Submitted:
05 July 2023
Posted:
07 July 2023
You are already at the latest version
Abstract
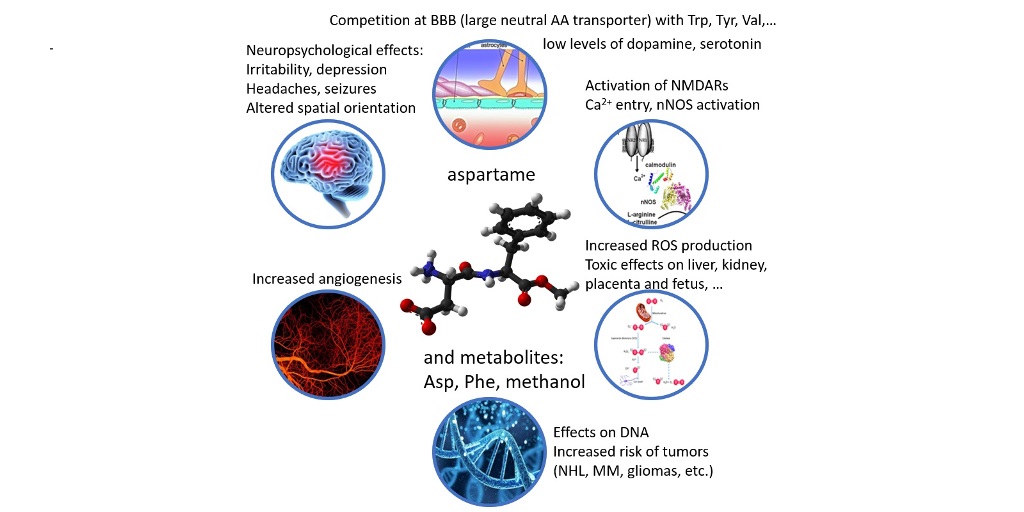
Keywords:
1. Introduction
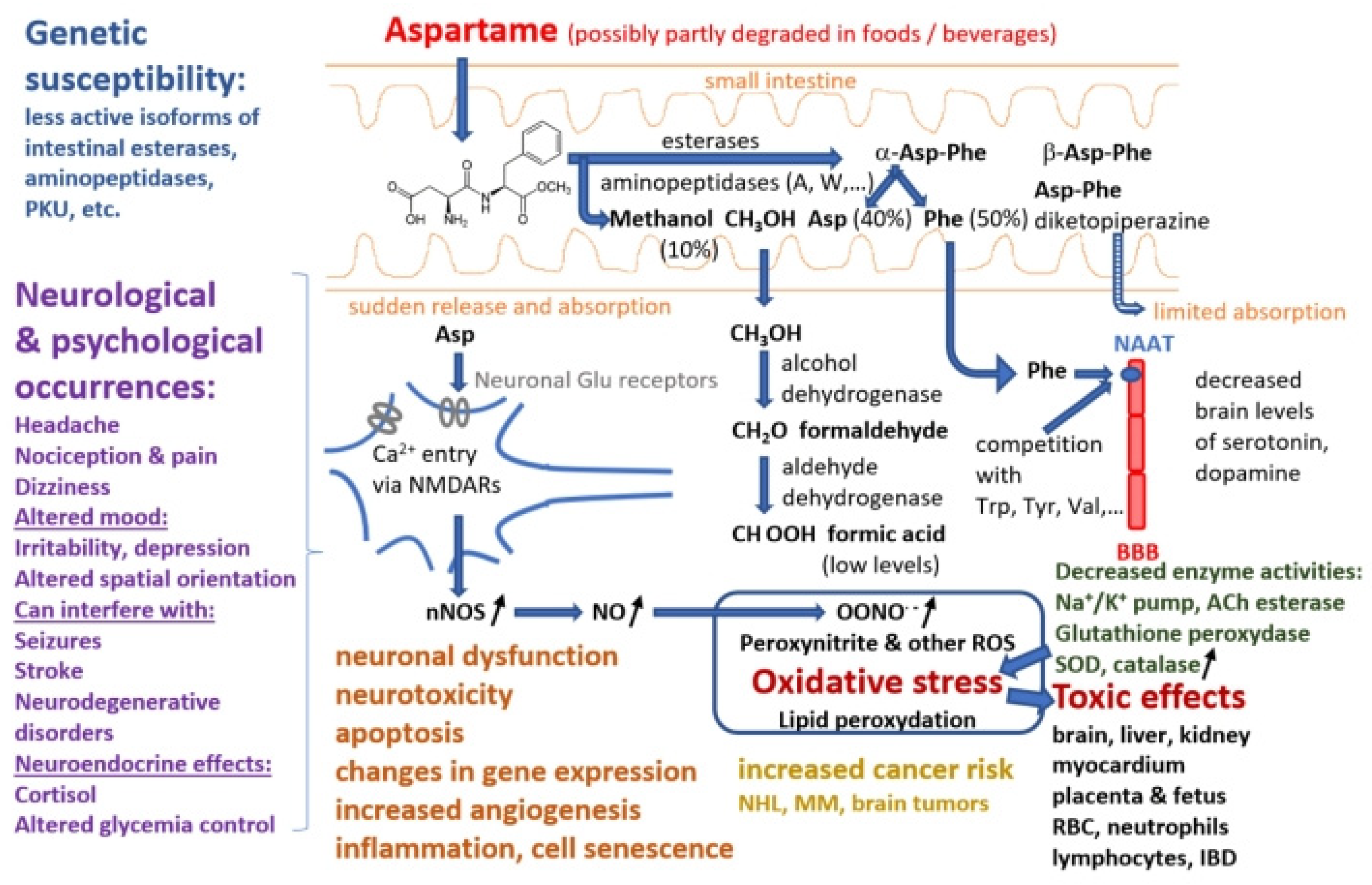
2. Chemical structure, digestion and metabolism
3. Mechanisms of toxicity of aspartame metabolism products
4. Neurological and cytotoxic effects by activation of NMDA and other glutamate receptors by aspartame or its metabolites
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Method | Experimental groups | Main results |
|---|---|---|---|
| Epidemiology studies | |||
| Lim et al. 2006 [109] | Clinical cohort prospective study to assess the risk of hematopoietic cancers or malignant gliomas associated with aspartame consumption | 285,079 men and 188,905 women aged 50 to 71 years with aspartame consumption assessed via a self-administered food frequency questionnaire, followed up for 5 years (1995–2000). | 1,888 hematological malignancies and 315 malignant gliomas were identified over the 5-year period; higher aspartame intake was not linked to an increased risk of hematological cancer (RR 0.98) or glioma (RR 0.73) in either men or women. |
| Schernhammer et al. 2012 [65] | Longitudinal prospective cohort study | 1324 non-Hodgkin lymphomas (NHL), 285 multiple myelomas (MM), and 339 leukemias were isolated from two large patient cohorts that were studied for more than 20 years; participants were divided into habitual diet soda drinkers (1 daily serving) and non-consumers. | In males but not in women, diet soda consumption was linked to an elevated risk of NHL (RR 1.31), and MM (RR 2.02). Men who drank more soda had a higher risk of developing NHL (RR 1.66). A coincidental association cannot be ruled out. |
| Maslova et al. 2013 [62] | Prospective longitudinal cohort study | 60,466 women enrolled during pregnancy in the prospective longitudinal Danish National Birth Cohort between 1996 and 2003; validated food frequency questionnaire adminstered at gestation week 25; child asthma evaluated at 18 months. | Childern of artificially-sweetened carbonated drinks consumers were more likely to have asthma (OR 1.30, 95%CI: 1.01-1.66) and take specific medication (OR 1.13, 95%CI: 0.98-1.29), as well as self-reported allergic rhinitis (OR 1.31, 95%CI: 0.98-1.74) during the first 7 years of follow-up. |
| Bassett et al. 2020 [63] | Prospective cohort study | 41,513 subjects aged 27 to 76 years at recruitment, of which 6,404 were excluded due to preexisting cancer, angina/heart attack, diabetes, placement in top/bottom 1% of energy consumption or missing data. | Over 19 years of follow-up, 4789 of the 35,109 selected participants developed cancers not related to obesity; subjects with high intake of artificially sweetened drinks had a significant hazard ratio of cancer development compared to control (HR 1.23; 95%CI: 1.02-1.48; p-trend 0.006). |
| Debras et al. 2022a [64] |
Longitudinal follow-up cohort study | NutriNet-Santé web-based cohort included 128,343 volunteers aged >18, of which 102,865 participants were selected and monitored over a median follow-up time of 7.8 years via daily 24h dietary records | High consumers of total artificial sweeteners had higher overall risk of cancers (HR 1.13, p-trend 0.002); for high-dose aspartame consumers HR 1.15, p =0.002; higher risks were detected for breast (HR 1.22, p=0.036) and obesity-related cancers (HR 1.15, p=0.026). |
| Debras et al. 2022b [55] |
Longitudinal follow-up cohort study | NutriNet-Santé web-based cohort included 103,388 selected participants, classified at baseline for diet, health, height and weight, lifestyle and socio-demographic data, physical activity, were monitored for dietary records and with biannual health questionnaires over the period 2009-2021 | Total artificial sweeteners (HR 1.09, p=0.03) and aspartame (HR 1.03, p=0.49) were associated with cardiovascular dieseases, coronary heart diseases and cerebrovascular diseases; aspartame intake was associated with cerebrovascular events (HR 1.17, p=0.02). |
| Clinical studies | |||
| Stegink et al. 1979 [36] | Randomized cross-over design | An overdose of aspartame (100 mg/kg body weight) was delivered to 6 fasting adult volunteers (3 men and 3 women) in both solution and slurry form. | Plasma aspartate increased from 0.16±0.05 (mean±SD) to 0.43±0.23 µM (solution) or 5.8 µM (slurry form), phenylalanine from 5 to 20.3±2.05 or 26.0±18.9 µM, methanol increased to 1.16±0.47 or 1.27±0.48 mg/dl at 60-90 min after loading, far below toxic levels. |
| Stegink et al. 1981 [32] | Randomized cross-over design | 30 healthy normal adult participants (15 men and 15 women) received different doses of aspartame p.o.: 12 subjects (6 male, 6 female) 34 mg/kg, and 6 subjects in each group were given 100, 150, and 200 mg/kg. | No differences on ophthalmologic examinations performed before and after aspartame loading, and no alterations in blood chemistry profile 24 hours after aspartame ingestion. |
| Stegink et al. 1983 [31] | Clinical study of blood methanol levels in one-year-old infants administered graded doses of aspartame | 24 infants aged between 8 and 14 months were tested in 3 stages: 10 subjects received aspartame 34 mg/kg, subsequently 6 subjects received 50 mg/kg, and 8 subjects 100 mg/kg body weight. | At 34 mg/kg blood methanol levels were below limit of detection (0.35mg/dl), at 50 mg/kg 0.30±0.10 mg/dl, at 100 mg/kg 1.02±0.28 mg/kg, non-toxic and similar to those observed in normal adults. |
| Stegink et al. 1987 [34] | Balanced Latin square design to test effects of co-ingestion of monosodium L-glutamate with aspartame | 12 healthy normal adult participants (6 males, 6 females) were adminsitered three distinct soup/beverage meals: meal A with no aspartame (APM) or monosodium L-glutamate (MSG); meal B with 50 mg MSG per kg body weight; meal C with 50 mg MSG and 34 mg APM/kg body weight. | Plasma glutamate levels increased significantly after meals B and C, and aspartate levels after meal C; plasma Glu+Asp mean levels increased from baseline (5.64±2.62 µM) to 23.1±7.29 µM (meal B) or 26.8±9.74 µM (meal C). |
| Koehler and Glaros 1988 [40] | Controlled double-blind randomized cross-over study on the frequency and intensity of migraine | 11 subjects with migraine history were exposed for 13 weeks to either 1200 mg aspartame/day or placebo and then switched regime after a wash-out period. | Aspartame ingestion by migraineurs caused a significant increase in headache frequency (p=0.0144). |
| Schiffman et al. 1987 [42] | Double-blind cross-over clinical trial | 40 subjects with history of headache/related neurological symptoms within 24h after aspartame intake were challenged with 30 mg/kg aspartame or placebo | The incidence of headache after aspartame ingestion (35%) or placebo (40%) were not different (p=0.5); the subjects with headache had lower plasma concentrations of epinephrine (p<0.0002) and norepinephrine (p<0.02). |
| Stegink et al. 1988 [35] | Two-stage clinical trial in a standard cross-over design | 8 healthy normal adult subjects (4 males, 4 females), were given in stage 1 3 servings of unsweetened beverage, and in stage 2 3 servings of beverage providing aspartame 10 mg/kg body weight each. | Addition of aspartame had no effect on plasma aspartate levels, and increased phenylalanine levels by 1.64-2.05 µM above baseline (5.09 ±0.82 µM), not exceeding normal postprandial values. |
| Stegink et al. 1989 [38] | Balanced cross-over design | 6 normal healthy adult subjects (3 male, 3 female) were given 8 beverage servings at 1h intervals, unsweetened or providing 600 mg aspartame per serving. | Plasma phenylalanine levels increased by 1.41-2.35 µM above baseline 30 min after ingestion of aspartame-containing drinks and reached steady-state after 4-5 servings. |
| Walton et al. 1993 [44] | Cross-over design | Initally designed to test 40 patients with unipolar depression and a similar control group; final groups were of 8 depression patients and 5 control subjects, given aspartame 30 mg/kg/day or placebo for 7 days. | Patiens with depression given aspartame experienced more frequenty symptoms like nausea, depression, insomnia, temper, nervousness, dizziness, trouble remembering, fatigue, malaise, irritability. |
| Van den Eeden et al. 1994 [43] | Double-blind cross-over study on volunteers with self-identified headaches | 18 subjects with headaches were randomly administered aspartame 30 mg/kg/day and placebo for 7 days in a 2-phase trial. | Headache incidence was 33% on aspartame regime vs. 24% on placebo (p=0.04), with no signifcant difference in length of headache or occurrence of associated side effects. |
| Shaywitz et al. 1994 [46] | Randomized double-blind placebo-controlled crossover study | Unmedicated children with attention deficit disorders (DSM3 criteria) given aspartame 34 mg/kg/day single morning dose or placebo for 2 weeks were tested by parents at home and teacher (at school); during a 2-day admission to a study center cognitive tests and blood sampling were performed. | No changes in cognitive and behavioral tests (matching familiar figures, children’s checking task, airplane, Wisconsin card sorting, subjects treatment emergent symptom scale, multgrade inventory for teachers, Conners behavior rating scale) and biochemical values, except for plasma Phe and Tyr |
| Stegink et al. 1995 [39] | Clinical trial including biochemistry and behavioral data on children described by their parents as sensitive to sugar vs. normal children | 25 normal preschool children (aged 3-5) and 23 school-aged sugar-sensitive children (aged 6–10) were fed diets with high sucrose, aspartame, or saccharin for 3 weeks each, with blood samples at baseline (while fasting) and within the last 3 days of each dietary session. | No biochemical or behavioral abnormalities, except for a subject with low plasma a-Asp-Phe hydrolase activity (> 2 SD below the mean) who experienced difficulty sitting still and paying attention towards the end of an aspartame diet session. |
| Spiers et al. 1998 [110] | Randomized double-blind placebo-controlled cross-over study | 48 healthy volunteers kept in control conditions for 1 month, then fed with high (45 mg/kg/day), low (15 mg/kg/day) or no aspartame (placebo or sucrose) for 20 days; neuropshychology and laboratory testing was performed at 10 and 20 days under each regime. | Plasma phenylalanine concentrations increased significantly during aspartame diet. By sex and by therapy, amino acid, insulin, and glucose readings, EEG, unfavorable experiences, and neuropsychological tests results were compared: there were no significant changes. |
| Newman and Lipton 2001 [41] | Clinical case reports |
Two recurrent migraine cases: 1. a 14-year-old male with a 2-year migraine history 2. a 36-year-old woman with migraines without an aura for 30 years. |
After receiving treatment with a rizatriptan formulation that contains aspartame (Maxalt-MLT), both patients experienced headache aggravation. |
| Lindseth et al. 2014 [105] |
Double-blinded repeated-measures within-subjects neurobehavioral study to determine differences in cognition, mood, depression, headaches following consumption of high vs. low amounts of aspartame |
28 healthy young adults (students) followed a study-prepared high-aspartame diet (25 mg/kg body weight/day) for 8 days and a low-aspartame diet (10 mg/kg body weight/day) for 8 days, with a 2-week washout period in between the diets. | Participants on high aspartame diets were more agitated (p=0.002, paired t test), showed greater signs of depression (p=0.001), and struggled on spatial orientation tests (p=0.03), but with no effect on working memory. |
| Solomi et al. 2019 [111] | Cross-over design to test the acute glycaemic effects of non-nutritive sweeteners aspartame and acesulfame-K | 10 healthy volunteers (4 males, 6 females), with a mean age and BMI of 27.2±6.9 years and 23.9±2.4 kg/m2) were tested for glycemia while fasting and at 15-min intervals for 2h after ingesting 236 mL of sucrose-sweetened cola with 125 mL of water, 25 g of glucose in 125 mL of water, and 25 g of glucose in 125 mL of water with 236 mL of diet cola. | None of the test beverages had significantly different glycaemic responses; co-consuming artificially sweetened diet cola with a drink containing glucose had no discernible impact on postprandial glycaemia. |
| In vivo studies | |||
| Neurological effects | |||
| Torii et al. 1986 [112] | Biochemistry and behavioral study in rats | Male Sprague-Dawley rats were fed with different combinations of casein, sucrose, corn starch, aspartame (5% of diet weight) for 2h (acute exposure) or for 3 weeks (chronic exposure). | Acute aspartame ingestion increased plasma and brain Phe and Tyr levels, but not Trp levels. Brain norepinephrine and dopamine levels were unaltered, serotonin levels were slightly increased on a protein-free diet. Chronic ingestion produced no signifcant chemical changes in brain. |
| Sharma and Coulombe 1987 [113] | Neurochemistry study in mice | Male CD-1 mice were given daily aspartame oral doses of 0, 13, 133, 650 mg/kg for 30 days. | Increases in adrenergic chemicals noticed after a single dose were not present upon chronic exposure. Serotonin was decreased in several brain regions, possibly due to the fact that increased Phe uptake decreased Trp uptake by brain tissue. |
| Goerss et al. 2000 [104] | Behavioral and neurochemistry study in rats | Aspartame (200-800 mg/kg i.p.) or a vehicle was given to adult male Long-Evans rats: 71 rats were used for behavioral testing, while 24 rats were used for a neurochemistry investigation. | High-dose aspartame dramatically reduced aggression, lengthened the intervals between attacks, reduced the number of bites per session, and markedly raised serotonin levels in the striatum. |
| Christian et al. 2004 [91] | Behavioral and neurochemistry study of chronic aspartame consumption in rats | Male Sprague-Dawley rats (225 g) that received aspartame (250 mg/kg/day) in water for three to four months vs. control rats. The animals were tested for latency to reward retrieval in a T-maze; after brain removal, membrane preparations from specific areas or whole brain were assessed for binding of [3H]quinuclidinyl benzilate and Na/K ATPase activity. | In aspartame-treated animals the number of muscarinic chlolinergic receptors increased by 31%; the frontal cortex, midcortex, posterior cortex, hippocampus, hypothalamus, and cerebellum all revealed substantial increases in muscarinic receptor densities, as well as significant increases in Na/K ATPase activity only in the midbrain. Latency to reward increased significantly in aspartame group after 3 and 4 months. |
| Collison et al. 2012 [101] | Behavioral and biochemistry study on spatial cognition, learning, memory, and insulin sensitivity in mice exposed to aspartame | C57Bl/6J mice of both sexes were exposed to aspartame or control diet since in utero until 17 weeks of age. | Male aspartame-fed mice gained weight, had higher fasting blood sugar levels (noticed also in females) and lower insulin sensitivity at 17 weeks compared to controls (p<0.05). Male aspartame-fed mice had longer escape latencies during spatial learning trials in the Morris water maze test. |
| Iyashwami et al. 2015 [95] | Neurobehavioral and biochemistry study in rats | Wistar male albino rats (200-220g, control or kept on folate-deficient diet 45 days and methotrexate-treated for 1 week) were given aspartame 40 mg/kg/day or saline p.o. for 90 days. Anxiety was assessed by open field and elevated maze plus tests. Subsequently animals were sacrificed, blood (for formate levels) and brain samples (for hydrogen peroxide, immunohistochemistry, Western blot immunoassay and RT-PCR for TNF-a, JNK3, Fas, Caspase 8 and 9 vs. b-actin) were collected. | Aspartame and methotrexate-treated rats showed immobility, fecal bolus and a clearly diminished level of ambulation, rearing, and grooming, increased anxiety, increased plasma formate, increased hydrogen peroxide generation in different brain areas, increased expression and neuronal staining with apoptosis markers. |
| Iyashwami et al. 2018 [51] |
Behavioral and neurobiochemistry study in rats | Three groups of male Wistar albino rats were selected randomly: saline control, folate-deficient (MTX-treated) control, folate-deficient (MTX-treated) group fed with aspartame 40 mg/kg for 3 months. | Aspartame-treated rats showed decline in memory tests (Morris water maze, Y maze), reduced body weight, increased plasma corticosterone levels, nitric oxide production, ACh-esterase activity, c-Fos, hsp70, iNOS and nNOS expression, microglia and astrocyte activation, as well as reduced Na/K ATPase activity and expression of NMDAR1, PSD95, synaptophysin, ERK, CaMKII, CREB in different brain regions. |
| Magalhães et al. 2019 [103] |
Behavioral and electrophysiology study in rats |
80 newborn Wistar rats were placed into 4 groups, each receiving aspartame 75 mg/kg/day or 125 mg/kg/day (groups ASP75 and ASP125), water (vehicle group), or no treatment (naïve group). | Early aspartame ingestion resulted in weight loss, anxiety (shorter times in open arms in elevated maze-plus test), and decreased cortical spreading depression (CSD) velocity via in vivo electrical brain recordings. |
| Neurological effects of aspartate | |||
| Park et al. 2000 [97] | Behavioral and histopathology study in mice | Male ICR mice (6-7 weeks, 25-30g) were administered a unique dose of 4.0 mg/g monosodium glutamate (MSG) or 0.5 mg/g aspartate (Asp) i.p., or the same amount of saline solution in the control groups. | MSG or Asp significantly damaged neurons in the arcuate nucleus of the hypothalamus, with no alterations in the cerebral cortex or hippocampus, or in any other brain regions. No significant changes were found in spontaneous motor activity, tail-flick response, but there was a decreased latency in passive avoidance test. |
| Vences-Mejía et al. 2006 [114] | Biochemistry study of liver and brain detoxifying enzymes in rat | 24 male Wistar rats (21 days old) were given by gavage Asp 75 and 125 mg/kg body weight daily for 30 days. Liver, cerebrum and cerebellum microsomes were assessed for activity of alkoxyresorufin O-dealkylase, 4-nitrophenol hydroxylase, erythromycin N –demethylase, and for different CytP450 isoforms by immunoblotting. | 75 mg/kg Asp reliably increased the activity of all seven enzymes in the cerebrum and cerebellum, but not at the same levels. |
| Errico et al. 2008 [102] | Behavioral, biochemistry, histology and electrophysiology study to assess the role of D-aspartate in regulating hippocampal synaptic plasticity | Male wild-type and mutant C57Bl/6 mice with targeted homozygous deletion of the D-aspartate oxidase (Ddo-/-) gene were fed with D-aspartate 20 mM p.o. for 1 month. | D-aspartate diet promoted long-term potentiation in hippocampal slices from both genetic and pharmacologically altered animal models, but it had no effect on the fundamental features of synaptic transmission; it slightly decreased spatial cognitive flexibility but not hippocampus-dependent learning and memory. |
| Free radicals production/oxidative stress | |||
| Mourad and Noor 2011 [90] | Biochemistry study in rats | Adult male Wistar albino rats (120-180 g) were given aspartame 40 mg/day p.o. for 2, 4 or 6 weeks, with equivalent control groups. Brain homogenate samples were subjected to biochemical tests. | A significant decrease in lipid peroxidation occurred after 2 weeks of aspartame diet, followed by a significant increase after 4 weeks; reduced glutathione levels and increased superoxide dismutase activity were recorded after 4 and 6 weeks, while catalase activity increased after 6 weeks of aspartame diet. |
| Iyaswamy and Rathinasamy 2012 [88] | Neurobiochemistry study in rats | Male albino Wistar rats (200–220 g) were split in 3 groups: a saline control group, a methotrexate-treated control group, and a methotrexate-treated aspartame-fed group (75 mg/kg p.o. for 90 days on a folate-deficient diet). | Aspartame induced a significant increase in lipid peroxidation, superoxide dismutase and catalase activity, decreased glutathione peroxidase activity, reduced glutathione and protein thiol levels, as well as detectable blood methanol levels. |
| Abdel-Salam et al. 2012 [85] | Neurobiochemistry study in mice | Male Swiss albino mice (20–22 g) were split into 10 groups: one saline control group (0.1 ml i.p.), 6 groups treated with lipopolysaccharide (LPS) 100 µg i.p. followed by aspartame 0, 0.625, 1.875, 5.625, 11.25, and 22.5 mg/kg s.c., and 3 groups treated with aspartame alone 11.25, 22.5, and 45 mg/kg s.c. | Aspartame after LPS decreased lipid peroxidation, reduced glutathione (GSH) and nitrite concentrations in brain and liver. Aspartame alone increased lipid peroxidation, TNF-a and decreased GSH. Serotonin, noradrenaline, and dopamine in the brain were inhibited by aspartame in a dose-dependent manner. Nitrite, GSH, AST, ALT, ALP levels in the liver were not affected by aspartame. Oxidative stress and inflammation increased in the brain, but not in the liver. |
| Abhilash et al. 2013 [86] | Neurobiochemistry and histopathology study in rats | Three groups of male Wistar rats, (150–175 g) were created at random; the first group received aspartame 500 mg/kg in 3 ml water, the second group 1000 mg/kg, and the control group only 3 ml water daily for 180 days. | The group that received 1000 mg/kg featured decreased brain concentrations of reduced glutathione (GSH) and glutathione reductase activity; the group fed with 500 mg/kg showed only a significant reduction in GSH. Histopathological examination revealed mild vascular congestion in the 1000 mg/kg group. |
| Prokić et al. 2014 [60] | A study of oxidative status in erythrocytes of rats on aspartame diet | There were two groups of animals: the experimental group received aspartame 40 mg/kg p.o. daily for 6 weeks, whereas the control group received only water. | Superoxide anion, hydrogen peroxide, peroxynitrite and lipid peroxides concentrations were significantly higher in the erythrocytes of the aspartame-treated group. Reduced glutathione (GSH) levels and catalase activity both increased under aspartame treatment. |
| Iyyaswamy and Rathinasamy 2014 [89] | Neurobiochemistry and histopathology study in rats | Adult male Wistar albino rats (200–220 g) were divided into three groups: saline control, folate-deficient (methotrexate-treated), and folate-deficient treated with aspartame 40 mg/kg p.o. for 90 days. Subsequently brain samples and homogenates were obtained. | Aspartame exposure led to significantly increased levels of protein carbonyl and decreased levels of protein thiol, increased lipid peroxidation, plasma methanol, and activity of superoxide dismutase, glutathione-S-transferase, glutathione peroxidase, catalase, significantly decreased levels of GSH, glutathione reductase. Proapoptotic gene expression was increased, as well as apoptosis markers caspase 3 and Bax, and CA1-3 pyramidal layer was depleted. |
| Choudhary and Rathinasamy 2014 [70] | Neurobehavioral and oxidative stress study in rats | Adult male Wistar albino rats (200-220 g) were fed a folate-deficient diet (FD) for 37 days and were given methotrexate (1 mg/kg) i.v. every second day for two weeks. The aspartame groups received aspartame 40 mg/kg/day p.o. 90 days. | After 90 days of aspartame administration, there was no discernible change in motor behavior, but there was a considerable decrease in membrane-bound ATPase activity and a decline in both enzymatic and non-enzymatic antioxidant levels in spinal cord lysates. |
| Alkafafy et al. 2015 [87] | Oxidative stress study on the rat liver | 25 male Wistar albino rats aged 7 weeks were separated into 5 groups. The first 2 groups were given aspartame p.o. (250 and 1000 mg/kg), while groups 3-4 received saccharin (25 and 100 mg/kg, respectively), daily for 8 weeks. | The aspartame-treated groups showed increased liver enzymes activities, decreased antioxidant levels; all sweetener-treated groups showed histological hepatotoxic effects, downregulation of the tumor suppressor gene P27 and overexpression of the main oncogene H-Ras, pointing to a possible risk of liver carcinogenesis. |
| Metabolic and other toxic effects | |||
| Abdel-Salam et al. 2009 [115] | A study on protective effects of citric acid and aspartame against CCl4-induced hepatic injury in rats | Adult Sprague-Dawley rats of both sexes (10 weeks, 120 g) were divided in 8 groups: 1 control group and 7 groups treated with CCl4 in olive oil (1:1 v/v) 2.8 mg/kg; these groups also received silymarin (25 mg/kg), aspartame (0.625 or 1.25 mg/kg), or citric acid (10 mg/kg, 100 mg/kg, or 1000 mg/kg) p.o. daily for 1 week. | At 0.625 or 1.25 mg/kg, aspartame decreased plasma ALT, AST, and ALP, respectively, by 39.8–52.0%, 43.2–52.4%, and 50.0–68.5%. On histology, 1.25 mg/kg aspartame significantly decreased CCl4-induced vacuolar degeneration and necrosis. |
| Kim et al. 2011 [116] |
Behavioral, biochemistry and histopathology study on zebrafish |
Zebrafish were given a high-cholesterol diet (HCD) along with aspartame or saccharin (5.2% and 3.6% of total food weight) for 12 days. | 30% of the aspartame-HCD group died (vs. 0% in the saccharin and control groups). The aspartame-fed group showed acute swimming defects, a significant rise in blood glucose (up to 125 mg/dl), with more inflammatory cells in brain and liver. |
| Choudhary and Rathinasamy 2016 [117] |
A study of expression of pro/antiapoptotic genes in immune organs of rats | Male Wistar albino rats (200- 220 g) were fed a folate-deficient diet for 37 days, followed by methotrexate (MTX) every second day for two weeks; subsequently, they received aspartame 40 mg/kg/day (or saline in control) for 90 days. | Aspartame treatment did not result in obvious DNA fragmentation in the spleen, thymus, or lymph nodes; it also did not significantly alter the mRNA levels of Bcl-2 and Bax in the immune organs, while Hsp70 expression increased significantly. |
| Gul et al. 2017 [118] | A study of metabolic syndrome based on intestinal alkaline phosphatase inhibition by Phe | 6-week old male mice fed a high-fat diet (HFD) or normal diet received aspartame in water (0.96 mg/ml) or regular water for 18 weeks. In an acute in vivo model, an intestinal pouch was created by isolating a 6-cm segment of small bowel and was injected with aspartame 34 mg/kg or control saline. In vitro experiments monitored alkaline phosphatase (ALP) activity in the presence or absence of regular or aspartame diet soda. | Mice on HFD+aspartame gained more weight than the HFD+water group (48.1 vs. 42.4, p=0.0001), and showed a higher glucose intolerance (p=0.008); both HFD and normal diet+aspartame groups showed increased TNF-a levels. Aspartame lowered ALP activity in both in vivo and in vitro experiments (p=0.02 and p=0.034, respectively). |
| Helal et al. 2019 [119] | Biochemistry study in rats | 30 male albino rats (100-120 g) were fed for 30 days with aspartame (50 mg/kg), acesulfame-K (15 mg/kg) or control diet. | Aspartame-treated rats had higher levels of serum glucose, insulin, creatinine, urea, lipid profiles (excepting HDL-C), higher ASAT and ALAT activities; serum testosterone, T3 and T4 levels decreased in the aspartame group, while total protein, albumin, and albumin/globulin ratio increased in both treated groups compared to control. |
| Nettleton et al. 2020 [120] |
Study of an obesogenic diet effects on metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring |
Following obesity induction, 150 female Sprague-Dawley rats were divided into three groups: 1. high fat/sucrose diet (HFS) + water (obese-WTR); 2. HFS + aspartame 5-7mg/kg/day (obese-APM); 3. HFS + stevia 2-3mg/kg/day (obese-STV). Infants were weaned onto a control diet and given water, then monitored for 18 weeks. | Despite no direct low-calorie sweetener consumption by children, maternal low-calorie sweetener use together with HFS may affect weight management, glucose homeostasis, gut microbiota in dams and their offspring, particularly in early life. Mesolimbic reward pathway was altered in offspring of aspartame or stevia-fed dams: increased ventral tegmental area dopamine transporter and tyrosine hydroxylase (only group2) mRNA levels, increased nucleus accumbens D2 and mu opioid receptor levels. |
| Ragi et al. 2021 [11] | Biochemistry and metabolism study in rats | Adult male Sprague-Dawley rats (7-week-old) were given a 1-week adaption time, followed by aspartame (0.05% w/w) or sucralose (0.016% w/w) administration in diet, water, or both, for 7 weeks. | Aspartame consumption considerably increased body weight and fat mass, mostly because of an improvement in energy efficiency. Rather than the method of intake, the impact was correlated with the dosage. Additionally, use of aspartame was linked to glucose intolerance. |
| Effects on immunity, inflammation, development | |||
| Pórtela et al. 2007 [77] | A morphometric and kariometric study in pregnant rats | 20 pregnant rats, distributed randomly in 4 groups, received either aspartame 14 mg/kg or control in water at normal temperature or 40°C; placentas, umbilical cords and fetal livers (1000 hepatocytes) were analyzed morphometrically by kariometry. | The groups on aspartame at room temperature or 40°C showed reduced placental, maternal and fetal weight, umbilical cord length, as well as altered hepatocyte kariometric parameters. |
| Choudhary et al. 2014 [59] | Biochemistry, oxidative stress and immune function study in rats | Adult male Wistar albino rats (12 weeks of age, 200–220 g), fed a folate-deficient diet for 37 days followed by methotrexate 1 mg/kg i.v. every second day for 2 weeks, were treated with aspartame 40 mg/kg p.o. for 15, 30 and 90 days, followed by venous blood collection. | In aspartame-fed groups there was a progressive decrease in RBC membrane-bound ATPase, G-6-PD and GR activity, increased lipid peroxidation and NO levels in RBC, neutrophils and lymphocytes, decreased neutrophil adhesion and phagocytic index, increased antibody titers and soluble immune complexes. |
| Shalaby et al. 2019 [78] | Histological/immu-nohistochemical study of the placenta in rats | 20 pregnant female rats received aspartame 14 mg/kg p.o. or control during days 9-11 of pregnancy. | Aspartame treatment decreased placental weight and thickness of labyrinth and basal zones, induced rupture of interhemal membranes, lysis of trophoblast cells, as well as increased VEGF staining of labyrinth and basal zones. |
| Fareed and Mostafa 2021 [121] |
Biochemical and histological study on renal maturation in rat offspring |
Pregnant rats were randomly divided into four groups: 1.control group; 2.aspartame group: 40 mg/kg/day until postnatal day 30; 3.caffeine group: 80 mg/kg/day; 4.aspartame and caffeine group. | Group 4 showed in kidneys a substantial increase in oxidative load (malondialdehyde), reduced antioxidant enzymes and total glutathione activity (superoxide dismutase and glutathione peroxidase). Renal tissues in group 4 matured faster than in groups 2 and 3, but with more pathological changes. |
| Genotoxic effects, mutagenic and carcinogenic potential | |||
| Bandyopadhyay et al. 2008 [122] | Genotoxicity study by comet assay in the bone marrow cells of mice | Swiss albino mice (8-10 weeks, 25 g) were orally administered aspartame (7, 14, 28, 35 mg/kg), acesulfame-K (150, 300, 600 mg/kg), and saccharin (50, 100, 200 mg/kg) individually. The animals were sacrificed after 18 h and the bone marrow cells were processed for comet assay. |
As evidenced by increased comet-tail extent and % DNA in the tail, sweetener-induced DNA strand breaks enhanced the comet characteristics of DNA in the bone marrow cells. Acesulfame-K and saccharin caused more DNA damage than aspartame. |
| Landrigan and Straif 2021 [19] | Immunohistochemical and morphological reevaluation of a study performed at Ramazzini Institute in 2006-2007 on rats and mice with aspartame-induced tumors | Reevaluation of studies BT6008 (aspartame 0-100000ppm since week 8 in rats), BT6009 (prenatal exposure in rats), BT6010 (prenatal exposure in Swiss mice) – a total of 2270 Sprague-Dawley rats and 852 Swiss mice. | In 92.3% of cases, the immunohistochemical and morphological reevaluation supported the initial diagnosis of malignancy; 3 lesions were reclassified as lymphoid hyperplasia and three as chronic inflammation with fibrosis out of the 6 lesions originally identified as lymphomas (8% of all HLTs). No signs of Mycoplasma infection were seen. |
| In vitro studies | |||
| Neurological effects | |||
| Fountain et al. 1988 [123] | In vitro study of hippocampal slice excitability and long-term potentiation (LTP) | Female Long-Evans rats aged 60–90 days used to prepare 400–450 µm thick hippocampal slices. | Exposure to 0.01, 0.1, 1, and 10 mM aspartame potentiated the electrical response of CA1 neurons (increased fEPSP slope and amplitude), similar to 0.1 mM aspartate, phenylalanine or its methyl ester, without effects on inhibitory systems (tested by double-pulse protocols) and LTP. |
| Pan-Hou et al. 1990 [84] | Ligand-receptor binding assay | Binding of NMDA, aspartate and aspartame in competition with 3H-glutamate on NMDARs from rat brain synaptic membrane preparations. | 0.1 mM NMDA, 1 mM aspartate and 1 mM aspartame displaced >50% of 3H-glutamate without modifying Vmax of glutamate binding, suggesting competitive binding. |
| Simintzi et al. 2007a [124] | Acetylcholine esterase activity in rat hippocampus and that of pure enzyme | Rat hippocampus homogenates or pure AChE enzyme were incubated with aspartame metabolites (aspartate 0.82-10 mM, phenylalanine 0.07-0.5 mM, methanol 0-0.8 mM). | Reduced AChE activity at high doses of metabolites (equivalent to consumption of aspartame 150-200 mg/kg). |
| Simintzi et al. 2007b [92] | Na/K ATPase activity in rat hippocampus and that of pure enzyme | Rat hippocampus homogenates or pure Na/K ATPase were incubated with aspartame metabolites (aspartate 0.82-10 mM, phenylalanine 0.07-0.5 mM, methanol 0-0.8 mM) | Reduced Na/K ATPase activity in homogenates (but increased activity of pure enzyme) at higher doses of metabolites (equivalent to consumption of aspartame 34-200 mg/kg). |
| Cytotoxic, genotoxic and carcinogenic effects | |||
| Kashanian et al. 2013 [125] | DNA binding study | Native calf thymus DNA interaction with aspartame at physiological pH was studied by spectrophotometry, spectrofluorimetric competition and circular dichroism; aspartame fluorescence quenching by DNA at various temperatures was used to estimate the number of binding sites per base pair. | The UV absorption band of aspartame exhibits hypochromism and red shift. Fluorescence quenching by DNA provided binding constants and corresponding number of binding sites; enthalpy (+181 kJ/mol) and entropy (+681 J/mol·K) changes were estimated. Iodide, methylene blue and competitive Hoechst 22358-aspartame quenching experiments proved minor groove DNA binding of aspartame, and lack of CD spectra changes with aspartame indicate a non-intercalative interaction. |
| Pandurangan et al. 2015 [74] |
Cytotoxicity study on human cervical carcinoma cells |
HeLa cells were seeded at a density of 2.2 x 105 cells/well in 6-well plates. After 24 h, the cells were treated with aspartame at different concentrations (10 µM, 100 µM, 1 mM, 10 mM, 20 mM) for 24 and 48 h. | After exposure to greater aspartame concentrations, cell viability was considerably changed. At greater aspartame exposure doses, ROS produced by mitochondria increased. DNA fragmentation occurred upon exposure to aspartame 10 or 20 mM. At 1–20 mM aspartame concentrations, apoptotic and necrotic bodies were discovered. |
| Park et al. 2019 [96] | Cytotoxicity study on embryonic mouse hypothalamic cell line mHypoE-N43/5 | mHypoE-N43/5 cells were cultured in medium containing either vehicle (saline) or 0.5, 5, 10, 20 mM of sucralose, aspartame, acesulfame-K, or rebaudioside A (all from Sigma) for 48 h. All non-nutritive sweetener stock solutions were made in sterile water. | When given in tolerable amounts for daily consumption, rebaudioside A did not cause ER stress, but sucralose, aspartame, and acesulfame-K did. Arcuate nucleus explants axon outgrowth was unaffected by sucralose, aspartame, or rebaudioside A, and aspartame had no impact on caspase 3/7 activity. |
| Maghiari et al. 2020 [66] |
Cytotoxicity study on HT-29 human colorectal carcinoma cells and chicken egg embryos |
HT-29 human colorectal carcinoma cells were cultured in specific medium, and incubated with different concentrations of aspartame (0.1, 0.25, 0.5, 1, 3, 6, 15, 30, or 50 mM) for 72 h, followed by Alamar blue cell viability assay. Chorioallantoic membranes of eggs were exposed to 10 µl aspartame solution placed inside a ring. | At the highest aspartame doses examined (15, 30 and 50 mM), there was a dose-dependent cytotoxic effect with a considerable reduction in viable cells, as well as morphological cellular alterations. Aspartame (15 and 30 mM) was shown to have a pro-angiogenic effect in ovo as well as a negligible irritating potential. |
| Çadirci et al. 2020 [126] |
An in vitro cytotoxicity, genotoxicity and oxidative stress study on cultured human blood cells |
Cytotoxicity was studied via MTT and lactate dehydrogenase release tests, genotoxic damage potential by using chromosome aberration (CA) assay, and antioxidant/oxidant activity by using total antioxidant capacity (TAC) and total oxidative stress analysis in cultured primary human whole blood cells. | Substantial, clearly concentration-dependent declines in cell viability were obtained upon aspartame exposure (3.125-100 mg/l). In aspartame-treated cells there was an increase in the frequency of CA, while TAC and TOS levels in whole blood cultures were not significantly altered. |
| Griebsch et al. 2023 [61] | Oxidative stress, membrane composition and mitochondrial damage study | The SH-SY5Y human neuroblastoma cell line was exposed to aspartame (271.7 μM) or its metabolites (Asp, Phe, methanol) | Aspartame and metabolites treatment altered mitochondiral integrity (assessed by transmission electron microscopy), increased total mitochondrial and lipid droplets area, decreased cardioliin levels to 56.7±5.6% (p=0.011), activated mitophagy and ROS release, increased expression of FIS1, PINK1, SOD1 and 2, increase in 7 triacylglycerol, phosphatidylcholine and phosphatidylethanolamine species |
| Angiogenesis effects | |||
| Alleva et al. 2011 [67] | In vitro angiogenesis and cytotoxicity study | Human endothelial cells were co-cultured with fibroblasts in a standardized angiogenesis model (Angio-Kit). Human umbilical vein endothelial cells (HUVEC) and fibroblasts were cultured in vitro. All cultures were exposed to aspartame 20, 40, 60, 80, or 100 µM dissolved in complete culture medium. | Exposure to aspartame stimulated angiogenesis, and also ROS production in endothelial cells associated with cytotoxicity (increased Erk1/2 and p38 activation and IL-6 secretion), but not in IMR-90 fibroblasts. |
| Yesildal et al. 2015 [69] | In vitro and in vivo angiogenesis and wound healing study | Male Sprague Dawley rats (8 weeks, 200–250 g) were used to remove two circular slices of skin (5 mm diameter). The wounds were treated with PBS or aspartame 50 mM for 7 days and collected on day 8, after calculating the surface area. Chicken eggs choriallantoic membrane (CAM) angiogenesis was tested by application of aspartame 6-60 mM, and HUVEC cells exposed to aspartame 20-100 µM were used for tube formation and 2,3-bis-2H-tetrazolium-5-carboxanilide (XTT) viability assays. | Aspartame increased CAM angiogenesis in a dose-dependent manner (p<0.001) and improved wound healing (p<0.05). Aspartame also slightly increased HUVEC cell proliferation (not statistically significant) and had no effect on tube formation. |
| Enuwosa et al. 2021 [68] |
In vitro study of effects of artificial sweeteners on VEGF-induced permeability of glomerular endothelium |
Human primary glomerular microvascular endothelial cells (GMVEC) in culture were treated with increasing concentrations (0.1-100 µM) of aspartame, saccharin, and sucralose for 24 h, and tested for endothelial monolayer permeability with dextran 20kDa-FITC upon VEGF exposure, VE-cadherin expression and intracellular cAMP level by ELISA, ROS production, and GC-MS for sucralose concentration in cell lysates. | All tested sweeteners had no effect on traditional VEGF signaling, but only saccharin and sucralose protected against VEGF-induced permeability, dependent on the sweet taste receptor T1R3. VEGF induced an increase in ROS production, which was not influenced by any sweetener. All sweeteners maintained VE-cadherin expression during VEGF exposure. In the absence of VEGF, aspartame significantly increased oxidative stress in GMVEC, while sucralose and saccharin had no effect. |
| Other effects | |||
| Manion et al. 2001 [127] | In vitro and clinical study of the efficacy of aspartame in treating sickle cell anemia | Twenty children with sickle cell anemia provided heparinized blood samples exposed to aspartame 1 or 2 mg/mL. 23 other patients with homozygous/heterozygous HbS or HbS/b0 were given aspartame 1.5, 3 or 6 mg/kg single-dose, and their blood samples were monitored up to 1440 min afterwards. | 1 mg/mL reduced sickled cells from 28% to 14%, and even further with 2 mg/mL. In 15 individuals with HbSS anemia, sickling was prevented by 6 mg/kg aspartame for at least 6 hours. |
| Rios et al. 2018 [128] | Comparison of enamel erosion produced by regular and light colas, with addition of aspartame sweetener |
60 bovine enamel blocks were exposed 2 min. 4 times daily for 5 consecutive days to erosion in 5 varieties of cola and kept in artificial saliva between exposures. Experimental groups: RC-regular cola-degassed (pH 2.6), RCpH-a base was added to raise the pH of RC (pH 3.0), RCAS aspartame added to RC (pH 2.6), LC-light cola (pH 3.0), LCpH-acid added to LC (pH 2.6). | The % surface hardness change of enamel after 1 day was similar for all 5 groups. LC caused less enamel loss than RC, but differences were not significant (p>.05) between erosion and erosion plus abrasion for LC. However, for RC erosion plus abrasion resulted in higher enamel loss than erosion alone. LCpH had an erosion effect similar to RC, while RCpH had similar effect to LC. |
| Pandurangan et al. 2014 [129] | An in vitro study of aspartame effects on preadipocyte differentiation | 3T3-L1 mouse preadipocytes were cultured and differentiated for 6 days in the absence and presence of aspartame 10 μg/ml. | The induction of p-PPARg, PPARg, SREBP1, and adipsin (by Western blot) and PPARγ, FABP4, and C/EBPα (RT-qPCR) was significantly reduced in aspartame-treated preadipocytes, as well as lipid accumulation (by Oil Red O staining). |
| Sun et al. 2019 [130] |
In vivo and in vitro study on small intestinal cell cycle and stimulating secretion and expression of glucagon-like peptide −2 (GLP-2) in pre-weaned lambs | Twelve 14-day lambs were randomly divided into two groups; control (n=6) fed on starter food, and aspartame-fed (200 mg/kg) (n=6) up to 49 days. At 56 days, 4 healthy lambs' jejunal tissue was used to dissociate and culture epithelial cells, treated with control, GLP-2 10 nM alone or with IGF-1R inhibitor picropodophyllin (PPP) 1 µM. | Aspartame-fed lambs showed higher GLP-2 plasma concentrations (p <.05), and larger jejunum weight/live body weight and jejunal crypt depth, as well as increased expression of cyclins D1, A2, CDK4 and 6, glucagon, IGF-1, GLP-2R in jejunum/ileum. Jejunal cells treated with GLP-2 (2h) showed increased proliferation (MTT test) and expression of IGF-1, cyclin D1, CDK6, which were decreased by PPP. |
| Chontzopoulou et al. 2021 [131] | In silico and experimental study on lipoxygenase (LOX) inhibition | Different LOX isoforms crystal structures were used for in silico studies: docking, molecular dynamics (MD) followed by QM/MM geometry optimization of ligand-receptor complexes. For in vitro assays of saturation transfer difference NMR (STD-NMR), a stock solution (10 mM) of the tested compound was prepared in DMSO. | In silico and in vitro assays confirmed strong aspartame binding to LOX-1 isoform (IC50=50 ± 3.0 μΜ) with functional inhibition. These results suggest that aspartame could serve as a novel starting point for drug design of LOX inhibitors. |
References
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners - a review. J Food Sci Technol 2014, 51, 611–621. [Google Scholar] [PubMed]
- Marinovich, M.; Galli, C.L.; Bosetti, C.; Gallus, S.; La Vecchia, C. Aspartame, low-calorie sweeteners and disease: regulatory safety and epidemiological issues. Food Chem Toxicol 2013, 60, 109–115. [Google Scholar]
- Mazur, R.H. Discovery of aspartame. In Aspartame: Physiology and Biochemistry, Stegink, L.D., Filer, L.J.J., Eds.; Marcel Dekker: New York, 1984; pp. 3–9. [Google Scholar]
- Mazur, R.H.; Schlatter, J.M.; Goldkamp, A.H. Structure-taste relationships of some dipeptides. J Am Chem Soc 1969, 91, 2684–2691. [Google Scholar]
- Tobey, N.A.; Heizer, W.D. Intestinal hydrolysis of aspartylphenylalanine--the metabolic product of aspartame. Gastroenterology 1986, 91, 931–937. [Google Scholar]
- Barceloux, D.G.; Bond, G.R.; Krenzelok, E.P.; Cooper, H.; Vale, J.A. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol 2002, 40, 415–446. [Google Scholar]
- EFSA Panel on Food Additives Nutrient Sources added to Food. Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA Journal 2013, 12, 3696. [Google Scholar]
- Soffritti, M.; Belpoggi, F.; Degli Esposti, D.; Lambertini, L.; Tibaldi, E.; Rigano, A. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environ Health Perspect 2006, 114, 379–385. [Google Scholar]
- Soffritti, M.; Belpoggi, F.; Tibaldi, E.; Esposti, D.D.; Lauriola, M. Life-span exposure to low doses of aspartame beginning during prenatal life increases cancer effects in rats. Environ Health Perspect. 2007, 115, 1293–1297. [Google Scholar]
- Collison, K.S.; Inglis, A.; Shibin, S.; Andres, B.; Ubungen, R.; Thiam, J.; Mata, P.; Al-Mohanna, F.A. Differential effects of early-life NMDA receptor antagonism on aspartame-impaired insulin tolerance and behavior. Physiol Behav 2016, 167, 209–221. [Google Scholar]
- Ragi, M.E.; El-Haber, R.; El-Masri, F.; Obeid, O.A. The effect of aspartame and sucralose intake on body weight measures and blood metabolites: role of their form (solid and/or liquid) of ingestion. Br J Nutr 2021, 128, 1–9. [Google Scholar]
- Sykes, M. The Aspartame Controversy of 1981, The Hidden Truth Behind the Not-So-Sweet Artificial Sweetner. 2015.
- Butchko, H.; Stargel, W.; Comer, C.; Mayhew, D.; Benninger, C.; Blackburn, G.; De Sonneville, L.; Geha, R.; Hertelendy, Z.; Kostner, A. Intake of aspartame vs the acceptable daily intake. Regul Toxicol Pharmacol 2002, 35, S13–S16. [Google Scholar]
- Fatibello-Filho, O.; Marcolino-Junior, L.H.; Pereira, A.V. Solid-phase reactor with copper (II) phosphate for flow-injection spectrophotometric determination of aspartame in tabletop sweeteners. Anal Chim Acta 1999, 384, 167–174. [Google Scholar]
- Fitch, C.; Keim, K. Position of the academy of nutrition and dietetics: use of nutritive and nonnutritive sweetener. J Acad Nutr Diet 2012, 112, 739–758. [Google Scholar]
- Monte, W.C. Aspartame: methanol and the public health. In Proceedings of the J Appl Nutr; 1984. [Google Scholar]
- Magnuson, B.A.; Burdock, G.A.; Doull, J.; Kroes, R.M.; Marsh, G.M.; Pariza, M.W.; Spencer, P.S.; Waddell, W.J.; Walker, R.; Williams, G.M. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev Toxicol 2007, 37, 629–727. [Google Scholar]
- National Research Council. Pesticides in the Diets of Infants and Children; The National Academies Press: Washington DC, 1993. [Google Scholar]
- Landrigan, P.J.; Straif, K. Aspartame and cancer - new evidence for causation. Environ Health 2021, 20, 42. [Google Scholar]
- Olney, J.W.; Farber, N.B.; Spitznagel, E.; Robins, L.N. Increasing brain tumor rates: is there a link to aspartame? J Neuropathol Exp Neurol 1996, 55, 1115–1123. [Google Scholar] [CrossRef]
- Czarnecka, K.; Pilarz, A.; Rogut, A.; Maj, P.; Szymańska, J.; Olejnik, Ł.; Szymański, P. Aspartame-True or False? Narrative Review of Safety Analysis of General Use in Products. Nutrients 2021, 13, 1957. [Google Scholar]
- Amchra, F.Z.; Al Faiz, C.; Chaouqi, S.; Khiraoui, A.; Benhmimou, A.; Guedira, T. Effect of Stevia rebaudiana, sucrose and aspartame on human health: A comprehensive review. Journal of Medicinal Plants Studies 2018, 6, 102–108. [Google Scholar]
- Singh, M.; Kumar, A.; Tarannum, N. Water-compatible 'aspartame'-imprinted polymer grafted on silica surface for selective recognition in aqueous solution. Anal Bioanal Chem 2013, 405, 4245–4252. [Google Scholar]
- Bell, L.N.; Labuza, T.P. Aspartame degradation kinetics as affected by pH in intermediate and low moisture food systems. Journal of food science 1991, 56, 17–20. [Google Scholar]
- Lipton, W.E.; Li, Y.N.; Younoszai, M.K.; Stegink, L.D. Intestinal absorption of aspartame decomposition products in adult rats. Metabolism 1991, 40, 1337–1345. [Google Scholar]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.P.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr Rev 2016, 74, 670–689. [Google Scholar] [PubMed]
- Hooper, N.M.; Hesp, R.J.; Tieku, S. Metabolism of aspartame by human and pig intestinal microvillar peptidases. Biochem J 1994, 298, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, X.H.; Yang, T.B.; Johnson, E.W.; Thacker, P.A. A comparison of the intestinal absorption of amino acids in piglets when provided in free form or as a dipeptide. Asian-Aus J Anim Sci 1999, 12, 939–943. [Google Scholar]
- Adibi, S.A. Intestinal transport of dipeptides in man: relative importance of hydrolysis and intact absorption. J Clin Invest 1971, 50, 2266–2275. [Google Scholar]
- Stegink, L.D. The aspartame story: a model for the clinical testing of a food additive. Am J Clin Nutr 1987, 46, 204–215. [Google Scholar]
- Stegink, L.D.; Brummel, M.C.; Filer, L.J., Jr.; Baker, G.L. Blood methanol concentrations in one-year-old infants administered graded doses of aspartame. J Nutr 1983, 113, 1600–1606. [Google Scholar]
- Stegink, L.D.; Brummel, M.C.; McMartin, K.; Martin-Amat, G.; Filer, L.J., Jr.; Baker, G.L.; Tephly, T.R. Blood methanol concentrations in normal adult subjects administered abuse doses of aspartame. J Toxicol Environ Health 1981, 7, 281–290. [Google Scholar]
- Stegink, L.D.; Filer Jr, L. Effects of Aspartame Ingestion on Plasma Aspartate, Phenylalanine and Methanol Concentrations in Potentially Sensitive Populations. In The clinical evaluation of a food additive: Assessment of aspartame; Tschanz, C., Butchko, H.H., Stargel, W., Kotsonis, F.N., Eds.; CRC Press: Boca Raton, 1996; pp. 87–113. [Google Scholar]
- Stegink, L.D.; Filer, L.J., Jr.; Baker, G.L. Plasma amino acid concentrations in normal adults ingesting aspartame and monosodium L-glutamate as part of a soup/beverage meal. Metabolism 1987, 36, 1073–1079. [Google Scholar]
- Stegink, L.D.; Filer, L.J., Jr.; Baker, G.L. Repeated ingestion of aspartame-sweetened beverage: effect on plasma amino acid concentrations in normal adults. Metabolism 1988, 37, 246–251. [Google Scholar]
- Stegink, L.D.; Filer, L.J., Jr.; Baker, G.L.; Brummel, M.C. Plasma and erythrocyte amino acid levels of adult humans given 100 mg/kg body weight aspartame. Toxicology 1979, 14, 131–140. [Google Scholar] [PubMed]
- Stegink, L.D.; Filer, L.J., Jr.; Baker, G.L.; McDonnell, J.E. Effect of an abuse dose of aspartame upon plasma and erythrocyte levels of amino acids in phenylketonuric heterozygous and normal adults. J Nutr 1980, 110, 2216–2224. [Google Scholar] [PubMed]
- Stegink, L.D.; Filer, L.J., Jr.; Bell, E.F.; Ziegler, E.E.; Tephly, T.R. Effect of repeated ingestion of aspartame-sweetened beverage on plasma amino acid, blood methanol, and blood formate concentrations in normal adults. Metabolism 1989, 38, 357–363. [Google Scholar] [PubMed]
- Stegink, L.D.; Lindgren, S.D.; Brummel, M.C.; Stumbo, P.J.; Wolraich, M.L. Erythrocyte L-aspartyl-L-phenylalanine hydrolase activity and plasma phenylalanine and aspartate concentrations in children consuming diets high in aspartame. Am J Clin Nutr 1995, 62, 1206–1211. [Google Scholar] [PubMed]
- Koehler, S.M.; Glaros, A. The effect of aspartame on migraine headache. Headache 1988, 28, 10–14. [Google Scholar] [CrossRef]
- Newman, L.C.; Lipton, R.B. Migraine MLT-down: an unusual presentation of migraine in patients with aspartame-triggered headaches. Headache 2001, 41, 899–901. [Google Scholar]
- Schiffman, S.S.; Buckley, C.E., 3rd; Sampson, H.A.; Massey, E.W.; Baraniuk, J.N.; Follett, J.V.; Warwick, Z.S. Aspartame and susceptibility to headache. N Engl J Med 1987, 317, 1181–1185. [Google Scholar]
- Van den Eeden, S.K.; Koepsell, T.D.; Longstreth, W.T., Jr.; van Belle, G.; Daling, J.R.; McKnight, B. Aspartame ingestion and headaches: a randomized crossover trial. Neurology 1994, 44, 1787–1793. [Google Scholar] [CrossRef]
- Walton, R.G.; Hudak, R.; Green-Waite, R.J. Adverse reactions to aspartame: double-blind challenge in patients from a vulnerable population. Biol Psychiatry 1993, 34, 13–17. [Google Scholar] [CrossRef]
- Ranney, R.E.; Oppermann, J.A. A review of the metabolism of the aspartyl moiety of aspartame in experimental animals and man. J Environ Pathol Toxicol 1979, 2, 979–985. [Google Scholar]
- Shaywitz, B.A.; Sullivan, C.M.; Anderson, G.M.; Gillespie, S.M.; Sullivan, B.; Shaywitz, S.E. Aspartame, behavior, and cognitive function in children with attention deficit disorder. Pediatrics 1994, 93, 70–75. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Lee, Y.Y. The debate over neurotransmitter interaction in aspartame usage. J Clin Neurosci 2018, 56, 7–15. [Google Scholar] [PubMed]
- Humphries, P.; Pretorius, E.; Naudé, H. Direct and indirect cellular effects of aspartame on the brain. Eur J Clin Nutr 2008, 62, 451–462. [Google Scholar] [PubMed]
- Reinhard, J.F.; Reinhard, J.F.J. Experimental evaluation of anticonvulsants. In Anticonvulsants, Vida, J.A., Ed.; Academic Press: New York, 1972; pp. 58–110. [Google Scholar]
- Maher, T.J.; Wurtman, R.J. Possible neurologic effects of aspartame, a widely used food additive. Environ Health Perspect 1987, 75, 53–57. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Kammella, A.K.; Thavasimuthu, C.; Wankupar, W.; Dapkupar, W.; Shanmugam, S.; Rajan, R.; Rathinasamy, S. Oxidative stress evoked damages leading to attenuated memory and inhibition of NMDAR-CaMKII-ERK/CREB signalling on consumption of aspartame in rat model. J Food Drug Anal 2018, 26, 903–916. [Google Scholar] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger principles of biochemistry, 5th edition ed.; WH Freeman & Co.: New York, 2008. [Google Scholar]
- Rycerz, K.; Jaworska-Adamu, J.E. Effects of aspartame metabolites on astrocytes and neurons. Folia Neuropathol 2013, 51, 10–17. [Google Scholar] [CrossRef]
- Ardalan, M.R.; Tabibi, H.; Attari, V.E.; Mahdavi, A.M. Nephrotoxic effect of aspartame as an artificial sweetener: A brief review. Iranian J Kidney Dis 2017, 11, 339. [Google Scholar]
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, S.F.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial sweeteners and risk of cardiovascular diseases: results from the prospective NutriNet-Santé cohort. BMJ 2022, 378, e071204. [Google Scholar]
- Alwaleedi, S.A. Alterations in antioxidant defense system in hepatic and renal tissues of rats following aspartame intake. J Appl Biol Biotechnol 2016, 4, 0–5. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine (5th edn.); Oxford University Press: 2015; p. 896.
- Anbara, H.; Kian, M.; Darya, G.H.; Sheibani, M.T. Long-term intake of aspartame-induced cardiovascular toxicity is reflected in altered histochemical parameters, evokes oxidative stress, and trigger P53-dependent apoptosis in a mouse model. Int J Exp Pathol 2022, 103, 252–262. [Google Scholar]
- Choudhary, A.K.; Rathinasamy, S.; Sundareswaran, L. Role of antioxidant enzymes in oxidative stress and immune response evaluation of aspartame in blood cells of wistar albino rats. Int Food Res J (Malaysia) 2014, 21, 2263–2272. [Google Scholar]
- Prokić, M.D.; Paunović, M.G.; Matić, M.M.; Djordjević, N.Z.; Ognjanović, B.I.; Štajn, A.S.; Saičić, Z.S. Prooxidative effects of aspartame on antioxidant defense status in erythrocytes of rats. J Biosci 2014, 39, 859–866. [Google Scholar] [PubMed]
- Griebsch, L.V.; Theiss, E.L.; Janitschke, D.; Erhardt, V.K.J.; Erhardt, T.; Haas, E.C.; Kuppler, K.N.; Radermacher, J.; Walzer, O.; Lauer, A.A.; et al. Aspartame and its metabolites cause oxidative stress and mitochondrial and lipid alterations in SH-SY5Y cells. Nutrients 2023, 15, 1467. [Google Scholar]
- Maslova, E.; Strøm, M.; Olsen, S.F.; Halldorsson, T.I. Consumption of artificially-sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoS One 2013, 8, e57261. [Google Scholar]
- Bassett, J.K.; Milne, R.L.; English, D.R.; Giles, G.G.; Hodge, A.M. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of cancers not related to obesity. Int J Cancer 2020, 146, 3329–3334. [Google Scholar] [PubMed]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, S.F.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med 2022, 19, e1003950. [Google Scholar]
- Schernhammer, E.S.; Bertrand, K.A.; Birmann, B.M.; Sampson, L.; Willett, W.C.; Feskanich, D. Consumption of artificial sweetener- and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am J Clin Nutr 2012, 96, 1419–1428. [Google Scholar]
- Maghiari, A.L.; Coricovac, D.; Pinzaru, I.A.; Macașoi, I.G.; Marcovici, I.; Simu, S.; Navolan, D.; Dehelean, C. High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells. Nutrients 2020, 12, 3600. [Google Scholar]
- Alleva, R.; Borghi, B.; Santarelli, L.; Strafella, E.; Carbonari, D.; Bracci, M.; Tomasetti, M. In vitro effect of aspartame in angiogenesis induction. Toxicol In Vitro 2011, 25, 286–293. [Google Scholar]
- Enuwosa, E.; Gautam, L.; King, L.; Chichger, H. Saccharin and sucralose protect the glomerular microvasculature in vitro against VEGF-induced permeability. Nutrients 2021, 13, 2746. [Google Scholar]
- Yesildal, F.; Aydin, F.N.; Deveci, S.; Tekin, S.; Aydin, I.; Mammadov, R.; Fermanli, O.; Avcu, F.; Acikel, C.H.; Ozgurtas, T. Aspartame induces angiogenesis in vitro and in vivo models. Hum Exp Toxicol 2015, 34, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Rathinasamy, S. Effect of aspartame in spinal cord and motor behavior in Wistar albino rats. J Behav Health 2014, 3, 107–111. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Pretorius, E. Revisiting the safety of aspartame. Nutr Rev 2017, 75, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, P.; Wang, Y.; Cui, W.; Li, D. The relationship between the use of artificial sweeteners and cancer: A meta-analysis of case-control studies. Food Sci Nutr 2021, 9, 4589–4597. [Google Scholar] [CrossRef] [PubMed]
- Guercio, B.J.; Zhang, S.; Niedzwiecki, D.; Li, Y.; Babic, A.; Morales-Oyarvide, V.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). PloS one 2018, 13, e0199244. [Google Scholar] [CrossRef]
- Pandurangan, M.; Enkhtaivan, G.; Mistry, B.; Chandrasekaran, M.; Noorzai, R.; Kim, D.H. Investigation of role of aspartame on apoptosis process in HeLa cells -->. Saudi J Biol Sci 2016, 23, 503–506. [Google Scholar] [CrossRef]
- Schiano, C.; Grimaldi, V.; Franzese, M.; Fiorito, C.; De Nigris, F.; Donatelli, F.; Soricelli, A.; Salvatore, M.; Napoli, C. Non-nutritional sweeteners effects on endothelial vascular function. Toxicology in Vitro 2020, 62, 104694. [Google Scholar] [CrossRef]
- Pandurangan, M.; Enkhtaivan, G.; Kim, D.H. Cytotoxic effects of aspartame on human cervical carcinoma cells. Toxicol Res (Camb) 2016, 5, 45–52. [Google Scholar] [CrossRef]
- Pórtela, G.S.; Azoubel, R.; Batigália, F. Effects of aspartame on maternal-fetal and placental weights, length of umbilical cord and fetal liver: a kariometric experimental study. Int J Morphol 2007, 25, 549–554. [Google Scholar] [CrossRef]
- Shalaby, A.M.; Ibrahim, M.; Aboregela, A.M. Effect of aspartame on the placenta of adult albino rat. A histological and immunohistochemical study. Ann Anat 2019, 224, 133–141. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, S.; Pira, E.; Ferrara, M.; Bugiani, M.; Papaleo, A.; Albera, R.; Palmi, S. Neurotoxic effects of aluminium among foundry workers and Alzheimer’s disease. Neurotoxicology 2002, 23, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969, 164, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W. Excitotoxic food additives--relevance of animal studies to human safety. Neurobehav Toxicol Teratol 1984, 6, 455–462. [Google Scholar]
- Choudhary, A.K.; Lee, Y.Y. Neurophysiological symptoms and aspartame: What is the connection? Nutr Neurosci 2017, 21, 306–316. [Google Scholar] [CrossRef]
- Pan-Hou, H.; Suda, Y.; Ohe, Y.; Sumi, M.; Yoshioka, M. Effect of aspartame on N-methyl-D-aspartate-sensitive L-[3H] glutamate binding sites in rat brain synaptic membranes. Brain Res 1990, 520, 351–353. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Salem, N.A.; Hussein, J.S. Effect of aspartame on oxidative stress and monoamine neurotransmitter levels in lipopolysaccharide-treated mice. Neurotoxicity research 2012, 21, 245–255. [Google Scholar] [CrossRef]
- Abhilash, M.; Sauganth Paul, M.; Varghese, M.V.; Nair, R.H. Long-term consumption of aspartame and brain antioxidant defense status. Drug Chem Toxicol 2013, 36, 135–140. [Google Scholar] [CrossRef]
- Alkafafy, M.E.-S.; Ibrahim, Z.S.; Ahmed, M.M.; El-Shazly, S.A. Impact of aspartame and saccharin on the rat liver: Biochemical, molecular, and histological approach. Int J Immunopathol Pharmacol 2015, 28, 247–255. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Rathinasamy, S. Effect of chronic exposure to aspartame on oxidative stress in the brain of albino rats. J Biosci 2012, 37, 679–688. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Rathinasamy, S. Biochemical responses and mitochondrial mediated activation of apoptosis on long-term effect of aspartame in rat brain. Redox Biol 2014, 2, 820–831. [Google Scholar]
- Mourad, I.M.; Noor, N.A. Aspartame (a widely used artificial sweetener) and oxidative stress in the rat cerebral cortex. Int J Pharm Biomed Sci 2011, 2, 4–10. [Google Scholar]
- Christian, B.; McConnaughey, K.; Bethea, E.; Brantley, S.; Coffey, A.; Hammond, L.; Harrell, S.; Metcalf, K.; Muehlenbein, D.; Spruill, W. Chronic aspartame affects T-maze performance, brain cholinergic receptors and Na+, K+-ATPase in rats. Pharmacol Biochem Behav 2004, 78, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Simintzi, I.; Schulpis, K.H.; Angelogianni, P.; Liapi, C.; Tsakiris, S. L-Cysteine and glutathione restore the reduction of rat hippocampal Na+, K+-ATPase activity induced by aspartame metabolites. Toxicology 2007, 237, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Choi, Y.-B.; Sucher, N.J.; Chen, H.-S.V. Neuroprotective versus neurodestructive effects of NO-related species. Biofactors 1998, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Naito, Y. What is oxidative stress ? Japan Medical Association Journal 2000, 124, 1549–1553. [Google Scholar]
- Iyaswamy, A.; Rathinasamy, S. Neurobehavioral changes and activation of neurodegenerative apoptosis on long-term consumption of aspartame in the rat brain. J Nutr Intermed Metabol 2015, 2, 76–85. [Google Scholar]
- Park, S.; Sethi, S.; Bouret, S.G. Non-nutritive Sweeteners Induce Hypothalamic ER Stress Causing Abnormal Axon Outgrowth. Front Endocrinol (Lausanne) 2019, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Choi, S.H.; Piao, Y.; Kim, S.; Lee, Y.J.; Kim, H.S.; Jeong, S.J.; Rah, J.C.; Seo, J.H.; Lee, J.H.; et al. Glutamate and aspartate impair memory retention and damage hypothalamic neurons in adult mice. Toxicol Lett 2000, 115, 117–125. [Google Scholar] [CrossRef]
- Lee, J.; Chung, M.K.; Ro, J.Y. Activation of NMDA receptors leads to phosphorylation of TRPV1 S800 by protein kinase C and A-Kinase anchoring protein 150 in rat trigeminal ganglia. Biochem Biophys Res Commun 2012, 424, 358–363. [Google Scholar] [CrossRef]
- Lee, J.; Saloman, J.L.; Weiland, G.; Auh, Q.S.; Chung, M.K.; Ro, J.Y. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain 2012, 153, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Nikfar, S.; Abdoli, N. Potentiation by nitric oxide synthase inhibitor and calcium channel blocker of aspartame-induced antinociception in the mouse formalin test. Fundam Clin Pharmacol 2001, 15, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Collison, K.S.; Makhoul, N.J.; Zaidi, M.Z.; Saleh, S.M.; Andres, B.; Inglis, A.; Al-Rabiah, R.; Al-Mohanna, F.A. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One 2012, 7, e31570. [Google Scholar] [CrossRef]
- Errico, F.; Nisticò, R.; Palma, G.; Federici, M.; Affuso, A.; Brilli, E.; Topo, E.; Centonze, D.; Bernardi, G.; Bozzi, Y.; et al. Increased levels of d-aspartate in the hippocampus enhance LTP but do not facilitate cognitive flexibility. Mol Cell Neurosci 2008, 37, 236–246. [Google Scholar] [CrossRef]
- Magalhães, P.C.G.; Abadie-Guedes, R.; da Costa Mendonça, M.A.B.; de Souza, A.D.; Guedes, R.C.A. Behavioral and electrophysiological brain effects of aspartame on well-nourished and malnourished rats. Metab Brain Dis 2019, 34, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Goerss, A.L.; Wagner, G.C.; Hill, W.L. Acute effects of aspartame on aggression and neurochemistry of rats. Life Sci 2000, 67, 1325–1329. [Google Scholar] [CrossRef]
- Lindseth, G.N.; Coolahan, S.E.; Petros, T.V.; Lindseth, P.D. Neurobehavioral effects of aspartame consumption. Res Nurs Health 2014, 37, 185–193. [Google Scholar] [CrossRef]
- Affairs, C.o.S. Aspartame. Review of safety issues. Council on Scientific Affairs. JAMA 1985, 254, 400–402. [Google Scholar]
- Malkan, S. Aspartame: Decades of Science Point to Serious Health Risks. 2022.
- Millstone, E.P.; Dawson, E. EFSA's toxicological assessment of aspartame: was it even-handedly trying to identify possible unreliable positives and unreliable negatives? Arch Public Health 2019, 77, 019–0355. [Google Scholar] [CrossRef]
- Lim, U.; Subar, A.F.; Mouw, T.; Hartge, P.; Morton, L.M.; Stolzenberg-Solomon, R.; Campbell, D.; Hollenbeck, A.R.; Schatzkin, A. Consumption of aspartame-containing beverages and incidence of hematopoietic and brain malignancies. Cancer Epidemiol Biomarkers Prev 2006, 15, 1654–1659. [Google Scholar] [CrossRef]
- Spiers, P.A.; Sabounjian, L.; Reiner, A.; Myers, D.K.; Wurtman, J.; Schomer, D.L. Aspartame: neuropsychologic and neurophysiologic evaluation of acute and chronic effects. Am J Clin Nutr 1998, 68, 531–537. [Google Scholar] [CrossRef]
- Solomi, L.; Rees, G.A.; Redfern, K.M. The acute effects of the non-nutritive sweeteners aspartame and acesulfame-K in UK diet cola on glycaemic response. Int J Food Sci Nutr 2019, 70, 894–900. [Google Scholar] [CrossRef]
- Torii, K.; Mimura, T.; Takasaki, Y.; Ichimura, M. Dietary aspartame with protein on plasma and brain amino acids, brain monoamines and behavior in rats. Physiol Behav 1986, 36, 765–771. [Google Scholar] [CrossRef]
- Sharma, R.P.; Coulombe, R.A., Jr. Effects of repeated doses of aspartame on serotonin and its metabolite in various regions of the mouse brain. Food Chem Toxicol. 1987, 25, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Vences-Mejía, A.; Labra-Ruíz, N.; Hernández-Martínez, N.; Dorado-González, V.; Gómez-Garduño, J.; Pérez-López, I.; Nosti-Palacios, R.; Camacho Carranza, R.; Espinosa-Aguirre, J.J. The effect of aspartame on rat brain xenobiotic-metabolizing enzymes. Hum Exp Toxicol 2006, 25, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Abdel Salam, O.M.; Shaffie, N.M.; Sleem, A.A. Hepatoprotective effects of citric acid and aspartame on carbon tetrachloride-induced hepatic damage in rats. EXCLI J 2009, 8, 41–49. [Google Scholar]
- Kim, J.Y.; Seo, J.; Cho, K.H. Aspartame-fed zebrafish exhibit acute deaths with swimming defects and saccharin-fed zebrafish have elevation of cholesteryl ester transfer protein activity in hypercholesterolemia. Food Chem Toxicol 2011, 49, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Rathinasamy, S. Effects of aspartame on hsp70, bcl-2 and bax expression in immune organs of Wistar albino rats. J Biomed Res 2016, 30, 427–435. [Google Scholar]
- Gul, S.S.; Hamilton, A.R.; Munoz, A.R.; Phupitakphol, T.; Liu, W.; Hyoju, S.K.; Economopoulos, K.P.; Morrison, S.; Hu, D.; Zhang, W.; et al. Inhibition of the gut enzyme intestinal alkaline phosphatase may explain how aspartame promotes glucose intolerance and obesity in mice. Appl Physiol Nutr Metab 2017, 42, 77–83. [Google Scholar] [CrossRef]
- Helal, E.G.; Abdelaziz, M.A.; Taha, N.M.; El-Gama, M.S. The influence of acesulfame-k and aspartame on some physiological parameters in male albino rats. The Egyptian Journal of Hospital Medicine 2019, 75, 1976–1981. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Noye Tuplin, E.; Chleilat, F.; et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut 2020, 69, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Fareed, S.A.; Mostafa, H.E. Could aspartame exacerbate caffeine effects on renal maturation in rat's offspring? A biochemical and histological study. Birth Defects Res 2021, 113, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Ghoshal, S.; Mukherjee, A. Genotoxicity testing of low-calorie sweeteners: aspartame, acesulfame-K, and saccharin. Drug Chem Toxicol 2008, 31, 447–457. [Google Scholar] [CrossRef]
- Fountain, S.B.; Hennes, S.K.; Teyler, T.J. Aspartame exposure and in vitro hippocampal slice excitability and plasticity. Fundam Appl Toxicol 1988, 11, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Simintzi, I.; Schulpis, K.H.; Angelogianni, P.; Liapi, C.; Tsakiris, S. The effect of aspartame on acetylcholinesterase activity in hippocampal homogenates of suckling rats. Pharmacol Res 2007, 56, 155–159. [Google Scholar] [CrossRef]
- Kashanian, S.; Khodaei, M.M.; Kheirdoosh, F. In vitro DNA binding studies of Aspartame, an artificial sweetener. J Photochem Photobiol B 2013, 120, 104–110. [Google Scholar] [CrossRef]
- Çadirci, K.; Tozlu, Ö.Ö.; Türkez, H.; Mardinoğlu, A. The in vitro cytotoxic, genotoxic, and oxidative damage potentials of the oral artificialsweetener aspartame on cultured human blood cells. Turkish J Med Sci 2020, 50, 448–454. [Google Scholar] [CrossRef]
- Manion, C.V.; Howard, J.; Ogle, B.; Parkhurst, J.; Edmundson, A. Aspartame effect in sickle cell anemia. Clin Pharmacol Ther 2001, 69, 346–355. [Google Scholar] [CrossRef]
- Rios, D.; Ionta, F.Q.; Rebelato, R.; Jordão, M.C.; Wang, L.; Magalhães, A.C.; Honório, H.M. The effect of aspartame and pH changes on the erosive potential of cola drinks in bovine enamel: An in vitro study. J Clin Exp Dent 2018, 10, e933–e937. [Google Scholar] [CrossRef]
- Pandurangan, M.; Park, J.; Kim, E. Aspartame downregulates 3T3-L1 differentiation. In Vitro Cell Dev Biol Anim 2014, 50, 851–857. [Google Scholar] [CrossRef]
- Sun, D.; Liu, L.; Mao, S.; Zhu, W.; Liu, J. Aspartame supplementation in starter accelerates small intestinal epithelial cell cycle and stimulates secretion of glucagon-like peptide-2 in pre-weaned lambs. J Anim Physiol Anim Nutr (Berl) 2019, 103, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Chontzopoulou, E.; Papaemmanouil, C.D.; Chatziathanasiadou, M.V.; Kolokouris, D.; Kiriakidi, S.; Konstantinidi, A.; Gerogianni, I.; Tselios, T.; Kostakis, I.K.; Chrysina, E.D.; et al. Molecular investigation of artificial and natural sweeteners as potential anti-inflammatory agents. J Biomol Struct Dyn 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





