Submitted:
05 July 2023
Posted:
06 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Semen Analysis
2.3. Measurement of ORP, 8-OHdG, and NOx
2.3.1. ORP Measurement
2.3.2. 8-OHdG Measurement
2.3.3. NOx Measurement
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Differences in OS or NS markers in each group
3.3. Correlation of OS or NS Markers with Sperm Parameters
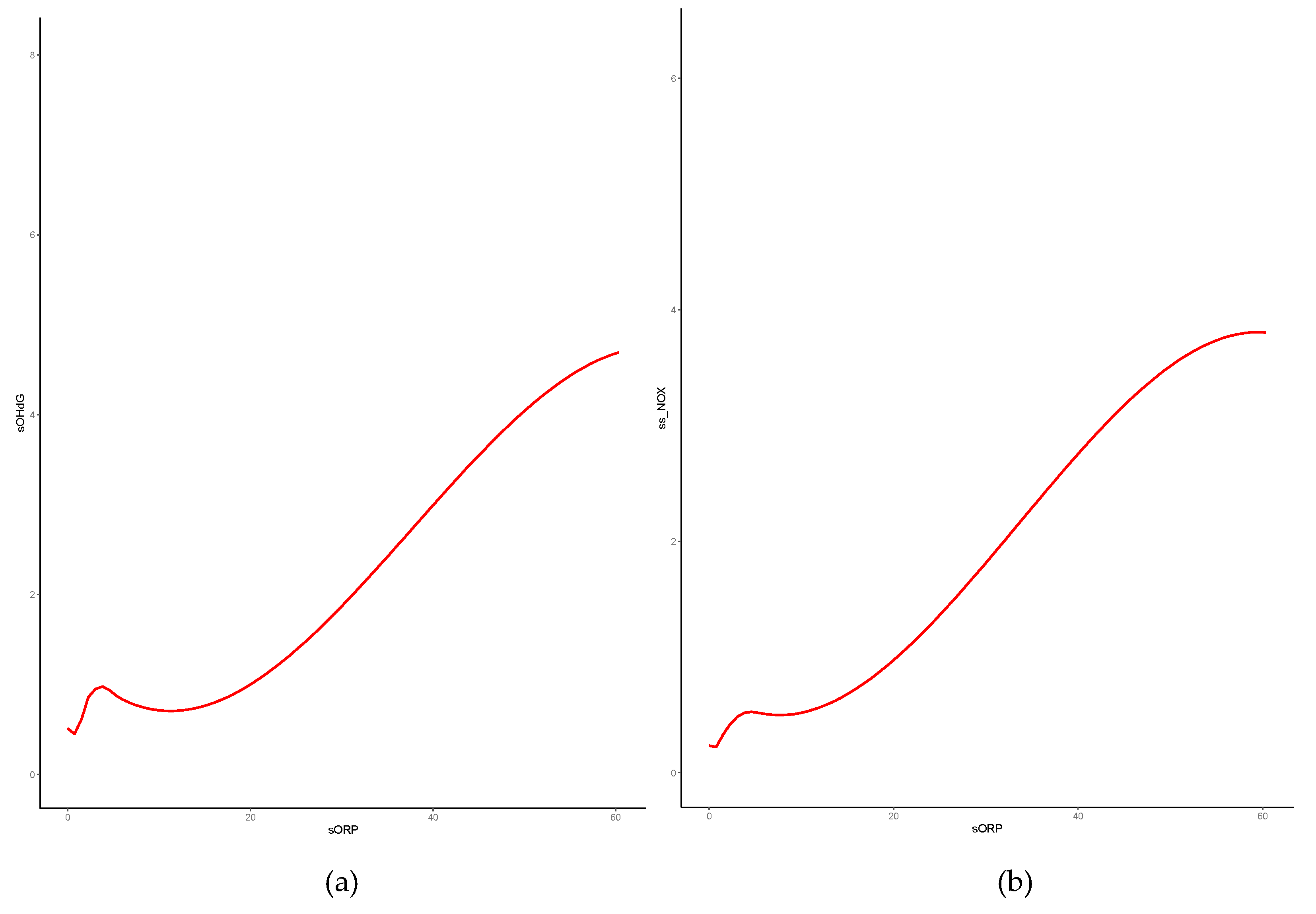
3.4. Correlation of OS or NS markers with ORP
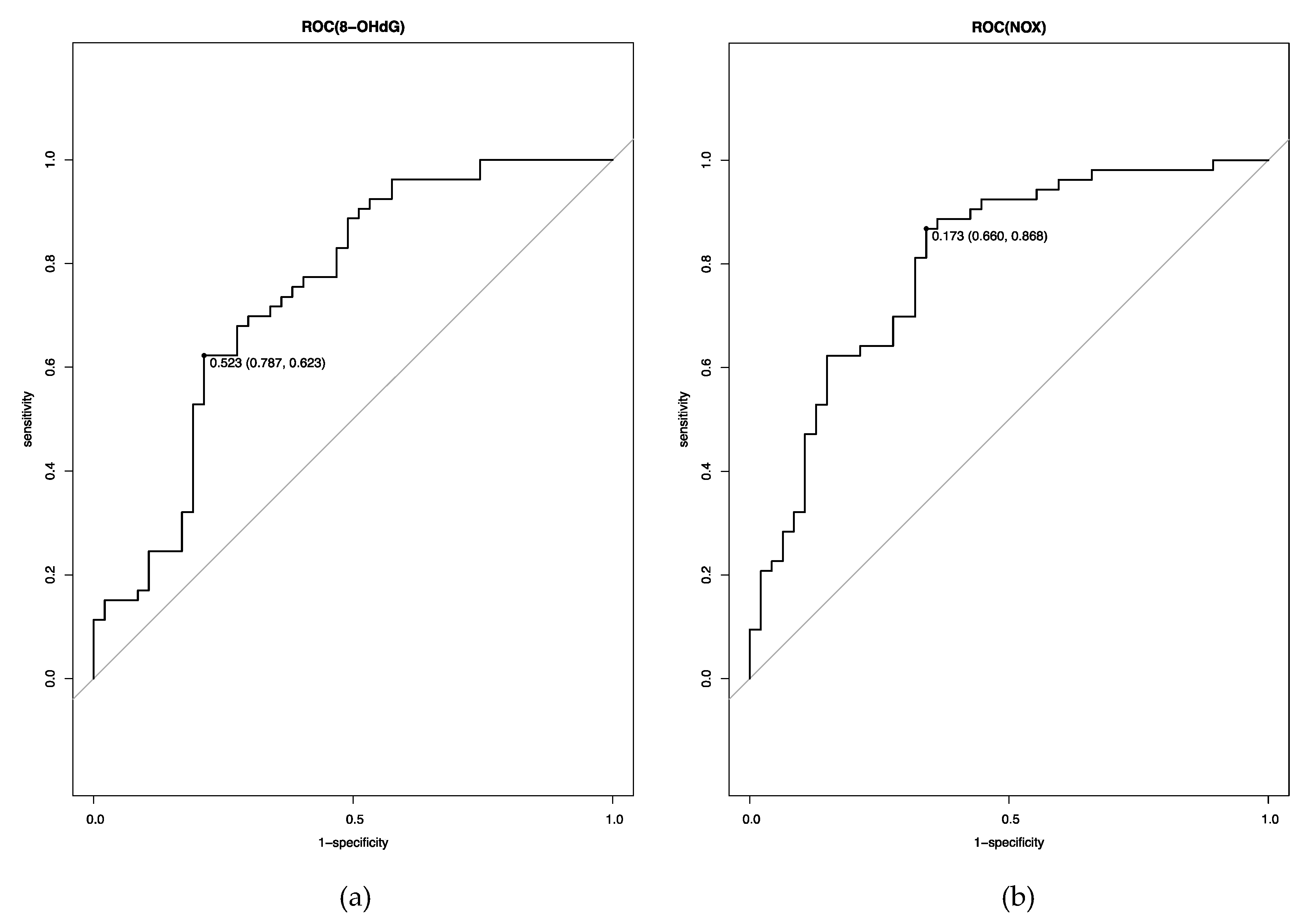
3.5. Cutoff Levels of OS and NS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clinical Biochemistry 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Yumura, Y.; Tsujimura, A.; Imamoto, T.; Umemoto, Y.; Kobayashi, H.; Shiraishi, K.; Shin, T.; Taniguchi, H.; Chiba, K.; Miyagawa, Y.; et al. Nationwide survey of urological specialists regarding male infertility: results from a 2015 questionnaire in Japan. Reproductive Medicine and Biology 2018, 17, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. The World Journal of Men's Health 2019, 37, 296–312. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Reviews of Reproduction 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. Journal of Assisted Reproduction and Genetics 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. Reproductive Medicine and Biology 2021, 20, 41–52. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertility and Sterility 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Tesarik, J.; Greco, E.; Mendoza, C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Human Reproduction (Oxford, England) 2004, 19, 611–615. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Jiang, H.; Chen, H.; Chen, Y.; Dai, Y. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: a meta-analysis. Journal of Assisted Reproduction and Genetics 2015, 32, 17–26. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertility and sterility 2014, 102, 998–1005. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Das, S.; Slama, P.; Roychoudhury, S. Reactive Nitrogen Species and Male Reproduction: Physiological and Pathological Aspects. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sharma, R.; Roychoudhury, S.; Du Plessis, S.; Sabanegh, E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertility and Sterility 2016, 106, 566–573. [Google Scholar] [CrossRef]
- Henkel, R.; Morris, A.; Vogiatzi, P.; Saleh, R.; Sallam, H.; Boitrelle, F.; Garrido, N.; Arafa, M.; Gül, M.; Rambhatla, A.; et al. Predictive value of seminal oxidation-reduction potential analysis for reproductive outcomes of ICSI. Reproductive Biomedicine Online 2022, 45, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Udayanga, K.G.S.; Satoh, M.; Hashimoto, S.; Morimoto, Y. Relation between semen oxidative reduction potential in initial semen examination and IVF outcomes. Reproductive Medicine and Biology 2023, 22, e12501. [Google Scholar] [CrossRef]
- WHO laboratory manual for the examination and processing of human semen 6th edition. 2021.
- Guz, J.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Zarakowska, E.; Siomek, A.; Szpila, A.; Kotzbach, M.; Kotzbach, R.; Olinski, R. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS One 2013, 8, e68490. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm oxidative stress during in vitro manipulation and its effects on sperm function and embryo development. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Gholinezhad, M.; Aliarab, A.; Abbaszadeh-Goudarzi, G.; Yousefnia-Pasha, Y.; Samadaian, N.; Rasolpour-Roshan, K.; Aghagolzadeh-Haji, H.; Mohammadoo-Khorasani, M. Nitric oxide, 8-hydroxydeoxyguanosine, and total antioxidant capacity in human seminal plasma of infertile men and their relationship with sperm parameters. Clinical and Experimental Reproductive Medicine 2020, 47, 54–60. [Google Scholar] [CrossRef]
- Escada-Rebelo, S.; Ramalho-Santos, J. Oxidative and nitrosative stress detection in human sperm using fluorescent probes. Methods in molecular Biology (Clifton, N.J.) 2023, 2566, 45–52. [Google Scholar] [CrossRef]
- Uribe, P.; Meriño, J.; Manquemilla, E.; Villagrán, C.; Vega, E.; Zambrano, F.; Schulz, M.; Pezo, F.; Villegas, J.V.; Boguen, R.; et al. Multiparameter flow cytometry assay for analysis of nitrosative stress status in human spermatozoa. Cytometry A 2020, 97, 1238–1247. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Baskaran, S.; O'Connell, S.; Almajed, W.; Hellstrom, W.J.G.; Sikka, S.C. Association between Seminal Oxidation-Reduction Potential and Sperm DNA Fragmentation-A Meta-Analysis. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Arafa, M.; Agarwal, A.; Majzoub, A.; Panner Selvam, M.K.; Baskaran, S.; Henkel, R.; Elbardisi, H. Efficacy of Antioxidant Supplementation on Conventional and Advanced Sperm Function Tests in Patients with Idiopathic Male Infertility. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Amiri, I.; Karimi, J.; Piri, H.; Goodarzi, M.T.; Tavilani, H.; Khodadadi, I.; Ghorbani, M. Association between nitric oxide and 8-hydroxydeoxyguanosine levels in semen of diabetic men. Systems Biology in Reproductive Medicine 2011, 57, 292–295. [Google Scholar] [CrossRef]
- Yamasaki, K.; Uchida, M.; Watanabe, N.; Ihana, T.; Ishiguro, Y.; Kuroda, S.; Takeshima, T.; Yumura, Y.; Mieno, M.; Yoshida, K.; et al. Effects of antioxidant co-supplementation therapy on spermatogenesis dysfunction in relation to the basal oxidation-reduction potential levels in spermatozoa: A pilot study. Reproductive Medicine and Biology 2022, 21, e12450. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Rao, K.A.; Thalaivarasai Balasundaram, S. Deterioration of semen quality and sperm-DNA integrity as influenced by cigarette smoking in fertile and infertile human male smokers-A prospective study. Free Radical Research 2019, 120, 11784–11793. [Google Scholar] [CrossRef] [PubMed]
- Homa, S.T.; Vessey, W.; Perez-Miranda, A.; Riyait, T.; Agarwal, A. Reactive Oxygen Species (ROS) in human semen: determination of a reference range. Journal of Assisted Reproduction and Genetics 2015, 32, 757–764. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, R.L. Possible role of nitric oxide on fertile and asthenozoospermic infertile human sperm functions. Free Radical Research 1996, 25, 347–354. [Google Scholar] [CrossRef]
- Lee, N.P.; Cheng, C.Y. Nitric oxide/nitric oxide synthase, spermatogenesis, and tight junction dynamics. Biology of Reproduction 2004, 70, 267–276. [Google Scholar] [CrossRef]
- Shen, H.M.; Chia, S.E.; Ni, Z.Y.; New, A.L.; Lee, B.L.; Ong, C.N. Detection of oxidative DNA damage in human sperm and the association with cigarette smoking. Reproductive Toxicology 1997, 11, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reproductive Biomedicine Online 2017, 34, 48–57. [Google Scholar] [CrossRef]

| Overall | OS | non-OS | p value | ||
| n | 100 | 53 | 47 | ||
| Age, years (SD) | 35.5 (6.6) | 35.8 (6.9) | 35.1 (6.5) | 0.64 | |
| BMI, kg/m2 (SD) | 23.6 (3.2) | 23.6 (3.2) | 23.6 (3.2) | 0.95 | |
| smoking, n (%) | 0.77 | ||||
| current smoker | 20 (20.0) | 9 (17.0) | 11 (23.4) | ||
| past smoker | 23 (23.0) | 13 (24.5) | 10 (21.3) | ||
| non-smoker | 52 (52.0) | 29 (54.7) | 23 (48.9) | ||
| unknown | 5 (5.0) | 2 (3.8) | 3 (6.4) | ||
| disease, n (%) | 0.63 | ||||
| idiopathic | 55 (55.0) | 32 (60.4) | 23 (48.9) | ||
| varicocele | 26 (26.0) | 13 (24.5) | 13 (27.7) | ||
| post anticancer drug | 11 (11.0) | 5 (9.4) | 6 (12.8) | ||
| other | 8 (8.0) | 3 (5.7) | 5 (10.6) | ||
| T, ng/ml (SD) | 4.9 (1.7) | 5.1 (1.7) | 4.7 (1.7) | 0.31 | |
| LH, mIU/ml (SD) | 4.9 (1.8) | 4.7 (1.7) | 5.0 (1.8) | 0.43 | |
| FSH, mIU/ml (SD) | 5.6 (3.4) | 5.8 (2.7) | 5.4 (4.2) | 0.58 | |
| semen volume, ml (SD) | 2.7 (1.4) | 2.7 (1.6) | 2.7 (1.2) | 0.86 | |
| concentration, million/ml (SD) | 40.1 (43.9) | 22.8 (21.3) | 59.6 (53.9) | <0.01 | |
| motility, % (SD) | 30.6 (19.8) | 25.8 (15.8) | 36.0 (22.5) | 0.01 | |
| OS | non-OS | p | |
| sORP [IQR], mV/106 spermataozoa/mL |
2.99 [2.11, 5.30] |
0.37 [0.14, 0.88] |
< 0.001 |
| s8-OHdG [IQR], mol/dL/106 spermatozoa/mL |
0.68 [0.30, 1.22] |
0.24 [0.12, 0.47] |
< 0.001 |
| sNOx [IQR], µM/106 spermatozoa/mL |
0.39 [0.20, 0.62] |
0.14 [0.09, 0.25] |
< 0.001 |
| correlation coefficient | overall | OS | non-OS | |
| s8-OHdG | 0.41* | 0.17 | 0.02 | |
| log-transformed | 0.38* | 0.39* | 0.04 | |
| sNOx | 0.54* | 0.26 | 0.17 | |
| log-transformed | 0.53* | 0.55* | 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





