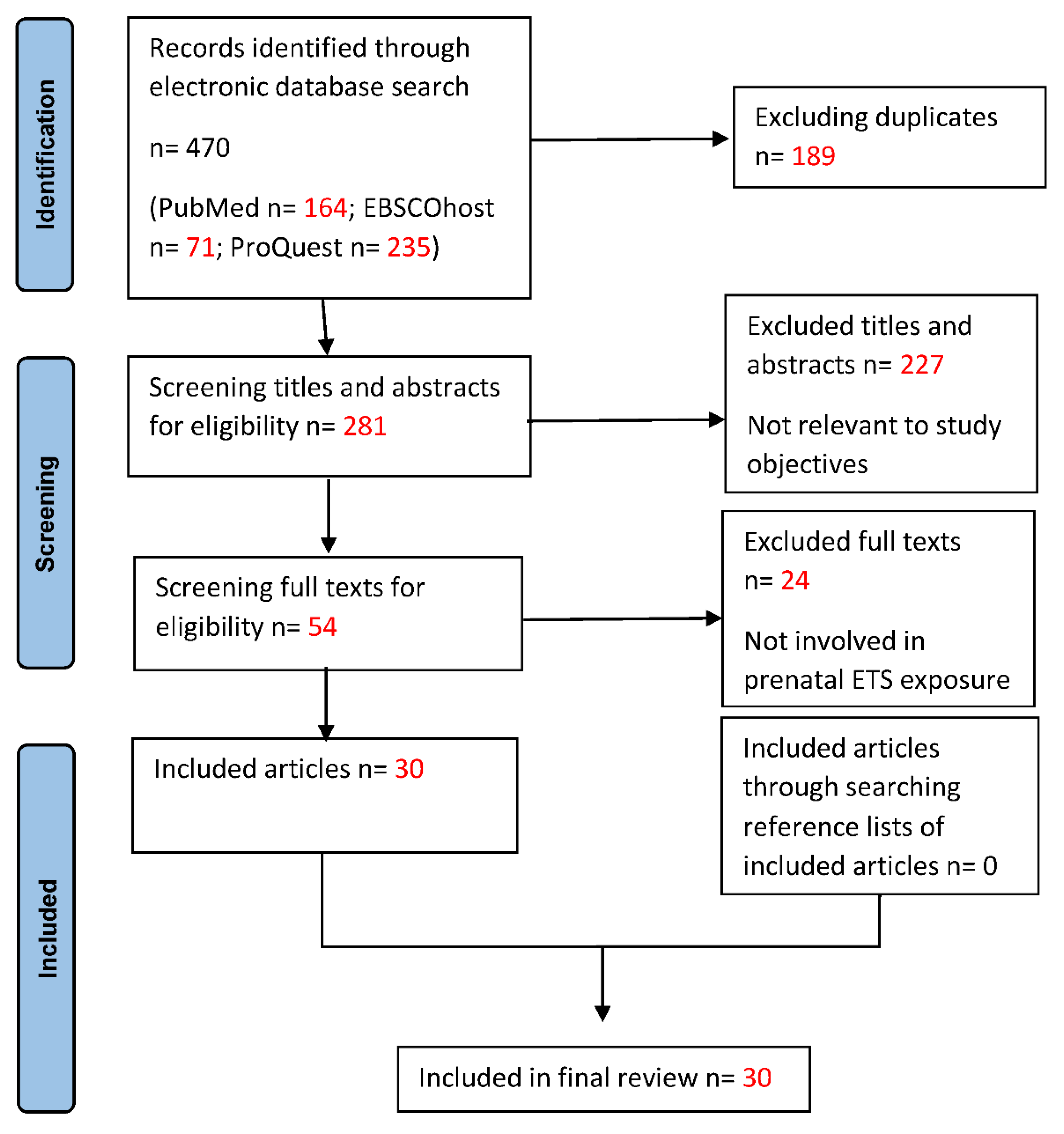
Submitted:
04 July 2023
Posted:
06 July 2023
You are already at the latest version
Abstract
Keywords:
1. Background
2. Materials and Methods
Eligibility Criteria
Type of Resource
Search Strategy
Selection of Eligible Studies
Data Charting
Critical Appraisal
Synthesis of Results
Characteristics and Quality of the Included Studies
Health Outcomes
Hypertension
Foetal and Children’s Development
Birth Outcomes
Orofacial Clefts (OFCs)
Neural Tube Defects (NTDs)
Congenital Heart Disease (CHD)
Developmental Coordination Disorder (DCD)
Developmental Delay
Behavioural Disorders
ADHD
Autism Behaviour
Other Disorders
Respiratory Outcomes
Others
Astigmatism
Sleep Disorder
3. Discussion
4. Limitation
5. Conclusion
References
- Ahmed Sakran, K., Mutahar Abotaleb, B., Khaled Al-Rokhami, R., Hsieh, T.-yen, Al-Wesabi, M. A., Mohammed, A. A., Al-Sharani, H. M., Shi, P., & He, D. (2022). Analysis of environmental exposures for nonsyndromic cleft lip and/or palate: a case-control study. Iranian Journal of Public Health, (20220313). [CrossRef]
- Chao, M. R., Cooke, M. S., Kuo, C. Y., Pan, C. H., Liu, H. H., Yang, H. J., Chen, S. C., Chiang, Y. C., & Hu, C. W. (2018). Children are particularly vulnerable to environmental tobacco smoke exposure: Evidence from biomarkers of tobacco-specific nitrosamines and oxidative stress. Environment International, 120, 238–245. [CrossRef]
- Chen, H., Zhang, Y., Zhang, L., Liu, J., Jin, L., Ren, A., & Li, Z. (2023). Indoor air pollution from coal combustion and tobacco smoke during the periconceptional period and risk for neural tube defects in offspring in five rural counties of shanxi province, china, 2010-2016. Environment International, 171. [CrossRef]
- Chen, J., Li, X., & Fang, P. (2020). Influence of family resources on secondhand smoking in pregnant women: A cross-sectional study in the border and minority urban areas of Northwest China. BMC Pregnancy and Childbirth, 20(1), 642. [CrossRef]
- Chen, M. M., Chiu, C.-H., Yuan, C.-P., Liao, Y.-C., & Guo, S.-E. (2020). Influence of Environmental Tobacco Smoke and Air Pollution on Fetal Growth: A Prospective Study. International Journal of Environmental Research and Public Health, 17(15), E5319. [CrossRef]
- Chen, X., Huang, L., Zhong, C., Li, Q., Chen, R., Sun, G., Jin, Z., Yang, X., Hao, L., Yang, H., & Yang, N. (2021). Association between environmental tobacco smoke before and during pregnancy and the risk of adverse birth outcomes: a birth cohort study in Wuhan, China. Environmental Science and Pollution Research, 28(21), 27230–27237. [CrossRef]
- Dai, S., & Chan, K. C. C. (2020). Household environmental tobacco smoke exposure in healthy young children in Hong Kong: Prevalence and risk factors. PLOS ONE, 15(1), e0227733. [CrossRef]
- Deng, C., Pu, J., Deng, Y., Xie, L., Yu, L., Liu, L., Guo, X., Sandin, S., Liu, H., & Dai, L. (2022). Association between maternal smoke exposure and congenital heart defects from a case–control study in china. Scientific Reports, 12(1). [CrossRef]
- DiGiacomo, S. I., Jazayeri, M.-A., Barua, R. S., & Ambrose, J. A. (2018). Environmental Tobacco Smoke and Cardiovascular Disease. International Journal of Environmental Research and Public Health, 16(1), E96. [CrossRef]
- Dong, G.-H., Wang, D., Yang, Z.-H., Zhang, P.-F., Ren, W.-H., Zhao, Y.-D., & He, Q.-C. (2011). Gender-specific differences in effects of prenatal and postnatal environmental tobacco smoke exposure on respiratory symptoms in 23,474 children with and without allergic predisposition: results from 25 districts of northeast China. International Journal of Environmental Health Research, 21(3), 173–188. [CrossRef]
- Greenhalgh, E. M., Scollo, M. M. & Winstanley, M. H. (2022). Tobacco in Australia: Facts and issues. Melbourne: Cancer Council Victoria. www.TobaccoInAustralia.org.au.
- He, Y., Luo, R., Wang, T., Gao, J., & Liu, C. (2018). Prenatal Exposure to Environmental Tobacco Smoke and Early Development of Children in Rural Guizhou Province, China. International Journal of Environmental Research and Public Health, 15(12), E2866. [CrossRef]
- Huang, L., Tian, F.-Y., Fan, L., He, Y.-H., Peng, D., Xie, C., Tao, L., Yuan, S.-X., Jia, D.-Q., & Chen, W.-Q. (2020). Appetite during the second and third trimesters mediates the impact of prenatal environmental tobacco smoke exposure on symmetric full-term low birth weight. The Journal of Maternal-Fetal & Neonatal Medicine, 33(9), 1544–1553. [CrossRef]
- Hu, L.-W., Yang, M., Chen, S., Shah, K., Hailegiorgis, Y., Burgens, R., Vaughn, M., Huang, J., Xaverius, P., Paul, G., Morawska, L., Lu, T., Lin, S., Zhong, S.-Q., Kong, M.-L., Xie, Y.-Q., Hao, Y.-T., Zeng, X.-W., Qian, Z., & Dong, G.-H. (2017). Effects of in utero and Postnatal Exposure to Secondhand Smoke on Lung Function by Gender and Asthma Status: The Seven Northeastern Cities (SNEC) Study. Respiration, 93(3), 189–197. [CrossRef]
- Lee, N. L., Samet, J. M., Yang, G., Zhou, M., Yang, J., Correa, A., & Lees, P. S. J. (2012). Prenatal Secondhand Smoke Exposure and Infant Birth Weight in China. International Journal of Environmental Research and Public Health, 9(10), 3398–3420. 10. [CrossRef]
- Lee, S. L., Lam, T. H., Leung, T. H., Wong, W. H. S., Schooling, M., Leung, G. M., & Lau, Y. L. (2012). Foetal exposure to maternal passive smoking is associated with childhood asthma, allergic rhinitis, and eczema. The Scientific World Journal, 2012, 542983. [CrossRef]
- Leonardi-Bee, J., Britton, J., & Venn, A. (2011). Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: A meta-analysis. Pediatrics, 127(4), 734–741. [CrossRef]
- Leung, C. Y., Leung, G. M., & Schooling, C. M. (2015). Early second-hand smoke exposure and child and adolescent mental health: evidence from Hong Kong’s ‘Children of 1997’ birth cohort. Addiction, 110(11), 1811–1824. [CrossRef]
- Li, C.-G., Yang, G.-Y., Schmid, K. L., Huang, L.-H., He, G.-H., Liu, L., Ruan, Z.-L., & Chen, W.-Q. (2019). Associations between Environmental Tobacco Smoke Exposure in Early Life and Astigmatism among Chinese Preschool Children. International Journal of Environmental Research and Public Health, 16(19), E3725. [CrossRef]
- Lin, L.-Z., Xu, S.-L., Wu, Q.-Z., Zhou, Y., Ma, H.-M., Chen, D.-H., Chen, G.-B., Yu, H.-Y., Yang, B.-Y., Zeng, X.-W., Hu, L.-W., & Dong, G.-H. (2021). Association of Prenatal, Early Postnatal, or Current Exposure to Secondhand Smoke With Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Network Open, 4(5), e2110931. [CrossRef]
- Lin, L.-Z., Xu, S.-L., Wu, Q.-Z., Zhou, Y., Ma, H.-M., Chen, D.-H., Dong, P.-X., Xiong, S.-M., Shen, X.-B., Zhou, P.-E., Liu, R.-Q., Chen, G., Yu, H.-Y., Yang, B.-Y., Zeng, X.-W., Hu, L.-W., Zhou, Y.-Z., & Dong, G.-H. (2021). Exposure to second-hand smoke during early life and subsequent sleep problems in children: a population-based cross-sectional study. Environmental Health, 20(1), 127. [CrossRef]
- Lin, Q., Xiang-Yu, H., Xiao-Na, Y., Guo-Min, W., Sun, D., Xian, D.-X., Fan, L., Jiang, H., Jin, J., Yu, J., Chuan-An, W., & Wei-Qing, C. (2017). Prenatal Exposure to Environmental Tobacco Smoke and Hyperactivity Behavior in Chinese Young Children. International Journal of Environmental Research and Public Health, 14(10), Article 10. [CrossRef]
- Li, Q., Hsia, J., & Yang, G. (2011). Prevalence of Smoking in China in 2010. New England Journal of Medicine, 364(25), 2469–2470. [CrossRef]
- Liu, J., Leung, P. W. L., McCauley, L., Ai, Y., & Pinto-Martin, J. (2013). Mother’s environmental tobacco smoke exposure during pregnancy and externalizing behavior problems in children. Neurotoxicology, 34, 167–174. [CrossRef]
- Liu, W., Huang, C., Cai, J., Wang, X., Zou, Z., & Sun, C. (2018). Household environmental exposures during gestation and birth outcomes: A cross-sectional study in Shanghai, China. Science of The Total Environment, 615, 1110–1118. [CrossRef]
- Liu, X.-C., Strodl, E., Huang, L.-H., Hu, B.-J., & Chen, W.-Q. (2022). Effect of prenatal exposure to household air pollution from multiple sources on risk of preterm birth. Atmosphere, 13(12), 2022–2022. [CrossRef]
- Öberg, M., Jaakkola, M. S., Woodward, A., Peruga, A., & Prüss-Ustün, A. (2011). Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. The Lancet, 377(9760), 139–146. [CrossRef]
- Pereira, P. P. da S., Da Mata, F. A. F., Figueiredo, A. C. G., de Andrade, K. R. C., & Pereira, M. G. (2017). Maternal Active Smoking During Pregnancy and Low Birth Weight in the Americas: A Systematic Review and Meta-analysis. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 19(5), 497–505. [CrossRef]
- Peters, M. D. J., Godfrey, C. M., Khalil, H., McInerney, P., Parker, D., & Soares, C. B. (2015). Guidance for conducting systematic scoping reviews. JBI Evidence Implementation, 13(3), 141–146. [CrossRef]
- Pi, X., Li, Z., Jin, L., Liu, J., Zhang, Y., Zhang, L., Wang, L., & Ren, A. (2018). Secondhand smoke during the periconceptional period increases the risk for orofacial clefts in offspring. Paediatric and Perinatal Epidemiology, 32(5), 423–427. [CrossRef]
- Ren, S., Xie, S., Li, X., Li, G., Wang, Y., Liu, W., & Wang, L. (2020). The association between maternal exposure to secondhand smoke during pregnancy and their children’s cerebral palsy, Shandong, China. Tobacco Induced Diseases, 18, 87. [CrossRef]
- Rethlefsen, M. L., & Page, M. J. (2022). PRISMA 2020 and PRISMA-S: Common questions on tracking records and the flow diagram. Journal of the Medical Library Association : JMLA, 110(2), 253. [CrossRef]
- Sabbagh, H. J., Hassan, M. H. A., Innes, N. P. T., Elkodary, H. M., Little, J., & Mossey, P. A. (2015). Passive smoking in the etiology of non-syndromic orofacial clefts: A systematic review and meta-analysis. PloS One, 10(3), e0116963. [CrossRef]
- Snodgrass, A. M., Tan, P. T., Soh, S. E., Goh, A., Shek, L. P., van Bever, H. P., Gluckman, P. D., Godfrey, K. M., Chong, Y. S., Saw, S. M., Kwek, K., Teoh, O. H., & GUSTO Study Group. (2016). Tobacco smoke exposure and respiratory morbidity in young children. Tobacco Control, 25(e2), e75–e82. [CrossRef]
- Song, X., Li, Q., Diao, J., Li, J., Li, Y., Zhang, S., Zhao, L., Chen, L., Wei, J., Shu, J., Liu, Y., Sun, M., Huang, P., Wang, T., & Qin, J. (2022). Association of mthfd1 gene polymorphisms and maternal smoking with risk of congenital heart disease: a hospital-based case-control study. Bmc Pregnancy and Childbirth, 22(1). [CrossRef]
- The Joanna Briggs Institute. (2020). Template for scoping review protocols. https://jbi.global/scoping-review-network/resources.
- The Joanna Briggs Institute. (2015). The Joanna Briggs Institute Reviewers’ Manual 2015, Methodology for JBI scoping reviews. https://nursing.lsuhsc.edu/JBI/docs/ReviewersManuals/Scoping-.pdf.
- U.S. Department of Health and Human Services. (2014). The health consequences of smoking—50 years of progress: A report of the Surgeon General. https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf.
- Vanker, A., Gie, R. P., & Zar, H. J. (2017). The association between environmental tobacco smoke exposure and childhood respiratory disease: A review. Expert Review of Respiratory Medicine, 11(8), 661–673. [CrossRef]
- Wang, L., Deng, Y., Yang, Y., Liu, F., Xu, Q., Peng, Z., He, Y., Wang, Y., Xu, J., Zhang, H., Zhang, Y., Wang, Q., Shen, H., Zhang, Y., Yan, D., & Ma, X. (2022). Paternal smoking and preterm birth: a population-based retrospective cohort study among non-smoking women aged 20–49 years in rural china. Reproductive Health, 19(1). [CrossRef]
- Wang, R., Sun, T., Yang, Q., Yang, Q., Wang, J., Li, H., Tang, Y., Yang, L., & Sun, J. (2020). Low birthweight of children is positively associated with mother’s prenatal tobacco smoke exposure in Shanghai: A cross-sectional study. BMC Pregnancy and Childbirth, 20, 1–9.
- Wang, T., Chen, L., Ni, B., Sheng, X., Huang, P., Zhang, S., & Qin, J. (2022). Maternal pre-pregnancy/early-pregnancy smoking and risk of congenital heart diseases in offspring: a prospective cohort study in central china. Journal of Global Health, 12, 11009–11009. [CrossRef]
- Wang, Y., Hu, D., Chen, W., Xue, H., & Du, Y. (2019). Prenatal Tobacco Exposure Modulated the Association of Genetic variants with Diagnosed ADHD and its symptom domain in children: A Community Based Case-Control Study. Scientific Reports, 9(1), 4274. [CrossRef]
- World Health Organization. (2000). Air Quality Guidelines for Europe (2nd ed.). World Health Organization. Regional Office for Europe.
- Wu, M., Williams, G. J., Chen, G., Zhang, L., Hu, C., Dai, X., Du, W., & Hua, J. (2022). Prenatal second-hand smoke exposure and the risk of suspected developmental coordination disorder in preschoolers: a nationwide retrospective cohort study in china. Frontiers in Public Health, 10, 993471–993471. [CrossRef]
- World Health Organization. (2010). Global Adult Tobacco Survey (GATS) – China Fact Sheet 2010. https://www.tobaccofreekids.org/assets/global/pdfs/en/GATS_china_2010.pdf.
- Xiao, L., Jiang, Y., Zhang, J., & Parascandola, M. (2020). Secondhand Smoke Exposure among Nonsmokers in China. Asian Pacific Journal of Cancer Prevention: APJCP, 21(S1), 17–22. [CrossRef]
- Yang, J.-H., Strodl, E., Wu, C.-A., Yin, X.-N., Wen, G.-M., Sun, D.-L., Xian, D.-X., Chen, J.-Y., Chen, Y.-J., Chen, J., & Chen, W.-Q. (2021). Association between environmental tobacco smoke exposure in early life and autistic-like behaviors in Chinese preschoolers. Journal of Psychosomatic Research, 152, 110680. [CrossRef]
- Zhang, H., Yu, L., Wang, Q., Tao, Y., Li, J., Sun, T., Zhang, Y., & Zhang, H. (2020). In utero and postnatal exposure to environmental tobacco smoke, blood pressure, and hypertension in children: The Seven Northeastern Cities study. International Journal of Environmental Health Research, 30(6), 618–629. [CrossRef]
- Zhou, S., Rosenthal, D. G., Sherman, S., Zelikoff, J., Gordon, T., & Weitzman, M. (2014). Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Current Problems in Pediatric and Adolescent Health Care, 44(8), 219–241. [CrossRef]
- Zhuge, Y., Qian, H., Zheng, X., Huang, C., Zhang, Y., Li, B., Zhao, Z., Deng, Q., Yang, X., Sun, Y., Zhang, X., & Sundell, J. (2020). Effects of parental smoking and indoor tobacco smoke exposure on respiratory outcomes in children. Scientific Reports, 10(1), 4311. [CrossRef]
| Authors | Publication Year | Location | Aims/Purpose | Methodology | Sample Size | Study Population | Data Collection and Measurement | Study Quality |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. | 2020 | Liaoning Province | Evaluate the association of ETS exposure with hypertension and blood pressure (BP) in children. | Cross-sectional study | 9354 | School-aged children (5-17years) | Questionnaire | Moderate |
| Chen et al. | 2020 | Taiwan (Chaiyi) | Evaluated the influences of air quality, including ETS and particulate matter (PM), on foetal development. | Longitudinal correlation study | 74 | Non-smoking pregnant women | Questionnaire & Laboratory result | High |
| Wang et al. | 2020 | Shanghai | Explore if the LBW in children is positively associated with mothers’ prenatal cigarette smoke exposure. | Cross-sectional study | 8586 | Kindergarten children | Interview & Questionnaire | Moderate |
| Liu et al. | 2013 | Jiangsu Province (Changzhou) | Examine the association between maternal ETS exposure during pregnancy and child behaviour problems. | Cross-sectional study | 646 | Mother-child pairs | Questionnaire & Evaluation tool | Moderate |
| He et al. | 2018 | Guizhou Province | Examines the association between prenatal exposure to ETS and the development of children in their first two years of life. | Cross-sectional study | 446 | Children | Questionnaire | Moderate |
| Lin et al. | 2017 | Shenzhen City | Examine the association between prenatal ETS exposure and hyperactivity behaviours in young children. | Cross-sectional study | 21,243 | Preschool children | Questionnaire & Evaluation tool | Moderate |
| Lee, Samet, et al. | 2012 | Beijing and Changchun Province | Examine the magnitude of association between maternal SHS exposure during pregnancy and reduction in infant birth weight in China. | Cross-sectional study | 2770 | Non-smoking postpartum women | Interview & Questionnaire | Moderate |
| Wang et al. | 2019 | Liuzhou City | Test the hypothesis that prenatal tobacco smoking exposure (PSE) could modulate the association of genetic variants with ADHD. | Case-control study | 401 (168 cases & 233 controls) | Children aged 6-12years | Interview & Questionnaire | High |
| Pi et al. | 2018 | Shanxi Province | Examine whether exposure to SHS during the periconceptional period among nonsmoking women is associated with an increased risk for orofacial clefts (OFCs) in offspring. | Case-control study | 1660 (240 cases & 1420 controls) | Newborns | Interview & Questionnaire | Moderate |
| Ren et al. | 2020 | Shandong Province | Assessed the association between maternal exposure to SHS during pregnancy and children’s cerebral palsy (CP). | Cross-sectional study | 5067 | Mother-child pairs | Questionnaire | Moderate |
| Chen et al. | 2021 | Wuhan City | Clarify the association of ETS before and during pregnancy with the risk of adverse birth outcomes. | Cohort study | 7,147 | Mothers-infant pairs | Questionnaire & Delivery records | High |
| Dong et al. | 2011 | Liaoning Province | Assess the interaction of ETS exposure and allergic predisposition regarding respiratory health. | Cross-sectional study | 23,474 | Children from elementary schools | Questionnaire | Moderate |
| Hu et al. | 2017 | Liaoning Province | Investigate whether gender or asthma status modifies the association of SHS exposure with lung function. | Cross-sectional study | 6,740 | School-aged children | Questionnaire & Electronic spirometers | Moderate |
| Lee, Lam, et al. | 2012 | Hong Kong | Examine the association of foetal exposure to maternal passive smoking with childhood asthma, allergic rhinitis, and eczema. | Cross-sectional study | 7,393 | Children≤ 14Y | Questionnaire, interview & evaluation tool | Moderate |
| Lin, Xu, Wu, Zhou, Ma, Chen, Chen, et al. | 2021 | Liaoning Province | Evaluate the associations of prenatal, early postnatal, or current SHS exposure with ADHD symptoms and subtypes. | Cross-sectional study | 45,562 | School-aged children | Questionnaire & Evaluation tool | Moderate |
| Liu et al. | 2018 | Shanghai | Investigate the associations of household environmental factors during gestation with preterm birth, low birth weight, term low birth weight, and small for gestational age. | Cross-sectional study | 13,335 | Children aged 4-6 years | Questionnaire | Moderate |
| Huang et al. | 2020 | Guangdong Province (Foshan & Shenzhen) | Examine the relationship between prenatal environmental tobacco smoke (ETS) exposure and full-term low birth weight (FT-LBW). | Case-control study | 1632 (243 cases & 1389 controls) | Mothers-infant pairs | Interview & Medical records | High |
| Leung et al. | 2015 | Hong Kong | Estimated the associations of early SHS exposure during the prenatal and postnatal periods with several aspects of adolescent mental health. | Cohort study | 7,914 | “Children of 1997”birth cohort | Questionnaire & Evaluation tool | High |
| Li et al. | 2019 | Shenzhen City (Longhua) | Investigate the association between ETS during early life and early-onset astigmatism. | Cross-sectional study | 27,890 | Preschool children | Questionnaire & Medical diagnosis | Moderate |
| Lin, Xu, Wu, Zhou, Ma, Chen, Dong, et al. | 2021 | Liaoning Province | Evaluate the associations of early-life SHS exposure with sleep problems in children. | Cross-sectional study | 45,562 | School-aged children | Questionnaire & Evaluation tool | Moderate |
| Yang et al. | 2021 | Shenzhen City (Longhua) | Explore the association between children's exposure to ETS in early life and autistic-like behaviours. | Cross-sectional study | 65,243 | Preschool children | Questionnaire & Evaluation tool | Moderate |
| Zhuge et al. | 2020 | Urumqi, Taiyuan, Beijing, Nanjing, Shanghai, Wuhan, Chongqing, &Changsha | Analyse associations of ETS with dry night cough, croup, pneumonia, and the frequent common cold. | Cross-sectional study | 41,176 | Children aged 3-8 years | Questionnaires | Moderate |
| Liu et al. | 2022 | Shenzhen City (Longhua) | Explore the independent and joint effects of prenatal exposure to multiple household air pollution (HAP) sources on PTB | Cross-sectional study | 63,038 | Mother–child pairs | Questionnaire | Moderate |
| Chen et al. | 2023 | Shanxi Province (Pingding, Xiyang, Shouyang, Taigu, and Zezhou). |
Investigated the impact of maternal exposure to indoor air pollution from coal combustion and tobacco smoke on the risk for neural tube defects (NTDs) | Case-control study | 739 (222 cases & 517 controls) | women (during the periconceptional period) | Questionnaire & Evaluation tool | High |
| Wang, Deng et al. | 2022 | Rural China | Evaluate the association of paternal smoking and preterm birth (PTB). | Cohort study |
5,298,043 |
reproductive-aged couples | Questionnaire & Medical diagnosis | High |
| Song et al. | 2022 | Changsha, Hunan Province | Examined the role of MTHFD1 gene and maternal smoking on infant CHD risk and investigated their interaction effects in Chinese populations. | Case-control study | 968 (464 cases & 504 controls) | Mother–child pairs | Questionnaire & Laboratory result | Moderate |
| Wu et al. | 2022 | Mainland China (nationwide) | Investigate the association between prenatal SHS exposure and suspected DCD in preschoolers | Cross-sectional study | 149,005 | Preschoolers | Questionnaire & Evaluation tool | Moderate |
| Ahmed Sakran et al. | 2022 | Gansu Province | Identify the relationship between environmental factors and nonsyndromic cleft lip and/or palate (NSCL/P) in Northwest China | Case-control study | 1260 (600 cases & 660 controls) | Children and their parents | Interview | High |
| Deng et al. | 2022 | West China Second University Hospital | Examine the association of maternal ETS with foetal CHDs and the potentially moderating effect by maternal hazardous and noxious substances (HNS), periconceptional folate intake and paternal smoking. | Case-control study | 1629 (749 cases & 880 controls) | singleton pregnant women | Questionnaire, interview & Medical diagnosis | High |
| Wang, Chen et al. | 2022 | Central China | Estimate the associations of maternal active and passive smoking during the pre-pregnancy/early-pregnancy period with CHDs as well as its common phenotypes in offspring | Cohort study | 49158 | Pregnant women between the 8th and 14th weeks of gestation |
Interview & Medical diagnosis | High |
| Criterion | Total Score | Percentage (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (Year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Cross-sectional study | |||||||||||||
| Zhang et al. (2020) | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | n/a | n/a | n/a | 6 | 55% |
| Wang et al. (2020) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Liu et al. (2013) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| He et al. (2018) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Lin et al. (2017) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Lee, Samet, et al. (2012) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Ren et al. (2020) | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | n/a | n/a | n/a | 6 | 55% |
| Dong et al. (2011) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | n/a | n/a | n/a | 6 | 55% |
| Hu et al. (2017) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Lee, Lam, et al. (2012) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | n/a | n/a | n/a | 6 | 55% |
| Lin, Xu, Wu, Zhou, Ma, Chen, Chen, et al (2021) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Liu et al. (2018) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Li et al. (2019) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | n/a | n/a | n/a | 6 | 55% |
| Lin, Xu, Wu, Zhou, Ma, Chen, Dong, et al. (2021) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Yang et al. (2021) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Zhuge et al. (2020) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | n/a | n/a | n/a | 6 | 55% |
| Liu et al. (2022) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | n/a | n/a | n/a | 6 | 55% |
| Wu et al. (2022) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | n/a | n/a | n/a | 7 | 64% |
| Case-control study | |||||||||||||
| Wang et al. (2019) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | 9 | 82% |
| Pi et al. (2018) | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | 8 | 73% |
| Huang et al. (2020) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | 9 | 82% |
| Chen et al. (2023) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | 9 | 82% |
| Song et al. (2022) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | 9 | 82% |
| Ahmed Sakran et al. (2022) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | 9 | 82% |
| Deng et al. (2022) | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | 9 | 82% |
| Cohort study | |||||||||||||
| Chen et al. (2020) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | n/a | 1 | 9 | 82% |
| Chen et al. (2021) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 | 82% |
| Leung et al. (2015) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | 82% |
| Wang, Deng et al. (2022) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 | 82% |
| Wang, Chen et al. (2022) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | n/a | 1 | 9 | 82% |
| Authors (Publication Year) | Main Results | Exposure Sources | Exposure Timepoint |
|---|---|---|---|
| 1. Hypertension | |||
| Zhang et al. (2020) | Significant associations were observed for hypertension with ETS exposure in utero, with current major ETS exposure from fathers or anyone, and with intensity of ETS exposure greater than 1 cigarette per day. For SBP, significant associations were only observed in children with major ETS exposure from father and with cigarettes smoking >10/day. | Household (SHS from father or anyone) | In utero and within children's first 2 years of life |
| 2. Foetal and children’s development | |||
| 2.1. Birth outcomes | |||
| Wang et al. (2020) | The mean birthweight was 167.7 g and 66.1 g lower in children born to mothers with prenatally FHS and SHS exposure compared with those children whose mother were not exposed, respectively. | Maternal passive and active smoking | Prenatal exposure |
| Huang et al. (2020) | Significant association between prenatal ETS exposure and FT-LBW. | Maternal SHS exposure at home, workplace & public places | During pregnancy |
| Chen et al. (2020) | ETS exposure decreased birth length by ≥1 cm, and potentially is an independent risk factor for foetal growth restriction. | Maternal SHS exposure at home, workplace & public places | During pregnancy |
| Chen et al. (2021) | Significant association between exposure to ETS during pregnancy with PTB, but not LBW or SGA births. | Maternal ETS exposure (indoor & outdoor) | Before and/or during pregnancy. |
| Liu et al. (2018) | Positive relation between paternal smoking during gestation and PB, LBW, and SGA, but not significant associations. | Household (paternal and maternal smoking) | During pregnancy |
| Lee, Samet, et al. (2012) | No deficit in mean birth weight was observed with exposure from all sources of SHS combined. | Maternal ETS exposure at home, workplace & public places | During pregnancy |
| Liu et al. (2022) | Prenatal exposure to ETS increased the risk of PTB and the PTB risk increased with the average level of daily ETS exposure |
Household (Maternal exposure) | Prenatal exposure |
| Wang, Deng et al. (2022) | Paternal smoking and preconception paternal smoking was independently positively associated with PTB risk. // The HRs of PTB also increased with the increment of paternal smoking and preconception paternal smoking categories | Paternal and maternal smoking | Preconception exposure |
| 2.2. Orofacial clefts | |||
| Pi et al. (2018) | Maternal SHS exposure during the periconceptional period increases the risk for OFCs in offspring among nonsmoking mothers, and there was a dose-response relationship. | Maternal SHS exposure at home & indoor public places | Prenatal exposure |
| Ahmed Sakran et al.(2022) | Maternal passive smoking was found to be a significant risk factor for NSCLP incidence. | Paternal and maternal smoking | the 1st trimester of gestation |
| 2.3. Neural tube defects (NTDs) | |||
| Chen et al. (2023) | Significant association between passive smoking and neural tube defects (NTDs) occurrence. // dosage respond// | Household | during the periconceptional period (1 month before to 2 months after conception) |
| 2.4. Congenital heart disease (CHD) | |||
| Song et al. (2022) | Increased risk of CHD in offspring whose mothers were exposed to secondhand smoke during 3 months before pregnancy and in the first trimester of pregnancy | At home and/or at work/school | during 3 months before pregnancy and in the first trimester of pregnancy |
| Deng et al. (2022) | Maternal exposure to ETS in first trimester was associated with increased risk of CHDs in a dose– response gradient. | Maternal exposure | in the first trimester of pregnancy |
| Wang, Chen et al. (2022) | Significantly higher risks of CHDs were detected in offspring exposed to maternal cigarette smoking in 3 months before pregnancy. // Maternal cigarette smoking in early pregnancy was also independently associated with risk of CHDs in offspring. | Maternal exposure | 3 months before pregnancy & in early pregnancy |
| 2.5. Developmental coordination disorder (DCD) | |||
| Wu et al. (2022) | Prenatal SHS exposure had the strong negative association with the total score of LDCDQ and increased the risk of suspected DCD | At home and/or at work | Prenatal & postnatal exposure |
| 2.6. Developmental delay | |||
| Ren et al. (2020) | Children born to mothers exposed to SHS during pregnancy had a higher risk of CP, and the risk increased with exposure time. | Maternal SHS exposure | Prenatal exposure |
| He et al. (2018) | Prenatal ETS exposure was associated with lower cognition scores and language scores. And the frequency of prenatal ETS exposure was negatively associated with language development before children reached two years old. | Maternal ETS exposure | Prenatal exposure |
| 3. Behavioural disorders | |||
| 3.1. ADHD | |||
| Wang et al. (2019) | Prenatal tobacco smoke exposure was a significant risk factor for ADHD even after adjusting for other potential confounders. The risk of the genetic variants in ADHD was increased significantly if the child had prenatal tobacco exposure. | Household & workplace | Prenatal & postnatal exposure |
| Lin, Xu, Wu, Zhou, Ma, Chen, Chen, et al. (2021) | Significant association between SHS exposure from pregnancy to childhood with having ADHD symptoms and subtypes. | SHS exposure | Prenatal, postnatal (ie, first 2 years of life) & current periods |
| Lin et al. (2017) | Prenatal ETS exposure was significantly associated with an increased risk of hyperactivity behaviours in young children, and there was a dose-response relationship. | Household (Maternal exposure) | Prenatal exposure |
| 3.2. Autism Behaviour | |||
| Yang et al. (2021) | Significant association between being exposed to ETS during gestation and autistic-like behaviours. // dosage respond | Household | During pregnancy, from birth to one year & from one to three years |
| 3.3. Other disorders | |||
| Liu et al. (2013) | ETS exposure was associated with a higher risk of externalizing behaviour problems in offspring of exposed mothers. However, it was not associated with internalizing or total behaviour problems. And no dose-response relationship was found. | Maternal ETS exposure at home, the workplace, and other places | Prenatal exposure |
| Leung et al. (2015) | Significant association between prenatal SHS exposure from non-parental sources and behavioural problems// Association between paternal smoking and maternal smoking and mental health problems. | Non-parental and parental exposure | Prenatal and postnatal |
| 4. Respiratory diseases | |||
| Zhuge et al. (2020) | Associations between most respiratory health outcomes and parental smoking except for the frequent common cold // Stronger association for father smoking // Insignificant effect for maternal smoking//Most association were insignificant after adjustment. | Parental smoking (mother only, father only, both) | During pregnancy, during the first year of life & current periods |
| Dong et al. (2011) | Significant association between ETS exposure in utero and the prevalence of respiratory morbidities// dosage respond// | Household (current & maternal) | Prenatal, postnatal (ie, first 2 years of life) & current periods |
| Lee, Lam, et al. (2012) | Significant association between foetal exposure to maternal passive smoking with wheeze ever, current wheeze, allergic rhinitis ever, and eczema ever //dose response | Household (maternal passive & active smoking) | During pregnancy |
| Hu et al. (2017) | Significant association between in utero exposure to SHS decreased lung function// Childhood asthma mediated the effects. | Household SHS exposure and maternal passive & active smoking | In utero & during early childhood |
| 5. Others | |||
| Li et al. (2019) | No significant association between exposed to ETS during pregnancy only and astigmatism. // Significant associations were found when exposed to ETS during pregnancy+from one to three years, or during pregnancy+from birth to one year+from one to three years old. | Household | During pregnancy, from birth to one year & from one to three years |
| Lin, Xu, Wu, Zhou, Ma, Chen, Dong, et al. (2021) | Significant association between being exposed during pregnancy and sleep problems. | Household | Pregnancy & the first 2 years of life |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





