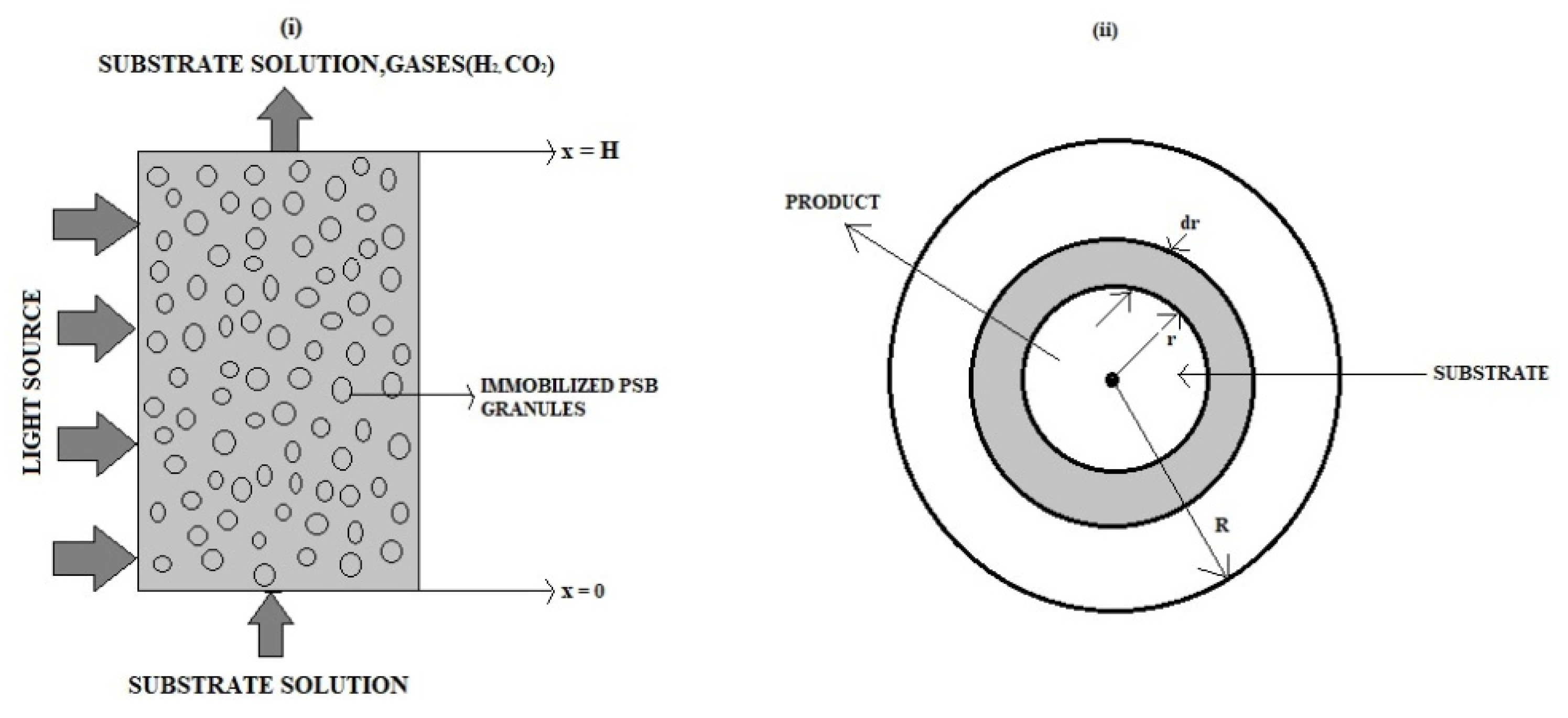
Nonlinear equations in the immobilized-cell photobioreactor are analytically solved. The approximate analytical expressions of concentrations of glucose and hydrogen inside the gel and granule in addition to approximate analytical expressions of steady-state source terms of liquid and gas phases are derived using Taylor series (TSM) and Akbari-Ganji (AGM) methods.
The reaction-diffusion equations representing the packed bed photobioreactor with immobilized-cell were solved using the Adomian decomposition method (ADM) [
2] and the homotopy perturbation method (HPM) [
3]. Interstingly, the semi-analytical expressions of the concentrations of substrate and product obtained by the ADM, HPM, and AGM were identical (Equations (20) and (26)) for all values of parameters.
To examine the accuracy of the two proposed analytical approaches, we compared their results with numerical results obtained from the reliable MATLAB pdepe function (
Appendix A) and with analytical results of other methods available in the literature. The approximate analytical and numerical concentrations of substrate and product for various parameters are summarized in
Table 1,
Table 2,
Table 3,
Table 4,
Table 5 and
Table 6. Even though both methods gave satisfactory results, TSM is notably more accurate. The maximum relative error average is 0.6% for the TSM and 5% for the AGM. Comparisons of normalized steady-state source terms of both liquid and gas phases for various values of parameters
and
are given in
Table 7 and
Table 8.
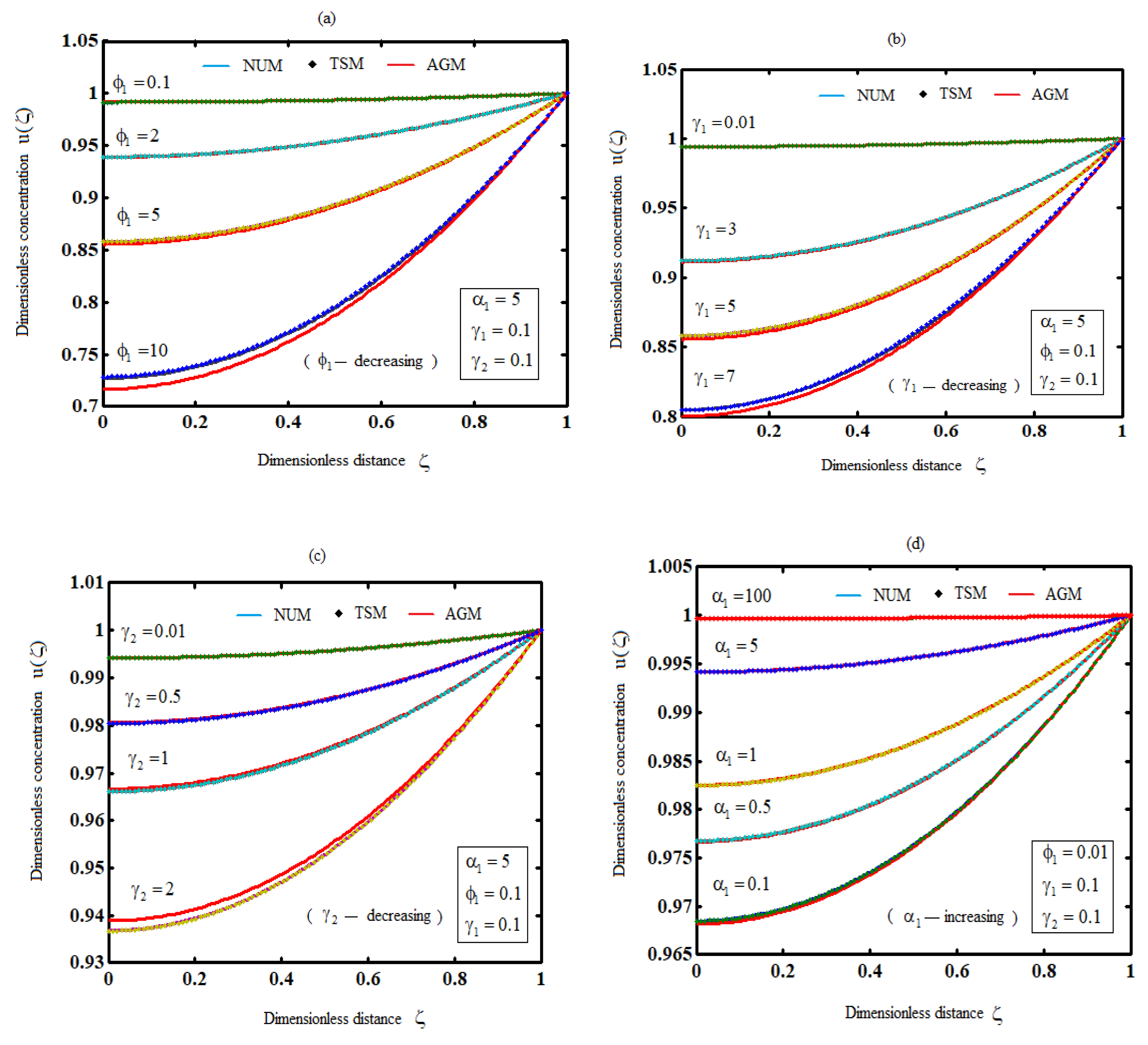
Figures 2(a)-2(c) illustrate the behavior of the biodegradation of substrate for different values of the parameters. It is noticed that as any of the parameters
decreases, the substrate concentration increases. In contrast,
Figure 2(d) confirms a direct relationship between the parameter
and the substrate concentration.
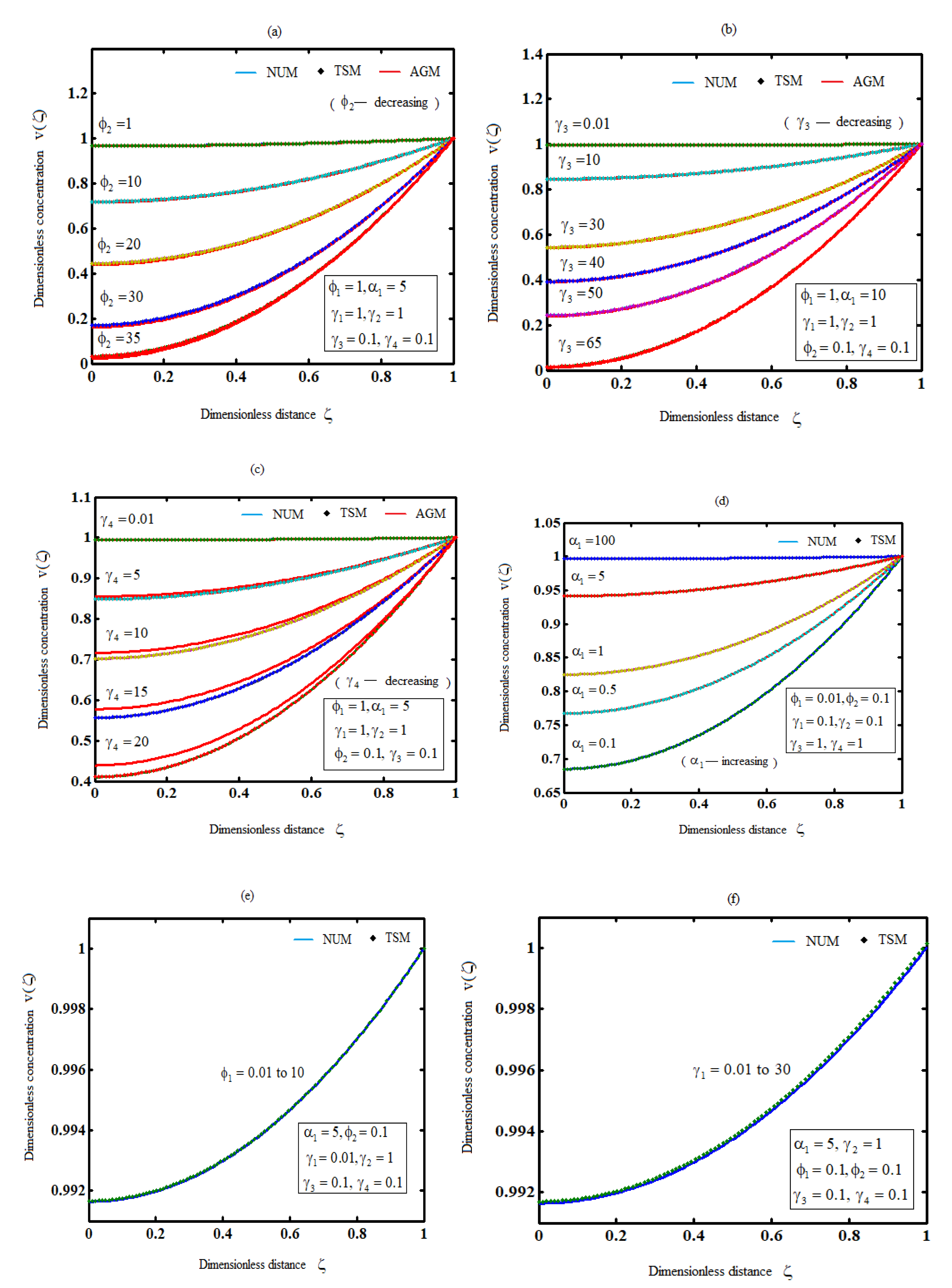
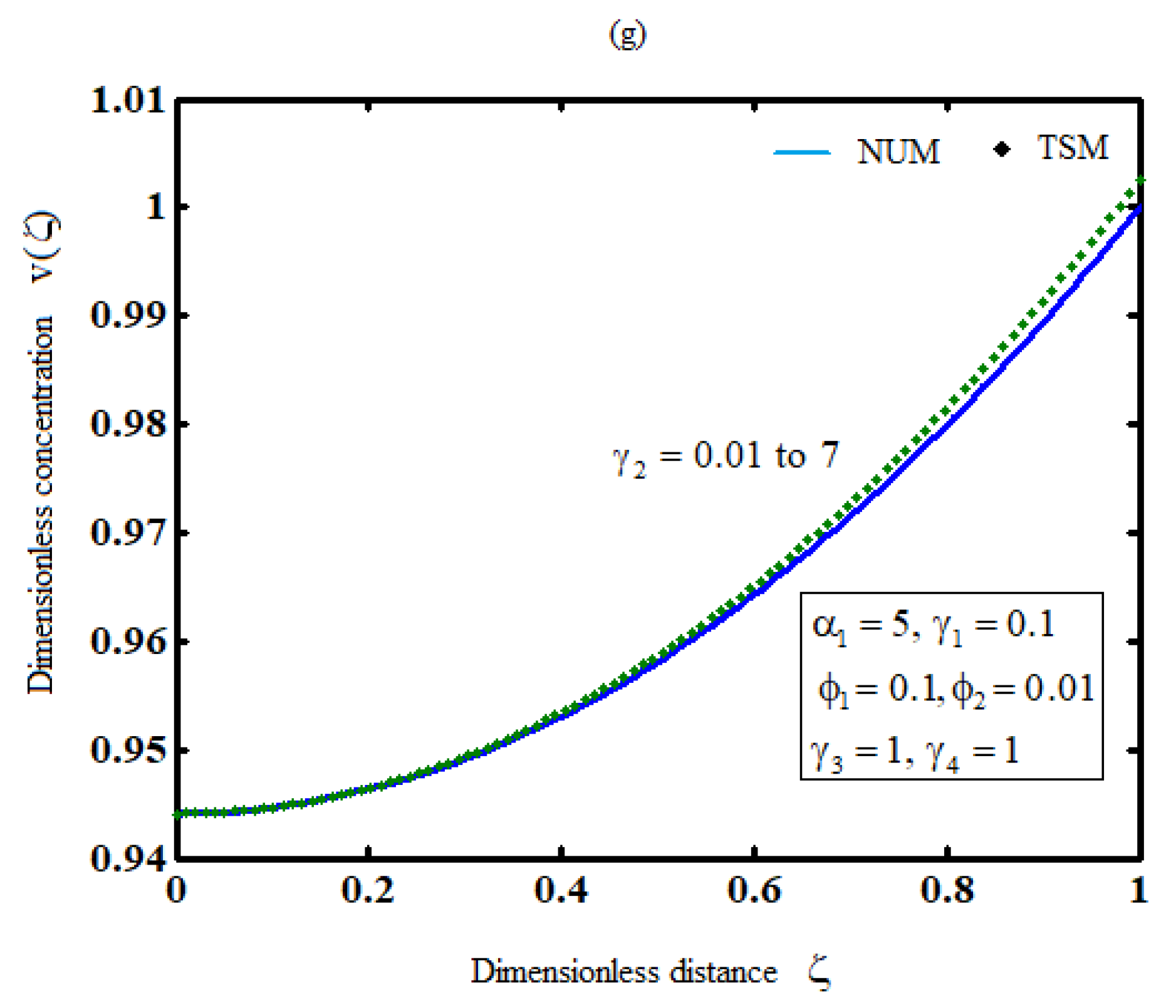
The effects of all parameters on the hydrogen production profiles are shown in Figures 3(a)-3(g),where it is noticed that the concentration of hydrogen increases the parameters
decreases or the parameter
increases. However, the concentration of hydrogen is independent of any of the parameters
.
Figure 2.
Plot of substrate concentration for various values of parameters (Equations (20) and (35)).
Figure 2.
Plot of substrate concentration for various values of parameters (Equations (20) and (35)).
Figure 3.
Plot of product concentration,, for various values of the parameters and (Equations (26) and (41)).
Figure 3.
Plot of product concentration,, for various values of the parameters and (Equations (26) and (41)).
Table 1.
Comparison beteween numerical and analytical results for dimensionless concentration of substrate for various values of parameter when and.
Table 1.
Comparison beteween numerical and analytical results for dimensionless concentration of substrate for various values of parameter when and.
|
|
|
| Num.Equation (9) |
AGMEq.(20) |
TSMEq.(35) |
Error % ofAGMEq.(20) |
Error % of TSMEquation (35) |
NumEquation (9) |
AGMEq.(20) |
TSMEq.(35) |
Error % ofAGMEq.(20) |
Error % of TSMEquation (35) |
| 0 |
0.73 |
0.72 |
0.73 |
1.37 |
0.00 |
0.60 |
0.58 |
0.61 |
3.33 |
1.67 |
| 0.2 |
0.74 |
0.73 |
0.74 |
1.35 |
0.00 |
0.62 |
0.59 |
0.63 |
4.84 |
1.61 |
| 0.4 |
0.77 |
0.76 |
0.77 |
1.30 |
0.00 |
0.67 |
0.65 |
0.67 |
2.98 |
0.00 |
| 0.6 |
0.83 |
0.82 |
0.83 |
1.20 |
0.00 |
0.75 |
0.73 |
0.75 |
2.67 |
0.00 |
| 0.8 |
0.90 |
0.90 |
0.90 |
0.00 |
0.00 |
0.86 |
0.85 |
0.86 |
1.16 |
0.00 |
| 1 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
| |
Average error % |
0.87 |
0.00 |
Average error % |
2.50 |
0.55 |
Table 2.
Comparison beteween numerical and analytical results for dimensionless concentration of substrate for various values of parameter when and.
Table 2.
Comparison beteween numerical and analytical results for dimensionless concentration of substrate for various values of parameter when and.
|
|
|
| Num.Equation (9) |
AGMEq.(20) |
TSMEq.(35) |
Error % ofAGMEq.(20) |
Error % of TSMEquation (35) |
NumEquation (9) |
AGMEq.(20) |
TSMEq.(35) |
Error % ofAGMEq.(20) |
Error % of TSMEquation (35) |
| 0 |
0.80 |
0.80 |
0.80 |
0.00 |
0.00 |
0.60 |
0.58 |
0.61 |
3.33 |
1.67 |
| 0.2 |
0.81 |
0.81 |
0.81 |
0.00 |
0.00 |
0.62 |
0.59 |
0.63 |
4.84 |
1.61 |
| 0.4 |
0.84 |
0.83 |
0.84 |
1.20 |
0.00 |
0.67 |
0.65 |
0.67 |
2.98 |
0.00 |
| 0.6 |
0.88 |
0.87 |
0.88 |
1.14 |
0.00 |
0.75 |
0.73 |
0.75 |
2.67 |
0.00 |
| 0.8 |
0.93 |
0.93 |
0.93 |
0.00 |
0.00 |
0.86 |
0.85 |
0.86 |
1.16 |
0.00 |
| 1 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
| |
Average error % |
0.39 |
0.00 |
Average error % |
2.50 |
0.55 |
Table 3.
Comparison beteween numerical and analytical results for dimensionless concentration of substrate for various values of parameter when and.
Table 3.
Comparison beteween numerical and analytical results for dimensionless concentration of substrate for various values of parameter when and.
|
|
|
| Num.Equation (9) |
AGMEquation (20) |
TSMEq.(35) |
Error % ofAGMEq.(20) |
Error % of TSMEquation (35) |
NumEquation (9) |
AGMEq.(20) |
TSMEq.(35) |
Error % ofAGMEq.(20) |
Error % of TSMEquation (35) |
| 0 |
0.84 |
0.86 |
0.84 |
2.38 |
0.00 |
0.77 |
0.80 |
0.76 |
3.90 |
1.30 |
| 0.2 |
0.85 |
0.86 |
0.85 |
1.18 |
0.00 |
0.78 |
0.81 |
0.77 |
3.85 |
1.28 |
| 0.4 |
0.87 |
0.88 |
0.87 |
1.15 |
0.00 |
0.81 |
0.83 |
0.80 |
2.47 |
1.23 |
| 0.6 |
0.90 |
0.91 |
0.90 |
1.11 |
0.00 |
0.86 |
0.87 |
0.85 |
1.16 |
1.16 |
| 0.8 |
0.95 |
0.95 |
0.94 |
0.00 |
1.05 |
0.92 |
0.93 |
0.92 |
1.09 |
0.00 |
| 1 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
| |
Average error % |
0.97 |
0.17 |
Average error % |
2.09 |
0.83 |
Table 4.
Comparison beteween numerical and analytical results for dimensionless concentration of product for various values of parameter when and.
Table 4.
Comparison beteween numerical and analytical results for dimensionless concentration of product for various values of parameter when and.
|
|
|
| Num.Eq.(10) |
AGMEq.(26) |
TSMEq.(41) |
Error% ofAGMEq.(26) |
Error% ofTSMEquation (41) |
NumEq.(10) |
AGMEq.(26) |
TSMEq.(41) |
Error% ofAGMEq.(26) |
Error% ofTSMEquation (41) |
| 0 |
0.44 |
0.44 |
0.44 |
0.00 |
0.00 |
0.03 |
0.02 |
0.03 |
33.33 |
0.00 |
| 0.2 |
0.47 |
0.46 |
0.47 |
2.13 |
0.00 |
0.07 |
0.06 |
0.07 |
14.29 |
0.00 |
| 0.4 |
0.53 |
0.53 |
0.53 |
0.00 |
0.00 |
0.19 |
0.18 |
0.19 |
5.26 |
0.00 |
| 0.6 |
0.65 |
0.64 |
0.65 |
1.54 |
0.00 |
0.39 |
0.38 |
0.39 |
2.56 |
0.00 |
| 0.8 |
0.81 |
0.80 |
0.81 |
1.23 |
0.00 |
0.66 |
0.66 |
0.66 |
0.00 |
0.00 |
| 1 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
| |
Average error % |
0.82 |
0.00 |
Average error % |
9.24 |
0.00 |
Table 5.
Comparison beteween numerical and analytical results for dimensionless concentration of product for various values of parameter when and.
Table 5.
Comparison beteween numerical and analytical results for dimensionless concentration of product for various values of parameter when and.
|
|
|
| Num.Eq.(10) |
AGMEq.(26) |
TSMEq.(41) |
Error % ofAGMEq.(26) |
Error % of TSMEquation (41) |
NumEq.(10) |
AGMEq.(26) |
TSMEq.(41) |
Error % ofAGMEq.(26) |
Error % of TSMEquation (41) |
| 0 |
0.39 |
0.39 |
0.39 |
0.00 |
0.00 |
0.01 |
0.01 |
0.01 |
0.00 |
0.00 |
| 0.2 |
0.42 |
0.42 |
0.42 |
0.00 |
0.00 |
0.05 |
0.05 |
0.05 |
0.00 |
0.00 |
| 0.4 |
0.49 |
0.49 |
0.49 |
0.00 |
0.00 |
0.18 |
0.17 |
0.18 |
5.55 |
0.00 |
| 0.6 |
0.62 |
0.61 |
0.62 |
1.61 |
0.00 |
0.38 |
0.37 |
0.38 |
2.63 |
0.00 |
| 0.8 |
0.79 |
0.79 |
0.79 |
0.00 |
0.00 |
0.66 |
0.66 |
0.66 |
0.00 |
0.00 |
| 1 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
| |
Average error % |
0.27 |
0.00 |
Average error % |
1.36 |
0.00 |
Table 6.
Comparison beteween numerical and analytical results for dimensionless concentration of product for various values of parameter when and .
Table 6.
Comparison beteween numerical and analytical results for dimensionless concentration of product for various values of parameter when and .
|
|
|
| Num.Eq.(10) |
AGMEq.(26) |
TSMEq.(41) |
Error % ofAGMEq.(26) |
Error % of TSMEquation (41) |
NumEq.(10) |
AGMEq.(26) |
TSMEq.(41) |
Error % ofAGMEq.(26) |
Error % of TSMEquation (41) |
| 0 |
0.70 |
0.72 |
0.70 |
2.86 |
0.00 |
0.41 |
0.44 |
0.41 |
7.32 |
0.00 |
| 0.2 |
0.71 |
0.73 |
0.71 |
2.82 |
0.00 |
0.43 |
0.46 |
0.43 |
6.98 |
0.00 |
| 0.4 |
0.75 |
0.76 |
0.75 |
1.33 |
0.00 |
0.51 |
0.53 |
0.51 |
3.92 |
0.00 |
| 0.6 |
0.81 |
0.82 |
0.81 |
1.23 |
0.00 |
0.63 |
0.64 |
0.63 |
1.59 |
0.00 |
| 0.8 |
0.90 |
0.90 |
0.90 |
0.00 |
0.00 |
0.80 |
0.80 |
0.80 |
0.00 |
0.00 |
| 1 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
1.00 |
1.00 |
1.00 |
0.00 |
0.00 |
| |
Average error % |
1.37 |
0.00 |
Average error % |
3.30 |
0.00 |
Table 7.
Comparison between numerical and analytical normalized steady-state source terms of liquid phase for various values of parameter and when .
Table 7.
Comparison between numerical and analytical normalized steady-state source terms of liquid phase for various values of parameter and when .
|
|
|
| Num.Eq.(13) |
AGMEq.(27) |
TSMEq.(43) |
Error% ofAGMEq.(27) |
Error% ofTSMEquation (43) |
Num.Eq.(13) |
AGMEquation (27) |
TSMEq.(43) |
Error% ofAGMEq.(27) |
Error%ofTSMEquation (43) |
| 0 |
0.06 |
0.06 |
0.06 |
0.00 |
0.00 |
0.04 |
0.04 |
0.04 |
0.00 |
0.00 |
| 0.1 |
0.09 |
0.09 |
0.09 |
0.00 |
0.00 |
0.07 |
0.07 |
0.07 |
0.00 |
0.00 |
| 0.5 |
0.20 |
0.21 |
0.21 |
5.00 |
5.00 |
0.15 |
0.15 |
0.15 |
0.00 |
0.00 |
| 1 |
0.34 |
0.37 |
0.34 |
8.82 |
0.00 |
0.26 |
0.27 |
0.26 |
3.85 |
0.00 |
| 5 |
1.25 |
1.56 |
1.33 |
24.8 |
6.40 |
1.00 |
1.15 |
1.01 |
15.00 |
1.00 |
| 10 |
2.08 |
3.09 |
2.42 |
48.56 |
16.35 |
1.74 |
2.27 |
1.90 |
30.46 |
9.19 |
| 50 |
5.64 |
15.2 |
5.23 |
169.7 |
7.27 |
4.81 |
11.15 |
5.22 |
131.8 |
8.52 |
| 100 |
7.75 |
30.4 |
5.66 |
291.7 |
26.97 |
7.31 |
22.27 |
5.66 |
204.6 |
22.57 |
| |
Average error % |
68.68 |
7.75 |
Average error % |
48.22 |
5.16 |
Table 8.
Comparison between numerical and analytical normalized steady-state source terms of gas phase for various values of parameter and when and .
Table 8.
Comparison between numerical and analytical normalized steady-state source terms of gas phase for various values of parameter and when and .
|
|
|
| Num.Eq.(14) |
AGMEq.(28) |
TSMEq.(44) |
Error % ofAGMEq.(28) |
Error % of TSMEquation (44) |
Num.Eq.(14) |
AGMEquation (28) |
TSMEquation (44) |
Error % ofAGMEq.(28) |
Error % of TSMEquation (44) |
| 0 |
0.08 |
0.09 |
0.09 |
12.50 |
12.50 |
0.06 |
0.07 |
0.06 |
16.67 |
0.00 |
| 0.1 |
0.12 |
0.14 |
0.12 |
16.67 |
0.00 |
0.10 |
0.10 |
0.10 |
0.00 |
0.00 |
| 0.5 |
0.27 |
0.32 |
0.28 |
18.52 |
3.70 |
0.22 |
0.23 |
0.22 |
4.54 |
0.00 |
| 1 |
0.47 |
0.54 |
0.47 |
14.87 |
0.00 |
0.37 |
0.40 |
0.37 |
8.11 |
0.00 |
| 5 |
2.02 |
2.36 |
2.03 |
16.83 |
0.49 |
1.58 |
1.73 |
1.60 |
9.49 |
1.27 |
| 10 |
3.96 |
4.64 |
3.98 |
17.17 |
0.50 |
3.10 |
3.40 |
3.14 |
9.68 |
1.29 |
| 50 |
19.5 |
22.8 |
19.55 |
17.21 |
0.41 |
15.26 |
16.73 |
15.42 |
9.63 |
1.05 |
| 100 |
38.9 |
45.5 |
39.02 |
17.19 |
0.41 |
30.46 |
33.40 |
30.78 |
9.65 |
1.05 |
| |
Average error % |
16.37 |
2.25 |
Average error % |
8.47 |
0.58 |