Submitted:
05 July 2023
Posted:
06 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Manure and Soil Properties
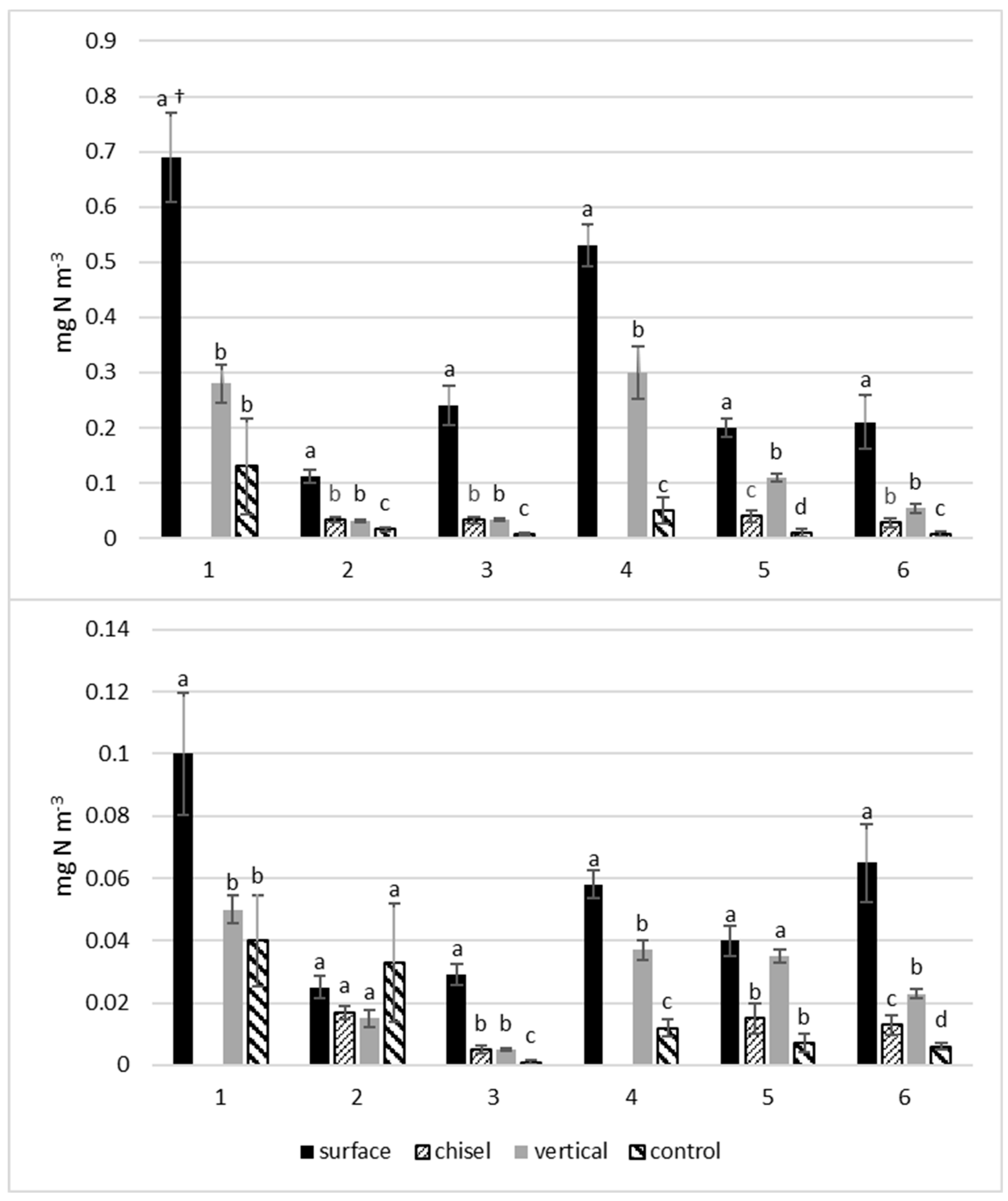
3.2. Influnece of Manure Incorporation on NH3-N Concentrations
3.3. Impact of Weather Conditions and Manure on NH3-N Concentrations
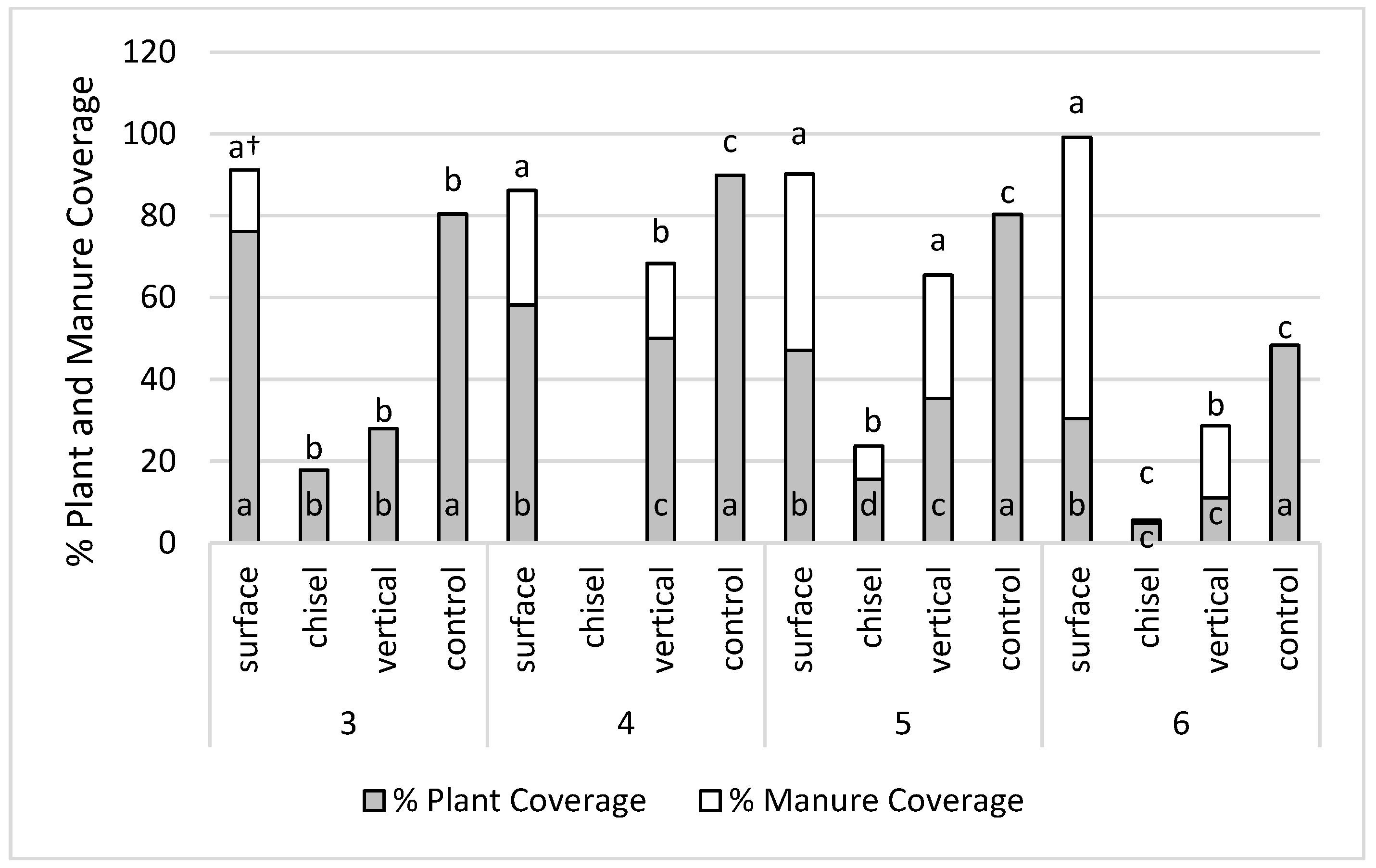
3.4. Impact of Crop Residue and Manure Coverage on NH3-N Concentrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apsimon, H.M.; Kruse, M.; Bell, J.N.B. 1987. Ammonia emissions and their role in acid deposition. Atmos. Environ. 1987, 21, 1939–1946. [Google Scholar] [CrossRef]
- IPCC. Guidelines for National Greenhouse Gas Inventories. Vol. 4, Agriculture, forestry and other land use; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2006. [Google Scholar]
- Renard, J.J.; Calidonna, S.E.; Henley, M.V. Fate of ammonia in the atmosphere - a review for applicability to hazardous releases. J. Hazard. Mater. 2004, B108, 29–60. [Google Scholar] [CrossRef]
- Webb, J.; Pain, B.; Bittman, S.; Morgan, J. The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response — a review. Agric. Ecosyst. Environ. 2010, 137, 39–46. [Google Scholar] [CrossRef]
- Wulf, S.; Maeting, M.; Clemens, J. Application technique and slurry co-fermentation effects on ammonia, nitrous oxide, and methane emissions after spreading: II. Greenhouse gas emissions. J. Environ. Qual. 2002, 31, 1795–1801. [Google Scholar] [CrossRef]
- Huijsmans, J.F.M.; Hol, J.M.G.; Vermeulen, G.D. Effect of application method, manure characteristics, weather and field conditions on ammonia volatilization from manure applied to arable land. Atmos. Environ. 2003, 37, 3669–3680. [Google Scholar] [CrossRef]
- Leytem, A.B.; Bjorneberg, D.L.; Sheffield, R.E.; de Haro Marti, M.E. Case study: On-farm evaluation of liquid dairy manure application methods to reduce ammonia losses. Prof. Anim. Sci. 2009, 25, 93–98. [Google Scholar] [CrossRef]
- Powell, J.M.; Jokela, W.E.; Misselbrook, T.H. Dairy slurry application method impacts ammonia emission and nitrate leaching in no-till corn silage. J. Environ. Qual. 2011, 40, 388–392. [Google Scholar] [CrossRef]
- Dell, C.J.; Kleinman, P.J.A.; Schmidt, J.P.; Beegle, D.B. Low-disturbance manure incorporation effects on ammonia and nitrate loss. J. Environ. Qual. 2012, 41, 928–937. [Google Scholar] [CrossRef]
- Webb, J.; Thorman, R.E.; Fernanda-Aller, M.; Jackson, D.R. Emission factors for ammonia and nitrous oxide emissions following immediate manure incorporation on two contrasting soil types. Atmos. Environ. 2014, 82, 280–287. [Google Scholar] [CrossRef]
- Sherman, J.F.; Young, E.O.; Cavadini, J. Tillage and liquid dairy manure effects on overland flow nitrogen and phosphorus loss potential in an upper Midwest corn silage-winter triticale cropping system. Agronomy. 2021, 11, 1775. [Google Scholar] [CrossRef]
- Sherman, J.F.; Young, E.O.; Jokela, W.E.; Cavadini, J. Impacts of low-disturbance dairy manure incorporation on ammonia and greenhouse gas fluxes in a corn silage–winter rye cover crop system. J. Environ. Qual. 2021, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.; Young, E.O.; Jokela, W.E.; Cavadini, J. Impacts of low disturbance liquid dairy manure incorporation on alfalfa yield and fluxes of ammonia, nitrous oxide, and methane. Agriculture. 2021, 11, 750. [Google Scholar] [CrossRef]
- Pain, B.F.; Phillips, V.R.; Clarkson, C.R.; Klarenbeek, J.V. Loss of nitrogen through ammonia volatilisation during and following the application of pig or cattle slurry to grassland. J. Sci. Food Agric. 1989, 47, 1–12. [Google Scholar] [CrossRef]
- Thompson, R.B.; Pain, B.F.; Rees, Y.J. Ammonia volatilization from cattle slurry following surface application to grassland: II. Influence of application rate, wind speed and applying slurry in narrow bands. Plant Soil 1990, 125, 119–128. [Google Scholar] [CrossRef]
- Huijsmans, J.F.M.; Hol, J.M.G.; Hendriks, M.M.W.B. Effect of application technique, manure characteristics, weather and field conditions on ammonia volatilization from manure applied to grassland. Neth. J. Agric. Sci. 2001, 49, 323–342. [Google Scholar] [CrossRef]
- Hanna, H.M.; Bundy, D.S.; Lorimor, J.C.; Mickelson, S.K.; Melvin, S.W.; Erbach, D.C. Manure incorporation equipment effects on odor, residue cover, and crop yield. Appl. Eng. Agric. 2000, 16, 621–627. [Google Scholar] [CrossRef]
- Shelton, D.P. Crop residue cover and manure incorporation Part I: Reduction of percent cover. Appl. Eng. Agric. 2004, 20, 605–611. [Google Scholar] [CrossRef]
- Kovar, J.L.; Moorman, T.B.; Singer, J.W.; Cambardella, C.A.; Tomer, M.D. Swine manure injection with low-disturbance applicator and cover crops reduce phosphorus losses. J. Environ. Qual. 2011, 40, 329–336. [Google Scholar] [CrossRef]
- Frost, J.P. Effect of spreading method, application rate and dilution on ammonia volatilization from cattle slurry. Grass Forage Sci. 1994, 49, 391–400. [Google Scholar] [CrossRef]
- Sommer, S.G.; Hutchings, N.J. Ammonia emission from field applied manure and its reduction - invited paper. Eur. J. Agron. 2001, 15, 1–15. [Google Scholar] [CrossRef]
- Brunke, R.P.; Schuepp, A.P.; Gordon, R. Effect of meteorological parameters on ammonia loss from manure in the field. J. Environ. Qual. 1988, 17, 431–436. [Google Scholar] [CrossRef]
- Sommer, S.G.; Olesen, J.E. Effects of dry matter content and temperature on ammonia loss from surface applied cattle slurry. J. Environ. Qual. 1991, 20, 679–683. [Google Scholar] [CrossRef]
- Sommer, S.G.; Olesen, J.E.; Christensen, B.T. Effects of temperature, wind speed and air humidity on ammonia volatilization from surface applied cattle slurry. J. Agric. Sci. 1991, 117, 91–100. [Google Scholar] [CrossRef]
- Moal, J.F.; Martinez, J.; Guiziou, F.; Coste, C.M. Ammonia volatilization following surface applied pig and cattle slurry in France. J. Agric. Sci. 1995, 125, 245–252. [Google Scholar] [CrossRef]
- Huijsmans, J.F.M.; Hol, J.M.G.; Vermeulen, G.D. Effect of application method, manure characteristics, weather and field conditions on ammonia volatilization from manure applied to arable land. Atmos. Environ. 2003, 37, 3669–3680. [Google Scholar] [CrossRef]
- CTIC. National crop residue management survey. 2016, Conservation Tillage Information Center, West Lafayette, IN. 2016.
- Kern, J.S.; Johnson, M.G. Conservation tillage impacts on national soil and atmospheric carbon levels. Soil Sci. Soc. Am. J. 1993, 57, 200–210. [Google Scholar] [CrossRef]
- West, T.O.; Post, W.M. Soil organic carbon sequestration rates by tillage and crop rotation: A global data analysis. Soil Sci. Soc. Am. J. 2002, 66, 1930–1946. [Google Scholar] [CrossRef]
- Jacobs, A.; Rauber, R.; Ludwig, B. Impact of reduced tillage on carbon and nitrogen storage of two Haplic Luvisols after 40 years. Soil Till. Res, 2009, 102, 158–164. [Google Scholar] [CrossRef]
- Kushwaha, C.P.; Tripathi, C. P.; Singh, K.P. Soil organic matter and water-stable aggregates under different tillage and residue conditions in a tropical dryland agroecosystem. Appl. Soil Ecol. 2001, 16, 229–241. [Google Scholar] [CrossRef]
- Mueller, D.H.; Klemme, R.M.; Daniel, T.C. Short and long-term cost comparisons of conventional tillage systems in corn production. J. Soil Water Conserv. 1985, 40, 466–470. [Google Scholar]
- Schuler, R.T. Residue Management- Horizontal vs. Vertical Tillage. In Wisconsin Fertilizer, Aglime and Pest Management Conference. University of Wisconsin Extension. Vol 46. Madison, WI. 2007; pp. 179–181.
- Klingsberg, K.; Weisenbeck, C. . Shallow vertical tillage: Impact on soil disturbance and crop residue. In Wisconsin Crop Management Conference. Vol. 50, Madison, WI. 2011; pp. 46–49.
- Peters, J. 2003. Recommended methods of manure analysis. in: University of Wisconsin-Extension (Ed.), Madison, WI.
- Schulte, E.E.; Hopkins, B.G. . Estimation of soil organic matter by weight 3. Organic matter (LOI) loss-on-ignition. in: F. R. Magdoff, M. A. Tabatabai and E. A. Hanlon, Jr. (Eds.), Soil Organic Matter: Analysis and Interpretation, Soil Sci. Soc. Am., Madison, WI. 1996; pp. 21–31.
- 37. Lachat Instruments. Determination of Ammonia (Salicylate) in 2M KCl soil extracts by Flow Injection Analysis. 1993. QuikChem Method 12-107-06-2-A, L.
- Booth, D.T.; Cox, S.E.; Berryman, R.D. Point sampling digital imagery with SamplePoint. Environ. Monit. Assess. 2006, 123, 97–108. [Google Scholar] [CrossRef]
- Roadman, M.J.; Scudlark, J.R.; Meisinger, J.J.; Ullman, W.J. Validation of Ogawa passive samplers for the determinations of gaseous ammonia concentrations in agricultural settings. Atmospheric Environment 2003, 37, 2317–2325. [Google Scholar] [CrossRef]
- Lachat Instruments. Determination of ammonia by flow injection analysis. 2003. QuikChem Method 10-107-06-2-A, Lachat Instruments, Loveland, CO.
- SAS Institute Inc. SAS 9.4 Guide to Software Updates. 2013. SAS Institute Inc., Cary, NC.
- Scudlark, J.R.; Jennings, J.A.; Roadman, M.J.; Savidge, K.B.; Ullman, W.J. Atmospheric nitrogen inputs to the Delaware inland bays: the role of ammonia. Environ. Pollut. 2005, 135, 433–444. [Google Scholar] [CrossRef]
- Pleim, J. E.; Ran, L.; Appel, W.; Shephard, M. W.; Cady-Pereira, K. : New bidirectional ammonia flux model in an air quality model coupled with an agricultural model, J. Adv. Model. Earth Sy. 2019. 11, 2934–2957. [CrossRef]
- Zhang, T.; Liu, H.; Luo, J.; Wang, H.; Zhai, L.; Geng, Y.; Zhang, Y.; Li, J.; Lei, Q.; Bashir, M.A.; Wu, S.; Lindsey, S. Long-term manure application increased greenhouse gas emissions but had no effect on ammonia volatilization in a Northern China upland field. Sci. Total Environ. 2018, 633, 230–239. [Google Scholar] [CrossRef]
- Ramanantenasoa, M.M.J.; Génermont, S.; Gilliot, J.-M.; Bedos, C.; Makowski, D. Meta-modeling methods for estimating ammonia volatilization from nitrogen fertilizer and manure applications. J. Environ. Manag. 2019, 236, 195–205. [Google Scholar] [CrossRef]
- Miola, E.C.C.; Aita, C.; Rochette, P.; Chantigny, M.H.; Angers, D. A, Bertrand, N.; Gasser, M.O. Static chamber measurements of ammonia volatilization from manured soils: impact of deployment duration and manure characteristics. Soil Sci. Soc. Am. J.; 2014, 79, 305–313. [Google Scholar] [CrossRef]
- Lorimor, J.; Powers, W.; Sutton, A. Manure characteristics. 2006. Manure management system series. Michigan State University Extension. MWPS-18 Section 1, second Edition.

| Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|
| Trial | Date | Control | Surface | Chisel | Case IH 330 | Great Plains Turbo Max 1800 | Residue | Manure Type |
| 1 | 9/25/2013 | X | X | X | Silage Corn | Solid Pack | ||
| 2 | 7/2/2014 | X | X | X | X | Oats | Separated Liquid | |
| 3 | 8/11/2015 | X | X | X | X | Oats | Unagitated Separated Liquid | |
| 4 | 11/4/2015 | X | X | X | Grain Corn | Whole Dairy Slurry | ||
| 5 | 5/3/2016 | X | X | X | X | Grain Corn | Whole Dairy Slurry | |
| 6 | 5/17/2016 | X | X | X | X | Silage Corn | Whole Dairy Slurry | |
| Trial | Date | DM † | TN | TP | NH4-N | pH | Rate‡ | TN | TP | NH4-N |
|---|---|---|---|---|---|---|---|---|---|---|
 % %
|
Mg ha-1 |  |
kg ha-1 |  |
||||||
| 1 | 9/25/2013 | 29 ±0.9†‡ | 1.8 ±0.06 | 0.34 ±0.04 | 0.4 ±0.1 | 8.3 ±0.12 | 95.3 | 484 | 91 | 94 |
| 2 | 7/2/2014 | 1.5 ±0.05 | 6.8 ±0.20 | 1.09 ±0.00 | 4.5 ±0.2 | 7.6 ±0.05 | 53.6 | 82 | 13 | 54 |
| 3 | 8/11/2015 | 5.1 ±0.1 | 3.3 ±0.05 | 0.65 ±0.03 | 1.3 ±0.0 | 8.2 ±0.08 | 83.9 | 137 | 28 | 55 |
| 4 | 11/4/2015 | 22 ±1.8 | 1.6 ±0.15 | 0.30 ±0.00 | 0.7 ±0.1 | 8.1 ±0.05 | 110 | 412 | 80 | 185 |
| 5 | 5/3/2016 | 8.7 ±0.60 | 3.1 ±0.25 | 0.74 ±0.00 | 1.5 ±0.1 | 7.8 ±0.10 | 69.6 | 181 | 44 | 89 |
| 6 | 5/17/2016 | 6.5 ±0.1 | 3.1 ±0.08 | 0.79 ±0.01 | 1.3 ±0.05 | 7.8 ±0.20 | 90.9 | 180 | 46 | 74 |
| Trial | Date | pH | NH4-N † | Moisture | OM ‡ |
|---|---|---|---|---|---|
| mg kg-1 |
 g kg-1 g kg-1
|
||||
| 1 | 9/25/2013 | 7.2 ±0.05 | . †‡ | 184 ±2.4 | 27 ±1.7 |
| 2 | 7/2/2014 | 7.0 ±0.16 | 3.7 ±1.5 | 329 ±26 | 32 ±1.4 |
| 3 | 8/11/2015 | 7.0 ±0.08 | 3.2 ±0.8 | 317 ±17 | 30 ±3.4 |
| 4 | 11/4/2015 | 6.3 ±0.20 | 2.6 ±0.8 | 346 ±19 | 30 ±0.5 |
| 5 | 5/3/2016 | 6.8 ±0.12 | 3.4 ±0.2 | 401 ±38 | 33 ±0.8 |
| 6 | 5/17/2016 | 6.7 ±0.05 | 3.7 ±1.2 | 289 ±6.2 | 35 ±0.5 |
| Trial | Date | Relative Humidity | Temperature | Total Rainfall | Wind Speed | Wind Gusts |
|---|---|---|---|---|---|---|
| % | °C | mm |
 m s-1 m s-1
|
|||
| 1 | 9/25/2013 | 89 | 13.9 | 0.0 | 2.55 | 7.38 |
| 2 | 7/2/2014 | 60 | 16.4 | 0.0 | 1.31 | 3.21 |
| 3 | 8/11/2015 | 73 | 20.0 | 0.0 | 0.93 | 2.30 |
| 4 | 11/4/2015 | 85 | 14.1 | 8.4 | 2.80 | 5.19 |
| 5 | 5/3/2016 | 62 | 9.40 | 7.1 | 2.03 | 4.19 |
| 6 | 5/17/2016 | 52 | 11.1 | 1.3 | 0.43 | 1.69 |
| Pearson Correlation Coefficients | ||||||
| first 24 h | 0.55 ** | -0.51 | 0.13 | 0.49 * | 0.56 ** | |
| second 24 h | 0.39 | -0.27 | 0.10 | 0.43 * | 0.55 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





