed. In this regard, two new ways to prepare alternative porcelain tiles have been developed. One is to add a green body enhancer [
28,
29] to produce porcelain tiles with less or no plastic minerals and the other is to incorporate bulk urban/industrial waste [
4,
5,
10,
30,
31,
32,
33,
34,
35,
36] in the porcelain tile manufacturing process. It is worth noting that for porcelain tiles prepared with less or no plastic minerals (such as clay mineral) the phase composition/ratio will differ significantly from that of traditional MPTs. Producing porcelain tiles with bulk urban/industrial waste is a promising approach due to the bulk stock and considerable proportion of useful ingredients. In addition, reusing waste has beneficial effects on both the environment and the economy. Future approaches to porcelain tile production with less/no clay minerals should also aim to provide decorative appearance, large size, better mechanical properties, lower firing temperature and shorter firing time in order to reduce energy consumption and carbon dioxide emissions.
2.2. Anorthite-Based Porcelain Tile
The composition of an APT body can comprise around 20% clay mineral, 25% wollastonite, 30% alumina, 20% quartz and 5% basic magnesium carbonate [
37]. The anorthite-based porcelain tile and hard porcelain have been studied by Capoglu and coworkers [
37,
38,
39,
40]. The composition is made up of three main oxides, SiO
2, Al
2O
3 and CaO, and thus they belong to the CaO-Al
2O
3-SiO
2 system. These oxides are supplied by wollastonite, clay mineral, alumina and quartz. MgO has been used a sintering aid [
41] to produce fired materials and enable it easily vitrify and densified. Furthermore, investigations have been carried out suggesting that wollastonite is the best CaO source considering Ca(OH)
2, CaCO
3, marble powder, gypsum mold waste, dolomite, wollastonite and calcite [
42,
43]. However, Ibañez and Sandoval reported the relatively low availability of natural wollastonite deposits [
44]. The same authors cited other papers which addressed applications of wollastonite-clay-feldspar mixtures for high and low porosity ceramic products, but not porcelain tiles. Wollastonite can reduce the drying and firing shrinkage and improve the dried and fired mechanical strength, but it narrows the sintering interval [
44,
45]. In recent years, new wollastonite deposits have been explored to supply a superior low-temperature fast-firing mineral, attracting considerable attention not only from academic researchers but also the ceramics industry.
The clay minerals and quartz minerals used in the APT preparation are the same as those used for MPT. Wollastonite and calcite have been used as calcium-containing minerals. It can be inferred from the description above that clay minerals with high Al
2O
3 content, minerals with high CaO content and near zero LOI and quartz minerals with almost 100% SiO2 all are ideal minerals for APT preparation. High-grade minerals will provide APT with low water absorption, high density, lower firing shrinkage and enhanced mechanical properties, as described in the section on MPT. APT was successfully developed by Tai et al. [
46] using aluminous cement as binder instead of traditional plastic raw materials. Wu et al. [
47] used diopside and magnesia clay as a source of MgO and CaO. Tarhan [
48] added 12% of a diopside-based frit to a porcelain tile composition. In both cases, anorthite and diopside were crystallized in the final body. Selli [
7] added 1.0–1.5% of CaO and 4–6% of Al
2O
3 to a regular porcelain tile composition and obtained anorthite and mullite as crystallized phases. In these cases, the reference system was CaO-MgO-Al
2O
3-SiO
2.
Another way to describe or analyze a porcelain tile composition is with regard to the oxide components. It has been noted that the content of SiO
2 should be 60–70%. When the SiO
2 content is in excess of 75% the porcelain tile can crack due to the transformation of β-SiO
2 to α-SiO
2 in the quartz [
49] during cooling. The appropriate Al
2O
3 content is above 20%. However, production experiences have shown that increasing the Al
2O
3 content in the formula can increase the firing temperature and expand the sintering temperature range. With an Al
2O
3 content of less than 18%, although the porcelain can be fired, the sintering temperature range is narrow and product deformation can easily occur. In the MPT formula, mullite is derived from the clay/feldspar components [
50]. If the K
2O (Na
2O) content in the formula is too low (less than 5%), the product will be difficult to sinter while a content that is too high (more than 10%) will significantly reduce the firing temperature and product deformation can easily occur. It has been verified that too much CaO (excess 15%) will significantly narrow the firing temperature range, resulting in difficulties during industrial production.
Based on scientific research, from the economical perspective, the less variety of raw minerals used in the composition of porcelain tiles the better. In addition, a large number of components makes analysis difficult to carry out. From the perspective of engineering applications, multi-variety of raw minerals used in the formulation of porcelain tiles is preferable. Also, when more types are used the content of each is smaller. The raw minerals can be replaced with similar minerals and the properties of the product will remain almost unchanged, thereby significantly reducing the limitation of mineral resource type.
3. Processing
Porcelain tile production lies within the category of powder technology processes. The milling process can be dry or wet and extrusion or pressing can be used as a forming stage. Tunnel or roller kilns can be used for firing. Despite other pathways being available, wet milling, pressing and roller kiln are the most common processes and they are widely used worldwide. Wet milling, as well as powder pressing, can be used in batch or continuous processes. Batch firing is mainly limited to laboratory size, whereas continuous and fast firing are more widely used due to productivity and cost considerations. The type of product (glazed or unglazed, small or large, thin or thick) and the target market (high or low added value; residential, commercial or industrial applications) determine the section of the process options. Also, the availability of raw materials can sometimes be a deciding factor. Unglazed porcelain tiles, for example, cannot be made with low-whiteness raw materials. In all circumstances, firing is the main step.
The choice of dry versus wet milling is one of the most crucial aspects of establishing the flow chart for a new plant. Both processes can create glazed porcelain tiles that are nearly identical [
51]. When taking the dry route, the main objective is usually to obtain a low-cost product. However, the milling technology is only one factor to consider and other factors of the product design, such as the quality of the raw materials and the glazed surface, also need to be taken into account.
Dry-processing is the simplest method available to produce porcelain tiles. Some authors show that this approach is less energy-intensive than the wet route [
52,
53,
54]. Ball or pendulum mills are used to blend and mill the dried raw materials. Following milling, the mixes are subjected to a particle size-enlargement process, for instance, using as a muller-mixer, to granulate the powder by adding 9% to 11% moisture [
51].
The wet-processing route of obtaining the granulated powder composition is more expensive. Ball milling is used to reduce the size of the particles in the water suspension in this case. A typical solid load in the slurry is 60% to 65% by weight. Inorganic or organic ionic dispersants are commonly employed to produce the highest possible solids loading in order to save energy in the spray-drying process. Spray-dried powder is used to dry and granulate the particles at the same time. When compared to the dry route, the wet route produces more homogenous mixing between particles. Spray-dried powder is well-rounded, with a moisture content between 5.5% and 7.5%.
The granulates are pressed onto a green porcelain tile body and then fired. The porcelain tile can then be cut, edged, polished and lastly packaged as a saleable product. In terms of processing route, there are no substantial distinctions between MPT and APT.
The sintering of porcelain tiles is mainly attributed to the diffusion behavior driven by thermodynamics. During sintering, both solid-phase mass transfer and the formation of a high-temperature liquid phase are conducive to particle rearrangement, filling open pores and completing the densification of sintering. Seen from its appearance changes, the porcelain body exhibits volume shrinkage, a decrease in open porosity and an increase in both strength and bulk density during sintering.
4. Phase Evolution
To better understand the differences between MPT and APT, a comparison of chemical compositions was given in
Table 1. APT green body has higher Al
2O
3, extremely higher CaO, MgO and lower SiO
2 than MPT’s; both green bodies contain kaolin and quartz phases, besides MPT green body comprises feldspar while APT green body comprise wollastonite and corundum phases. Variances in the source components and their contents of MPT and APT greatly effects their firing behavior, phase evolution and eventually determines the final microstructure and mechanical properties. Since with the rapid development of the porcelain industry, clay minerals, which are the most important component used in the porcelain tile preparation, will soon be depleted. Thus, APT was developed as alternative composition. The final microstructure and properties of ceramic materials are established during firing, which is the most important step in their processing. The main reaction is the densification through-out sintering. Many other reactions occur in addition to sintering, including clay mineral and other hydroxide dehydroxylation, organic matter volatilization and oxidation, phase transitions, thermal expansion, melting and crystallization [
55]. In the case of porcelain tile manufacturing, liquid phase sintering is usually the dominant mechanism. Distinct final phase kind, content and distribution, which is determined directly by the raw materials kind, batch formula and processing procedure feature, will significantly affect the mechanical properties of final product. Hereby, we investigated the final phase composition of two kinds of porcelain tile, to understand and interpret the mechanical property differences (namely bending strength) between them.
4.1. Mullite-Based Porcelain Tile
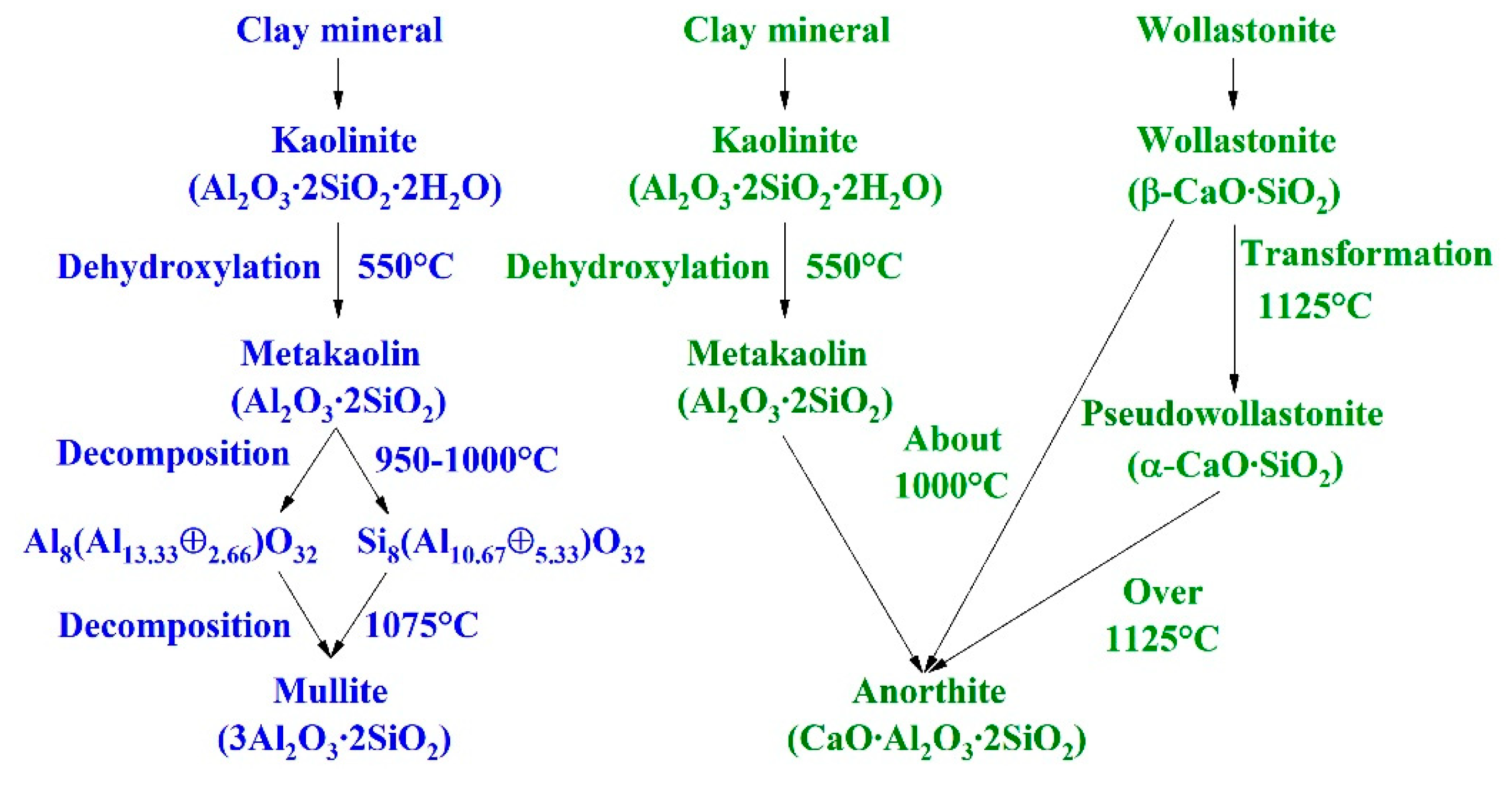
The sintering temperature of MPT is around 1200 °C. With the elevation of sintering temperature, a glassy matrix is gradually formed by the melting of potassium-rich clays and alkaline-rich rocks such as feldspars at around 1100 °C. Residual feldspar particles can be present in the final microstructure depending on the process time and temperature. Quartz is present in the amounts of 20–25% as α-quartz and the particles remain unreacted, with the exception of those below 6 µm, which can be partially or completely dissolved by the liquid phase. The main reaction involving quartz is its allotropic transition β → α at 573 °C during the cooling step. This transition generates thermal stress and can collapse the body if the cooling rate is too fast (cooling stage, around 573 °C, less than 30°C/min). Mullite, after quartz, is the second richest crystalline phase in the porcelain tile microstructure (7–16%). In some cases, small amounts of corundum or zirconite are also found [
56]. Mullite phase can be presented in two stoichiometric forms [
57], 3Al
2O
3‧2SiO
2 or 2Al
2O
3‧SiO
2, and in MPT, it usually stands for the former. In general, mullite is developed in a routine MPT sintering process with the following steps [
58]: (a) dehydroxylation of hydroxyl groups contained in kaolinite (Al
2O
3‧2SiO
2‧2H
2O), introduced by clay or kaolin, to form metakaolin (Al
2O
3‧2SiO
2) at around 550°C; (b) metakaolin decomposition to form a spinel-type structure (Al
8(Al
13.33⊕
2.66)O
32 or Si
8(Al
10.67⊕
5.33)O
32), while amorphous free silica is released at around 950–1000°C; and (c) the spinel phase transforms to mullite phase at above 975°C. Notably, a MPT body comprises two kinds of mullite: primary mullite and secondary mullite. Primary mullite is comprised of very small crystals (with low aspect ratio, approximately 1–3:1) derived from clay at a lower temperature [
59]; secondary mullite consists of needle-like crystals (with high aspect ratio, from 5:1 to 20:1) and grows through the liquid phase while primary mullite serves as a seed for the secondary mullite crystallization [
15,
60]. In the K
2O-Al
2O
3-SiO
2 system, primary mullite forms at around 985°C [
61] under a fast rection. Secondary mullite formation is much slower in comparison with primary mullite and its completion is dependent on the process time and temperature. In a typical porcelain tile microstructure, the primary mullite accounts for nearly all of the mullite [
15,
27].
4.2. Anorthite-Based Porcelain Tile
In the case of APT, anorthite is the main phase with a representative composition of around 52% anorthite, 12% corundum, 8% cristobalite and 28% glassy phases. Anorthite is formed from the CaO introduced by wollastonite, Al
2O
3 introduced by clay minerals and/or alumina and SiO
2 introduced by quartz [
37]. The formation of anorthite begins with the dehydroxylation of kaolinite (Al
2O
3‧2SiO
2‧2H
2O, introduced by clay minerals) to form metakaolin (Al
2O
3‧2SiO
2) and ends with the reaction of metakaolin (Al
2O
3‧2SiO
2) and wollastonite (CaO‧SiO
2). The formation temperature of anorthite is around 1000°C [
62] in the CaO-Al
2O
3-SiO
2 system. In addition, anorthite can also be formed from metakaolin and CaO [
63], or mullite, SiO
2 and CaO [
64].
The firing time appears to affect the form of the anorthite phase with a rounded shape, or low aspect ratio, for a short time [
48] and needle-like crystals for a longer time, showing similar firing behavior as the mullite phase. The anorthite phase is a congruent melting compound in the CaO-Al
2O
3-SiO
2 system, just the same as the mullite phase in the K
2O-Al
2O
3-SiO
2 system. More specifically, they show a preferred orientation of c-axis growth under constant heating for a relative long time. It is worth noting that the formation velocity of the anorthite phase in a typical APT body is between those of the primary and secondary mullite phases in a typical MPT body under the same processing condition. The anorthite phase formation velocity is competitive with both types of mullite, especially secondary mullite. This point is of interest, since the K
2O-Al
2O
3-SiO
2 and CaO-Al
2O
3-SiO
2 systems could be joined together with the final phase of primary mullite, the glassy phase and the anorthite phase (to replace secondary mullite). This new combination could probably lead to a new mullite-anorthite porcelain with distinct performance compared with MPT or APT, and it could achieve some unexpected properties. Attempts to determine the raw composition should be made, since the processes of anorthite and mullite formation are competitive reactions. Brasileiro et al. [
65] reported a reduction in mullite content from 7.7% to around 3.8% when wollastonite or diopside were added to an industrial MPT composition.
For these two types of porcelain tile, the phase compositions were determined by the types of raw material and their contents, indicating different ternary systems (K2O-Al2O3-SiO2 or CaO-Al2O3-SiO2). Indeed, the main components (e.g., oxide) in a formula determine the peak firing temperature, firing temperature range and firing behavior. The firing temperature was mainly defined by the content of Al2O3 and its particle diameter was affected by the proportion of oxide flux. Although Fe2O3 and TiO2 are not expected to be present in a green porcelain body, they will promote sintering and densification to some extent.
A comparison of the mullite development in MPT and anorthite development in APT is given in
Figure 1. For mullite formation, kaolin minerals are the most critical, and for the formation of anorthite, kaolin and wollastonite minerals are both indispensable.
Table 2 shows a comparison of the fundamental physical properties of typical crystal phases that could form in a porcelain material [
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78]. It can be seen that the corundum phase has much higher thermal conductivity (nearly 6 times that of mullite). This indicates that if a material with high thermal conductivity is prepared, corundum phase is far more suitable than mullite phase as a target phase.
5. Phase Evolution
Vickers microhardness (HV), bending strength (σf), fracture toughness (KIC) and Young's modulus (E) are all mechanical properties of porcelain tiles that are closely related to the phase composition and microstructure features [
79]. The bending strength, along with the water absorption (Wa), are key aspects for determining the quality of porcelain tiles.
The mechanical properties of all varieties of porcelain tile are influenced by porosity (P), which is one of the most important microstructural parameters [
80]. Low porosity increases the E value and decreases the flaw size, which increases the KIC and σf values. The mechanical characteristics improved with interactions between the processing parameters that lead to reduced porosity [
56]. The interactions between the particle size distribution, moisture and forming pressure promote sintering due to the improved green density. Finer particles increase the sintering rate, especially if particle packaging [
81] is not reduced significantly, reducing the final porosity, pore size and flaw size. The most common pores in porcelain tiles can be divided into two types: connected open pores with irregular shapes and closed pores with regular shapes (usually round and oval).
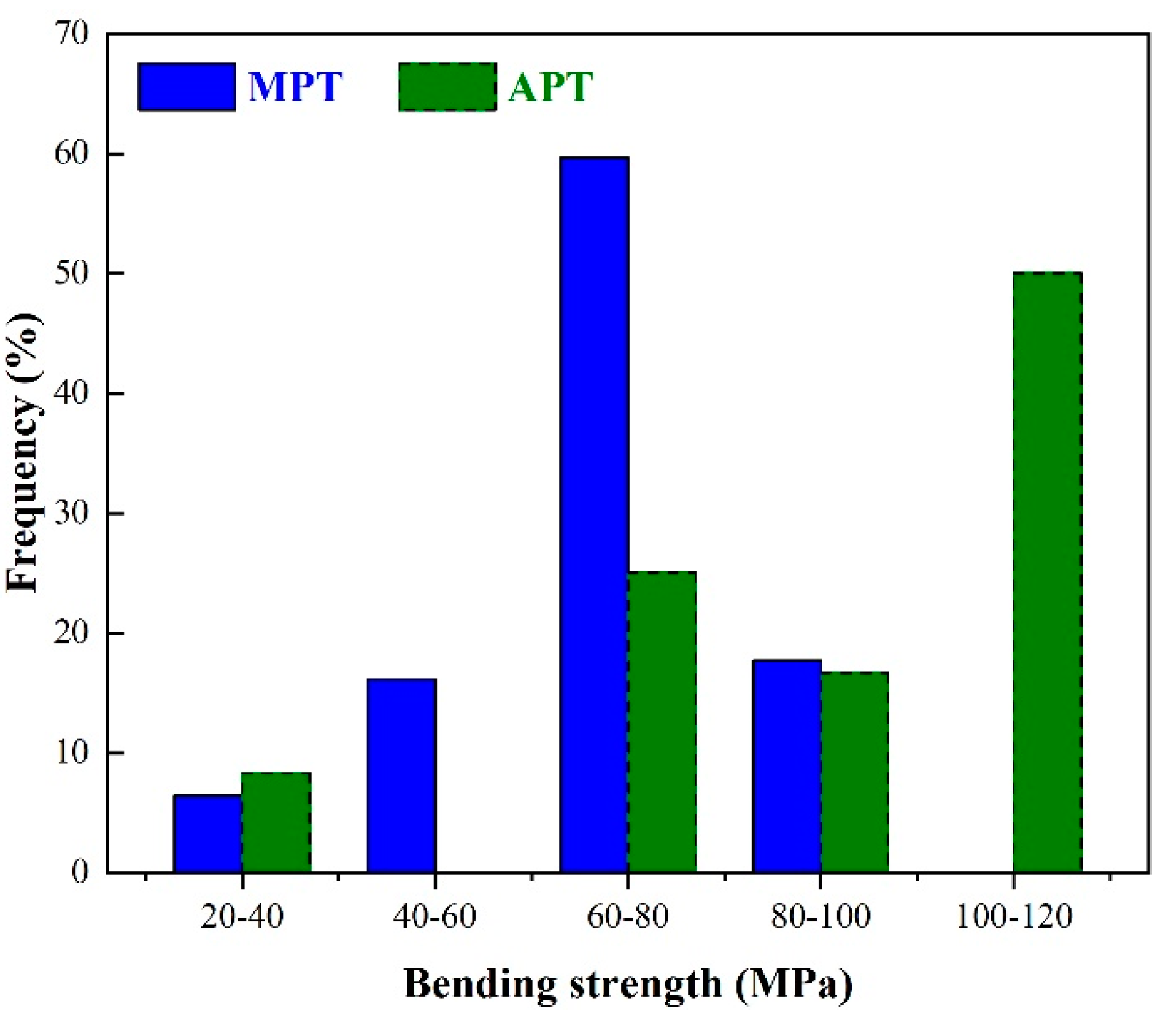
The chemical and mineralogical composition of porcelain tiles have a strong effect on the mechanical properties. A component that increases the sintering rate and thus lowers the porosity and pore size distribution will also improve the mechanical properties. Other effects are dependent on the dispersed crystalline particles and clusters that remain or form during the sintering. The role of such components are the key aspects that differentiate mullite from anorthite porcelain tiles. In generally, high-strength porcelain tiles have the following characteristics: few or no open/connected pores, a small number of regular-shaped closed pores, dense cross-sections without obvious cracks or defects, and a crystal phase that is tightly surrounded by the glass phase or matrix. The frequency histogram in
Figure 2 shows bending strength data taken from the literature on MPT and APT [
9,
13,
21,
27,
37,
39,
43,
46,
74,
77,
79,
80,
82,
83,
84,
85,
86,
87]. In many cases the values are similar, but APT appears to have higher bending strength values than MPT.
5.1. Mullite-Based Porcelain Tile
The crack deflection and glass matrix stress effect are the major reinforcement mechanisms in porcelain tiles. Every dispersed crystalline phase or particle can cause crack deflection, enhancing the fracture toughness. Finer particles, when compared to coarser particles, have a more beneficial effect because they result in a shorter mean free path between particles [
88]. Finer particles can help reduce the flaw size, improving the bending strength. This is one of the beneficial effects of secondary and primary mullite clusters. The holding time is usually inadequate (usually 10–15 mins) to form a significant amount of interconnected secondary mullite. The same beneficial effects can be achieved with finer quartz particles [
89]. Coarse quartz particles can form clusters and increase the flaw size, while reducing the bending strength [
90].
According to Selsing's model [
91], particles with thermal expansion mismatch generate microscopic residual stress due to glass matrix stress effects. Quartz particles have a higher thermal expansion coefficient (TEC) than the glass matrix, causing tangential compressive stress across the matrix. As a result, the fracture energy and the bending strength increase [
92]. The size of the quartz particles affects (positively or negatively) the mechanical properties. Particles larger than ~45 µm are surrounded by microcracks that have detached from the matrix and are unable to generate stress in the matrix. The Young's modulus [
93] and bending strength can both be reduced by the cracks. Microcracks partially surround particles above ~6 µm and below ~45 µm, causing compressive stress in the glass matrix. Particles below ~6 µm are partially dissolved and generate tensile stress, which lowers the fracture energy by forming a glass silica-rich interface with a lower TEC than the glass matrix [
92]. Mullite also has a lower TEC than the glass matrix, which reduces the fracture energy.
The dispersed crystalline phase and particles in mullite porcelain tiles have a notable antagonistic effect on the mechanical properties. In addition to this complex relationship, the product is subjected to a fast-cooling rate, which results in macroscopical residual stress increasing the bending strength [
94]. A fast-cooling rate can also have a negative impact on the mechanical characteristics, increasing the flaw size [
94].
Finding a balance between the positive and negative effects of each reinforcing mechanism is a key factor in relation to improving the mechanical properties. The data indicate that the bending strength of mullite porcelain tiles could be increased from ~60 MPa to ~90 MPa using this approach [
27,
95].
5.2. Anorthite-Based Porcelain Tile
CaO will greatly narrow the firing temperature range, and overfired deformation will be observed. Thus, the CaO content should be strictly controlled. During sintering, the glassy phase formed with CaO has lower viscosity than that of K2O or Na2O, leading to easier particle rearrangement and the filling in of pores, thus accelerating the densification process. Relatively low viscosity means less open porosity and less glassy phase, leading to high mechanical strength. On the other hand, an accelerated densification process shortens the period from melting to completion and narrows the acceptable temperature range.
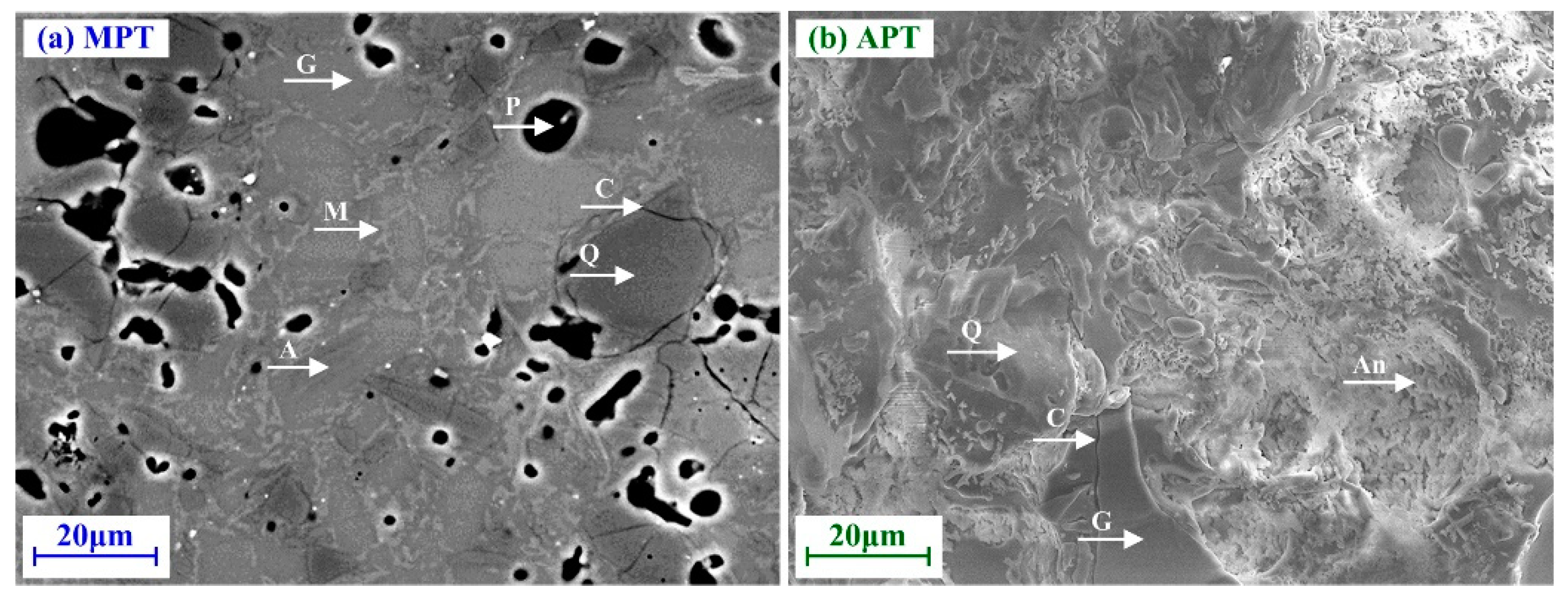
Figure 3 shows the typical microstructures of MPT and APT. In the case of MPT, the micrograph is of an industrial sample. The cross-section was polished with 2 μm alumina paste. No sputtering of a conducting element was applied. The SEM (Philips XL30 CP, The Netherlands) setup was: 20 kV, spot 5.8, BSE detector, 0.2 mBar and 800× magnification. The original photo was adjusted by balancing the brightness, contrast and sharpness using image editing software.
In the case of APT, the micrograph is of a laboratory sample. The fractured surface was subjected to Au sputtering, without polishing and etching. The SEM (Zeiss Ultra Plus, Carl Zeiss AG) setup was: 15 kV, spot 1.0, BSE detector, 5×10−6 mBar, 1000× magnification. The original photo was treated using image editing software (the same used in the case of MPT).
The MPT microstructure is formed by a glass matrix (G), closed pores (P), quartz particles (Q) surrounded by cracks (C), unmelted albite (A) and closed pores (P). Primary mullite clusters (M) can be observed surrounding quartz particles and the glass matrix originated from albite particle melting. In this case, these cluster do not appear to be interconnected, because of the low amount of kaolinite in the starting composition [
81]. The APT microstructure is formed by interconnected anorthite clusters embedded in a glass matrix (G), surrounded by quartz particles (Q) and cracks (C). The absence of irregular pores and closed pores is probably due to a relatively long firing cycle which led to the redistribution of particles and the elimination of pores. The criss-crossing anorthite and surrounding quartz phases probably contribute to the strength of the fired body.
A comprehensive comparison of MPT and APT is given in
Table 3, based on the above discussions.
The crystalline phase type, the content of each phase and the microstructure of the anorthite-based porcelain tile show the same behavior when compared with the mullite-based porcelain tile. However, in contrast to MPT, the bending strength of APT is governed by the anorthite content, glassy phase, reinforcing phase (such as corundum and zirconite) and open porosity and is affected by the prestress obtained and blisters.
The most striking difference between MPT and APT is the representative phase formed as well as the way it is distributed. In the literature, APT reportedly has a higher strength than MPT and this difference probably originates from the ratio of crystalline to amorphous phase: which for MPT is 2:4 (~55 MPa) while for APT it is close to 3:1 (~110 MPa). A high content of CaO will greatly narrow the firing temperature range, and overfired deformation will be observed. Differs from the MPT body contain K2O and Na2O, APT body contains CaO will form a soluble calcium glass phase at high temperature which will significantly decrease the viscosity of the melt, leading much narrower firing temperature range. Thus, the CaO content should be strictly controlled. During sintering, the glassy phase formed with CaO has lower viscosity than that of K2O or Na2O, leading to easier particle rearrangement and the filling in of pores, thus accelerating the densification process. Relatively low viscosity means less open porosity and less glassy phase, leading to high mechanical strength. On the other hand, an accelerated densification process shortens the period from melting to completion and narrows the acceptable temperature range.
Figure 3 shows the typical microstructures of MPT and APT. In the case of MPT, the micrograph is of an industrial sample. The cross-section was polished with 2 μm alumina paste. No sputtering of a conducting element was applied. The SEM (Philips XL30 CP, The Netherlands) setup was: 20 kV, spot 5.8, BSE detector, 0.2 mBar and 800× magnification. The original photo was adjusted by balancing the brightness, contrast and sharpness using image editing software.
In the case of APT, the micrograph is of a laboratory sample. The fractured surface was subjected to Au sputtering, without polishing and etching. The SEM (Zeiss Ultra Plus, Carl Zeiss AG) setup was: 15 kV, spot 1.0, BSE detector, 5×10−6 mBar, 1000× magnification. The original photo was treated using image editing software (the same used in the case of MPT).
The MPT microstructure is formed by a glass matrix (G), closed pores (P), quartz particles (Q) surrounded by cracks (C), unmelted albite (A) and closed pores (P). Primary mullite clusters (M) can be observed surrounding quartz particles and the glass matrix originated from albite particle melting. In this case, these cluster do not appear to be interconnected, because of the low amount of kaolinite in the starting composition [
81]. The APT microstructure is formed by interconnected anorthite clusters embedded in a glass matrix (G), surrounded by quartz particles (Q) and cracks (C). The absence of irregular pores and closed pores is probably due to a relatively long firing cycle which led to the redistribution of particles and the elimination of pores. The criss-crossing anorthite and surrounding quartz phases probably contribute to the strength of the fired body.
A comprehensive comparison of MPT and APT is given in
Table 3, based on the above discussions.
6. Prospects and Outlook
The raw material, processing, phase evolution and mechanical behavior of MPT and APT were discussed. The conclusions could be draw as follows:
1. Typically, APT is prepared using 50% clay, 40% feldspar and 10% quartz and it can be attributed to SiO2-Al2O3-K2O ternary system; an MPT can be prepared using 20% clay mineral, 25% wollastonite, 30% alumina, 20% quartz and 5% basic magnesium carbonate and it can be attributed to SiO2-Al2O3-CaO ternary system. Variances in the source components and their contents of MPT and APT greatly effects their firing behavior, phase evolution and eventually determines the final microstructure and mechanical properties. The insufficient reserves of wollastonite in major porcelain tile manufacture countries affects the industrial application of APT.
2. MPT and APT has no substantial distinctions in processing route except sintering temperature, sintering temperature range and holding time. The mature similar parameters are: mean powder particle size 5–6 μm, fine particle 10% and coarse particle 90%, granulating powder moisture 5–7%, forming pressure 33–45 MPa, and cold to cold time 35–60 mins. The average sintering temperature of APT is 40°C lower than that of MPT whereas its sintering temperature range is more than 40°C narrower than MPT’s. Much narrow sintering temperature range is the main obstacle the industrial application of APT. A combined system of SiO2-Al2O3-K2O and SiO2-Al2O3-CaO ternary system, as well as flux consist of both feldspar and magnesia-contain component, may improve the firing behavior and furthermore promote the industrial application of APT.
3. Mullite is the feature phase in MPT, and anorthite is the feature phase in APT. Due to a larger ratio of crystalline to amorphous phase, the crystalline phase type and a higher CaO content, APT has a mechanical strength two times higher than that of MPT. APT and MPT have comparable whiteness.
4. MPT has dominated the porcelain tile market to date and its in-process behavior is better understood compared to that of APT. However, APT represents a promising option for replacing, or for use in combination with, MPT on a large scale, in order to achieve better results.
Author Contributions
Conceptualization, Kun Li and Agenor De Noni Junior; writing—original draft preparation, Kun Li, Eloise Cordeiro and Agenor De Noni Junior; data collection, Eloise Cordeiro; writing—review and editing, Kun Li, Eloise Cordeiro and Agenor De Noni Junior; supervision, Agenor De Noni Junior; project administration, Kun Li; funding acquisition, Kun Li and Agenor De Noni Junior. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Science and Technology of Shaanxi Province, grant number 2020GY-314; Shaanxi University of Technology, grant number SLGRCQD2021; and Brazilian National Council of Scientific and Technological Development CNPQ, grant number 302555/2020-0.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Department of Science and Technology of Shaanxi Province [grant number 2020GY-314], Shaanxi University of Technology [grant number SLGRCQD2021] and Brazilian National Council of Scientific and Technological Development CNPQ [grant number 302555/2020-0].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amorós, J.L.; Blasco, E.; Moreno, A.; Feliu, C. Kinetics of the transformations occurring during the firing process of an industrial spray-dried porcelain stoneware body. Ceram. Int. 2022. Ceram. Int. 2022, 48, 17611–17620. [Google Scholar]
- la Garza, D.A.A.-D.; Guzmán, A.M.; Gómez-Rodríguez, C.; Martínez, D.I.; Elizondo, N. Influence of Al2O3 and SiO2 nanoparticles addition on the microstructure and mechano-physical properties of ceramic tiles. Ceram. Int. 2022, 48, 12712–12720. [Google Scholar]
- Demarch, A.; Waterkemper, A.; Pasini, D.; Ruzza, S.; Montedo, O.R.; Angioletto, E. Effects of roughness parameters on slip resistance for different methods used to determine the coefficient of friction for ceramic floor tiles. Ceram. Int. 2021, 47, 24281–24286. [Google Scholar]
- Dana, K.; Das, S.; Das, S.K. Effect of substitution of fly ash for quartz in triaxial kaolin-quartz-feldspar system. J. Eur. Ceram. Soc. 2004, 24, 3169–3175. [Google Scholar]
- Serra, M.F.; Conconi, M.S.; Suarez, G.; Aglietti, E.F.; Rendtorff, N.M. Volcanic ash as flux in clay based triaxial ceramic materials, effect of the firing temperature in phases and mechanical properties. Ceram. Int. 2015, 41, 6169–6177. [Google Scholar]
- Bhattarai, J.; Okada, K. Characterization of clay raw materials in Nepal and their applicability for porcelain raw material. Clay Sci. 1992, 8, 393–402. [Google Scholar]
- Selli, N.T. Development of anorthite based white porcelain stoneware tile compositions. Ceram. Int. 2015, 41, 7790–7795. [Google Scholar]
- Cheng, X.; Ke, S.; Wang, Q.; Wang, H.; Shui, A.; Liu, P. Characterization of transparent glaze for single-crystalline anorthite porcelain. Ceram. Int. 2012, 38, 4901–4908. [Google Scholar]
- Ozturk, Z.B. Microstructural characterization of mullite and anorthite-based porcelain tile using regional clay. J Ceram. Process. Res. 2016, 17, 555–559. [Google Scholar]
- Andreola, F.; Barbieri, L.; Corradi, A.; Lancellotti, I.; Manfredini, T. Utilisation of municipal incinerator grate slag for manufacturing porcelainized stoneware tiles manufacturing. J. Eur. Ceram. Soc. 2002, 22, 1457–1462. [Google Scholar] [CrossRef]
- Romero, M.; Martín-Márquez, J.; Rincón, J.M. Kinetic of mullite formation from a porcelain stoneware body for tiles production. J. Eur. Ceram. Soc. 2006, 26, 1647–1652. [Google Scholar]
- Martín-Márquez, J.; Rincón, J.M.; Romero, M. Effect of firing temperature on sintering of porcelain stoneware tiles. Ceram. Int. 2008, 34, 1867–1873. [Google Scholar]
- Martín-Márquez, J.; Rincón, J.M.; Romero, M. Effect of microstructure on mechanical properties of porcelain stoneware. J. Eur. Ceram. Soc. 2010, 30, 3063–3069. [Google Scholar]
- Martín-Márquez, J.; De la Torre, A.G.; Aranda, M.A.; Rincón, J.M.; Romero, M. Evolution with temperature of crystalline and amorphous phases in porcelain stoneware. J. Am. Ceram. Soc. 2010, 92, 229–234. [Google Scholar]
- Martín-Márquez, J.; Rincón, J.M.; Romero, M. Mullite development on firing in porcelain stoneware bodies. J. Eur. Ceram. Soc. 2010, 30, 1599–1607. [Google Scholar]
- Pérez, J.M.; Rincón, J.M.; Romero, M. Effect of moulding pressure on microstructure and technological properties of porcelain stoneware. Ceram. Int. 2012, 38, 317–332. [Google Scholar]
- Pérez, J.M.; Romero, M. Microstructure and technological properties of porcelain stoneware tiles moulded at different pressures and thicknesses. Ceram. Int. 2014, 40, 1365–1377. [Google Scholar]
- Kamseu, E.; Leonelli, C.; Boccaccini, D.N.; Veronesi, P.; Miselli, P.; Pellacani, G.; Melo, U.C. Characterisation of porcelain compositions using two china clays from Cameroon. Ceram. Int. 2007, 33, 851–857. [Google Scholar]
- Ferrari, S.; Gualtieri, A.F. The use of illitic clays in the production of stoneware tile ceramics. Appl. Clay Sci. 2006, 32, 73–81. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.K.; Ghatak, S.; Maiti, H.S. Effect of pyrophyllite on the mullitization in triaxial porcelain system. Ceram. Int. 2009, 35, 1493–1500. [Google Scholar]
- Sokolář, R.; L.Keršnerová; M.Šveda, The effect of different fluxing agents on the sintering of dry pressed porcelain bodies. J. Asian Ceram. Soc. 2017, 5, 290–294.
- Frizzo, G.R.; Zaccaron, A.; de Souza Nandi, V.; Bernardin, A.M. Pyroplasticity on porcelain tiles of the albite-potassium feldspar-kaolin system: A mixture design analysis. J. Build. Eng. 2020, 31, 101432. [Google Scholar]
- Kamseu, E.; Bakop, T.; Djangang, C.; Melo, U.C.; Hanuskova, M.; Leonelli, C. Porcelain stoneware with pegmatite and nepheline syenite solid solutions: Pore size distribution and descriptive microstructure. J. Eur. Ceram. Soc. 2013, 33, 2775–2784. [Google Scholar]
- Esposito, L.; Salem, A.; Tucci, A.; Gualtieri, A.; Jazayeri, S.H. The use of nepheline-syenite in a body mix for porcelain stoneware tiles. Ceram. Int. 2005, 31, 233–240. [Google Scholar]
- Bragança, S.R.; Lengler, H.C.M.; Bergmann, C.P. Spodumene-bearing rock as flux for triaxial ceramic bodies. Adv. Appl. Ceram. 2011, 110, 293–300. [Google Scholar]
- Azarov, G.M.; Vlasov, A.S.; Maiorova, E.V.; Oborina, M.A. Diopside: raw material for porcelain production. Glass Ceram. 1995, 52, 216–218. [Google Scholar]
- Magagnin, D.; Santos, C.M.F.D.; Wanderlind, A.; Jiusti, J.; Junior, A.D.N. ; Effect of kaolinite, illite and talc on the processing properties and mullite content of porcelain stoneware tiles. Mater. Sci. Eng. A 2014, 618, 533–539. [Google Scholar]
- Deng, T.; Wang, Y.; Dufresne, A.; Lin, N. Simultaneous enhancement of elasticity and strength of Al2O3-based ceramics body from cellulose nanocrystals via gel-casting process. Carbohydr. Polym. 2018, 181, 111–118. [Google Scholar] [PubMed]
- Lee, H.J.; Park, H.Y.; Kim, E.H.; Choi, H.H.; Jin, J.; Choi, J.; Yang, S.; Jung, Y.G. Relationship between mechanical properties of ceramic green body and structures of photo-cured acrylate polymer for ceramic 3D printing based on photo polymerization. Ceram. Int. 2021, 47, 3867–3875. [Google Scholar]
- Zhao, L.H.; Wei, W.; Bai, H.; Zhang, X.; Cang, D.Q. Synthesis of steel slag ceramics: chemical composition and crystalline phases of raw materials. Int. J. Miner. Metall. Mater. 2015, 22, 325–333. [Google Scholar]
- Tarhan, B.; Tarhan, M.; Aydin, T. Reusing sanitaryware waste products in glazed porcelain tile production. Ceram. Int. 2017, 43, 3107–3112. [Google Scholar] [CrossRef]
- Filho, J.E.S.; Aurich, J.C.; Sousa, F.J.P.; Nascimento, R.M.; Paskocimas, C.A.; Silva, A.H.A. Polishing performance of eco-friendly porcelain stoneware tiles reusing bricks and roof tiles wastes. J. Cleaner Prod. 2020, 256, 120362. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, T.; Liu, T.; Huang, J. Preparation and characterization of red porcelain tiles with hematite tailings. Constr. Build. Mater. 2013, 38, 1083–1088. [Google Scholar] [CrossRef]
- Yang, H.; Chen, C.; Pan, L.; Lu, H.; Sun, H.; Hu, X. Preparation of double-layer glass-ceramic/ceramic tile from bauxite tailings and red mud. J. Eur. Ceram. Soc. 2009, 29, 1887–1894. [Google Scholar] [CrossRef]
- Dana, K.; Dey, J.; Das, S.K. Synergistic effect of fly ash and blast furnace slag on the mechanical strength of traditional porcelain tiles. Ceram. Int. 2005, 31, 147–152. [Google Scholar] [CrossRef]
- Wei, Q.; Gao, W.; Sui, X. ; Synthesis of low-temperature; fast, single-firing body for porcelain stoneware tiles with coal gangue. Waste Manage. Res. 2010, 28, 944–950. [Google Scholar]
- Taskiran, M.U.; Demirkol, N.; Capoglu, A. A new porcelainised stoneware material based on anorthite. J. Eur. Ceram. Soc. 2005, 25, 293–300. [Google Scholar] [CrossRef]
- Taskiran, M.U.; Demirkol, N.; Capoglu, A. Influence of mixing/milling on sintering and technological properties of anorthite based porcelainised stoneware. Ceram. Int. 2006, 32, 325–330. [Google Scholar] [CrossRef]
- Capoglu, A. A novel low-clay translucent whiteware based on anorthite. J. Eur. Ceram. Soc. 2011, 31, 321–329. [Google Scholar] [CrossRef]
- Yildirim, H.; Azakli, Y.; Tarakci, M.; Capoglu, A. The effect of surface polishing on the flexural strength of anorthite-based porcelainised stoneware. Acta Phys. Pol. A. 2015, 127, 1336–1341. [Google Scholar] [CrossRef]
- Montanaro, L.; Perrot, C.; Esnouf, C.; Thollet, G.; Fantozzi, G.; Negro, A. Sintering of industrial mullites in the presence of magnesia as a sintering aid. J. Am. Ceram. Soc. 2000, 83, 189–196. [Google Scholar] [CrossRef]
- Kurama, S.; Ozel, E. The influence of different CaO source in the production of anorthite ceramics. Ceram. Int. 2009, 35, 827–830. [Google Scholar] [CrossRef]
- Ke, S.; Cheng, X.; Wang, Y.; Wang, Q.; Wang, H. ; Dolomite, wollastonite and calcite as different CaO sources in anorthite-based porcelain. Ceram. Int. 2013, 39, 4953–4960. [Google Scholar] [CrossRef]
- Ibañez, A.; Sandoval, F. ; Wollastonite; Properties, synthesis and ceramic uses. Bol. Soc. Esp. Ceram. Vidrio 1993, 32, 349–361. [Google Scholar]
- Sletson, L.C.; Reed, J.S. Microstructure development in a vitrified anorthite porcelain. Am. Ceram. Soc. Bull. 1988, 67, 1403–1408. [Google Scholar]
- Tai, W.P.; Kimura, K.; Jinnai, K. A new approach to anorthite porcelain bodies using nonplastic raw materials. J. Eur. Ceram. Soc. 2002, 22, 463–470. [Google Scholar] [CrossRef]
- Wu, J.F.; Li, K. X.H. Xu, Y.X. Zhang, X.Y. Xu, X.B. Lao, White porcelain material based on diopside. Int. J. Appl. Ceram. Technol. 2017, 3, 454–560.
- Tarhan, M. Whiteness improvement of porcelain tiles incorporated with anorthite and diopside phases. J. Therm. Anal. Calorim. 2019, 138, 929–936. [Google Scholar] [CrossRef]
- Hatch, D.M.; Ghose, S. The α-β phase transition in cristobalite. SiO2, Phys. Chem. Miner. 1991, 17, 554–562. [Google Scholar] [CrossRef]
- Lee, W.E.; Souza, G.P.; McConville, C.J.; Tarvornpanich, T.; Iqbal, Y. Mullite formation in clays and clay-derived vitreous ceramics. J. Eur. Ceram. Soc. 2008, 28, 465–471. [Google Scholar] [CrossRef]
- Conserva, L.R.D.S.; Melchiades, F.G.; Nastri, S.; Boschi, A.O.; Dondi, M.; Guarini, G.; Raimondo, M.; Zanelli, C. Pyroplastic deformation of porcelain stoneware tiles: Wet vs. dry processing. J. Eur. Ceram. Soc. 2017, 37, 333–342. [Google Scholar] [CrossRef]
- Melchiades, F.G.; Daros, M.T.; Boschi, A.O. Porcelain tiles by the dry route. Bol. Soc. Esp. Ceram. Vidrio 2010, 49, 221–226. [Google Scholar]
- Mezquita, A.; Monfort, E.; Ferrer, S.; Gabaldón-Estevan, D. How to reduce energy and water consumption in the preparation of raw materials for ceramic tile manufacturing: Dry versus wet route. J. Cleaner Prod. 2017, 168, 1566–1570. [Google Scholar] [CrossRef]
- Soldati, R.; Zanelli, C.; Guarini, G.; Fazio, S.; Bignozzi, M.C.; Dondi, M. Characteristics and rheological behaviour of spray-dried powders for porcelain stoneware slabs. J. Eur. Ceram. Soc. 2018, 38, 4118–4126. [Google Scholar] [CrossRef]
- Minlheiro, F.A.C.; Freire, M.N.; Silva, A.G.P.; Holanda, J.N.F. Densification behaviour of red firing Brazilian kaolinitic clay. Ceram. Int. 2005, 31, 757–763. [Google Scholar] [CrossRef]
- Dondi, M.; Ercolani, G.; Melandri, C.; Mingazzini, C.; Marsigli, M. The chemical composition of porcelain stoneware tiles and its influence on microstructural and mechanical properties. Interceram 1999, 48, 75–83. [Google Scholar]
- Abdullayev, A.; Klimm, D.; Kamutzki, F.; Gurlo, A.; Bekheet, M.F. AlF3–assisted flux growth of mullite whiskers and their application in fabrication of porous mullite-alumina monoliths. Open Ceram. 2021, 7, 100145. [Google Scholar] [CrossRef]
- W.M. Carty, U. Senapati, Porcelain—raw materials, processing, phase evolution, and mechanical behavior, J. Am.
Ceram. Soc. 1998, 81, 3–20.
- Iqbal, Y.; Lee, W.E. Microstructural evolution in triaxial porcelain. J. Am. Ceram. Soc. 2000, 83, 3121–3127. [Google Scholar] [CrossRef]
- Schuller, K.H. Reactions between mullite and glassy phase in porcelains. Trans. Br. Ceram. Soc. 1964, 64, 103–117. [Google Scholar]
- Lecomte, G.; Pateyron, B.; Blanchart, P. Experimental study and simulation of a vertical section mullite-ternary eutectic (985°C) in the SiO2–Al2O3–K2O system. Mater. Res. Bull. 2004, 39, 1469–1478. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kato, E. Low—temperature fabrication of anorthite ceramics. J. Am. Ceram. Soc. 1994, 77, 833–834. [Google Scholar] [CrossRef]
- Kenzour, A.; Belhouchet, H.; Kolli, M.; Djouallah, S.; Ramesh, S. Sintering behavior of anorthite-based composite ceramics produced from natural phosphate and kaolin. Ceram. Int. 2019, 45, 20258–20265. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C.; Yang, S. Recycling of lime mud and fly ash for fabrication of anorthite ceramic at low sintering temperature. Ceram. Int. 2015, 41, 5648–5655. [Google Scholar] [CrossRef]
- Brasileiro, C.T.; Conte, S.; Contartesi, F.; Melchiades, F.G.; Zanelli, C.; Dondi, M.; Boschi, A.O. Effect of strong mineral fluxes on sintering of porcelain stoneware tiles. J. Eur. Ceram. Soc. 2021, 41, 5755–5767. [Google Scholar] [CrossRef]
- Yoon, Y.G.; Car, R.; Srolovitz, D.J.; Scandolo, S. Thermal conductivity of crystalline quartz from classical simulations. Phys. Rev. B 2004, 70, 2199–2208. [Google Scholar] [CrossRef]
- Ackermann, R.J.; Sorrell, C.A. Thermal expansion and the high–low transformation in quartz. I. High-temperature X-ray studies. J. Appl. Crystallogr. 1974, 7, 461–467. [Google Scholar] [CrossRef]
- Schneider, H.; Eberhard, E. Thermal expansion of mullite. J. Am. Ceram. Soc. 1990, 73, 2073–2076. [Google Scholar] [CrossRef]
- Aldebert, P.; Traverse, J.P. αAl2O3: A high-temperature thermal expansion standard. High Temp. High Pressure 1984, 16, 127–135. [Google Scholar]
- Horai, K.I.; Simmons, G. Thermal conductivity of rock-forming minerals. Earth Planet. Sci. Lett. 1969, 6, 359–368. [Google Scholar] [CrossRef]
- Stewart, D.B. D. Von Limbach. Thermal expansion of low and high albite. Am. Mineral. 1967, 52, 389–413.
- Hovis, G.L.; Medford, A.; Conlon, M.; Tether, A.; Romanoski, A. Principles of thermal expansion in the feldspar system. Am. Mineral. 2010, 95, 1060–1068. [Google Scholar] [CrossRef]
- Taylor, D.; Henderson, C.M.B. The thermal expansion of the leucite group of minerals. Am. Mineral. 1968, 53, 1476–1489. [Google Scholar]
- Pal, M.; Das, S.; Das, S.K. ; Anorthite porcelain: synthesis, phase and microstructural evolution. Bull. Mater. Sci. 2015, 38, 551–555. [Google Scholar] [CrossRef]
- Cukierman, M.; Uhlmann, D.R. Viscosity of liquid anorthite. J. Geophys. Res. 1973, 78, 4920–4923. [Google Scholar] [CrossRef]
- Barbieri, L.; Bondioli, F.; Lancellotti, I.; Leonelli, C.; Montorsi, M.; Ferrari, A.M.; Miselli, P. The anorthite-diopside system: structural and devitrification study. Part II: crystallinity analysis by the rietveld-RIR method. J. Am. Ceram. Soc. 2005, 88, 3131–3136. [Google Scholar] [CrossRef]
- Ikawa, H.; Otagiri, T.; Imai, O.; Suzuki, M.; Urabe, K.; Udagawa, S. Crystal structures and mechanism of thermal expansion of high cordierite and its solid solutions. J. Am. Ceram. Soc. 1986, 69, 492–498. [Google Scholar] [CrossRef]
- Cameron, M.; Sueno, S.; Prewitt, C.T.; Papike, J.J. High-temperature crystal chemistry of acmite, diopside, hedenbergite jadeite, spodumene and ureyite. Am. Mineral. 1973, 58, 594–618. [Google Scholar]
- Sánchez, E.; Ibáñez, M.J.; García-Ten, F.J.; Quereda, M.F.; Xu, Y.M.; Hutchings, I.M. Porcelain tile microstructure: implications for polished tile properties. J. Eur. Ceram. Soc. 2006, 26, 2533–2540. [Google Scholar] [CrossRef]
- Gültekin, E.E.E. The effects of heating rate and sintering temperature on the strength, firing shrinkage, and bulk density of porcelain tiles. J. Aust. Ceram. Soc. 2018, 54, 39–46. [Google Scholar] [CrossRef]
- Junior, A.D.N.; Hotza, D.; Soler, V.C. E.S. Vilches. Influence of composition on mechanical behaviour of porcelain tile. Part I: Microstructural characterization and developed phases after firing. Mater. Sci. Eng. A 2010, 527, 1730–1735. [CrossRef]
- Matthew, G.O.; Fatile, B.O. Characterization of vitrified porcelain tiles using feldspar from three selected deposits in Nigeria. Res. J. Recent Sci. 2014, 3, 67–72. [Google Scholar]
- Cheng, X.S.; Ke, S.J.; Wang, Q.H.; Wang, H.; Shui, A.Z.; Liu, P.A. Fabrication and characterization of anorthite-based ceramic using mineral rawmaterials. Ceram. Int. 2012, 38, 3227–3235. [Google Scholar] [CrossRef]
- Dana, K.; Das, S.K. Partial substitution of feldspar by B.F. slag in triaxial porcelain: phase andmicrostructural evolution. J. Eur. Ceram. Soc. 2004, 25, 3833–3839. [Google Scholar] [CrossRef]
- Manfredini, T.; Romagnoli, M.; Hanuskova, M. Wollastonite as sintering aid for porcelain tile bodies. Int. Ceram. J. 2000, 61–67. [Google Scholar]
- Wang, S.; Qi, X.; Hu, J.; X.Tian, Characterization of anorthite-based porcelain prepared by using wollastonite as a calcium source. J. Ceram. Process. Res. 2015, 16, 361–365.
- Darolt, R.D. Estudo do efeito da moagem de alta energia no comportamento mecânico de porcelanato, Master Thesis, Universidade do Extremo Sul Catarinense, Brazil, 2019. [Google Scholar]
- Bragança, S.R.; Bergmann, C.P. A view of whitewares mechanical strength and microstructure. Ceram. Int. 2003, 29, 801–806. [Google Scholar] [CrossRef]
- Junior, A.D.N.; Hotza, D.; Soler, V.C.; Vilches, E.S. Analysis of the development of microscopic residual stresses on quartz particles in porcelain tile. J. Eur. Ceram. Soc. 2008, 28, 2629–2637. [Google Scholar]
- Ece, O.I.; Nakagawa, Z.E. Bending strength of porcelains. Ceram. Int. 2002, 28, 131–140. [Google Scholar] [CrossRef]
- Selsing, J. Internal stresses in ceramics. J. Am. Ceram. Soc. 1961, 44, 419–419. [Google Scholar] [CrossRef]
- Junior, A.D.N.; Hotza, D.; Soler, V.C.; Vilches, E.S. Influence of composition on mechanical behaviour of porcelain tile. Part II: Mechanical properties and microscopic residual stress. Mater. Sci. Eng. A 2010, 527, 1736–1743. [Google Scholar]
- De Oliveira, A.P.N.; Vilches, E.S.; Soler, V.C.; Villegas, F.A.G. Relationship between Young's modulus and temperature in porcelain tiles. J. Eur. Ceram. Soc. 2012, 32, 2853–2858. [Google Scholar] [CrossRef]
- Junior, A.D.N.; Hotza, D.; Soler, V.C.; Vilches, E.S. Influence of macroscopic residual stresses on the mechanical behavior and microstructure of porcelain tile. J. Eur. Ceram. Soc. 2008, 28, 2463–2469. [Google Scholar]
- Junior, A.D.N.; Hotza, D.; Soler, V.C.; Vilches, E.S. Influence of composition on mechanical behaviour of porcelain tile. Part III: Effect of the cooling rate of the firing cycle. Mater. Sci. Eng. A 2011, 528, 3330–3336. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).