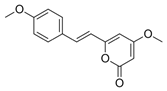
1. Introduction
Tianeptine is an antidepressant approved for prescription use in Europe, Asia, and Latin America [
1]. Tianeptine is a synthetic tricyclic compound originally patented by Malen et. al at the French Society of Medical Research in 1973 [
2]. The patent for the synthesis of tianeptine was issued to Biophore India Pharmaceuticals in 2010 [
3]. The international patent describes the purpose of the drug as enhancing serotonin reuptake, but does not mention the specific working mechanism [
3]. Tianeptine has a tricyclic structure, but it is classified as an atypical antidepressant because its mechanism of action is not consistent with other tricyclic antidepressants. Specifically, tianeptine aids in regulation of plasticity in the amygdala, attenuation of stress-induced glutamate release, and reversal of stress-induced dystrophy of hippocampal CA3 dendrite [4-7]. With a dosage of 25-50 mg per day, tianeptine is as effective as classic antidepressants such as sertraline and fluoxetine [
5]. The side effects of this drug are similar to selective serotonin reuptake inhibitors (SSRIs) in that it can cause headaches, dizziness, nausea, and abdominal pain [
5].
Tianeptine has been shown to be a full μ-receptor opioid agonist that can lead to severe psychological or physical dependence. Experimentally, tianeptine appears to be approximately five times less potent than morphine [
6], its propensity for dependence is a characteristic of a United States Food and Drug Administration (FDA) Schedule II Controlled Substance commensurate; a list that includes morphine, methamphetamine, cocaine, fentanyl, phencyclidine (PCP) and others [
6,
8]. Mechanistically, as a full µ-opioid receptor agonist, tianeptine is thought to possibly cause increased dependence by stimulating the release of dopamine in the limbic system. This is carried out by the inhibition of GABAergic interneurons in the ventral tegmental area, leading to disinhibition of dopaminergic neurons [
9]. Between 2011 and 2012 a multitude of fatal tianeptine overdoses influenced law makers in Turkey to classify tianeptine as a controlled substance and ban its use in the country [
10]. A study from 2014-2017 by the Centers for Disease Control in the United States regarding incidents of tianeptine abuse revealed numerous concerning case studies of tianeptine overdose resulting in death [
11,
12]. By 2018, the reports of tianeptine addiction in psychiatric patients and incidents of overdose were prevalent in literature [
13,
14]. The Drug Enforcement Agency (DEA) in the United States classifies tianeptine as not approved for medical use according to the FDA; however, it is not classified as a controlled substance in most states and is not regulated under the controlled substance act, despite μ-opioid receptor agonism and propensity for addiction [
15]. At time of this publication, only the states of Michigan, Alabama, Minnesota, Tennessee, and Oklahoma have classified tianeptine as a Schedule II drug [
16]. Tianeptine is not approved for distribution in the United States as a prescription drug and does not meet the classification of a dietary supplement; however, it is sold in the over the counter supplement Tianaa™ [
17].
There are three Tianaa™ products, differentiated by the names White, Red, and Green. The Tianaa™ products all contain tianeptine, and can be differentiated from one another by the addition of select organic material in their formulation. Tianaa
TM White promotes “energy” and contains L-⍺-glycerylphosphorylcholine (⍺-GPC), a compound that promotes release of the neurotransmitter acetylcholine in the brain, and cytidine 5′-diphosphocholine (CDP choline), a molecule found naturally in the body that stabilizes cell membranes [
18,
19]. Tianaa™ Red is advertised to promote “
rest and stress relief.” This supplement has kava extract, which contains kavapyrones, used for their psychoactive effect to alleviate anxiety, but cautioned due to hepatotoxic side effects. Tianaa™ Green is marketed to induce “
mellow moods,” causing a sedating, calming, relaxing effect aimed at alleviating stress and anxiety.
The health risks posed by tianeptine usage in the United States have been recognized by several states, leading to the classification of the drug as a Schedule II substance. Tianeptine can be purchased online or over the counter in most states in the US within Tianaa™ products. Tianaa™ causes consumers to experience delirium, autonomic dysfunction and increased dependence when taken in high doses. Severe withdrawal symptoms are most prevalent among frequent users of Tianaa™ products, whose dosage escalates with increased tolerance. Characteristic symptoms of withdrawal from TianaaTM are agitation, insomnia, yawning, headache, restlessness, autonomic hyperactivity, and nausea. The principal reason for Tianaa™ usage is individuals seeking relief from chronic pain, which has not been relieved by surgical or pharmacologic interventions. The current investigation targeted a quantitative understanding of tianeptine content across Tianaa™ products to gain insight into the alarming dependence profile exhibited by daily users seeking help from their addiction.
2. Materials and Methods
Extraction of Tianeptine from Tianaa™
capsules. One capsule from each class of Tianaa™ (Red, Green, and White) was divided equally into three samples and placed in centrifuge tubes. The samples were dissolved in three mL of 95% ethanol (Decon, UN1170), in which its solubility is 10 mg/mL [
20] vortexed for 30 seconds, then centrifuged for five minutes at 4000 rpm. The superficial ethanol layer, was extracted via pipet and then filtered into new centrifuge tubes. The three mL solutions were divided into two – one mL microcentrifuge tubes and placed in a centrifugal, reduced pressure evaporator to remove the solvent (1 hr). Added to each microcentrifuge tube was 250 μL of 100% ethanol (Spectrum Chemical, E1028), followed by vortex (4 min) to uniformly mix the solution, and finally transfer to 2.0 mL autosampler vials for liquid chromatography-mass spectrometry (LC-MS) analysis.
Tianeptine Identification and Quantification. Tianeptine was identified and quantified in Tianaa™ samples using OpenLab CDS 2 software operating an Agilent 1260 Infinity II high pressure liquid chromatography system coupled to a diode array detector and single quadrupole electrospray ionization detector (HPLC-DAD-MS), and commercially available standards. Samples were injected on a Waters Xterra MS C18 column (5 μm, 2.1 x 150 mm) maintained at 40 ℃ at a volume of 5 μL. The mobile phase solvent system consisted of 0.1% trifluoroacetic acid (TFA) in water (solvent A) and 0.1% TFA in acetonitrile (solvent B) with a 0.5 mL/min flow rate. For Tianaa™ analysis, the gradient elution started at 15% solvent B and increased to 50% solvent B over 10 min. After 10 min, the solvent composition was returned to 15% solvent B between minutes 10 and 15. The electrospray ionization source was operated under the following conditions: positive ion-mode, 4000 to -4000 V between capillaries, 7.0 L/min N2 drying gas at 300 ℃ and nebulizer pressure of 15 psi. Tianeptine was quantified by standard curve using a tianeptine sodium salt hydrate (>98% purity, CAS RN 30123-17-2) purchased from TCI America diode array detector (DAD) measuring absorbance at 220 nm.
3. Results
3.1. TianaaTM Product Comparison – Red, Green, and White
3.1.1. Tianeptine and Kavapyrone Analysis
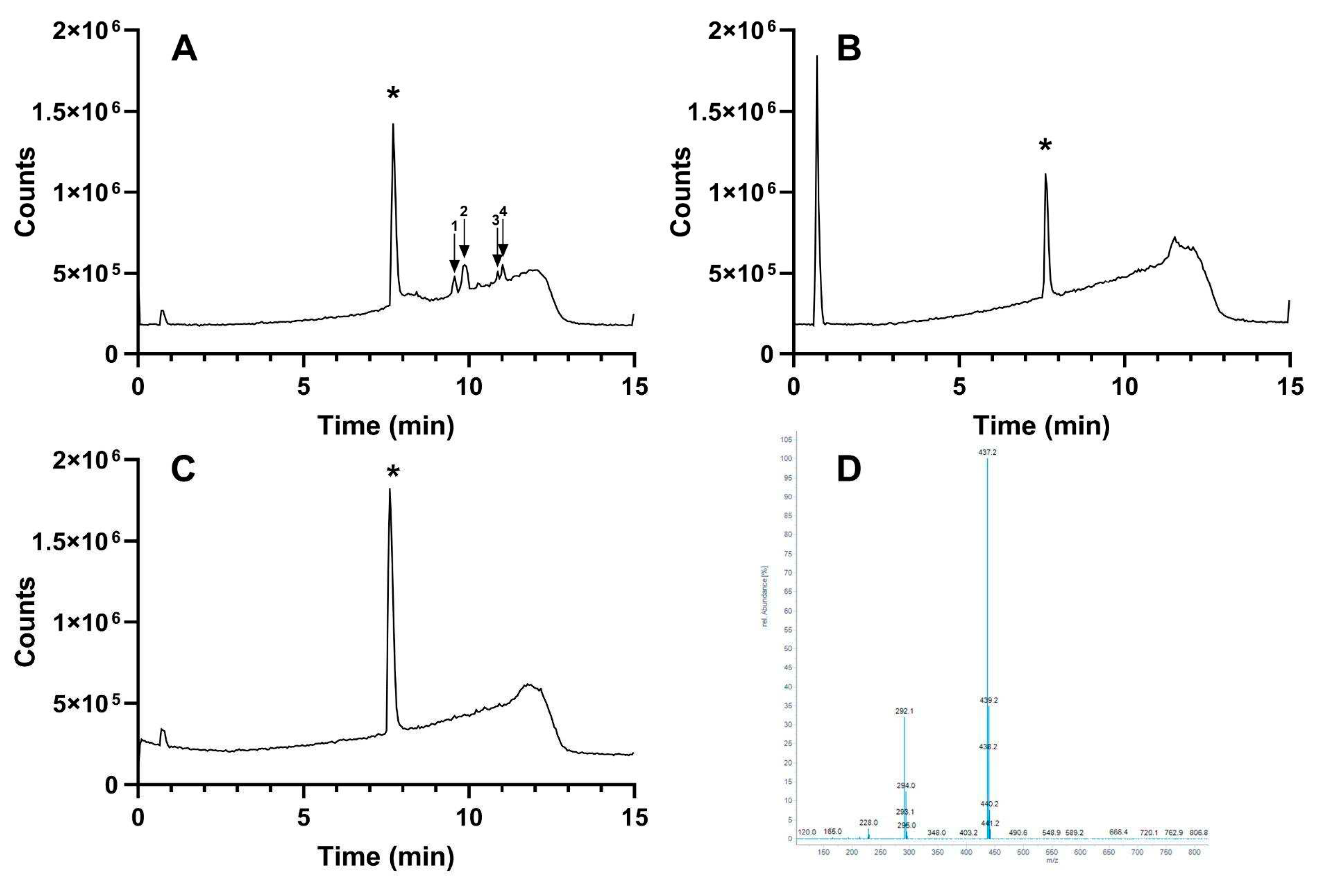
To identify and quantify tianeptine in each type of Tianaa
TM, a commercially available tianeptine standard was used as a direct comparison for Tianaa
TM sample extracts. Tianeptine was identified in each sample with a retention time between 7.616-7.716 min and [M+H]
+ of 437.2 m/z (
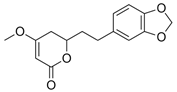
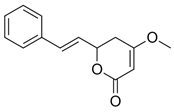
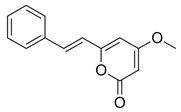
Figure 1). Although tianeptine was the focus of this study, mass spectral data suggest that additional compounds including dihydromethysticin, kavain, desmethoxyyangonin, and yangonin were present within the ethanolic extract of Tianaa
TM Red (
Figure 1A and
Table 1). The identity of these compounds is inconsistent with results from previous published work [
21]. The identified compounds are constituents of kava leaf extract that is added to Tianaa
TM Red to reduce anxiety. Tianeptine concentration across Tianaa
TM products was determined by standard curve (R
2 = 0.9999), where the limit of detection and limit of quantification for tianeptine was 0.51 mg/L and 1.55 mg/L, respectively. On average, the mass of tianeptine per capsule for Tianaa
TM Red, Tianaa
TM Green, and Tianaa
TM White was 8.06 ± 0.03 mg, 8.08 ± 0.03 mg and 4.55 ± 0.04 mg, respectively (
Table 2).
3.1.2. Capsule-to-Capsule Comparison
Following the identification of tianeptine in Tianaa
TM, a capsule-to-capsule comparison was performed to investigate quantity variation of tianeptine within each Tianaa
TM product. The total capsule mass ranged between 445-601 mg whereas the reported capsule mass from the manufacturer is 531 mg (
Table 3). The percent mass of tianeptine compared to the total mass of the capsule ranged between 0.6-2.4%, however, the positive and negative deviations of tianeptine mass from the averaged mass were as high as 36% and 33%, respectively. Altogether, these data suggest that the mass of tianeptine within each capsule was inconsistent within and between each type of Tianaa
TM, and not dependent upon total mass of the capsule substrate.
4. Discussion
The mass of tianeptine present in capsules for each class of Tianaa
TM deviated between 0-36% from their respective average values. From the analysis of eight capsules from each Tianaa
TM class where tianeptine content in each capsule was measured in triplicate, it was determined that tianeptine mass in Tianaa
TM White was statistically (p-value <0.01) different than found in Tianaa
TM Red or Tianaa
TM Green. This difference in tianeptine content was not due to differences in the total mass of each capsule. Between 0.6-2.4% of a capsule’s mass was tianeptine (see
Table 4). The calculated percent variance of tianeptine was based on an average, because the manufacturer does not report the mass of tianeptine per capsule. The difference in mass between individual capsules and an average capsule mass was consistently greater than 10%, with positive and negative deviations of 36% and 33%, respectively. The calculated capsule masses had low standard deviations (0.02-0.2 mg), suggesting that variation in tianeptine mass is likely due to manufacturing process.
The mass of Tianaa
TM capsules varied between and within Tianaa™ products. In the case of Tianaa™ White, measurement of mass across three capsules varied by ≥15% compared to the reported value on the bottle, which exceeds the United States GMP standards [
18]. Tianaa™ Red and Green capsules varied in mass between randomly selected capsules between 5-16% across at least three capsules tested. The US GMP standards report that no more than two capsules may exceed 15% if the capsules are over 300 mg in mass; the bottle label for Tianaa™ products lists the capsule mass to be approximately 531 mg [
22].
The results of the current study suggest that Tianaa™ brand tianeptine supplements do not conform with United States GMP standards. The opioid activity of tianeptine and observed variability in drug content and capsule mass suggest that enhanced regulatory oversight is warranted. In countries where tianeptine is legal for medical use, a prescribed daily dose is 13.5-50 mg per day. Based on our analysis of Tianaa
TM Red and Green products, a median tianeptine level of approximately 8.06 ± 0.03 mg per capsule will deliver a therapeutic dose of the drug in two to six capsules [
23]. Lauhan et al. described a compilation of studies investigating tianeptine dependence or abuse where the average daily dose of tianeptine was on the order of 1924 mg across 65 patients, further solidifying a basis for risk of tianeptine addiction due to overconsumption of Tianaa
TM [
24]. Further investigation of the quantities of tianeptine in supplements may assist in informing clinicians of patient-regulated tianeptine use.
The importance of this research comes back to what is seen being seen in medical facilities. Clinically, Tianna™ has been correlated with severe withdrawal symptoms. The increased tolerance associated with Tianna™ and increased risk of dependence from the supplement is most likely due to a multitude of effects induced by tianeptine on receptors, including glutaminergic, dopaminergic and GABA, in various neuronal tracts in the brain. Additionally, other natural products in the formulation of TianaaTM supplements may attenuate the effects on neural circuits. This study draws attention to the risk of unregulated tianeptine containing supplements and the variation in active component that may exist within and between capsules of the same or related products.
5. Conclusions
Analysis of Tianaa™ products reveals inconsistent tianeptine content and capsule mass, which exceed good manufacturing practice standards. The product variation for this nutraceutical supplement, coupled with concerning addiction potential for tianeptine, are expected to lead to inevitable increases in incidents of overdose and addition. The long-term repercussions of tianeptine addiction include increased tolerance to Tianaa™, which requires more capsules for daily consumption, and severe withdrawal symptoms that include intensive agitation, nausea, headache, and autonomic hyperactivity. Patients requiring medical intervention for tianeptine abuse are treated with medication regimens that include administration of methadone, buprenorphine or clonidine. To justify labeling Tianaa™ products as dietary supplements, nutrients like kava extract, Tribulus terrestris fruit, niacin, and others are added, but masking tianeptine is dangerous for consumers that are not aware of the risks for overdose and addiction.
Supplementary Materials
Not applicable
Author Contributions
Conceptualization, J.T.S., J.M.T.F. and O.M.M.; methodology, J.T.S and E.A.G.; validation, J.T.S., E.A.G., J.M.T.F. and O.M.M.; formal analysis, J.T.S., E.A.G., J.M.T.F. and O.M.M.; investigation, J.T.S and E.A.G.; resources, O.M.M.; data curation, O.M.M.; writing—original draft preparation, J.T.S and E.A.G.; writing—review and editing, J.M.T.F. and O.M.M.; visualization, J.T.S and E.A.G.; supervision, O.M.M.; project administration, J.M.T.F. and O.M.M.; funding acquisition, O.M.M. All authors have read and agreed to the published version of the manuscript.”
Funding
Please add: This research and the APC was funded by the Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grants #P20GM103408, P20GM109095, and 1C06RR020533. We also acknowledge support from The Biomolecular Research Center at Boise State, BSU-Biomolecular Research Center, RRID:SCR_019174, with funding from the National Science Foundation, Grants #0619793 and #0923535; the M. J. Murdock Charitable Trust; Lori and Duane Stueckle, and the Idaho State Board of Education.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Data supporting reported results can be requested from the corresponding author at owenmcdougal@boisestate.edu.
Acknowledgments
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- Medicines and Healthcare products Regulatory Agency (MHRA). The British Pharmacopeia, 2022. Monographs: Tianeptine Sodium [Internet]. London: The Stationary Office; 2022.
- Malen et al. (1973). Tricyclic Compounds. Patent #3758528. United States Patent and Trademark Office. https://patentimages.storage.googleapis.com/94/81/93/24ca0b63a45d8a/US3758528.pdf.
- Rangisetty, J. B.; Pallagurla, M. R.; Bhudeti, R. Novel Process for the Preparation of 7-((3-Chloro-6-methyI-5,5-dioxo-6,11-dihydro dibenzo(c,f) (1,2) thiazepin-11-yl)amino) heptanoate. International Patent WO 2010/070667A2, June 24, 2010.
- Banasr, M.; Dwyer, J.; Duman, R. Cell atrophy and loss in depression: Reversal by antidepressant treatment. Current Opinion in Cell Biology 2011, 23(6), 730-737. [CrossRef]
- Wagstaff, A. J.; Ormrod, D.; Spencer, C. M. Tianeptine: a review of its use in depressive disorders. CNS Drugs 2001, 15(3), 231-259. [CrossRef]
- Samuels, B.; Nautiyal, K.; Kruegel, A.; et al. The Behavioral Effects of the Antidepressant Tianeptine Require the Mu-Opioid Receptor. Neuropsychopharmacology 2017, 42(10), 2052-2063. [CrossRef]
- McEwen B. S.; Chattarji S.; Diamond D. M.; et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Molecular Psychiatry. 2010 Mar;15(3):237-49. Epub 2009 Aug 25. PMID: 19704408; PMCID: PMC2902200. [CrossRef]
- Gassaway, M. M.; Rives, M.; Kruegel, A.; et al. The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Translational Psychiatry 2014, 4(7), 1-5. [CrossRef]
- Valentino, R. J. & Volkow, N. Untangling the complexity of opioid receptor function. Neuropsychopharmacology 43, 2514–2520 (2018). [CrossRef]
- Durmus, N.; Ozbilen, G.; Kasap, Y.; et al. Risk Management in Tianeptine Abuse in Turkey: A National Experience. Klinik Psikofarmakoloji Bulteni 2013, 23(2), 149-154. [CrossRef]
- Bakota, E.; Samms, W.; Gray, T.; et al. Case reports of fatalities involving tianeptine in the United States. Journal of Analytical Toxicology 2018, 42(7), 503-509. [CrossRef]
- El Zahran, T.; Schier, J.; Glidden, E.; et al. Characteristics of Tianeptine Exposures Reported to the National Poison Data System - United States 2000-2017. MMWR Morbidity and Mortality Weekly Report 2018, 67(30), 815-818. [CrossRef]
- Springer, J. & Cubała, W. J. Tianeptine Abuse and Dependence in Psychiatric Patients: A Review of 18 Case Reports in the Literature. Journal of Psychoactive Drugs 2018, 50 (3), 275–280. [CrossRef]
- Marraffa, J. M.; Stork, C. M.; Hoffman, R. S.; et al.. K. Poison control center experience with tianeptine: an unregulated pharmaceutical product with potential for abuse. Clinical Toxicology 2018, 56 (11), 1155–1158. [CrossRef]
- Drug Enforcement Administration, Diversion Control Division. Drug & Chemical Evaluation Section. Tianeptine 05.2019.
- Felton, R. An Illegal Dietary Supplement Named Tianeptine Is Being Sold to Americans—and the FDA Knows It. Consumer Reports. February 4, 2021.
- Food and Drug Administration. Tianeptine in Dietary Supplements. Food and Drug Administration, 2018. https://www.fda.gov/food/dietary-supplement-products-ingredients/tianeptine-dietary-supplements.
- Parker, A.; Byars, A.; Purpura, M.; et al.. The effects of alpha-glycerylphosphorylcholine, caffeine or placebo on markers of mood, cognitive function, power, speed, and agility. Journal of the International Society of Sports Nutrition 2015, 12(1), 41. [CrossRef]
- Fioravanti, M.; Buckley, A. Citicoline (Cognizin) in the treatment of cognitive impairment. Clinical Interventions in Aging 2006, 1(3), 247-251. [CrossRef]
- Cayman Chemical. Tianeptine (sodium salt). Item No. 17561 2022. https://cdn.caymanchem.com/cdn/insert/17561.pdf.
- Shao, Y.; He, K.; Zheng, B.; et al. Reversed-phase high-performance liquid chromatographic method for quantitative analysis of the six major kavalactones in Piper methysticum. Journal of Chromatography A 1998, 825(1), 1-8. [CrossRef]
- U.S Department of Health & Human Services, Food & Drug Administration. CFR - Code of Federal Regulations Title 21 [21CFR210.1], last revised July 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=210.1.
- Smith, K. E.; Rogers, J. M.; Strickland, J. C.; et al. (2021). When an obscurity becomes trend: social-media descriptions of tianeptine use and associated atypical drug use. American Journal of Drug Alcohol Abuse 2021, 47(4), 455–466. [CrossRef]
- Lauhan, R.; Hsu, A.; Alam, A.; et al. Tianeptine Abuse and Dependence: Case Report and Literature Review. Psychosomatics 2018, 59(6), 547-553. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).