1. Introduction
Rice is one of the most critical staple crops in the world, serving as a primary source of sustenance for over half of the world’s population. It provides millions of people in developing countries with basal carbohydrates, vitamins, and minerals. However, the widespread occurrence of cadmium (Cd) pollution in soil-rice ecosystems has been a negative impact on the production of safe rice, which is particularly acute in China [
1]. The rapid industrial development and inadequate environmental protection over the past thirty years have caused widespread Cd pollution, particularly in areas surrounding smelting facilities and metal mining sites. In China, millions hectare areas of arable land have been contaminated by Cd mainly by irrigation of industrial wastewaters [
2]. Meanwhile, rice plant is an efficient crop for the uptake Cd from contaminated paddy soils, leading to the accumulation of excessive levels of Cd in the grains. Consumption of the contaminated the rice grains has become a major source of Cd exposure for the general population. This raises serious concerns about food safety in China, where the quantity and quality of food supply is already a pressing issue [
3]
.
Application of lime is an efficient way to control the translocation of soil Cd into rice grains. Lime materials, including quicklime and limestone, are low-cost and easily accepted by farmers, which have a significant effect on increasing soil pH and reducing available Cd of soil [
4]. Meanwhile, appropriate application of lime can also recover the Ca content in acidified soil. It has long been known that calcium (Ca) is an essential element for plants, crucial in maintaining cell wall structure, membrane stability, and participating in plant signal transduction [
5]. Since Ca and Cd have similar ionic radii, these two elements may exhibit antagonistic effects at multiple levels in plants. Maintaining and supplementing a certain amount of Ca nutrition may also improve the Cd resistance of rice.
However, to date, the antagonistic mechanism between Ca and Cd in plants, especially for rice, has not been thoroughly understood. According to the mode of Ca and Cd, their uptake and translocation may be joint in the following aspects, including exchange absorption on root, plant cell wall composition, co-transporter gene expression, transpiration inhibition. Thus, this review systematically elaborates the migration process of Cd in the soil-plant system, and then discusses the possible mechanisms of Ca in inhibiting Cd translocation in rice plants. The aim of the paper is to provide theoretical and practical support for utilization of Ca materials to prevent Cd accumulation in rice and improving the food safety.
2. Pathways of cadmium in soil-plant system
2.1. Cadmium source and fate in environment
Cd, rarely existing in its pure form, is commonly associated with zinc sulfide, lead and copper ores. The natural source of Cd includes volcanic activity and the weathering of parent rocks [
6]. Anthropogenic sources such as the use of phosphate fertilizers and soil amendments, wastewater irrigation, smelting, deposition of airborne Cd from mining, and fossil fuel combustion also contribute to the elevation of soil Cd [
7], which has been evidenced in many regions of China with extensive nonferrous metal mining, smelting, and other related industrial operations [
8].
The mobility and bioavailability of Cd in soil are influenced by a variety of factors, including soil organic matter (SOM) and soil pH. SOM immobilize Cd through the formation of stable with large negatively charged interfaces, thereby decreasing the phyto-availability to plants [
9]. However, SOM, such as fulvic/humic acids and dissolved organic carbon (DOC), can form soluble chelates with Cd and increase its mobility under certain soil conditions [
10]. Therefore, amending organic materials have been utilized in controlling the bioavailability of soil Cd either in decreasing crop accumulation or increasing extraction of hyperaccumulators [
11]. Soil pH, another key factor, have a fundamental impact on the Cd mobility and bioavailability [
12], mainly because of the competition between Cd and H
+ for adsorption sites [
13]. Generally, the bioavailability of Cd is restrained when soil pH is higher than 6.5, since it leads to the increase in Cd adsorption on the negatively charged soil surface.
2.2. Migration pathways of cadmium in plants
2.2.1. Cd in rhizosphere
Plant roots significantly influence the environment of rhizosphere, hence impact their ability to uptake of Cd. Generally, organic acid excreted from roots can change the Cd solubility by chelation and ligand exchange reactions [
14]. Furthermore, by the means of the proton excretion, the rhizosphere is acidified and can promote the release of Cd from the solid phase. Numerous studies have found the positive role of organic acids in Cd bioavailability. For example, significant correlation between oxalic acids and Cd accumulation was observed in rice under Cd-contaminated condition [
15]. Secreting oxalate acid by tomato roots could be an important detoxification mechanism by excluding the Cd entry into the root cell membrane. High excretion of oxalate from the root of
Sedum alfredii Hance potentially enhance Cd uptake and accumulation [
16]. Other negatively charged anions, such as citric, malic and acetic acid, have also been shown to be incapable of forming stable Cd complexes, which influences plant Cd uptake [
17]. Under iron (Fe) deficiency, Cd stress caused greater phytosiderophores production by maize roots, but this release failed to protect maize plants from Cd toxicity [
18,
19].
Furthermore, different nitrogen forms differentially affect Cd uptake and accumulation. Compared with NO
3- fertilization, NH
4+ fertilization caused a significant enhancement of soil acidity, which was due to the proton release resulting from the absorption of NH
4+ by the plant roots. The soil acidity concurred with significant increase in Cd uptake [
20].
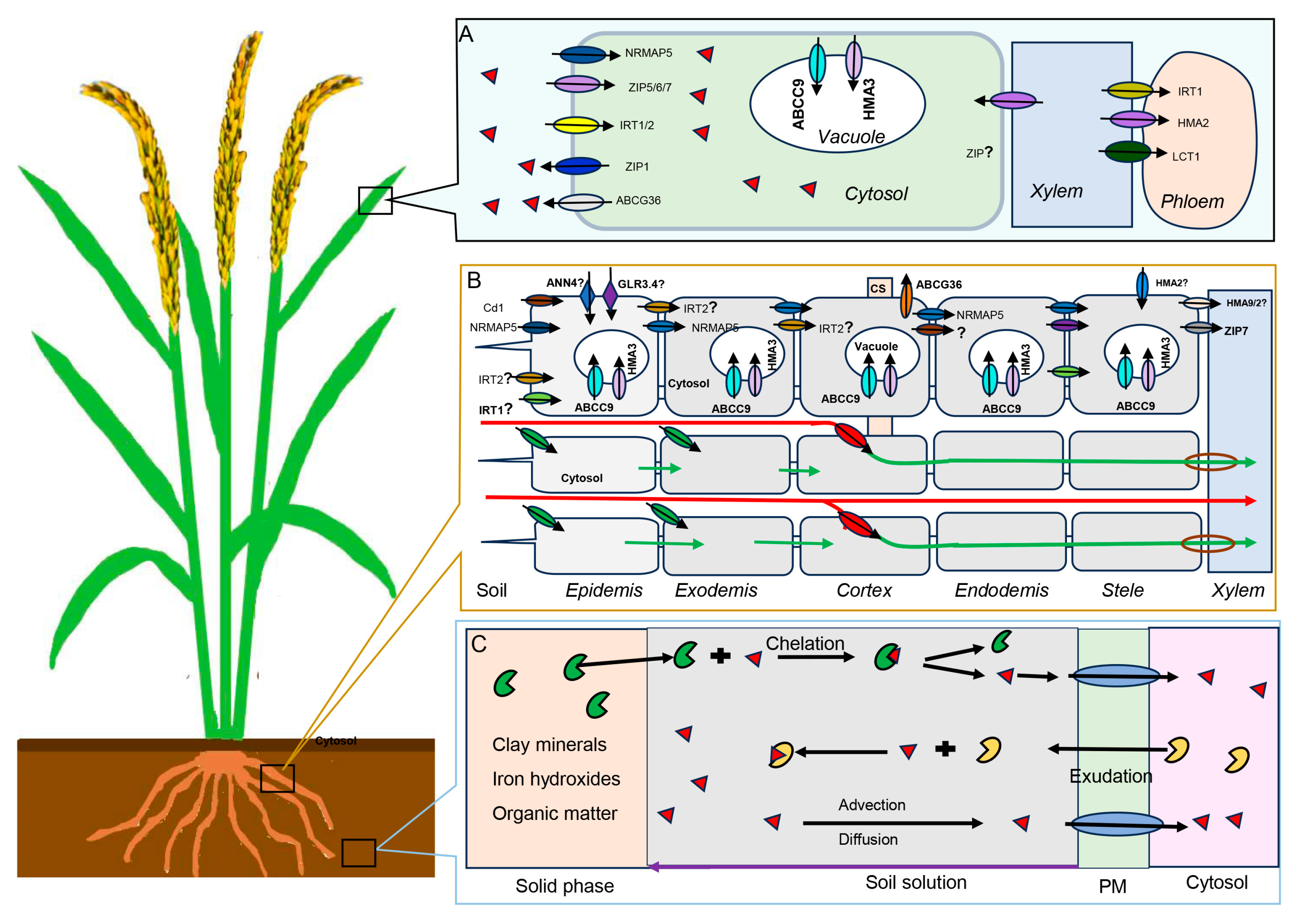
(A) Transporters involved in cadmium uptake and translocation in plant leaves. (B) Schematic representation of plant root with the proteins involved in Cd transport. CS, Casparian strip; The green arrows and red arrows indicates symplastic and apoplastic pathways of radial transport of Cd ions across the root, respectively. (C) Principal reactions and processes controlling cadmium availability at the plant–soil interface. PM: Plasma membrane; The red triangle, yellow shape, and green shape represent cadmium and organic ligand originating from the soil, organic ligand originating from rhizodeposition, respectively; For simplification, the cell wall is not presented and the vacuoles in some cells are not shown.
2.2.2. Root morphology
Plants with thin, hairy roots are able to absorb and accumulate higher levels Cd [
21]. The Cd assimilation is based on the root morphological structure [
22], surface area [
23], physiological characteristics [
21], and plant growth stages [
24]. For example, maize plants with a greater root average diameter inhibit more Cd uptake [
25]. Generally, Cd influx was much higher at the root tip than at the root base, as has been observed in wheat [
26], sunflower [
27], rice [
28], etc. Hence, plants with fewer tips exhibit lower Cd translocation [
29], which can be used for pre-screen the low-Cd-accumulating cultivars [
30]. This may be due to elevated activity of transport systems close to the root tip. Incomplete development of apoplastic barriers near the root apex may also contribute to the higher influx of Cd, as it may favor apoplastic Cd uptake [
22]. Based on the modeling analysis conducted by Laporte et al. [
31], the total surface area of the root may be a more influential parameter in determining the extent of Cd uptake by the root system.
2.2.3. Cell wall
As the outermost structure of plant cells, cell wall functions the first line of defense against Cd invasion. The negatively charged sites on cellulose, hemicellulose, and pectin chains in plant cell walls allow for the Cd absorption. Energy-dispersive X-ray micro-analysis (EDX) in the root cortex of
Arabidopsis thaliana revealed that Cd accumulate in the cell walls together with phosphate ions [
32]. In contrast, in the central cylinder of the root, Cd was found to be present as Cd/ sulfur (S) granular deposits in the middle lamella of the pericycle, suggesting that Cd may also form complexes with sulfur-containing biomolecules or proteins in this region [
32]. The binding capacity of the cell wall for Cd varies depending on the plant species and the specific structural characteristics of the cell wall matrix. In leaves of oilseed rape, only a small fraction (11%) of the Cd accumulated in cell walls [
33], indicating that other cellular compartments such as vacuoles or organelles may play a more significant role in Cd sequestration in this species. While in
S. alfredii, more than 60% of Cd was found in the cell wall fraction [
34]. In rice, 70-90% of total root Cd was found in the cell walls [
35].
Under Cd stress, the proportion of cell wall components and their binding capacity to Cd are commonly altered. Reactive oxygen species (ROS) production induced by Cd stress impacts the cell wall composition through the regulation of gene expression of cinnamyl-CoA reductase and cinnamyl alcohol dehydrogenase, promoting pectin biosynthesis and demethylation [
36]. This molecular regulation augments the number of functional groups in pectin, such as hydroxyl and carboxylic groups, enhancing its binding capacity to Cd and initiating xylem development procedures [
36,
37]. It has been found that Cd stress in rice roots triggers the production of H
2O
2 which promotes the biosynthesis of pectin. Through demethylation, pectin releases -OH and -COOH, enhancing the binding of Cd to pectin components in root cell walls [
36].
It should be noted that the method used for the determination of Cd in cell walls in many studies may be questionable. The use of homogenization and fractionation methods in the liquid phase may alter the original distribution of Cd in the plant tissue due to diffusion or release of the metal from one fraction to another. This can lead to an overestimation or underestimation of the amount of Cd present in each fraction and inaccuracies in the interpretation of Cd distribution in plant cells. Therefore, alternative methods such as cryo-sectioning [
38] or laser microdissection [
39] are recommended because they can preserve the integrity of plant cells and avoid the diffusion of metals during the extraction process.
2.2.4. Transporters
Understanding the transporter protein families of Cd is crucial for developing strategies to reduce Cd accumulation in crops. Several transporter protein families have been identified including Natural Resistance-Associated Macrophage Protein (NRAMP), Zinc and Iron regulated transporter Protein (ZIP), Heavy Metal-transporting ATPases (HMA), ATP-Binding Cassette (ABC), H
+/cation-antiporters (CAXs) families, etc (See
Figure 1).
NRAMP proteins are widely present in plants, and mainly functions in the transport of Cd and other metal ions, such as Fe, manganese (Mn), aluminium (Al), etc. This gene was first reported in the model plant Arabidopsis, while was mainly studied in rice among the food corps.
OsNRAMP1, a transporter localized in the plasma membrane mediating xylem loading, is mainly expressed in the roots. By heterologous expression of
OsNRAMP1 in Arabidopsis,
OsNRAMP1 increased the accumulation of As and Cd [
40]. Knockout of
OsNRAMP1 resulted in decreasing Cd uptake by the rice roots and the accumulation in the leaves and grains, while overexpression of
OsNRAMP1 in rice reduced Cd accumulation in the roots, but increased it in the leaves [
41,
42].
OsNRAMP2 mediated Cd efflux from the vacuoles in the vegetative tissues, as noted that knockout of
OsNRAMP2 significantly decreased the Cd content in rice grains [
43].
OsNRAMP5, localized at the distal part of the exodermis and endodermis of root cells, is accountable for influx of Mn and Cd into root cells from external solutions [
44].
Several types of ZIP proteins were identified, each with a different role in regulating Cd transport.
OsZIP1, mainly expressed in the endoplasmic reticulum and the plasma membrane of roots, functions in the Cd efflux transporter. Overexpression of
OsZIP1 resulted in accumulation of zinc (Zn), copper (Cu), and Cd in rice plants [
45].
OsZIP9 had influx transporter activity that functioned synergistically in the Cd/Zn uptake of rice [
46]. Knockout of
OsZIP7 resulted in Cd retention in rice roots, hindering Cd upward transmission and xylem loading and delivery of Cd into the rice grains [
47].
HMA transporters, mainly localized on the plasma membrane and tonoplast, regulate the uptake and translocation of Cd through the roots and shoot tissues. For instance,
OsHMA2 is involved in Cd across in cell membrane and root to shoot translocation [
48].
OsHMA3 regulates the sequestration of Cd in vacuoles to limit the accumulation of Cd in the cytosol [
49].
OsHMA9 is mainly expressed in the root epidermis and outer cortical cells where it functions to transport Cd out of the root for sequestration or storage [
50].
ABC transporters are one of the largest known superfamilies, with over 120 members in both Arabidopsis and rice plants. They play a crucial role in the transport of a wide range of substances across membranes including Cd. In rice,
OsABCC9 is predominantly expressed in the root stele, mediating Cd accumulation by sequestering Cd into the vacuoles [
51].
OsABCG36 is localized in plasma membrane, functions as a Cd extrusion pump. Knockout of it induced significantly higher Cd accumulation in root cell sap and significantly increased sensitivity to Cd of rice [
52].
Cd can also be translocated into the vacuole by CAXs by using the proton gradient to mediate Cd storage in the vacuole of plant cells. Detailed information will be expanded in the following Ca/Cd section.
2.2.5. Translocation
By means of the Cd transporters, Cd can cross the exodermis and/or the endodermis cell layers within the symplastic route, where the apoplastic route is blocked by the barriers, such as Casparian strip [
22]. However, Cd may also get into the xylem via apoplastic pathway where the barrier is incomplete or lacking, such as root tip, emergence sites of lateral root and stage I endodermis [
53] (See
Figure 1).
After being taken up by the roots and loaded into the root xylem, Cd is transported to the above-ground plant parts through the xylem sap flow, which is primarily driven by plant transpiration and the water potential gradient between the soil and atmosphere. Numerous studies have confirmed this mechanism. Spraying the transpiration inhibitor in leaves, such as abscisic acid (ABA), dramatically reduced Cd accumulation in
lndian Mustard’s leaves [
54]. Using isotopic analysis, it was found that in wheat roots, higher transpiration rates were positively related to higher Cd accumulation [
55]. By determination of Cd level among 69 rice accessions, Cd levels in the xylem sap were found that strongly correlated to the shoots and grains [
56].
After long-distance transport, Cd is unloaded from the xylem vessels, which may be similar to the nutrient process via a symplastic pathway. This process occurs through a high branch network of veins that cross the leaf blade. However, there is limited knowledge regarding the transport mechanisms accountable for unloading Cd ions from the xylem. Cd may enter the leaf cells through nutrient transport proteins, such as Fe, Mn, and Zn, which is equally what happens during root uptake. But the roles in the transporting pathways to the epidermis, the storage and distribution of Cd in the shoot still remain unknown [
57].
2.2.6. Cd Redistribution
Phloem determines the Cd redistribution between the aerial parts of the plant, particularly in the sink organs such as from leaves into seeds. For example, it has been observed that 91% to 100% of Cd accumulated in rice grains is transported via the phloem [
58]. The Cd content in rice grains was correlated with the Cd level of phloem sap, but not with the concentration in xylem sap [
59]. Drawing on the example of Ca as a benchmark for phloem transportation, it can be inferred that phloem served as the principal conduit for Cd transport to sunflower seeds [
60]. Similarly in wheat, 50-60% Cd in mature grains was found by re-mobilization through phloem from leaves and stem [
61]. Rice nodes were the important transfer stations when Cd can be loading Cd into the rice phloem, especially for the first node [
62]. In addition, it was suggested that
OsHMA2, highly expressed in the nodes and close to the vascular bundles, could be involved in mediating Cd transference from the xylem sap to the phloem [
63].
2.3. Cadmium toxicity to plants
The toxic mechanisms of Cd in plants has been proposed as following aspects: (1) imbalance of nutrients uptake and resulting in reduced absorption at the root surface; (2) direct combination with sulfhydryl (-SH) group, which impair protein structure thereby interfere various physiological processes, such as respiration [
64], photosynthesis [
65], cell division [
66] and ROS production/scavenging [
67]. Since a number of review papers have fully discussed the toxic mechanisms of Cd [
68,
69,
70,
71], here, some hot topics about Cd-plant research are suggested.
2.3.1. Hormesis of Cd on plant growth
High levels of Cd exposure to plants have been shown to cause necrotic lesions, leaf chlorosis, inhibition of root elongation, wilting, reduced biomass, and potential death [
72,
73,
74]. Cd also negatively affect seed germination, but it can be reversed after rinsing, indicating the Cd toxicity was due to seeds not achieving sufficient water rather than direct photo-toxicity [
75]
.
Interestingly, few studies found that seed germination can be stimulated by the low concentration of Cd stress [
76]. Recent evidence for the hormesis of Cd on plant growth has been rapidly accumulating. For instance, 5 mg kg
-1 Cd treatment increased the biomass, height, and chlorophyll content in
Lonicera japonica Thunb, a Cd-hyperaccumulator [
77]. Similar finding was also noted in
Polygonatum sibiricum under 1 mg Cd kg
-1 [
78] and tomato under 6.9 mg Cd kg
-1 stresses [
79]. The hormesis induced by low-dose Cd stress might be attributed to the overproduction of ROS that intensify the signaling role in cell cycle activity [
80]. However, the underlying effects on plant metabolisms remain insufficiently studied.
2.3.2. Ionomics of Cd with elements
The assimilation of plant nutrients is greatly interfered by Cd toxicity. To date, Cd interaction with essential mineral elements, including nitrogen (N), phosphorus (P), potassium (K), silicon (Si), magnesium (Mg), S, Zn, Fe, Ca, boron (B), Mn, Cu, selenium (Se), etc, have been intensively investigated [
81]
. Under Cd stress, the reduced uptake of nutrient is mainly due to the inhibition of transporters that responsible for loading elements into the aerial parts of plants [
82]. As mentioned above, such as NRAMPs for Fe/Mn/Cd, HMAs for Cu/cobalt (Co)/Zn/Cd, ZIPs for Fe/Zn/Cd, and CAXs for Ca/Cd, the function of co-transporters will continue to be unveiled. This is a promising field for ionomics research that involves the comprehensive analysis of the elemental composition of biological systems, providing a powerful tool for understanding the impact of Cd toxicity to plants.
In addition to general competition, some elements have specific mechanisms that help reduce the toxicity of Cd in plants. For example, Fe can form a layer of Fe oxide on rice roots, known as iron plaque, to sequester Cd and reduce its bioavailability to rice plants [
83]. Moreover, it can induce the synthesis of metallothioneins (MTs), which are small, cysteine-rich proteins that bind to and detoxify Cd [
84]. For Si and B, they can promote the deposition of Cd in cell walls, thereby limiting its translocation from roots to shoots by creating a barrier in the endodermis [
85,
86]. For S, it can reduce the toxicity of Cd in plants by forming thiol compounds such as glutathione (GSH) and phytochelatins (PCs) that help sequester and detoxify Cd [
87]. It can also modify the physicochemical properties of the rhizosphere, which affects the availability and mobility of Cd in soil and, consequently, its uptake by plants [
88]. Furthermore, Ca has been shown to interact with Cd in various ways, as detailed in the following sections.
2.3.3. Detoxication of Cd by glutathione
The primary toxicity of Cd in plants is the induction of ROS production, leading to oxidative damage [
89]. Although Cd does not directly participate in cellular redox reactions, it disrupts electron transport, damages antioxidant enzyme structures, and interferes with antioxidant molecule synthesis, leading to elevated ROS levels in the cell.
Cd induces ROS production in plants, causing oxidative damage. Although Cd does not directly participate in cellular redox reactions, it disrupts electron transport, Among the antioxidant molecules, glutathione (g-Glu-Cys-Gly, GSH) is one of the most important reducing equivalents, protecting plants against Cd-induced oxidative damage. Furthermore, it is also a key molecular compound or a basic component of PCs involved in Cd chelation and thereby confines Cd to less sensitive organelles, such as vacuoles [
90]. Increase in the demand for Cd detoxification usually leads to rapid depletion of GSH levels and a loss of antioxidative defense [
91]. Numerous studies have been done on the antioxidative and chelating roles of GSH under Cd stress, and signaling pathways that regulate these two roles are comprehensive and not well studied.
A number of genetic reports have demonstrated possible links between phytohormone signaling and GSH metabolism. For instance, the
cat2 Arabidopsis mutant, which has high levels of salicylic acid (SA), shows increased GSH levels and SA-dependent responses [
92]. Conversely, the SA-deficient mutant
sid2 had much lower GSH levels than wild-type plants [
93]. This signaling role of SA may be related to the production of GSH by serine acetyltransferase (SAT) and the recovery of GSH by glutathione reductase1 (GR1) [
93]. Furthermore, ethylene signaling was found to be involved in GSH biosynthesis. Arabidopsis roots can produce ethylene, which activates ethylene signaling in leaves and induces GSH biosynthesis in response to Cd stress [
94]. In another study, the accumulation of endogenous jasmonic acid (JA) in Cd-stressed
Lycium chinense plants affected the expression of glutathione reductase (GR), a key enzyme in GSH accumulation and Cd tolerance [
95]. Similarly, auxin was shown to activate glutathione-S-transferase (GST) in barley roots under Cd stress [
96]. Understanding these signaling pathways can help in the development of strategies to enhance the antioxidant capacity of cells and prevent Cd-induced toxicity.
3. Mechanisms of Ca-mediated restriction in Cd translocation in rice
Calcium, one of essential element for plants, is required in relatively large quantities (0.1%-5%) because it is involved in a multitude of structural and biochemical functions, such as cell wall development, membrane function, enzyme activation, signal transduction, stomatal regulation, nutrient uptake, etc [
5,
97]. It also plays a critical role in protecting plants against various abiotic stresses. Ca helps to maintain ion homeostasis by regulating ion channels and transporters in cell membranes [
98]
. Ca also regulate the production and scavenging of ROS, reducing oxidative stress [
99]. In addition, Ca signaling can activate various stress-responsive proteins that help plants to prevent abiotic damage [
100]. Besides of the general protecting roles as mentioned above, Ca displays some special resistant mechanisms when plants are exposed to Cd stress.
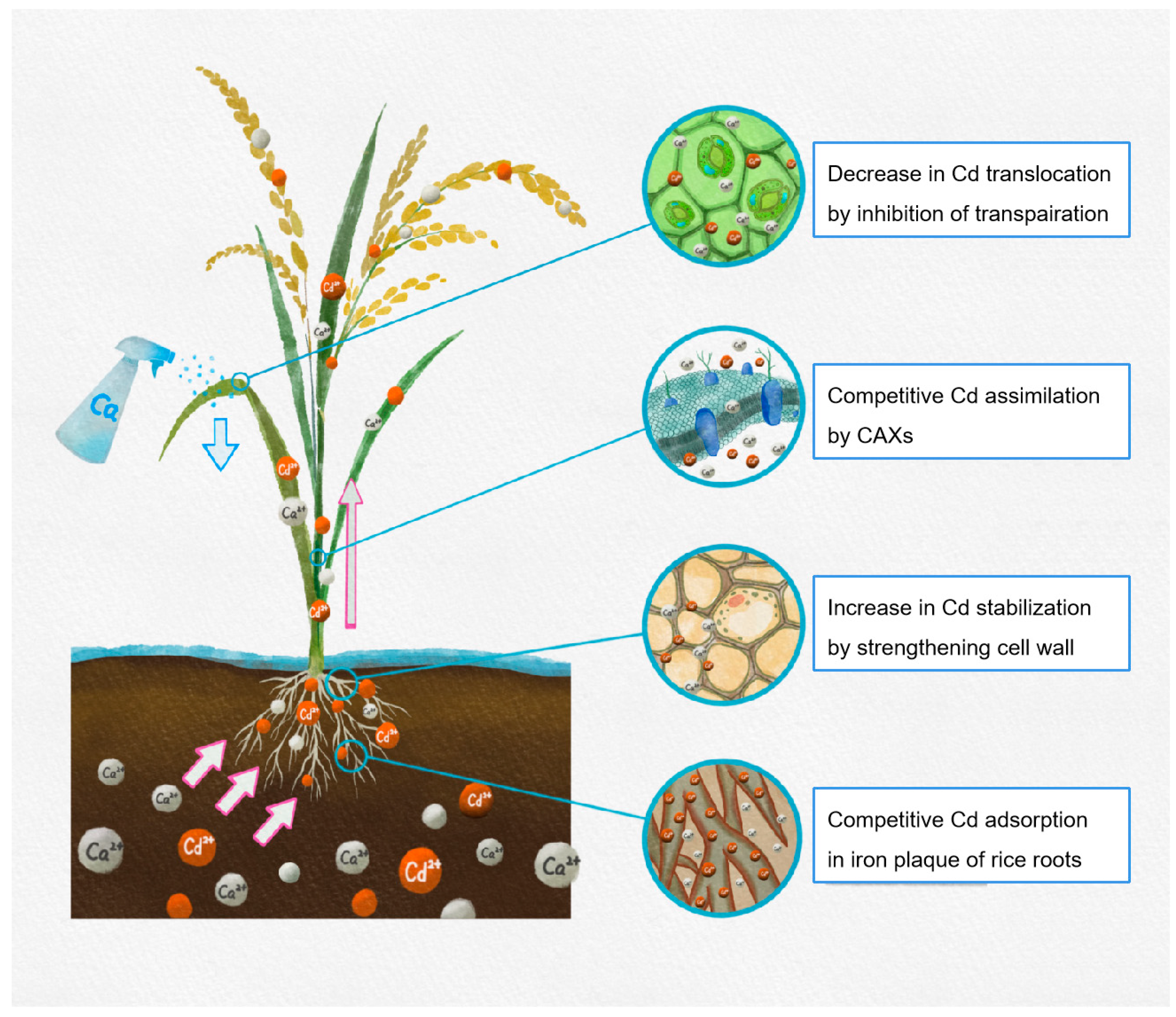
3.1. Liming
Lime addition has been proven to be an efficient and cost-effective way to reduce the bioavailability of Cd in soil. This technique increases soil pH, which leads to the formation of Cd(OH)
2 in soil solution, effectively slowing down the movement of soluble Cd in soil. In southern China, the recommended application rate of CaO is between 0.75 t ha
-1 to 1.50 t ha
-1 before soil tillage, resulting in a soil pH increase of 0.50 unit and a significant decrease of 35% in Cd concentration in rice grains [
101]
. However, the effectiveness of CaO addition may not be consistent due to the relatively small amount used, which can be difficult to distribute uniformly on the topsoil. As an example, in the study by Wang et al. [
102], only a modest increase of 0.28 units in soil pH and a 15% decrease in grain Cd were observed, which the effects were much lower than the similar doses used by Zhu et al. [
101]. Pot experiments have shown more significant changes in soil pH due to liming than field experiments, which may be due to better control of cultivation conditions [
103].
Different types of lime, such as burnt lime (CaO), hydrated lime (Ca(OH)
2) and limestone (CaCO
3) have different chemical properties that determine their corresponding recommended doses, which vary greatly, ranging from 0.50 t ha
-1 to 180 t ha
-1 [
104,
105]. CaCO
3 has a lower effect on increasing soil pH compared to Ca(OH)
2 and CaO. Thus, the recommended amount of CaCO
3 is usually higher than that of Ca(OH)
2 or CaO [
103]. However, some studies have observed that the lime effect on soil pH was independent of the amount added, potentially due to soil buffer capacity [
104]. As soil properties, such as pH, soil organic matter (SOM), cation exchange capacity (CEC), and clay content, typically relate to the buffering capacity, it is important to carefully compare and quantify the type and amount of lime based on soil conditions [
103].
3.2. Iron plaque
As an aquatic crop, rice is capable of delivering oxygen to its roots to support the respiration. The excess oxygen in the roots can be discharged from aerenchyma, then, oxidizing Fe
2+ in submerged soils to Fe
3+ oxides, resulting reddish-brown precipitate on the root surface. This is called iron plaque, an amphoteric colloid with strong physical and chemical adsorption capacity that can affect nutrient and metal uptake by plants [
106]. Some studies have shown that iron plaque effectively sequester Cd from the surroundings, reducing its mobility and bioavailability to plants [
107]. For example, a hydroponical research revealed that the formation of iron plaque on rice root reduced Cd concentration in root by 34% [
108]. The decline in Cd accumulation in rice grains was linked to the enhanced formation of ion plaque on the root surfaces [
109]. However, the barrier effect of ion plaque has a threshold based on its thickness. When the adsorbed Cd reaches a certain level, it may penetrate into the root and cause Cd accumulation in rice plant. Several studies have suggested that this threshold can range from 20 to 27.3 g kg
-1, with the average being about 23.5 g kg
-1[
110,
111].
As a bivalent ion, Ca is commonly used in exchange adsorption studies because it is abundant in soils and can readily exchange with other ion cations. For instance, the CaCl
2 solution ranged from 10 to 100 mM has been widely utilized as a extractor for soil available Cd [
112]. A 5 mM CaCl
2 solution could even recover 99% Cd from the extraplasmic bodies of wheat roots [
113]. However, a previous study indicated that exogenous Se
4+ and Se
6+ solutions failed to affect the adsorption of Cd on iron plaque, possibly due to the different valence states between Se and Cd [
114]. Since both Cd and Ca are bivalent ions with similar ionic radii, the desorption of Cd on the iron plaque by exogenous Ca may play an important role in preventing the Cd migration in rice roots. However, this hypothesis remains unconfirmed.
3.3. Cell wall synthesis
Recently, there has been a growing interest in using nutrient elements as exogenous substances to reinforce the cell wall structure and prevent Cd migration in cells. Silicon (Si), for example, mainly accumulates in cell walls in the form of a wall-bound organosilicon compound. During in situ examination of cellular fluxes of Cd in suspension cells, it was observed that cells treated with Si exhibited a significant inhibition of net Cd influx compared to cells without Si treatment [
115]. Signal investigation revealed that K reduced the expression levels of brassinolide synthesis genes in Cd-stressed Notoginseng Radix roots. As a result, the biosynthesis of brassinolides was hindered, leading to a reduced expression of the pectin methylesterase gene (PME) and then caused an increase in pectin methylation, which ultimately results in reduced Cd accumulation [
116]. Applying boron (B) to the roots increases pectin content by modifying biosynthesis pathways, inhibiting pectinase activity, and reducing the expression levels of associated genes. This leads to an increase in chelation of Cd onto cell walls and a decrease in Cd uptake by organelles via enhanced pectin demethylation. B application also normalizes the levels of cellulose and hemicellulose in the cell walls and enhances gene expression from the expansin, xyloglucan endotransglucosylase, and a-xylosidase families, thus strengthening cell wall integrity and root flexibility. As a result, accumulation of reactive oxygen species (ROS) is curbed and damage to the root surface structure is mitigated, leading to an increase in root viability [
117].
As an essential nutrient element for plants, Ca plays a significant role in maintaining the structural stability of plant cell walls. Through the binding of galacturonic acid residues, Ca forms a pectin calcium gel, which connects adjacent cells and increases cell toughness as a component of both the cell wall and intercellular layer [
118]. Spraying CaCl
2 onto grapevines resulted in the downregulation of the PG1 and PG2 genes encoding polygalacturonase, while the cellulose synthase family gene CesA3 in grape peel was unaffected. These findings highlight the vital role of Ca in inhibiting the degradation of pectin components and stabilizing the structure of the cell wall [
119].
The Ca
2+ signaling pathway in plants is intricately linked to active oxygen metabolism [
120], in a process called ROS-mediated Ca
2+ signaling. Exogenous Ca prevented the accumulation of superoxide radicals induced by Cd in mesophyll cells of pea plants, suggesting Ca regulates the cellular response to the Cd exposure [
121]. Low ROS levels stimulate Ca
2+ channels, allowing for a rapid influx of Ca
2+ into the cytosol of the cell. This influx then triggers the activation of downstream signaling pathways, which can lead to a range of physiological responses, such as regulating the synthesis of cell wall components, particularly pectin. However, under conditions of high oxidative stress, excessive ROS production can overwhelm the Ca
2+ signaling pathway, leading to the loss of Ca
2+ homeostasis and negative impacts on cell viability [
122,
123]. To date, research on the interplay between Ca/Cd in the synthesis and modification of cell wall components relating ROS production has been relatively limited.
3.4. Calcium carrier proteins (CAXs) family
Cd is a non-essential element in plants, and its active transport is mainly facilitated by divalent cation transporters with relatively low specificity, such as Zn transporter (ZRT), Fe transporter (IRT), Fe/Mn/Zn cotransporter (NRAMP), etc. Since Cd
2+ and Ca
2+ have comparable ionic radii, which leads to competition between these two elements for ion channels and carrier proteins on the root surface of plants. This competition can inhibit Ca absorption by plants. For example, Cd induces the depolarization of wheat root tip cells, resulting in a decrease in the amount of Ca adsorbed by the cells, and subsequently reducing the net content of Ca in the root [
124]. The addition of La
3+ (a Ca channel inhibitor) to S. alfredii suspension cell system showed significant inhibition of Cd transport to protoplasts [
125].
The Ca
2+/H
+ reverse carrier protein family, also known as CAXs is an ion channel protein that facilitates Ca/Cd co-transport. The ATPase on the vacuolar membrane generates an H
+ electrochemical potential gradient, which drives the CAXs function. The cooperation between CAXs and the HMA protein family is responsible for transporting Cd from the cytoplasm to the vacuole [
126], and achieving Cd segregation. The N terminus of CAXs contains an autoinhibitory region, while two conserved regions (c-1 and c-2) are primarily responsible for ion selectivity. The difference in these regions determines which ions the CAXs family members can select [
127].
Currently, CAX family genes involved in Cd uptake and transport have been cloned and identified in different plants. However, their capacity of Cd transport remains a point of debate. Arabidopsis, for instance, has six AtCAXs family members, which has been previously suggested that AtCAX2 and AtCAX4-coded proteins have the highest ability to transport Cd [
128]. However, recent Quantitative Trait Loci (QTL) mapping studies reveal that loss of AtCAX1 gene function leaded to high sensitivity of Arabidopsis to Cd toxicity [
129]. Similarly, the rice OsCAXs family has six members, namely OsCAX1a, OsCAX1b, OsCAX1c, OsCAX2, OsCAX3 and OsCAX4 [
130]. Among them, the loss of OsCAX2 function resulted in increasing Cd toxicity, while the up-regulation of its expression significantly inhibited Cd accumulation in rice [
131]. There is preliminary evidence from yeast heterologous system showed that OsCAX1a, OsCAX1c and OsCAX4 had Cd transport activity in rice [
130]
. However, the extent to which each member of the OsCAX family can transport Cd and their potential synergistic effects remain subjects for further investigation.
3.5. Transpiration
The Cd translocation from root to shoot are mainly via two routes: apoplast (xylem-passive transport) and symplastic (phloem-active transport). The apoplast route, relying on transpiration, accounts for over 90% of Cd transport [
132]. Thus, all the external factors that influence plant transpiration (e.g., temperature, light, ABA, transpiration inhibitors, etc.) affect Cd transport to the shoot [
133]. For example, when the leaves of India mustard were subjected to 100 M ABA for 24 hours, stomatal diffusion resistance increased by nearly 50 times, leading to a substantial decrease in transpiration rate. This caused Cd transport to the shoot to nearly cease, possibly due to the Ca
2+ signal transduction [
132]
. Shading significantly reduced transpiration rate in tobacco leaves, and leading to a 73.5% decrease in Cd accumulation [
133]. Similarly, shading rice leaves by intercropping Sesbania significantly inhibited both transpiration and Cd translocation in rice grains [
110].
Similar to Cd, the Ca transport in plants is also mainly through the apoplast pathway with water serving as the carrier. To facilitate the stable function of Ca
2+ signals, plants have evolved regulatory pathways for water transport (transpiration) in response to changes in Ca
2+ concentration. The process may involve the reverse regulation of aquaporins (AQPs) in leaf guard cells. When the cytosolic Ca
2+ content is low, AQPs are activated and Ca
2+ from the apoplast along with water flow into the cytoplasm. Conversely, when the cytosolic Ca
2+ content is high, AQPs shuts down and reduces the hydraulic conductivity of leaves to prevent excessive Ca
2+ accumulation in the cytoplasm [
134]. Thus, the Ca ion can be utilized as transpiration regulator for plants, as a previous study have has observed that spraying CaCl
2 on tobacco leaves significantly reduces the transpiration together with the Cd accumulation. However, the underlying mechanism has not been thoroughly investigated [
133].
4. Conclusion and perspectives
In recent decades, the cycle of Ca through the soil and ecosystem has been significantly affected by rising temperatures and changes in precipitation patterns. Increased temperatures speed up the decomposition of organic matter in soils, alongside Ca release from the soil, making it less available to plants. In addition, droughts, dry spells and heavy rainfall events lead to Ca leaching, as soil water moves through the soil and carries the nutrient away. The loss of Ca has far-reaching consequences for ecosystems. Plants may experience Ca deficiency, which reduce their growth, productivity and resistance to abiotic stresses, including Cd toxicity. For this reason, the possible roles of Ca in mitigating Cd translocation in rice are reviewed here, which may arouse the concern about Ca research in plants.
Ca is known for its role in cell wall composition, transporter gene expression, and transpiration, which have a crucial role in Cd tolerance. Hence, the special proposed mechanisms in this review include desorption of Cd on the iron plaque of rice roots, maintaining the structural stability of cell wall, co-transport of Cd by CAXs, and inhibiting Cd translocation by regulating transpiration (see
Figure 2). Besides the mentioned mechanisms, several general functions concerning ion homeostasis, ROS regulation, synthesis of stress-responsive proteins should also be further elaborated. Furthermore, an increasing number of studies show that the function of Ca is mediated by signaling messengers, such as plant hormones, nitric oxide (NO), and ROS. Thus, the crosstalk between Ca and signaling messengers may be an important research aspect in the mechanisms of Ca-mediated restriction in Cd translocation in plants.
Author Contributions
writing—original draft preparation, Junli Liu, Xiaoyu Feng, and Bin Guo; Conceptualization, Gaoyang Qiu; writing—review and editing, Junli Liu, Bin Guo and Xiaoyu Feng; visuali-zation, Hua Li; supervision, Qinlin Fu and Bin Guo; validation, Xiaodong Chen and Yuan Wang; funding acquisition, Bin Guo, Xiaodong Chen, Gaoyang Qiu and Junli Liu. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of China (42007120, 41001184, 42007085, and 42207356), Zhejiang Provincial Natural Science Foundation of China (LQ21D010003), Fundamental Research Funds for the Central Universities (226-2023-00077), and State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABA |
Abscisic acid |
| ABC |
ATP-Binding Cassette |
| Al |
Aluminium |
| AQPs |
Aquaporins |
| B |
Boron |
| Ca |
Calcium |
| CAX |
H+/cation-antiporters Exchanger |
| Cd |
Cadmium |
| CEC |
Cation exchange capacity |
| Co |
Cobalt |
| CS |
Casparian strip |
| Cu |
Copper |
| DOC |
Dissolved organic carbon |
| EDX |
Energy-dispersive X-ray micro-analysis |
| Fe |
Iron |
| GR |
Glutathione reductase |
| GR1 |
Glutathione reductase1 |
| GST |
Glutathione-S-transferase |
| HMA |
Heavy Metal-transporting ATPases |
| JA |
Jasmonic acid |
| K |
Potassium |
| Mg |
Magnesium |
| Mn |
Manganese |
| MTs |
Metallothioneins |
| N |
Nitrogen |
| NO |
Nitric oxide |
| NRAMP |
Natural Resistance-Associated Macrophage Protein |
| P |
Phosphorus |
| PCs |
Phytochelatins |
| PM |
Plasma membrane |
| PME |
Pectin methylesterase gene |
| QTL |
Quantitative Trait Loci |
| ROS |
Reactive oxygen species |
| S |
Sulfur |
| S |
Salicylic acid |
| SAT |
Serine acetyltransferase |
| Se |
Selenium |
| Si |
Silicon |
| -SH |
Sulfhydryl |
| SOM |
Soil organic matter |
| ZIP |
Zinc and Iron regulated transporter |
| Zn |
Zinc |
References
- Zou, M.; Zhou, S.; Zhou, Y.; Jia, Z.; Guo, T.; Wang, J. Cadmium Pollution of Soil-Rice Ecosystems in Rice Cultivation Dominated Regions in China: A Review. Environmental Pollution 2021, 280, 116965. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil Heavy Metal Pollution and Food Safety in China: Effects, Sources and Removing Technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Chen, C.; Kou, M.; Liu, Z.; Wang, Z.; Cai, J.; Tan, W. Heavy Metal Concentrations in Rice That Meet Safety Standards Can Still Pose a Risk to Human Health. Commun Earth Environ 2023, 4, 84. [Google Scholar] [CrossRef]
- Inkham, R.; Kijjanapanich, V.; Huttagosol, P.; Kijjanapanich, P. Low-Cost Alkaline Substances for the Chemical Stabilization of Cadmium-Contaminated Soils. Journal of Environmental Management 2019, 250, 109395. [Google Scholar] [CrossRef]
- White, P.J. Calcium in Plants. Annals of Botany 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Pacyna, J.M. Quantitative Assessment of Worldwide Contamination of Air, Water and Soils by Trace Metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef]
- Williams, P.N.; Lei, M.; Sun, G.; Huang, Q.; Lu, Y.; Deacon, C.; Meharg, A.A.; Zhu, Y.-G. Occurrence and Partitioning of Cadmium, Arsenic and Lead in Mine Impacted Paddy Rice: Hunan, China. Environ. Sci. Technol. 2009, 43, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, X.; Bai, J.; Shih, K.; Zeng, E.Y.; Cheng, H. Assessing Heavy Metal Pollution in the Surface Soils of a Region That Had Undergone Three Decades of Intense Industrialization and Urbanization. Environ Sci Pollut Res 2013, 20, 6150–6159. [Google Scholar] [CrossRef]
- Parvin, A.; Moniruzzaman, M.; Hossain, M.K.; Saha, B.; Parvin, A.; Suchi, P.D.; Hoque, S. Chemical Speciation and Potential Mobility of Heavy Metals in Organic Matter Amended Soil. Applied and Environmental Soil Science 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Salati, S.; Quadri, G.; Tambone, F.; Adani, F. Fresh Organic Matter of Municipal Solid Waste Enhances Phytoextraction of Heavy Metals from Contaminated Soil. Environmental Pollution 2010, 158, 1899–1906. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metal(Loid)s Contaminated Soils – To Mobilize or to Immobilize? Journal of Hazardous Materials 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Wielgusz, K.; Praczyk, M.; Irzykowska, L.; Świerk, D. Fertilization and Soil PH Affect Seed and Biomass Yield, Plant Morphology, and Cadmium Uptake in Hemp (Cannabis Sativa L.). Industrial Crops and Products 2022, 175, 114245. [Google Scholar] [CrossRef]
- Argüello, D.; Chavez, E.; Gutierrez, E.; Pittomvils, M.; Dekeyrel, J.; Blommaert, H.; Smolders, E. Soil Amendments to Reduce Cadmium in Cacao (Theobroma Cacao L.): A Comprehensive Field Study in Ecuador. Chemosphere 2023, 324, 138318. [Google Scholar] [CrossRef] [PubMed]
- Ubeynarayana, N.; Jeyakumar, P.; Bishop, P.; Pereira, R.C.; Anderson, C.W.N. Effect of Soil Cadmium on Root Organic Acid Secretion by Forage Crops. Environmental Pollution 2021, 268, 115839. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman, M.; Bani Mfarrej, M.F.; Usman, M.; Azhar, M.; Rizwan, M.; Alharby, H.F.; Bamagoos, A.A.; Alshamrani, R.; Ahmad, Z. Exogenous Application of Low and High Molecular Weight Organic Acids Differentially Affected the Uptake of Cadmium in Wheat-Rice Cropping System in Alkaline Calcareous Soil. Environmental Pollution 2023, 329, 121682. [Google Scholar] [CrossRef]
- Tao, Q.; Hou, D.; Yang, X.; Li, T. Oxalate Secretion from the Root Apex of Sedum Alfredii Contributes to Hyperaccumulation of Cd. Plant Soil 2016, 398, 139–152. [Google Scholar] [CrossRef]
- Sakouhi, L.; Kharbech, O.; Ben Massoud, M.; Munemasa, S.; Murata, Y.; Chaoui, A. Exogenous Oxalic Acid Protects Germinating Chickpea Seeds Against Cadmium Injury. J Soil Sci Plant Nutr 2022, 22, 647–659. [Google Scholar] [CrossRef]
- Hill, K.A.; Lion, L.W.; Ahner, B.A. Reduced Cd Accumulation in Zea Mays : A Protective Role for Phytosiderophores? Environ. Sci. Technol. 2002, 36, 5363–5368. [Google Scholar] [CrossRef]
- Meda, A.R.; Scheuermann, E.B.; Prechsl, U.E.; Erenoglu, B.; Schaaf, G.; Hayen, H.; Weber, G.; Von Wirén, N. Iron Acquisition by Phytosiderophores Contributes to Cadmium Tolerance. Plant Physiol. 2007, 143, 1761–1773. [Google Scholar] [CrossRef]
- Argüello, D.; Chavez, E.; Gutierrez, E.; Pittomvils, M.; Dekeyrel, J.; Blommaert, H.; Smolders, E. Soil Amendments to Reduce Cadmium in Cacao (Theobroma Cacao L.): A Comprehensive Field Study in Ecuador. Chemosphere 2023, 324, 138318. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace Elements in Soil-Vegetables Interface: Translocation, Bioaccumulation, Toxicity and Amelioration - A Review. Science of The Total Environment 2019, 651, 2927–2942. [Google Scholar] [CrossRef]
- Redjala, T.; Zelko, I.; Sterckeman, T.; Legué, V.; Lux, A. Relationship between Root Structure and Root Cadmium Uptake in Maize. Environmental and Experimental Botany 2011, 71, 241–248. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-Driven Removal of Heavy Metals from Soil: Uptake, Translocation, Tolerance Mechanism, Challenges, and Future Perspectives. Environ Monit Assess 2016, 188, 206. [Google Scholar] [CrossRef] [PubMed]
- Manousaki, E.; Kalogerakis, N. Phytoextraction of Pb and Cd by the Mediterranean Saltbush (Atriplex Halimus L.): Metal Uptake in Relation to Salinity. Environ Sci Pollut Res 2009, 16, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, I.; Kastori, R.; Krstić, L.; Luković, J. Steady Presence of Cadmium and Nickel Affects Root Anatomy, Accumulation and Distribution of Essential Ions in Maize Seedlings. Biologia plant. 2007, 51, 589–592. [Google Scholar] [CrossRef]
- Farrell, R.E.; McArthur, D.F.E.; Van Rees, K.C.J. Net Cd 2+ Flux at the Root Surface of Durum Wheat ( Triticum Turgidum L. Var. Durum ) Cultivars in Relation to Cultivar Differences in Cd Accumulation. Can. J. Plant Sci. 2005, 85, 103–107. [Google Scholar] [CrossRef]
- Piñeros, M.A.; Shaff, J.E.; Kochian, L.V. Development, Characterization, and Application of a Cadmium-Selective Microelectrode for the Measurement of Cadmium Fluxes in Roots of Thlaspi Species and Wheat1. Plant Physiology 1998, 116, 1393–1401. [Google Scholar] [CrossRef]
- Chen, X.; Ouyang, Y.; Fan, Y.; Qiu, B.; Zhang, G.; Zeng, F. The Pathway of Transmembrane Cadmium Influx via Calcium-Permeable Channels and Its Spatial Characteristics along Rice Root. Journal of Experimental Botany 2018, 69, 5279–5291. [Google Scholar] [CrossRef]
- Huang, L.; Hansen, H.; Chr, B.; Wang, H.; Mu, J.; Xie, Z.; Zheng, L.; Hu, Z. Effects of Sulfate on Cadmium Uptake in Wheat Grown in Paddy Soil - Pot Experiment. Plant Soil Environ. 2019, 65, 602–608. [Google Scholar] [CrossRef]
- Anwen, X.; Danting, C.; Chin, L.W.; Zhihong, Y. Root Morphology and Anatomy Affect Cadmium Translocation and Accumulation in Rice. Rice Science 2021, 28, 594–604. [Google Scholar] [CrossRef]
- Laporte, M.A.; Denaix, L.; Pagès, L.; Sterckeman, T.; Flénet, F.; Dauguet, S.; Nguyen, C. Longitudinal Variation in Cadmium Influx in Intact First Order Lateral Roots of Sunflower (Helianthus Annuus. L). Plant Soil 2013, 372, 581–595. [Google Scholar] [CrossRef]
- Van Belleghem, F.; Cuypers, A.; Semane, B.; Smeets, K.; Vangronsveld, J.; d’Haen, J.; Valcke, R. Subcellular Localization of Cadmium in Roots and Leaves of Arabidopsis Thaliana. New Phytologist 2007, 173, 495–508. [Google Scholar] [CrossRef]
- Carrier, P.; Baryla, A.; Havaux, M. Cadmium Distribution and Microlocalization in Oilseed Rape (Brassica Napus) after Long-Term Growth on Cadmium-Contaminated Soil. Planta 2003, 216, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tao, Q.; Shohag, M.J.I.; Yang, X.; Sparks, D.L.; Liang, Y. Root Cell Wall Polysaccharides Are Involved in Cadmium Hyperaccumulation in Sedum Alfredii. Plant Soil 2015, 389, 387–399. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fahad, S.; Wang, X. Nano-Silicon Mediated Alleviation of Cd Toxicity by Cell Wall Adsorption and Antioxidant Defense System in Rice Seedlings. Plant Soil 2023, 486, 103–117. [Google Scholar] [CrossRef]
- Wang, K.; Yu, H.; Zhang, X.; Ye, D.; Huang, H.; Wang, Y.; Zheng, Z.; Li, T. Hydrogen Peroxide Contributes to Cadmium Binding on Root Cell Wall Pectin of Cadmium-Safe Rice Line (Oryza Sativa L.). Ecotoxicology and Environmental Safety 2022, 237, 113526. [Google Scholar] [CrossRef]
- Yu, M.; Zhuo, R.; Lu, Z.; Li, S.; Chen, J.; Wang, Y.; Li, J.; Han, X. Molecular Insights into Lignin Biosynthesis on Cadmium Tolerance: Morphology, Transcriptome and Proteome Profiling in Salix Matsudana. Journal of Hazardous Materials 2023, 441, 129909. [Google Scholar] [CrossRef]
- Delgado, L.; Martínez, G.; López-Iglesias, C.; Mercadé, E. Cryo-Electron Tomography of Plunge-Frozen Whole Bacteria and Vitreous Sections to Analyze the Recently Described Bacterial Cytoplasmic Structure, the Stack. Journal of Structural Biology 2015, 189, 220–229. [Google Scholar] [CrossRef]
- Serova, T.A.; Tikhonovich, I.A.; Tsyganov, V.E. Analysis of Nodule Senescence in Pea (Pisum Sativum L.) Using Laser Microdissection, Real-Time PCR, and ACC Immunolocalization. Journal of Plant Physiology 2017, 212, 29–44. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and Cellular Localization Reveal Involvement of Rice NRAMP, OsNRAMP1, in Arsenic Transport and Tolerance: OsNRAMP1 in Arsenic Transport and Tolerance. Plant Cell Environ 2014, 37, 140–152. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. Role of the Iron Transporter OsNRAMP1 in Cadmium Uptake and Accumulation in Rice. Plant Signaling & Behavior 2011, 6, 1813–1816. [Google Scholar] [CrossRef]
- Chang, J.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F. OsNRAMP1 Transporter Contributes to Cadmium and Manganese Uptake in Rice. Plant Cell Environ 2020, 43, 2476–2491. [Google Scholar] [CrossRef]
- Chang, J.-D.; Xie, Y.; Zhang, H.; Zhang, S.; Zhao, F.-J. The Vacuolar Transporter OsNRAMP2 Mediates Fe Remobilization during Germination and Affects Cd Distribution to Rice Grain. Plant Soil 2022, 476, 79–95. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 Is a Major Transporter Responsible for Manganese and Cadmium Uptake in Rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Cao, H.W.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 Functions as a Metal Efflux Transporter Limiting Excess Zinc, Copper and Cadmium Accumulation in Rice. BMC Plant Biol 2019, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Y.; Liu, Z.; Tian, J.; Liang, L.; Qiu, Y.; Wang, G.; Du, Q.; Cheng, D.; Cai, H.; et al. A High Activity Zinc Transporter OsZIP9 Mediates Zinc Uptake in Rice. Plant J 2020, 103, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 Functions in Xylem Loading in Roots and Inter-Vascular Transfer in Nodes to Deliver Zn/Cd to Grain in Rice. Biochemical and Biophysical Research Communications 2019, 512, 112–118. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Feng Ma, J. Preferential Delivery of Zinc to Developing Tissues in Rice Is Mediated by P-Type Heavy Metal ATPase OsHMA2. Plant Physiology 2013, 162, 927–939. [Google Scholar] [CrossRef]
- Wiggenhauser, M.; Aucour, A.-M.; Bureau, S.; Campillo, S.; Telouk, P.; Romani, M.; Ma, J.F.; Landrot, G.; Sarret, G. Cadmium Transfer in Contaminated Soil-Rice Systems: Insights from Solid-State Speciation Analysis and Stable Isotope Fractionation. Environmental Pollution 2021, 269, 115934. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.-Y.; Lee, Y.; An, G. Rice P1B-Type Heavy-Metal ATPase, OsHMA9, Is a Metal Efflux Protein. Plant Physiology 2007, 145, 831–842. [Google Scholar] [CrossRef]
- Yang, G.; Fu, S.; Huang, J.; Li, L.; Long, Y.; Wei, Q.; Wang, Z.; Chen, Z.; Xia, J. The Tonoplast-Localized Transporter OsABCC9 Is Involved in Cadmium Tolerance and Accumulation in Rice. Plant Science 2021, 307, 110894. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Lu, Y.; Zhang, X.; Yang, G.; Chao, D.; Wang, Z.; Shi, M.; Chen, J.; Chao, D.-Y.; Li, R.; et al. The ABC Transporter ABCG36 Is Required for Cadmium Tolerance in Rice. Journal of Experimental Botany 2019, 70, 5909–5918. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. The Pathways of Calcium Movement to the Xylem. Journal of Experimental Botany 2001, 52, 891–899. [Google Scholar] [CrossRef]
- Prince, R.C. Mechanisms of Cadmium Mobility and Accumulation in Lndian Mustard’. 1995. [Google Scholar]
- Van Der Vliet, L.; Peterson, C.; Hale, B. Cd Accumulation in Roots and Shoots of Durum Wheat: The Roles of Transpiration Rate and Apoplastic Bypass. Journal of Experimental Botany 2007, 58, 2939–2947. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-Shoot Cd Translocation via the Xylem Is the Major Process Determining Shoot and Grain Cadmium Accumulation in Rice. Journal of Experimental Botany 2009, 60, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mishra, A.; Küpper, H. Protein Biochemistry and Expression Regulation of Cadmium/Zinc Pumping ATPases in the Hyperaccumulator Plants Arabidopsis Halleri and Noccaea Caerulescens. Front. Plant Sci. 2017, 8, 835. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujimaki, S.; Fujiwara, T.; Yoneyama, T.; Hayashi, H. Quantitative Estimation of the Contribution of the Phloem in Cadmium Transport to Grains in Rice Plants ( Oryza Sativa L.). Soil Science and Plant Nutrition 2007, 53, 72–77. [Google Scholar] [CrossRef]
- Kato, M.; Ishikawa, S.; Inagaki, K.; Chiba, K.; Hayashi, H.; Yanagisawa, S.; Yoneyama, T. Possible Chemical Forms of Cadmium and Varietal Differences in Cadmium Concentrations in the Phloem Sap of Rice Plants ( Oryza Sativa L.). Soil Science and Plant Nutrition 2010, 56, 839–847. [Google Scholar] [CrossRef]
- Liñero, O.; Cornu, J.-Y.; De Diego, A.; Bussière, S.; Coriou, C.; Thunot, S.; Robert, T.; Nguyen, C. Source of Ca, Cd, Cu, Fe, K, Mg, Mn, Mo and Zn in Grains of Sunflower (Helianthus Annuus) Grown in Nutrient Solution: Root Uptake or Remobilization from Vegetative Organs? Plant Soil 2018, 424, 435–450. [Google Scholar] [CrossRef]
- Yan, B.-F.; Nguyen, C.; Pokrovsky, O.S.; Candaudap, F.; Coriou, C.; Bussière, S.; Robert, T.; Cornu, J.Y. Contribution of Remobilization to the Loading of Cadmium in Durum Wheat Grains: Impact of Post-Anthesis Nitrogen Supply. Plant Soil 2018, 424, 591–606. [Google Scholar] [CrossRef]
- Kobayashi, N.I.; Tanoi, K.; Hirose, A.; Nakanishi, T.M. Characterization of Rapid Intervascular Transport of Cadmium in Rice Stem by Radioisotope Imaging. Journal of Experimental Botany 2013, 64, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Ma, J.F. Node-Controlled Allocation of Mineral Elements in Poaceae. Current Opinion in Plant Biology 2017, 39, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Screening of Tomato ( Lycopersicon Esculentum ) Cultivars against Cadmium through Shotgun Approach. Journal of Plant Interactions 2009, 4, 187–201. [Google Scholar] [CrossRef]
- Lysenko, E.A.; Klaus, A.A.; Pshybytko, N.L.; Kusnetsov, V.V. Cadmium Accumulation in Chloroplasts and Its Impact on Chloroplastic Processes in Barley and Maize. Photosynth Res 2015, 125, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-Induced Morphogenic Responses: Growing out of Trouble? Trends in Plant Science 2007, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium Toxicity in Maize (Zea Mays L.): Consequences on Antioxidative Systems, Reactive Oxygen Species and Cadmium Accumulation. Environ Sci Pollut Res 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- DalCorso, G.; Farinati, S.; Furini, A. Regulatory Networks of Cadmium Stress in Plants. Plant Signaling & Behavior 2010, 5, 663–667. [Google Scholar] [CrossRef]
- Sanità Di Toppi, L.; Gabbrielli, R. Response to Cadmium in Higher Plants. Environmental and Experimental Botany 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicology and Environmental Safety 2021, 211, 111887. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium Stress in Plants: A Critical Review of the Effects, Mechanisms, and Tolerance Strategies. Critical Reviews in Environmental Science and Technology 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Zhou, Y.; Núñez-Delgado, A.; Wang, X. Boron Application Mitigates Cd Toxicity in Leaves of Rice by Subcellular Distribution, Cell Wall Adsorption and Antioxidant System. Ecotoxicology and Environmental Safety 2021, 222, 112540. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.Z.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sabir, M.; Qayyum, M.F. Effect of Inorganic Amendments for in Situ Stabilization of Cadmium in Contaminated Soils and Its Phyto-Availability to Wheat and Rice under Rotation. Environ Sci Pollut Res 2015, 22, 16897–16906. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Rahman, A.; Ansary, Md.M.U.; Watanabe, A.; Fujita, M.; Tran, L.-S.P. Hydrogen Sulfide Modulates Cadmium-Induced Physiological and Biochemical Responses to Alleviate Cadmium Toxicity in Rice. Sci Rep 2015, 5, 14078. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Marañón, T.; Murillo, J.M.; Redondo-Gómez, S. Response of Holm Oak (Quercus Ilex Subsp. Ballota) and Mastic Shrub (Pistacia Lentiscus L.) Seedlings to High Concentrations of Cd and Tl in the Rhizosphere. Chemosphere 2011, 83, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Gapper, C.; Dolan, L. Control of Plant Development by Reactive Oxygen Species. Plant Physiology 2006, 141, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Gao, X.; Zhang, S.; Fu, X.; Le, Y.; Wang, L. Cadmium Stimulated Cooperation between Bacterial Endophytes and Plant Intrinsic Detoxification Mechanism in Lonicera Japonica Thunb. Chemosphere 2023, 325, 138411. [Google Scholar] [CrossRef]
- Mengdi, X.; Wenqing, C.; Haibo, D.; Xiaoqing, W.; Li, Y.; Yuchen, K.; Hui, S.; Lei, W. Cadmium-Induced Hormesis Effect in Medicinal Herbs Improves the Efficiency of Safe Utilization for Low Cadmium-Contaminated Farmland Soil. Ecotoxicology and Environmental Safety 2021, 225, 112724. [Google Scholar] [CrossRef]
- Nogueira, M.L.; Carvalho, M.E.A.; Ferreira, J.M.M.; Bressanin, L.A.; Piotto, K.D.B.; Piotto, F.A.; Marques, D.N.; Barbosa, S.; Azevedo, R.A. Cadmium-Induced Transgenerational Effects on Tomato Plants: A Gift from Parents to Progenies. Science of The Total Environment 2021, 789, 147885. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis in Plants: Its Common Occurrence across Stresses. Current Opinion in Toxicology 2022, 30, 100333. [Google Scholar] [CrossRef]
- He, S.; Yang, X.; He, Z.; Baligar, V.C. Morphological and Physiological Responses of Plants to Cadmium Toxicity: A Review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium Stress in Rice: Toxic Effects, Tolerance Mechanisms, and Management: A Critical Review. Environ Sci Pollut Res 2016, 23, 17859–17879. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.B.; Rahman, M.M.; Islam, Md.R.; Naidu, R. Influences of Soil PH, Iron Application and Rice Variety on Cadmium Distribution in Rice Plant Tissues. Science of The Total Environment 2022, 810, 152296. [Google Scholar] [CrossRef] [PubMed]
- Dundar, E.; Sonmez, G.D.; Unver, T. Isolation, Molecular Characterization and Functional Analysis of OeMT2, an Olive Metallothionein with a Bioremediation Potential. Mol Genet Genomics 2015, 290, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of Silicon-Mediated Alleviation of Heavy Metal Toxicity in Plants: A Review. Ecotoxicology and Environmental Safety 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Yang, G.; Rizwan, M.; Ali, S.; Zhou, Y.; Wang, Q.; Deng, L.; Wang, Y.; et al. Boron Supply Alleviates Cadmium Toxicity in Rice (Oryza Sativa L.) by Enhancing Cadmium Adsorption on Cell Wall and Triggering Antioxidant Defense System in Roots. Chemosphere 2021, 266, 128938. [Google Scholar] [CrossRef]
- Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Protective Mechanisms of Sulfur against Arsenic Phytotoxicity in Brassica Napus by Regulating Thiol Biosynthesis, Sulfur-Assimilation, Photosynthesis, and Antioxidant Response. Plant Physiology and Biochemistry 2022, 188, 1–11. [Google Scholar] [CrossRef]
- Rajendran, M.; Shi, L.; Wu, C.; Li, W.; An, W.; Liu, Z.; Xue, S. Effect of Sulfur and Sulfur-Iron Modified Biochar on Cadmium Availability and Transfer in the Soil–Rice System. Chemosphere 2019, 222, 314–322. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F.; et al. Osmoregulation and Antioxidant Production in Maize under Combined Cadmium and Arsenic Stress. Environ Sci Pollut Res 2016, 23, 11864–11875. [Google Scholar] [CrossRef]
- Seth, C.S.; Remans, T.; Keunen, E.; Jozefczak, M.; Gielen, H.; Opdenakker, K.; Weyens, N.; Vangronsveld, J.; Cuypers, A. Phytoextraction of Toxic Metals: A Central Role for Glutathione: Metal Phytoextraction and Glutathione. Plant, Cell & Environment 2012, 35, 334–346. [Google Scholar] [CrossRef]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-Induced Changes in Antioxidative Systems, Hydrogen Peroxide Content, and Differentiation in Scots Pine Roots. Plant Physiology 2001, 127, 887–898. [Google Scholar] [CrossRef]
- Chaouch, S.; Queval, G.; Vanderauwera, S.; Mhamdi, A.; Vandorpe, M.; Langlois-Meurinne, M.; Van Breusegem, F.; Saindrenan, P.; Noctor, G. Peroxisomal Hydrogen Peroxide Is Coupled to Biotic Defense Responses by ISOCHORISMATE SYNTHASE1 in a Daylength-Related Manner. Plant Physiology 2010, 153, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, C.; Li, H.; Yi, K.; Ding, N.; Li, N.; Lin, Y.; Fu, Q. Endogenous Salicylic Acid Is Required for Promoting Cadmium Tolerance of Arabidopsis by Modulating Glutathione Metabolisms. Journal of Hazardous Materials 2016, 316, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Hendrix, S.; Prinsen, E.; Vangronsveld, J.; Cuypers, A. Identifying the Pressure Points of Acute Cadmium Stress Prior to Acclimation in Arabidopsis Thaliana. IJMS 2020, 21, 6232. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; An, T.; Zhu, X.; Ji, J.; Wang, G.; Guan, C.; Jin, C.; Yi, L. GR1-like Gene Expression in Lycium Chinense Was Regulated by Cadmium-Induced Endogenous Jasmonic Acids Accumulation. Plant Cell Rep 2017, 36, 1457–1476. [Google Scholar] [CrossRef]
- Bočová, B.; Huttová, J.; Mistrík, I.; Tamás, L. Auxin Signalling Is Involved in Cadmium-Induced Glutathione-S-Transferase Activity in Barley Root. Acta Physiol Plant 2013, 35, 2685–2690. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The Language of Calcium Signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Bolat, I.; Kaya, C.; Almaca, A.; Timucin, S. Calcium Sulfate Improves Salinity Tolerance in Rootstocks of Plum. Journal of Plant Nutrition 2006, 29, 553–564. [Google Scholar] [CrossRef]
- Rentel, M.C.; Knight, M.R. Oxidative Stress-Induced Calcium Signaling in Arabidopsis. Plant Physiology 2004, 135, 1471–1479. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Sharma, K.; Bisht, D.; Sharma, S.; Sushmita, K.; Kateriya, S.; Pandey, G.K. Role of Calcium Sensor Protein Module CBL-CIPK in Abiotic Stress and Light Signaling Responses in Green Algae. International Journal of Biological Macromolecules 2023, 237, 124163. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of Soil Acidification and Liming on the Phytoavailability of Cadmium in Paddy Soils of Central Subtropical China. Environmental Pollution 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Chen, W. Manganese, Zinc, and PH Affect Cadmium Accumulation in Rice Grain under Field Conditions in Southern China. J. Environ. Qual. 2018, 47, 306–311. [Google Scholar] [CrossRef] [PubMed]
- He, L.-L.; Huang, D.-Y.; Zhang, Q.; Zhu, H.-H.; Xu, C.; Li, B.; Zhu, Q.-H. Meta-Analysis of the Effects of Liming on Soil PH and Cadmium Accumulation in Crops. Ecotoxicology and Environmental Safety 2021, 223, 112621. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.O.; Kim, S.Y.; Gutierrez, J.; Owens, V.N.; Kim, P.J. Comparison of Oyster Shell and Calcium Hydroxide as Liming Materials for Immobilizing Cadmium in Upland Soil. Biol Fertil Soils 2010, 46, 491–498. [Google Scholar] [CrossRef]
- Cao, X.; Hu, P.; Tan, C.; Wu, L.; Peng, B.; Christie, P.; Luo, Y. Effects of a Natural Sepiolite Bearing Material and Lime on the Immobilization and Persistence of Cadmium in a Contaminated Acid Agricultural Soil. Environ Sci Pollut Res 2018, 25, 22075–22084. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace Elements in the Soil-Plant Interface: Phytoavailability, Translocation, and Phytoremediation–A Review. Earth-Science Reviews 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Siddique, A.B.; Rahman, M.M.; Islam, Md.R.; Naidu, R. Varietal Variation and Formation of Iron Plaques on Cadmium Accumulation in Rice Seedling. Environmental Advances 2021, 5, 100075. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, X.; Shen, H. Root Iron Plaque Alleviates Cadmium Toxicity to Rice (Oryza Sativa) Seedlings. Ecotoxicology and Environmental Safety 2018, 161, 534–541. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Iron Plaque Decreases Cadmium Accumulation in Oryza Sativa L. and Serves as a Source of Iron. Plant Biol J 2016, 18, 1008–1015. [Google Scholar] [CrossRef]
- Guo, B.; Liu, J.; Liu, C.; Lin, Y.; Li, H.; Zhu, D.; Zhang, Q.; Chen, X.; Qiu, G.; Fu, Q.; et al. Shade and Iron Plaque of Sesbania Affect Cadmium Accumulation in Rice: A New Strategy for Safe Production in Contaminated Soil. Environmental Technology & Innovation 2023, 29, 102964. [Google Scholar] [CrossRef]
- Zandi, P.; Yang, J.; Darma, A.; Bloem, E.; Xia, X.; Wang, Y.; Li, Q.; Schnug, E. Iron Plaque Formation, Characteristics, and Its Role as a Barrier and/or Facilitator to Heavy Metal Uptake in Hydrophyte Rice (Oryza Sativa L.). Environ Geochem Health 2023, 45, 525–559. [Google Scholar] [CrossRef]
- Rivera, M.B.; Giráldez, M.I.; Fernández-Caliani, J.C. Assessing the Environmental Availability of Heavy Metals in Geogenically Contaminated Soils of the Sierra de Aracena Natural Park (SW Spain). Is There a Health Risk? Science of The Total Environment 2016, 560–561, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Buckley, W.T.; Buckley, K.E.; Huang, J.J. Root Cadmium Desorption Methods and Their Evaluation with Compartmental Modeling. New Phytologist 2010, 188, 280–290. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, S.; Wan, N.; Feng, B.; Wang, Q.; Huang, K.; Fang, Y.; Bao, Z.; Xu, F. Iron Plaque Effects on Selenium and Cadmium Stabilization in Cd-Contaminated Seleniferous Rice Seedlings. Environ Sci Pollut Res 2022, 30, 22772–22786. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, J.; He, C.; Li, X.; Zhang, W.; Xu, F.; Lin, Y.; Wang, L. Inhibition of Cadmium Ion Uptake in Rice (Oryza Sativa) Cells by a Wall-Bound Form of Silicon. New Phytologist 2013, 200, 691–699. [Google Scholar] [CrossRef]
- Liu, P.; Jin, Z.; Dai, C.; Guo, L.; Cui, X.; Yang, Y. Potassium Enhances Cadmium Resistance Ability of Panax Notoginseng by Brassinolide Signaling Pathway-Regulated Cell Wall Pectin Metabolism. Ecotoxicology and Environmental Safety 2021, 227, 112906. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, B.; Shen, C.; Zhou, W.; Liao, Q.; Chen, Y.; Xin, J. Boron Supplying Alters Cadmium Retention in Root Cell Walls and Glutathione Content in Capsicum Annuum. Journal of Hazardous Materials 2022, 432, 128713. [Google Scholar] [CrossRef] [PubMed]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front Plant Sci 2016, 7, 569. [Google Scholar] [CrossRef]
- Martins, V.; Garcia, A.; Costa, C.; Sottomayor, M.; Gerós, H. Calcium- and Hormone-Driven Regulation of Secondary Metabolism and Cell Wall Enzymes in Grape Berry Cells. Journal of Plant Physiology 2018, 231, 57–67. [Google Scholar] [CrossRef]
- Marcec, M.J.; Tanaka, K. Crosstalk between Calcium and ROS Signaling during Flg22-Triggered Immune Response in Arabidopsis Leaves. Plants (Basel) 2021, 11, 14. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmiño, D.M.; Testillano, P.S.; Risueño, M.C.; Del Río, L.A.; Sandalio, L.M. Cellular Response of Pea Plants to Cadmium Toxicity: Cross Talk between Reactive Oxygen Species, Nitric Oxide, and Calcium. Plant Physiology 2009, 150, 229–243. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Pittman, J.K. Shaping the Calcium Signature. New Phytologist 2009, 181, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating Rapid Long-distance Signaling in Plants with Ca 2+, ROS and Electrical Signals. The Plant Journal 2017, 90, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, J.; Zhu, M.; Zhao, F.; Luan, S. Cadmium Impairs Ion Homeostasis by Altering K+ and Ca2+ Channel Activities in Rice Root Hair Cells. Plant, Cell & Environment 2012, 35, 1998–2013. [Google Scholar] [CrossRef]
- Tian, S.; Xie, R.; Wang, H.; Hu, Y.; Ge, J.; Liao, X.; Gao, X.; Brown, P.; Lin, X.; Lu, L. Calcium Deficiency Triggers Phloem Remobilization of Cadmium in a Hyperaccumulating Species1. Plant Physiol 2016, 172, 2300–2313. [Google Scholar] [CrossRef]
- Li, Z.; Mei, X.; Li, T.; Yang, S.; Qin, L.; Li, B.; Zu, Y. Effects of Calcium Application on Activities of Membrane Transporters in Panax Notoginseng under Cadmium Stress. Chemosphere 2021, 262, 127905. [Google Scholar] [CrossRef]
- Kamiya, T.; Maeshima, M. Residues in Internal Repeats of the Rice Cation/H+ Exchanger Are Involved in the Transport and Selection of Cations. Journal of Biological Chemistry 2004, 279, 812–819. [Google Scholar] [CrossRef]
- Korenkov, V.; Hirschi, K.; Crutchfield, J.D.; Wagner, G.J. Enhancing Tonoplast Cd/H Antiport Activity Increases Cd, Zn, and Mn Tolerance, and Impacts Root/Shoot Cd Partitioning in Nicotiana Tabacum L. Planta 2007, 226, 1379–1387. [Google Scholar] [CrossRef]
- Baliardini, C.; Meyer, C.-L.; Salis, P.; Saumitou-Laprade, P.; Verbruggen, N. CATION EXCHANGER1 Cosegregates with Cadmium Tolerance in the Metal Hyperaccumulator Arabidopsis Halleri and Plays a Role in Limiting Oxidative Stress in Arabidopsis Spp. Plant Physiol. 2015, 169, 549–559. [Google Scholar] [CrossRef]
- Zou, W.; Chen, J.; Meng, L.; Chen, D.; He, H.; Ye, G. The Rice Cation/H+ Exchanger Family Involved in Cd Tolerance and Transport. Int J Mol Sci 2021, 22, 8186. [Google Scholar] [CrossRef]
- Zou, W.; Zhan, J.; Meng, L.; Chen, Y.; Chen, D.; Zhang, M.; He, H.; Chen, J.; Ye, G. The Cation/H + Exchanger OsCAX2 Is Involved in Cadmium Tolerance and Accumulation through Vacuolar Sequestration in Rice; Plant Biology, 2022. [Google Scholar]
- Prince, R.C. Center for Agricultural and Molecular Biology, Rutgers University, Cook College, New Brunswick, New Jersey 08903 (D.E.S., I.R.); Exxon Research and Engineering, Annandale, New Jersey 08801 (R.C.P.);and Stanford Synchrotron Radiation Laboratory, Stanford University, Stanford Linear Accelerator Center, Stanford, California 94309 (I.J.P.).
- Liu, H.; Wang, H.; Ma, Y.; Wang, H.; Shi, Y. Role of Transpiration and Metabolism in Translocation and Accumulation of Cadmium in Tobacco Plants (Nicotiana Tabacum L.). Chemosphere 2016, 144, 1960–1965. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium Delivery and Storage in Plant Leaves: Exploring the Link with Water Flow. Journal of Experimental Botany 2011, 62, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).