Submitted:
05 July 2023
Posted:
05 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
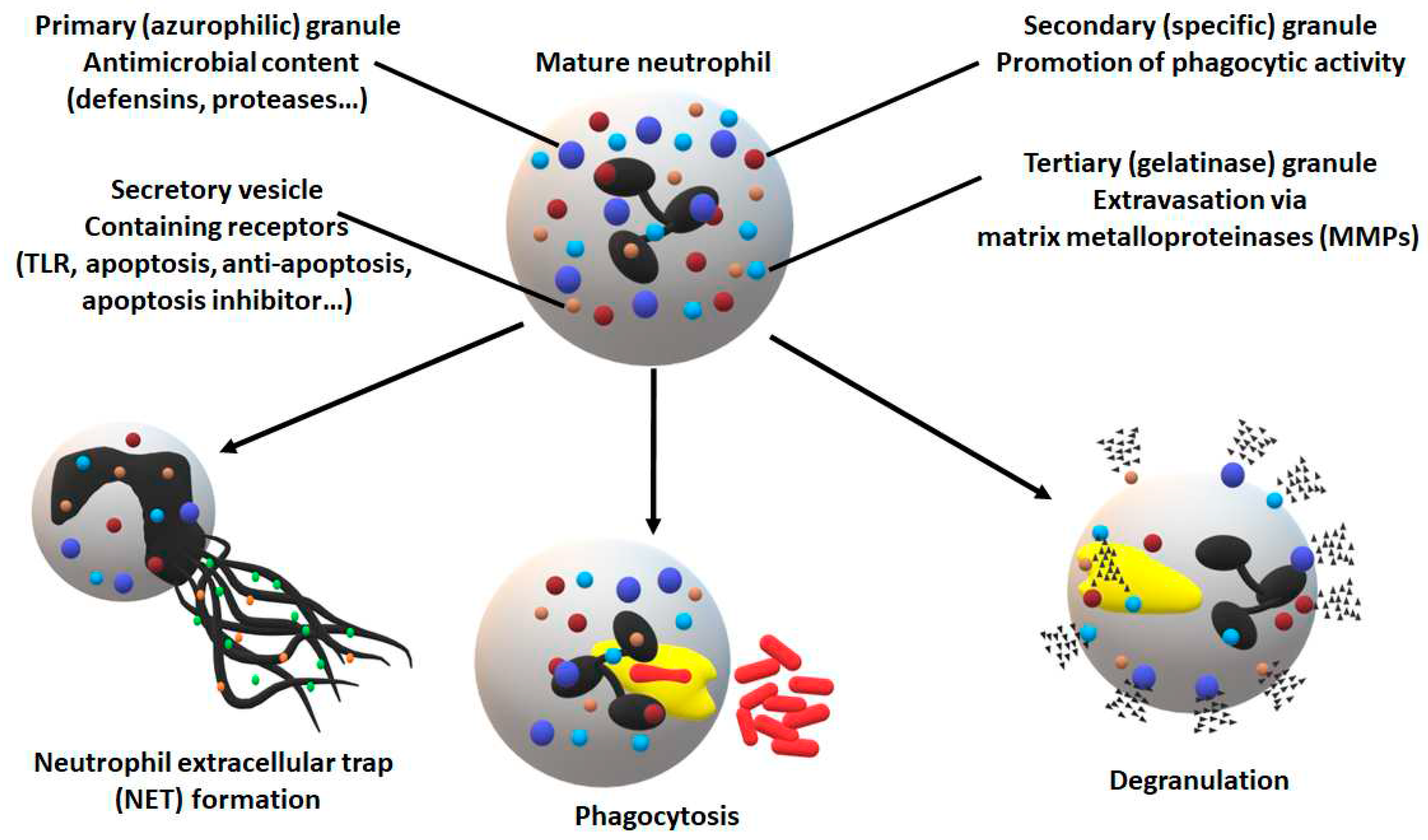
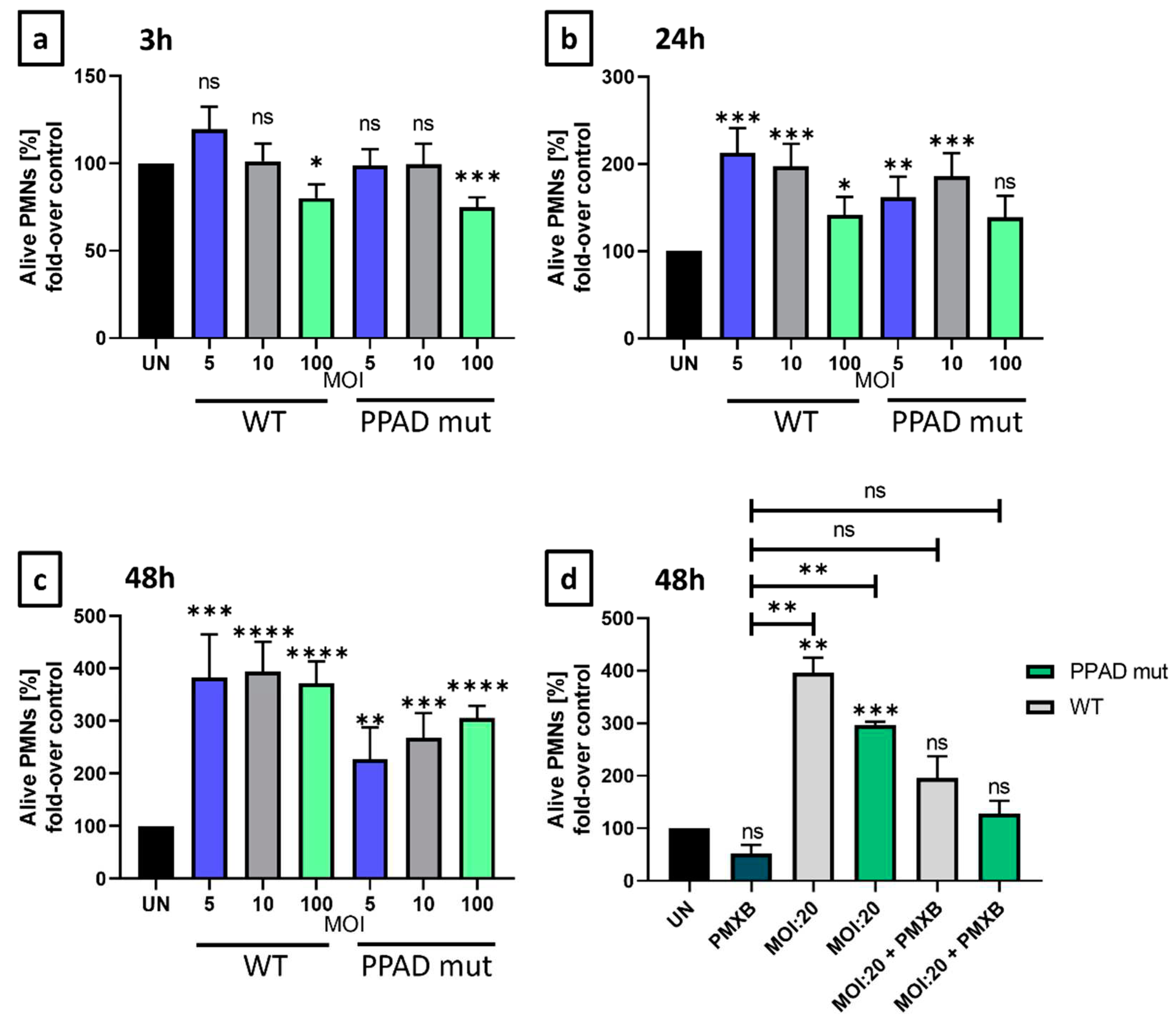
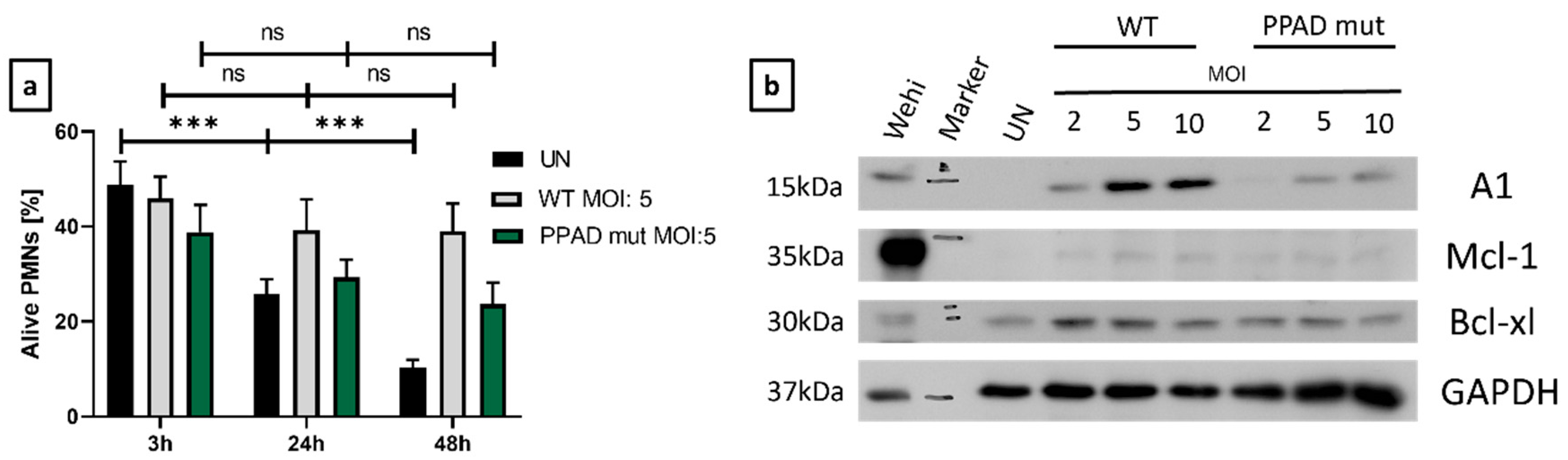
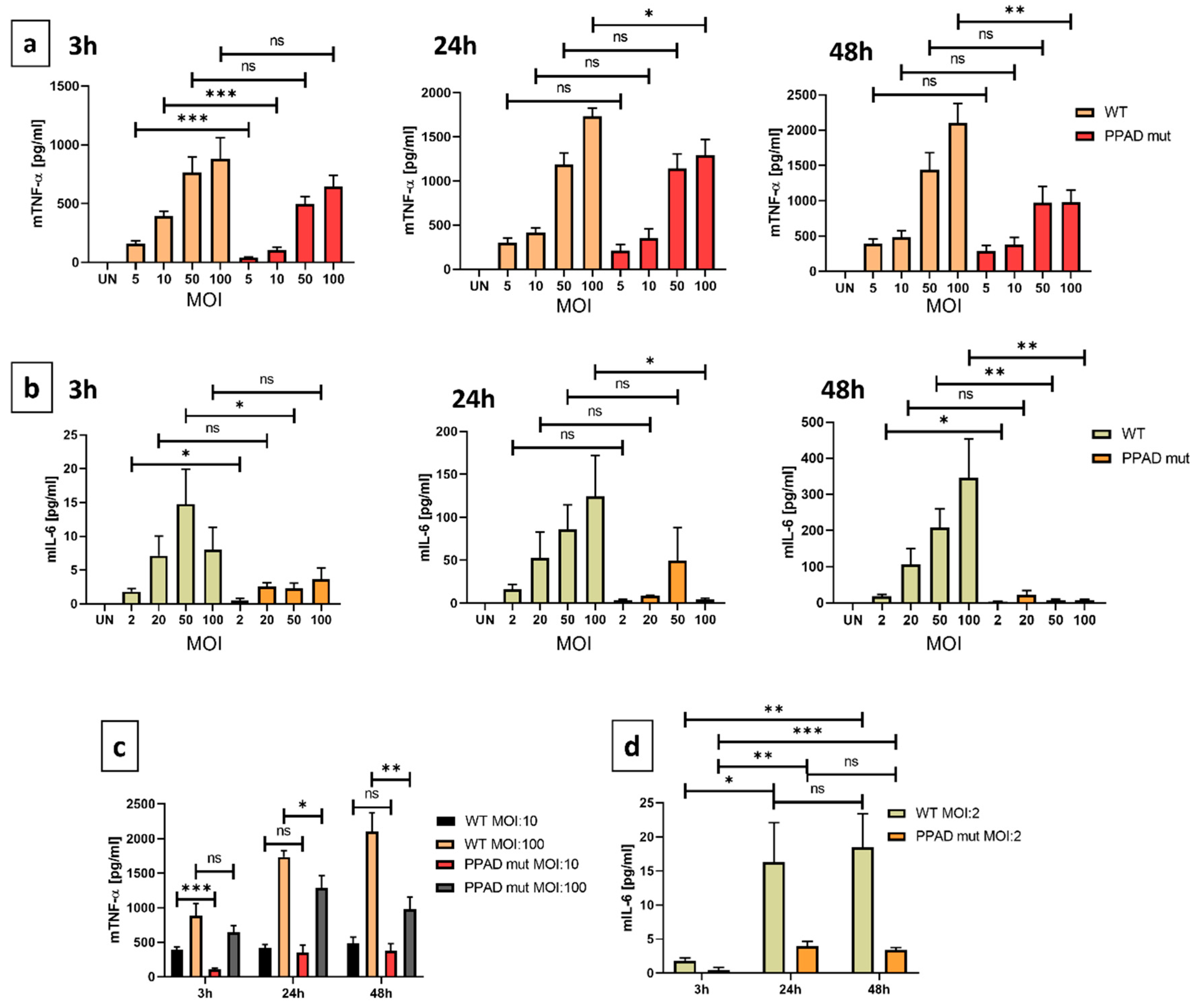
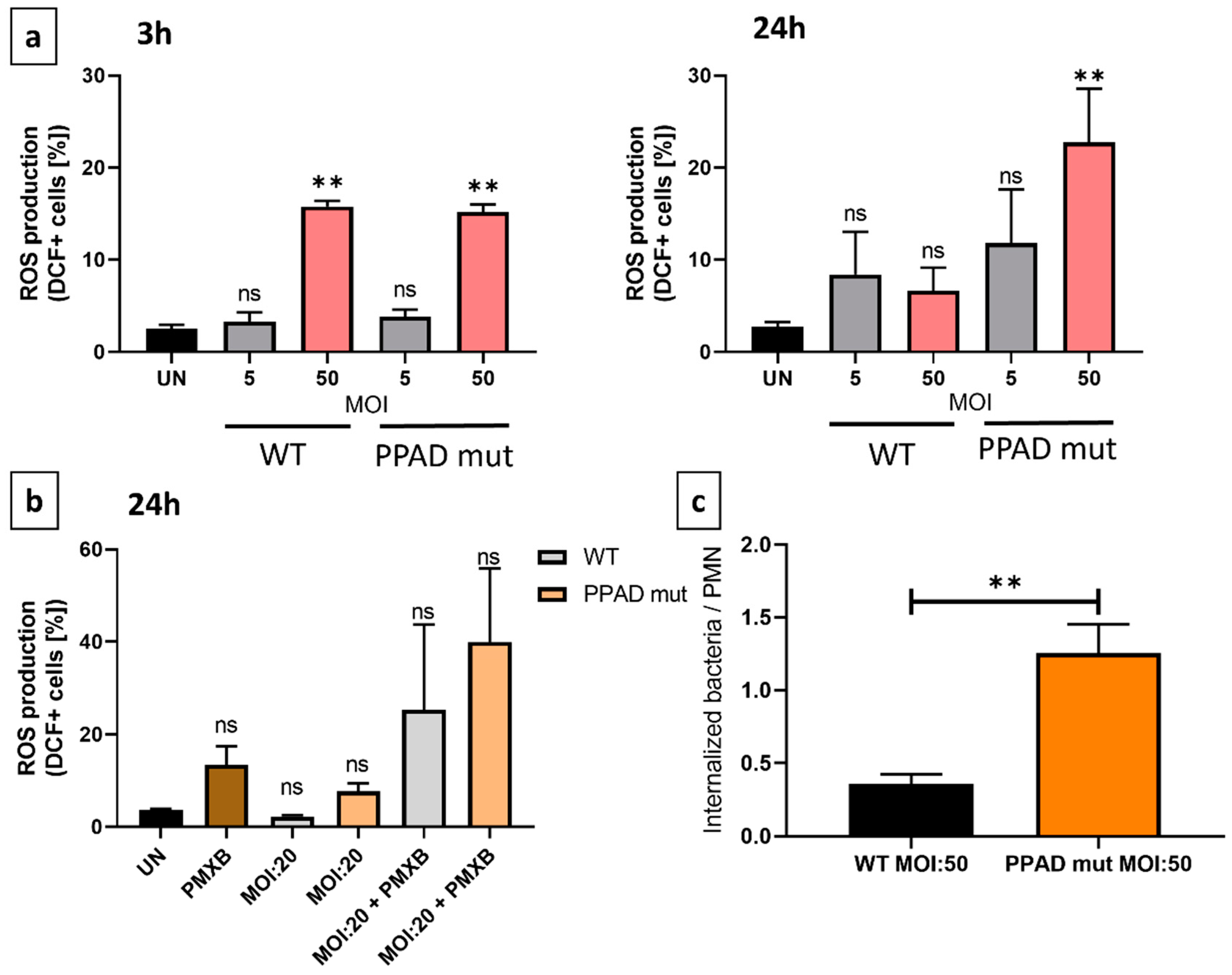
2. Results
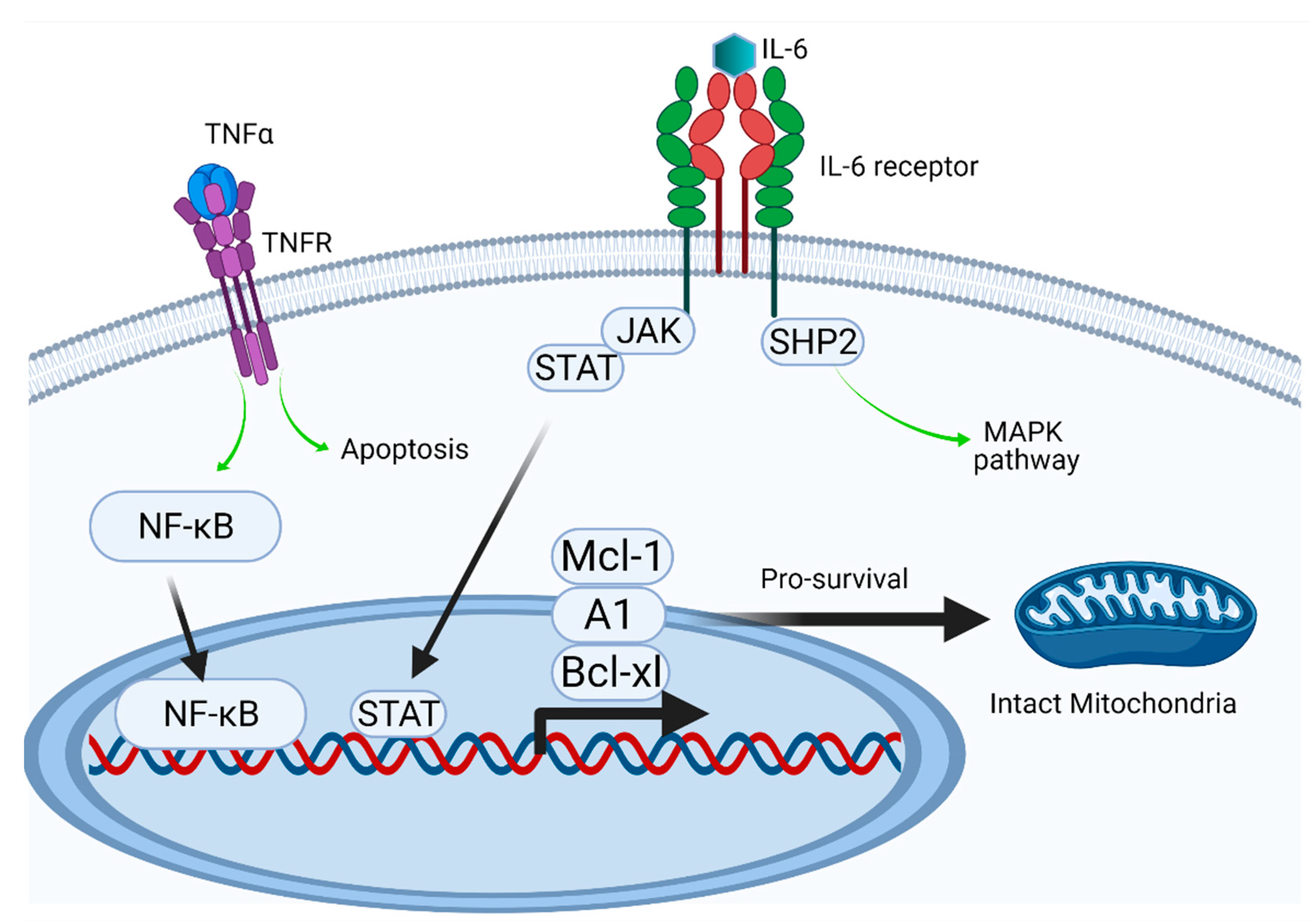
3. Discussion
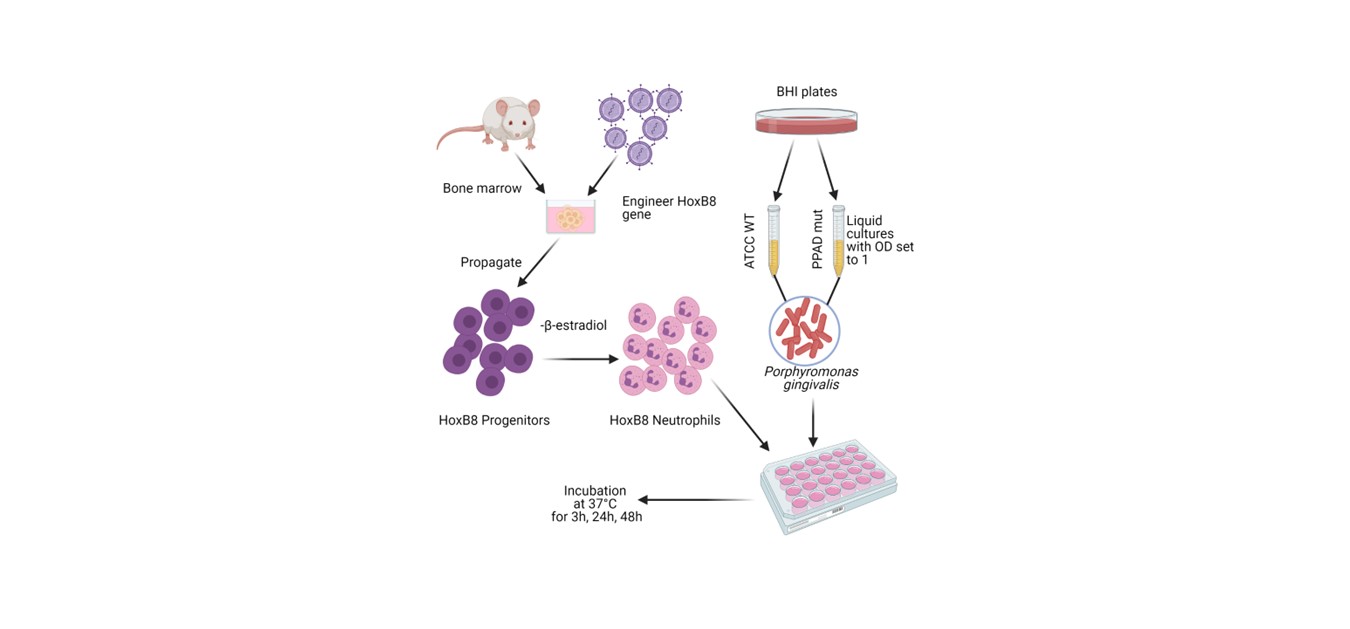
4. Materials and Methods
4.1. Cell lines and bacteria strains
4.2. Flow cytometry
4.3. Immunoblotting
4.4. Enzyme-linked immunosorbent assay (ELISA)
4.5. Invasion assay
4.6. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- “Oral health.” https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed Mar. 23, 2021).
- F. E. Dewhirst et al., “The human oral microbiome,” J Bacteriol, 2010. [CrossRef]
- T. E. van Dyke and D. Sheilesh, “Risk factors for periodontitis.,” Journal of the International Academy of Periodontology, vol. 7, no. 1. NIH Public Access, pp. 3–7, 2005. [CrossRef]
- G. Hajishengallis, R. P. Darveau, and M. A. Curtis, “The keystone-pathogen hypothesis,” Nature Reviews Microbiology, vol. 10, no. 10. NIH Public Access, pp. 717–725, Oct. 2012. [CrossRef]
- P. de Pablo, I. L. C. Chapple, C. D. Buckley, and T. Dietrich, “Periodontitis in systemic rheumatic diseases,” Nat Rev Rheumatol, vol. 5, no. 4, pp. 218–224, 2009. [CrossRef]
- J. Potempa, P. Mydel, and J. Koziel, “The case for periodontitis in the pathogenesis of rheumatoid arthritis,” Nature Reviews Rheumatology, vol. 13, no. 10. Nature Publishing Group, pp. 606–620, Aug. 24, 2017. [CrossRef]
- W. T. McGraw, J. Potempa, D. Farley, and J. Travis, “Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase,” Infect Immun, vol. 67, no. 7, pp. 3248–3256, 1999. [CrossRef]
- 8. G. Gabarrini et al., “The peptidylarginine deiminase gene is a conserved feature of Porphyromonas gingivalis,” Sci Rep, vol. 5, Sep. 2015. [CrossRef]
- S. N. Abdullah, E. A. Farmer, L. Spargo, R. Logan, and N. Gully, “Porphyromonas gingivalis peptidylarginine deiminase substrate specificity,” Anaerobe, vol. 23, pp. 102–108, Oct. 2013. [CrossRef]
- Olsen, S. K. Singhrao, and J. Potempa, “Citrullination as a plausible link to periodontitis, rheumatoid arthritis, atherosclerosis and Alzheimer’s disease,” J Oral Microbiol, vol. 10, no. 1, p. 1487742, Jan. 2018. [CrossRef]
- D. M. Vermilyea, G. K. Ottenberg, and M. E. Davey, “Citrullination mediated by PPAD constrains biofilm formation in P. gingivalis strain 381,” NPJ Biofilms Microbiomes, vol. 5, no. 1, p. 7, Dec. 2019. [CrossRef]
- J. Karkowska-Kuleta et al., “The activity of bacterial peptidylarginine deiminase is important during formation of dual-species biofilm by periodontal pathogen Porphyromonas gingivalis and opportunistic fungus Candida albicans,” Pathog Dis, vol. 76, no. 4, Jun. 2018. [CrossRef]
- N. Gully et al., “Porphyromonas gingivalis peptidylarginine deiminase, a key contributor in the pathogenesis of experimental periodontal disease and experimental arthritis,” PLoS One, vol. 9, no. 6, Jun. 2014. [CrossRef]
- N. Borregaard, “Neutrophils, from Marrow to Microbes,” Immunity, vol. 33, no. 5. Immunity, pp. 657–670, Nov. 24, 2010. [CrossRef]
- T. Li et al., “Neutrophil Extracellular Traps: Signaling Properties and Disease Relevance,” Mediators Inflamm, vol. 2020, pp. 1–14, Jul. 2020. [CrossRef]
- D. A. Scott and J. Krauss, “Neutrophils in periodontal inflammation.,” Front Oral Biol, vol. 15, pp. 56–83, 2012. [CrossRef]
- G. I. Vladimer, R. Marty-Roix, S. Ghosh, D. Weng, and E. Lien, “Inflammasomes and host defenses against bacterial infections.,” Curr Opin Microbiol, vol. 16, no. 1, pp. 23–31, Feb. 2013. [CrossRef]
- S. Elmore, “Apoptosis: A Review of Programmed Cell Death,” Toxicologic Pathology, vol. 35, no. 4. NIH Public Access, pp. 495–516, 2007. [CrossRef]
- C. Haslett, A. Lee, J. S. Savill, L. Meagher, and M. K. Whyte, “Apoptosis (programmed cell death) and functional changes in aging neutrophils: Modulation by inflammatory mediators,” in Chest, Elsevier, Mar. 1991, p. 6S. [CrossRef]
- J. S. Savill, P. M. Henson, and C. Haslett, “Phagocytosis of aged human neutrophils by macrophages is mediated by a novel ’charge-sensitive’recognition mechanism,” Journal of Clinical Investigation, vol. 84, no. 5, pp. 1518–1527, Nov. 1989. [CrossRef]
- P. M. Preshaw, R. E. Schifferle, and J. D. Walters, “Porphyromonas gingivalis lipopolysaccharide delays human polymorphonuclear leukocyte apoptosis in vitro,” J Periodontal Res, vol. 34, no. 4, pp. 197–202, 1999. [CrossRef]
- Y. Leira et al., “Periodontitis and vascular inflammatory biomarkers: an experimental in vivo study in rats,” Odontology, vol. 108, no. 2, pp. 202–212, Apr. 2020. [CrossRef]
- S. Zaric et al., “Impaired immune tolerance to Porphyromonas gingivalis lipopolysaccharide promotes neutrophil migration and decreased apoptosis,” Infect Immun, vol. 78, no. 10, pp. 4151–4156, Oct. 2010. [CrossRef]
- M. Sochalska et al., “Application of the in vitro HOXB8 model system to characterize the contributions of neutrophil–lps interaction to periodontal disease,” Pathogens, vol. 9, no. 7, pp. 1–13, 2020. [CrossRef]
- K. Kriebel et al., “Porphyromonas gingivalis Peptidyl Arginine Deiminase Can Modulate Neutrophil Activity via Infection of Human Dental Stem Cells,” J Innate Immun, vol. 10, no. 4, pp. 264–278, Sep. 2018. [CrossRef]
- Aliko et al., “Impact of Porphyromonas gingivalis Peptidylarginine Deiminase on Bacterial Biofilm Formation, Epithelial Cell Invasion, and Epithelial Cell Transcriptional Landscape,” Sci Rep, vol. 8, no. 1, Dec. 2018. [CrossRef]
- J. Zhang, J. He, J. Xia, Z. Chen, and X. Chen, “Delayed apoptosis by neutrophils from COPD patients is associated with altered bak, bcl-xl, and mcl-1 mRNA expression,” Diagn Pathol, vol. 7, no. 1, p. 65, Jun. 2012. [CrossRef]
- J. Vier, M. Groth, M. Sochalska, and S. Kirschnek, “The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling,” Cell Death Dis, vol. 7, no. 2, 2016. [CrossRef]
- J. G. Caton et al., “A new classification scheme for periodontal and peri-implant diseases and conditions – Introduction and key changes from the 1999 classification,” J Clin Periodontol, vol. 45, pp. S1–S8, Jun. 2018. [CrossRef]
- Z. Prucsi, A. Płonczyńska, J. Potempa, and M. Sochalska, “Uncovering the Oral Dysbiotic Microbiota as Masters of Neutrophil Responses in the Pathobiology of Periodontitis,” Front Microbiol, vol. 12, p. 2980, Oct. 2021. [CrossRef]
- M. M. Domingues, R. G. Inácio, J. M. Raimundo, M. Martins, M. A. R. B. Castanho, and N. C. Santos, “Biophysical characterization of polymyxin B interaction with LPS aggregates and membrane model systems.,” Biopolymers, vol. 98, no. 4, pp. 338–344, 2012. [CrossRef]
- D. A. Moulding, C. Akgul, M. Derouet, M. R. White, and S. W. Edwards, “BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis.,” J Leukoc Biol, vol. 70, no. 5, pp. 783–92, Nov. 2001. [CrossRef]
- G. L. Kelly and A. Strasser, “Toward Targeting Antiapoptotic MCL-1 for Cancer Therapy,” Annu Rev Cancer Biol, vol. 4, no. 1, pp. 299–313, Mar. 2020. [CrossRef]
- E. M. Cardoso, C. Reis, and M. C. Manzanares-Céspedes, “Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases,” Postgraduate Medicine, vol. 130, no. 1. Taylor and Francis Inc., pp. 98–104, Jan. 02, 2018. [CrossRef]
- S. Ji, J. Hyun, E. Park, B. L. Lee, K. K. Kim, and Y. Choi, “Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils,” J Periodontal Res, vol. 42, no. 5, pp. 410–419, Oct. 2007. [CrossRef]
- J. El-Benna, M. Hurtado-Nedelec, V. Marzaioli, J.-C. Marie, M.-A. Gougerot-Pocidalo, and P. M.-C. Dang, “Priming of the neutrophil respiratory burst: role in host defense and inflammation,” Immunol Rev, vol. 273, no. 1, pp. 180–193, Sep. 2016. [CrossRef]
- M. Fathalla et al., “Polymyxin-induced cell death of human macrophage-like THP-1 and neutrophil-like HL-60 cells associated with the activation of apoptotic pathways,” Antimicrob Agents Chemother, vol. 64, no. 9, Sep. 2020. [CrossRef]
- T. Stobernack et al., “Extracellular Proteome and Citrullinome of the Oral Pathogen Porphyromonas gingivalis,” J Proteome Res, vol. 15, no. 12, pp. 4532–4543, Dec. 2016. [CrossRef]
- Wielento et al., “TLR2 Activation by Porphyromonas gingivalis Requires Both PPAD Activity and Fimbriae,” Front Immunol, vol. 13, Apr. 2022. [CrossRef]
- Akgul, D. A. Moulding, and S. W. Edwards, “Molecular control of neutrophil apoptosis,” FEBS Letters, vol. 487, no. 3. John Wiley & Sons, Ltd, pp. 318–322, Jan. 05, 2001. [CrossRef]
- M. Sochalska, S. Tuzlak, A. Egle, and A. Villunger, “Lessons from gain- and loss-of-function models of pro-survival Bcl2 family proteins: Implications for targeted therapy,” FEBS Journal, vol. 282, no. 5. Blackwell Publishing Ltd, pp. 834–849, 2015. [CrossRef]
- J. Kale, E. J. Osterlund, and D. W. Andrews, “BCL-2 family proteins: Changing partners in the dance towards death,” Cell Death and Differentiation, vol. 25, no. 1. Nature Publishing Group, pp. 65–80, Nov. 17, 2018. [CrossRef]
- H. Dai, X. W. Meng, and S. H. Kaufmann, “Selective Inhibition of BFL1: It’s All about Finding the Right Partner,” Cell Chem Biol, vol. 27, no. 6, pp. 639–642, Jun. 2020. [CrossRef]
- X. Li, J. Dou, Q. You, and Z. Jiang, “Inhibitors of BCL2A1/Bfl-1 protein: Potential stock in cancer therapy,” Eur J Med Chem, vol. 220, p. 113539, Aug. 2021. [CrossRef]
- G. Salamone et al., “Promotion of Neutrophil Apoptosis by TNF-α,” The Journal of Immunology, vol. 166, no. 5, pp. 3476–3483, Mar. 2001. [CrossRef]
- C. Akgul and S. W. Edwards, “Regulation of neutrophil apoptosis via death receptors,” Cellular and Molecular Life Sciences, vol. 60, no. 11. Springer, pp. 2402–2408, Nov. 2003. [CrossRef]
- H. H. Lee, H. Dadgostar, Q. Cheng, J. Shu, and G. Cheng, “NF-κB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes,” Proc Natl Acad Sci U S A, vol. 96, no. 16, pp. 9136–9141, Aug. 1999. [CrossRef]
- R. Cherla, Y. Zhang, L. Ledbetter, and G. Zhang, “Coxiella burnetii inhibits neutrophil apoptosis by exploiting survival pathways and antiapoptotic protein Mcl-1,” Infect Immun, vol. 86, no. 4, Apr. 2018. [CrossRef]
- M. S. Hayden and S. Ghosh, “NF-κB in immunobiology,” Cell Research, vol. 21, no. 2. Cell Res, pp. 223–244, Feb. 2011. [CrossRef]
- M. S. Hayden and S. Ghosh, “Regulation of NF-κB by TNF family cytokines,” Seminars in Immunology, vol. 26, no. 3. Academic Press, pp. 253–266, 2014. [CrossRef]
- K. Rathinasamy, A. Ulaganathan, S. Ramamurthy, R. Ganesan, P. Saket, and S. Alamelu, “Estimation of TNF-α levels in saliva and serum of patients with periodontal health and chronic periodontitis: A case-control study,” Journal of Contemporary Dental Practice, vol. 21, no. 2, pp. 148–151, Feb. 2020. [CrossRef]
- H. Batool, A. Nadeem, M. Kashif, F. Shahzad, R. Tahir, and N. Afzal, “Salivary Levels of IL-6 and IL-17 Could Be an Indicator of Disease Severity in Patients with Calculus Associated Chronic Periodontitis,” Biomed Res Int, vol. 2018, 2018. [CrossRef]
- J. H. Ross, D. C. Hardy, C. A. Schuyler, E. H. Slate, T. W. Mize, and Y. Huang, “Expression of periodontal interleukin-6 protein is increased across patients with neither periodontal disease nor diabetes, patients with periodontal disease alone and patients with both diseases,” J Periodontal Res, vol. 45, no. 5, pp. 688–694, Oct. 2010. [CrossRef]
- C. Gabay, “Interleukin-6 and chronic inflammation,” Arthritis Res Ther, vol. 8, no. SUPPL. 2, p. S3, Jul. 2006. [CrossRef]
- W. L. Biffl, E. E. Moore, F. A. Moore, C. C. Barnett, V. S. Carl, and V. M. Peterson, “Interleukin-6 delays neutrophil apoptosis,” Archives of Surgery, vol. 131, no. 1, pp. 24–30, 1996. [CrossRef]
- P. C. Heinrich, I. Behrmann, S. Haan, H. M. Hermanns, G. Müller-Newen, and F. Schaper, “Principles of interleukin (IL)-6-type cytokine signalling and its regulation,” Biochemical Journal, vol. 374, no. 1. Portland Press, pp. 1–20, Aug. 15, 2003. [CrossRef]
- W. L. Biffl, E. E. Moore, F. A. Moore, and C. C. Barnett, “Interleukin-6 suppression of neutrophil apoptosis is neutrophil concentration dependent,” J Leukoc Biol, vol. 58, no. 5, pp. 582–584, 1995. [CrossRef]
- W. Pan, Q. Wang, and Q. Chen, “The cytokine network involved in the host immune response to periodontitis,” International Journal of Oral Science, vol. 11, no. 3. Springer Nature, pp. 1–13, Sep. 01, 2019. [CrossRef]
- T. Stobernack et al., “A secreted bacterial peptidylarginine deiminase can neutralize human innate immune defenses,” mBio, vol. 9, no. 5, pp. 1–15, 2018. [CrossRef]
- E. Bielecka et al., “Peptidyl arginine deiminase from porphyromonas gingivalis abolishes anaphylatoxin C5a activity,” Journal of Biological Chemistry, vol. 289, no. 47, pp. 32481–32487, Nov. 2014. [CrossRef]
- T. Maekawa et al., “Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis,” Cell Host Microbe, vol. 15, no. 6, pp. 768–778, Jun. 2014. [CrossRef]
- J. M. Goodson, “Diagnosis of Periodontitis by Physical Measurement: Interpretation From Episodic Disease Hypothesis.,” J Periodontol, vol. 63 Suppl 4S, no. 4s, pp. 373–382, Apr. 1992. [CrossRef]
- M. Elashiry, M. M. Meghil, R. M. Arce, and C. W. Cutler, “From manual periodontal probing to digital 3-D imaging to endoscopic capillaroscopy: Recent advances in periodontal disease diagnosis,” Journal of Periodontal Research, vol. 54, no. 1. Blackwell Munksgaard, pp. 1–9, Feb. 01, 2019. [CrossRef]
- F. Graziani, D. Karapetsa, B. Alonso, and D. Herrera, “Nonsurgical and surgical treatment of periodontitis: how many options for one disease?,” Periodontology 2000, vol. 75, no. 1. Blackwell Munksgaard, pp. 152–188, Oct. 01, 2017. [CrossRef]
- E. B. Hancock and D. H. Newell, “Preventive strategies and supportive treatment,” Periodontol 2000, vol. 25, no. 1, pp. 59–76, 2001. [CrossRef]
- J. Waerhaug, “Healing of the Dento-Epithelial Junction Following Subgingival Plaque Control: II: As Observed on Extracted Teeth,” J Periodontol, vol. 49, no. 3, pp. 119–134, Mar. 1978. [CrossRef]
- N. Institute of Dental and C. Research, “Periodontal (Gum) Disease: Causes, Symptoms, and Treatments.”.
- G. G. Wang, K. R. Calvo, M. P. Pasillas, D. B. Sykes, H. Häcker, and M. P. Kamps, “Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8,” Nat Methods, vol. 3, no. 4, pp. 287–293, Apr. 2006. [CrossRef]
- K. Gawron et al., “Peptidylarginine deiminase from Porphyromonas gingivalis contributes to infection of gingival fibroblasts and induction of prostaglandin E2-signaling pathway,” Mol Oral Microbiol, vol. 29, no. 6, pp. 321–332, Dec. 2014. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




