1. Introduction
In recent years, alumina has gained significant attention in high-value applications such as adsorbent [
1,
2,
3], ceramic [
4,
5], catalyst and catalyst carrier [
6,
7,
8,
9], etc. The application of alumina is not only dependent on particle size but also on particle shape. Different shapes of alumina, such as rod-like [
10], fibrous [
11], plate-like [
12], and spherical [
13], have been wildly used. Seyed [
14] got massive γ-aluminum with particle sizes between 0.5 μm and 0.9 μm, but the morphology was irregular. Dabbagh, et al. and Feng, et al [
15,
16] prepared rod-like, fibrous, and spherical alumina, but the process was complex and hard to control, resulting in poor dispersibility. Lv et al. [
17] synthesized spherical alumina with a highly spherical shape and uniform particle size by using the oil-ammonia drop method, but the particle size was large, which limited its application area. Using aluminum isopropoxide as precursor and Pluronic P123 (P123) as template, Wu et al. [
18] used aluminum isopropoxide as a precursor and P123 as a template to synthesize organized mesoporous alumina with a hierarchical structure. However, these methods suffer from limitations such as irregular morphology, poor dispersibility, and limited application areas.
Mesoporous materials are highly valued due to their higher specific surface area, organized pore structure, narrow pore size distribution, and continuous pore size, which make them important for adsorption and separation, as well as catalytic reactions [
19] Among various shapes of alumina, spherical alumina has high fluidity, making it less prone to gathering and producing channeling during catalytic processes, which significantly enhances catalyst activity. Therefore, the preparation of mesoporous alumina can greatly improve the application efficiency of mesoporous alumina spheres in adsorption, separation, and catalysis [
20].
Chitin ((C
8H
13O
5N)
n) is a naturally occurring biopolymer that is highly organized and abundant in the exoskeletons of crustaceans and insects. The primary chitin fibrils' structural arrangement varies among arthropod species, with some having helical structures called the Bouligand structure, which enhances photonic and mechanical properties. This structure resembles cholesteric lyotropic liquid crystals. The hierarchical structure of chitin fibrils makes them an excellent natural template for developing new materials. The use of biomass as a porous material can be biodegradable, achieve biocompatibility, and achieve green and sustainable development [
21,
22,
23]. Pluronic-P123 (P123) is a soft template with a symmetric triblock copolymer comprising poly (ethylene oxide) (PEO) alternating with poly (propylene oxide) (PPO), PEO-PPO-PEO. Its phases vary depending on the concentration and combination of solvents [
24,
25,
26,
27] and it is often used as a crystal structure modifier in the preparation of mesoporous materials [
28].
In this study, mesoporous alumina spheres were successfully prepared using alumina hydrate (AlOOH) as a precursor, (NH2)2CO as a precipitant, as well as Chitin powder and P123. The preparation process, which involved evaporation induced self-assembly (EISA), was green, low cost, and pollution-free. This method significantly improved the catalyst loading firmness and service life of mesoporous alumina spheres. The effects of synthesis temperature, time, and the addition of mixed templates on the structure and morphology of the products were investigated. The CO2 adsorption performance of spherical mesoporous alumina and the electrochemical performance of supported SnO2 were also evaluated.
3. Results and Discussion
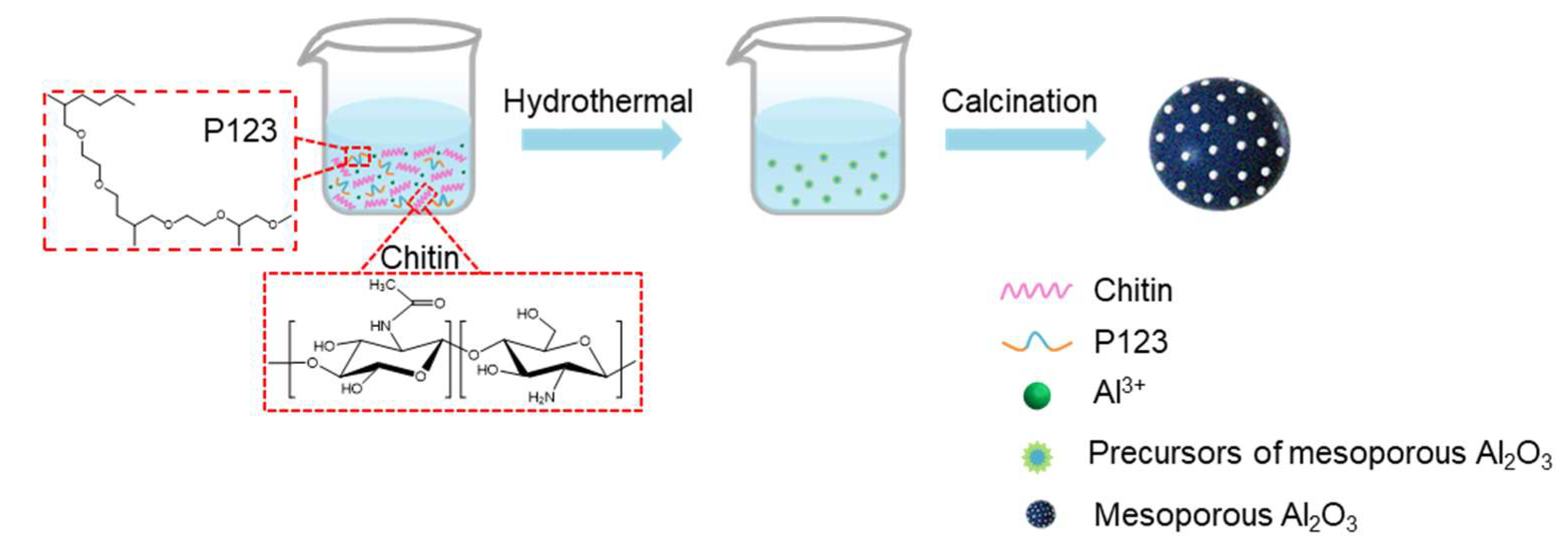
Synthesis of Mesoporous Alumina Spheres The synthesis of spherical macroporous alumina materials has been achieved through the hydrothermal method, using hydrated alumina (AlOOH) as the precursor, along with Chitin and P123 as the templates, and urea as a precipitant, while adapting the evaporation induced self-assembly (EISA) method, as depicted in
Figure 1. By systematically varying the reaction conditions, the impact of reaction temperature and time, as well as the Chitin:P123 weight ratio, on the morphology and properties of the resulting spherical mesoporous alumina materials was investigated. The Detailed process parameters are shown as fowling table. During the calcining process at 700 °C, the organic components, including Chitin and P123, underwent decomposition and evaporation, creating voids within the alumina matrix. This decomposition and evaporation of organic materials resulted in the formation of well-defined mesopores within the macroporous alumina microspheres.
Table 1.
Detailed process parameters for preparing spherical mesoporous alumina.
Table 1.
Detailed process parameters for preparing spherical mesoporous alumina.
| Sample |
Weight ratio
(Chitin:P123) |
Reaction temperature (°C) |
Reaction time (h) |
| R2:1T120H3
|
2:1 |
120 |
3 |
| R0:1T120H9
|
0:1 |
120 |
9 |
| R4:1T120H15
|
4:1 |
120 |
15 |
| R1:2T140H3
|
1:2 |
140 |
3 |
| R3:1T140H9
|
3:1 |
140 |
9 |
| R1:1T140H15
|
1:1 |
140 |
15 |
| R0:4T160H3
|
0:4 |
160 |
3 |
| R2:3T160H9
|
2:3 |
160 |
9 |
| R3:1T160H15
|
3:1 |
160 |
15 |
| R3:1T180H3
|
3:1 |
180 |
3 |
| R1:2T180H9
|
1:2 |
180 |
9 |
| R0:4T180H15
|
0:4 |
180 |
15 |
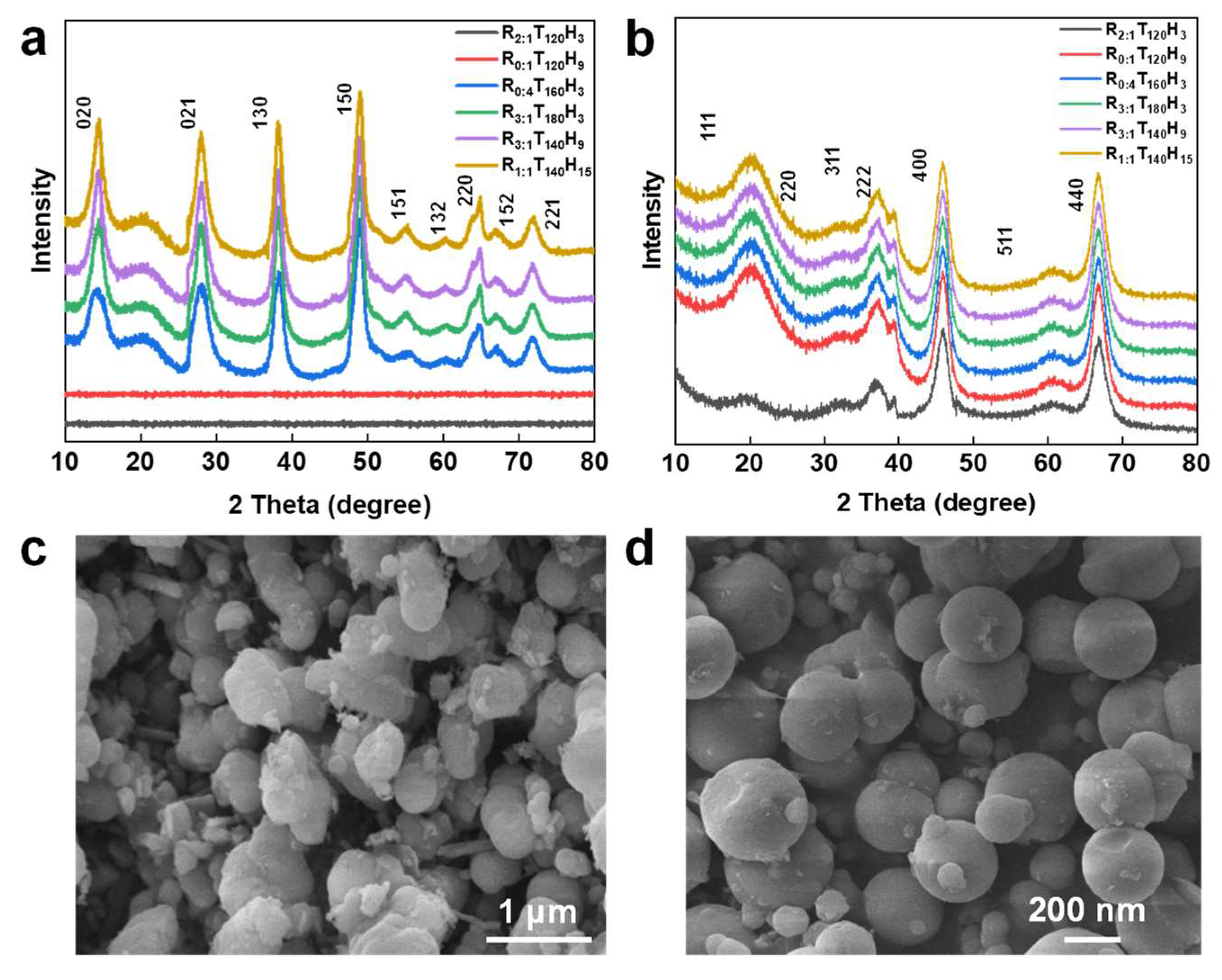
The effect of synthesis temperature and time on the morphology of spherical mesoporous alumina. Figure 2a displays the X-ray diffraction (XRD) map of the precursor synthesized under various temperatures and durations. The precursors of R
2:1T
120H
3 and R
0:1T
120H
9 are primarily amorphous products. However, as the synthesis temperature increases to 160 °C and 180 °C, The precursors of R
0:4T
160H
3 and R
3:1T
180H
3 start to crystallize and become AlOOH (JCPDS 01-072-0359). This finding suggests that the synthesis temperature has a significant impact on the crystallization of the precursor. At lower hydrothermal temperatures, the system energy is insufficient to facilitate the formation and transformation of crystals, leading to the amorphous state of the samples. In contrast, when the hydrothermal temperature increases, individual diffraction peaks emerge, and their intensity and width grow, indicating the increased crystallinity of the particles. The precursors of R
3:1T
140H
9 and R
1:1T
140H
15 are synthesized at 140 °C for 9 h and 15 h, respectively, providing sufficient energy for crystallization. Nevertheless, the diffraction peak intensity and width of precursors R
3:1T
140H
9 and R
1:1T
140H
15 are smaller compared to those of precursors R
0:4T
160H
3 and R
3:1T
180H
3 synthesized at higher temperatures. This difference implies that the crystallinity of particles in precursors of R
3:1T
140H
9 and R
1:1T
140H
15 is weaker than that in precursors R
0:4T
160H
3 and R
3:1T
180H
3. By controlling the growth rate of crystal faces, the crystal orientation can be influenced, enabling control over the morphology and crystal structure of products.
Figure 2b shows the mesoporous alumina XRD image after calcination at 600 °C. The amorphous precursor of R
2:1T
120H
3 and R
0:1T
120H
9 prepared at a lower synthesis temperature transforms into γ-Al
2O
3 under calcination at 700 °C; sample R
0:4T
160H
3, R
3:1T
180H
3, R
3:1T
140H
9 and R
1:1T
140H
15 are γ-Al
2O
3 (JCDPS 10-0425). With the increase of hydrothermal temperature, the position of each diffraction peak remains the same. However, the intensity and width of the diffraction peak grow larger, indicating an increase in the crystallinity of particles.
Figure 2c, d illustrates the SEM images of the mesoporous alumina samples prepared under different synthesis conditions. The images reveal that the temperature significantly influences the sample morphology, and 140 °C promotes the formation of spherical particles. When synthesized at 120 °C, the sample particles are massive and spherical (
Figure 2c), while at 140 °C, the particles are regularly spherical with a smooth surface and particle sizes ranging between 50 nm and 200 nm (
Figure 2d). As the synthesis temperature increases, more particles begin to crystallize and form crystalline solids. While these solids can completely transform into γ-Al
2O
3 at 700 °C, the amorphous precursor requires more energy to undergo phase transformation at this temperature. Thus, crystallization is relatively slow, which favors the preparation of spherical mesoporous alumina (
Figure 2a, b). However, at higher synthesis temperatures, the Chitin powder used as a template tends to carbonize and lose some of its templating function. As a result, the sample transforms into irregular and flocculent particles that agglomerate (Figure S1a-d). Moreover, longer synthesis times are also not conducive to the development of spherical alumina particles (Figure S1c, d). Such conditions promote the development of flocculent, strip, and irregular particles, which hinder the normal growth of spherical particles.
The Effect of Chitin:P123 Weight Ratio on Spherical Morphology. During the synthesis process, the amount of additive used has a significant impact on the morphology and structure of the final product.[
29,
30,
31] Table 1 displays the test results of spherical mesoporous alumina prepared using different Chitin:P123 weight ratios (Chitin quality:P123 quality).
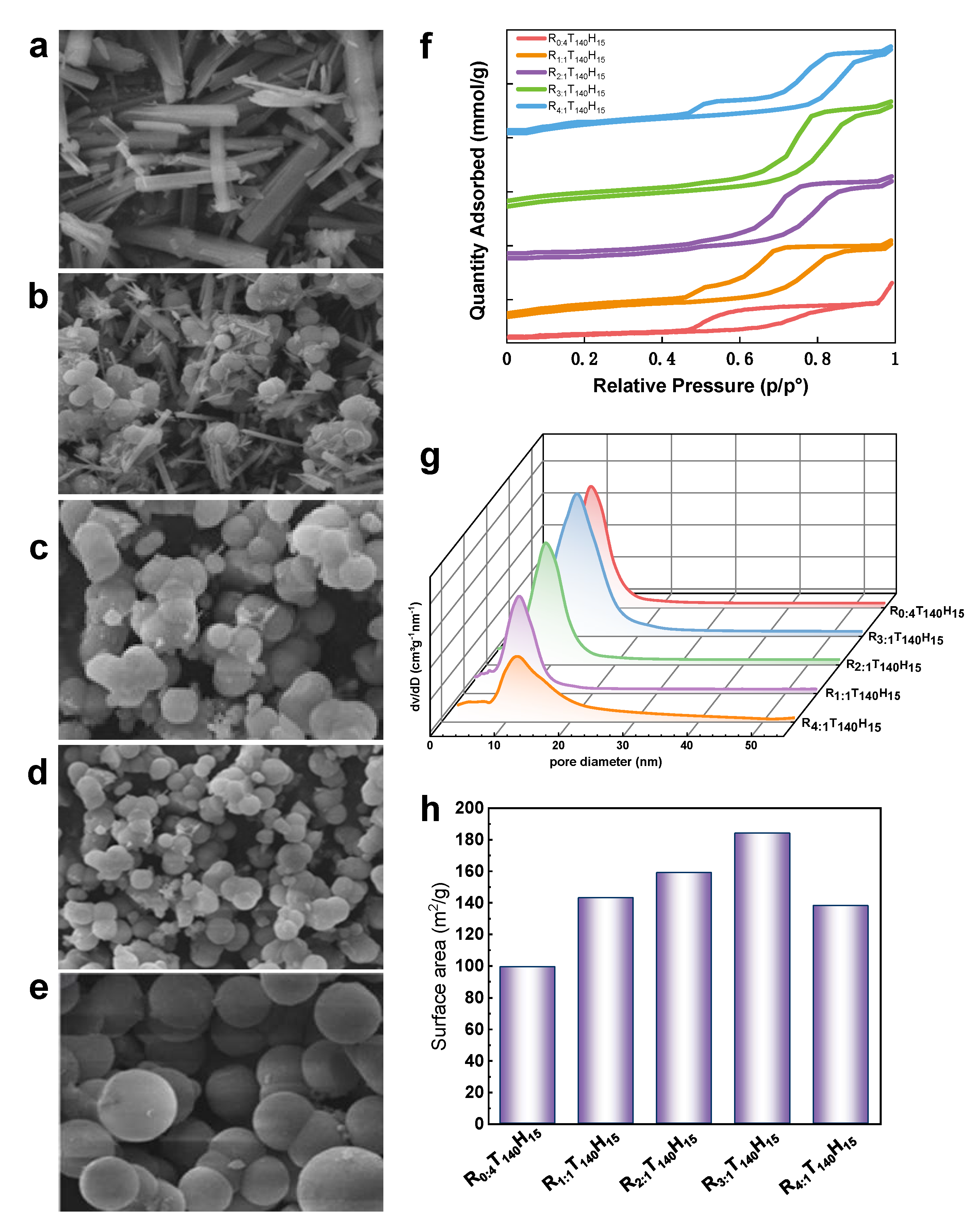
When Chitin/P123 weight ratio is 0 (R= 0:4), the predominant types of pores observed in the samples are ink-bottle type pores with a large opening and small diameter, as well as uneven crack-like pores, as shown in
Figure 3a. The nitrogen adsorption isotherm (see
Figure 3f) exhibits a type IV curve with a hysteresis loop between H2 and H3 in the mid-dle to high voltage range of 0.5 p/p
0 to 0.9 p/p
0. The adsorption amount in this range is limited to only 0.4 to 1 mmol/g. This is primarily due to the irregular stacking of the strip-shaped samples, which results in a low number of mesoporous structures being dis-tributed. As shown in
Figure 3g, the pore sizes are mainly distributed in the range of approximately 7 nm.
As the weight ratio increases to 3, 4 (R= 3:1, 4:1), the particle size of the sample becomes larger and the spherical shape becomes more regular (see
Figure 3d,e). The nitrogen adsorption isotherms show that in the middle-high pressure area, nitrogen adsorption capacity has further increased to about 7-8 mmol/g, as shown in
Figure 3f,g. The pore size has further increased to about 9.4 nm and the pore volume has increased to 0.7 cm
3/g. The type IV curve of nitrogen adsorption isotherms shows a hysteresis loop between type H1 and H2. The formation of inkbottle pores becomes more prominent, and the mesoporous structure becomes more developed. However, with the further increase of weight ratio, the nitrogen adsorption capacity gradually decreases, indicating that excessive chitin may hinder the formation of the mesoporous structure. Therefore, the optimal weight ratio of Chitin/P123 is 3, 4 (R= 3:1, 4:1).
As shown in
Figure 3d, at a weight ratio of 3, the sample formed regular, uniform, and monodisperse microspheres and the predominant pore structure is a regular cylindrical shape with uniform size and shape. The nitrogen adsorption isotherms for this sample show a steep type IV curve and an H1 hysteresis loop (see
Figure 3f), indicating that the sample has a uniform meso-porous structure. and pore sizes are mainly distributed between 7 nm and 9 nm. Notably, the pore size has increased by 8.6 nm compared to the previous weight ratio, and the pore volume has also increased by 0.3 cm
3/g.
In summary, the Chitin/P123 weight ratio has a significant effect on the morphology and structure of the mesoporous alumina sample. When the weight ratio is 0 (R= 0:4), the sample has irregular strips with few mesoporous structures. As the weight ratio increases to 3(R= 3:1), the sample forms regular and uniform microspheres with a uniform mesoporous structure, which leads to an increase in pore size, pore volume, and BET surface area. However, when the weight ratio is further increased to 4 (R= 4:1), the sample exhibits adhesion and aggregation, and the pore size, pore volume, and BET surface area decrease. This is due to the increase in viscosity of the solution, which affects the template space steric effect and weakens homogeneous nucleation.
Using the method, the experiment successfully prepared spherical mesoporous alumina with uniform pore size, good dispersion, and identical morphology, with a Chitin/P123 weight ratio of 3 (R= 3:1). The amorphous precursor was prepared at a lower temperature of 140 °C and for a shorter time of 3 hours. Upon calcination at 600 °C, inorganic Al3+ interacted slowly with and connected to the organic micelle interface via electrostatic interaction. This process allowed the aluminum ions to cover the entire particle surface and achieve consistent growth rates for all surfaces, ultimately resulting in the formation of spherical particles via homogeneous nucleation. The particle surface also formed a uniform mesoporous layer, resulting in the complete transformation of the precursor into spherical mesoporous Al2O3, as shown in Figure S2.
Figure S2 displays TEM images of spherical mesoporous alumina materials. The images clearly show that the alumina has a uniform and regular spherical form, as well as good dispersion. A 10 nm mesoporous layer uniformly covers the surface of the alumina particles, and the mesopore size is consistent throughout. The nitrogen sorption isotherms and corresponding pore size distributions of the spherical mesoporous alumina materials are also presented in Figure S2.
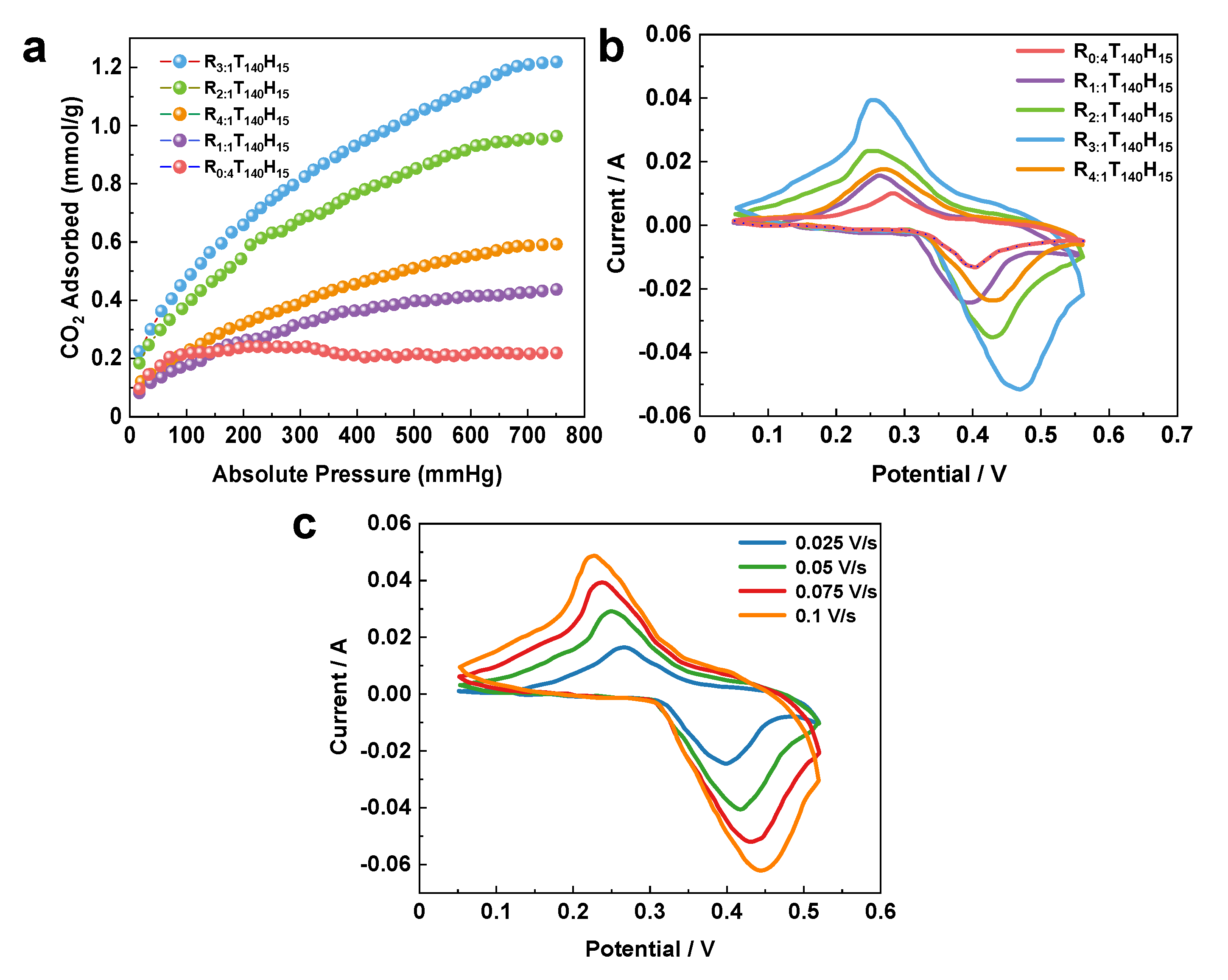
CO2 adsorption on spherical mesoporous alumina. Figure 4a displays the CO
2 gas adsorption isotherm curves of spherical mesoporous alumina, which was synthesized with varying mass ratios of Chitin to P123 at a temperature of 273 K. With its large specific surface area and pore volume, spherical mesoporous alumina exhibits a high potential for gas adsorption. In this study, we investigated the adsorption capacity of spherical mesoporous alumina, synthesized with different ratios of template agents (Chitin:P123), for carbon dioxide at 273 K.
The adsorption capacity of CO2 is highest (1.21 mmol/g) when the ratio of template agents is 3, resulting in the preparation of spherical mesoporous alumina with excellent dispersion and uniform pore size. Notably, the different mass ratios of template agents used in the synthesis produced alumina with distinct shapes, which significantly impacted their adsorption capacities.
Electrochemical performance of porous alumina supported SnO2. The electrochemical performance of porous alumina supported SnO2 was studied using spherical mesoporous alumina-supported SnO2 particle electrodes. The electrodes were prepared by a dip-calcination method, with a loading amount of 4%. Cyclic voltammetry tests were carried out using a three-electrode system with 5 mol/L KOH solution as the electrolyte, a mercury/mercury oxide electrode as the reference electrode, and a platinum electrode as the auxiliary electrode. The prepared electrode sheet was used as the working electrode.
The CV curves of spherical mesoporous alumina-supported SnO
2 prepared with different ratios of template agents (Chitin:P123) at a scan rate of 0.075 V/s were shown in
Figure 4b. The redox peak in the Figure 6a indicates the pseudocapacitive properties of the material. It was observed that when the ratio of template agent increases, the area of the cyclic voltammetry curve increases, and the specific capacitance increases significantly, indicating that the spherical mesoporous alumina has a better energy storage performance. However, when the ratio of template agent is too large (R= 4), the viscosity is viscosity of the solution increases significantly. This increase in viscosity can affect the steric effect of the template space and weaken the homogeneous nucleation, which can lead to poor dispersion of particles. Poor particle dispersion can result in a poor carrier effect, which can negatively impact the electrochemical performance of the material.
The CV curves at different scan rates when the ratio of template agent (Chitin:P123) is 3 and the loading of SnO
2 is 4% were shown in
Figure 4c. The CV curves were observed to be different with different scan rates, and with the increase of the test scan rate, the area of the closed graph also increased. This can be attributed to the high surface area and porous structure of the alumina support, which provides a large number of active sites for SnO
2 deposition and enhances the catalytic activity of SnO
2. Mesoporous alumina sphere can be an excellent electrocatalyst support due to its special mesoporous structure.
Periodic density functional theory (DFT). To gain deep insight on CO
2 adsorption, we performed periodic density functional theory (DFT) calculations (see Supplemental Information for computational details). The (110) and (100) surfaces of γ-Al
2O
3 were selected, as these two surfaces are predominant in γ-Al
2O
3, proved by XRD in
Figure 2b.[
1] The optmized structures of Al
2O
3(110) and Al
2O
3(100) surfaces are shown in Figure S3. The bare Al
2O
3(110) surface is terminated with two-coordinated O
(II), three-coordinated O
(III) and four-coordinated Al
(IV), while Al
2O
3(100) surface is exposed with three-coordinated O
(III) and five-coordinated Al
(V). Our results indicate that the surface energy of Al
2O
3(110) is lower than that of Al
2O
3(100) (1.41 J·m
-2 vs 2.45 J·m
-2), indicating that the Al
2O
3(110) surface is more stable.
The adsorption energies and optimized structures of CO
2 on Al
2O
3(110) and Al
2O
3(100) surfaces are shown in
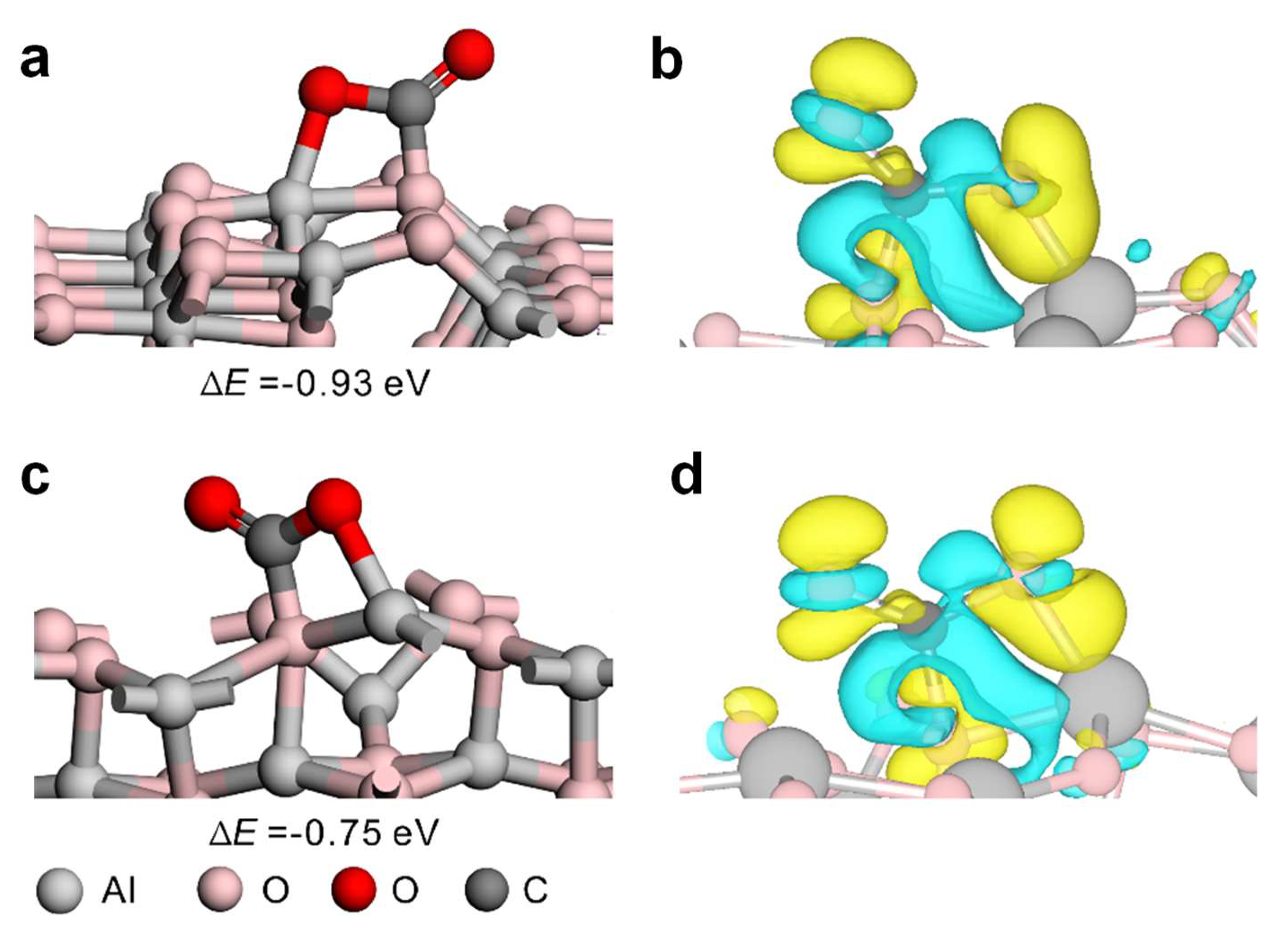
Figure 5 and Figure S4. DFT calculated results show that the formation of carbonate species is the most stable adsorption model, where CO
2 binds in abidentate configuration across the O-Al bridge sites with an adsorption energy of -0.93 eV and -0.75 eV on Al
2O
3(110) and Al
2O
3(100), respevtively. To obtain the insight into the nature of molecular adsorption behavior, the differences of charge density (Δρ) of the most stable adsorption CO
2 on Al
2O
3 surfaces are calculated. The results are clearly plotted in
Figure 5. It is evident that charges accumulate and deplet around the O and C of CO
2 on both surfaces, respectively. The net result is the transfer of electrons from Al
2O
3 surfaces to the adsorbed CO
2. The Bader charge analyses proved that the electronic charges transferred from the Al
2O
3(110) and Al
2O
3(100) surfaces to CO
2 are 0.28 a.u. and 0.29 a.u., respectively. These two surfaces achieve the purpose of CO
2 activation by transferring their electrons to the antibonding molecular orbital of CO
2. The former is more stable, mainly because there are more electrons near the Fermi level on the atoms of surface active site Al
(IV)-O
(II) on Al
2O
3(110) than that of Al
(V)-O
(III) on Al
2O
3(100) (see projected density of states in Figure S5). The above results demonstrate that both surfaces can strongly adsorb CO
2, which explains the experimental results. The difference in the amount of CO
2 adsorbed by Al
2O
3 synthesized under different conditions is mainly due to the different surface areas, which give different number of exposed active sites. The higher the crystallinity, the higher the surface content of Al
2O
3(110) [
32] .