3.1. Spectroscopic characterization of N, P-CDs
N, P-CDs show significant fluorescence emission at 510 nm-650 nm excitation (
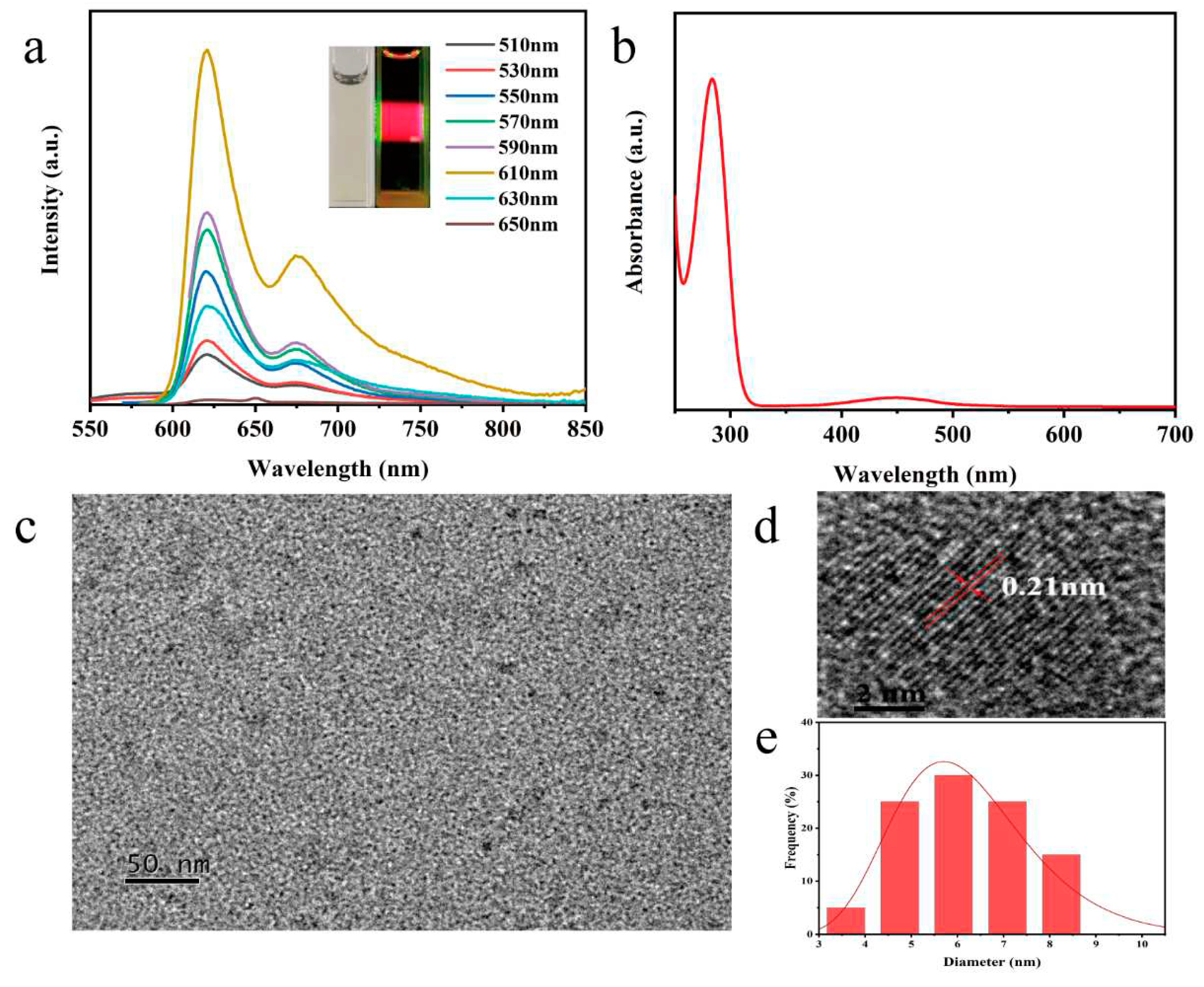
Figure 1a). With increasing excitation wavelengths, N, P-CDs show excellent red light emission at different excitation wavelengths. The maximum fluorescence intensity is at an excitation of 610 nm, corresponding to an emission wavelength of 626 nm, with a shoulder peak at around 675 nm. The N, P-CDs solution appears pale yellow under daily light and bright red fluorescence under UV (530 nm) irradiation. N, P-CDs have two peaks at 282 nm and 450 nm (
Figure 1b), where the absorption peak at 282 nm is a π-π* leap in the C=C bond in the aromatic ring, and the absorption peak at 450 nm may be attributed to the n-π* leap in the C=O bond and the π-π* leap in the aromatic fluorophore structure [
13].
Figure 1c–d shows the nearly spherical shape and good dispersion of the N, P-CDs, and the HRTEM test results illustrate the distinct lattice striations of the N, P-CDs. The calculated crystal plane spacing is 0.21 nm, which is consistent with the (100) crystal plane of graphene [
14]. The particle size of the N, P-CDs ranged from 4.3 nm to 7.5 nm with an average particle size of about 6.21 nm.
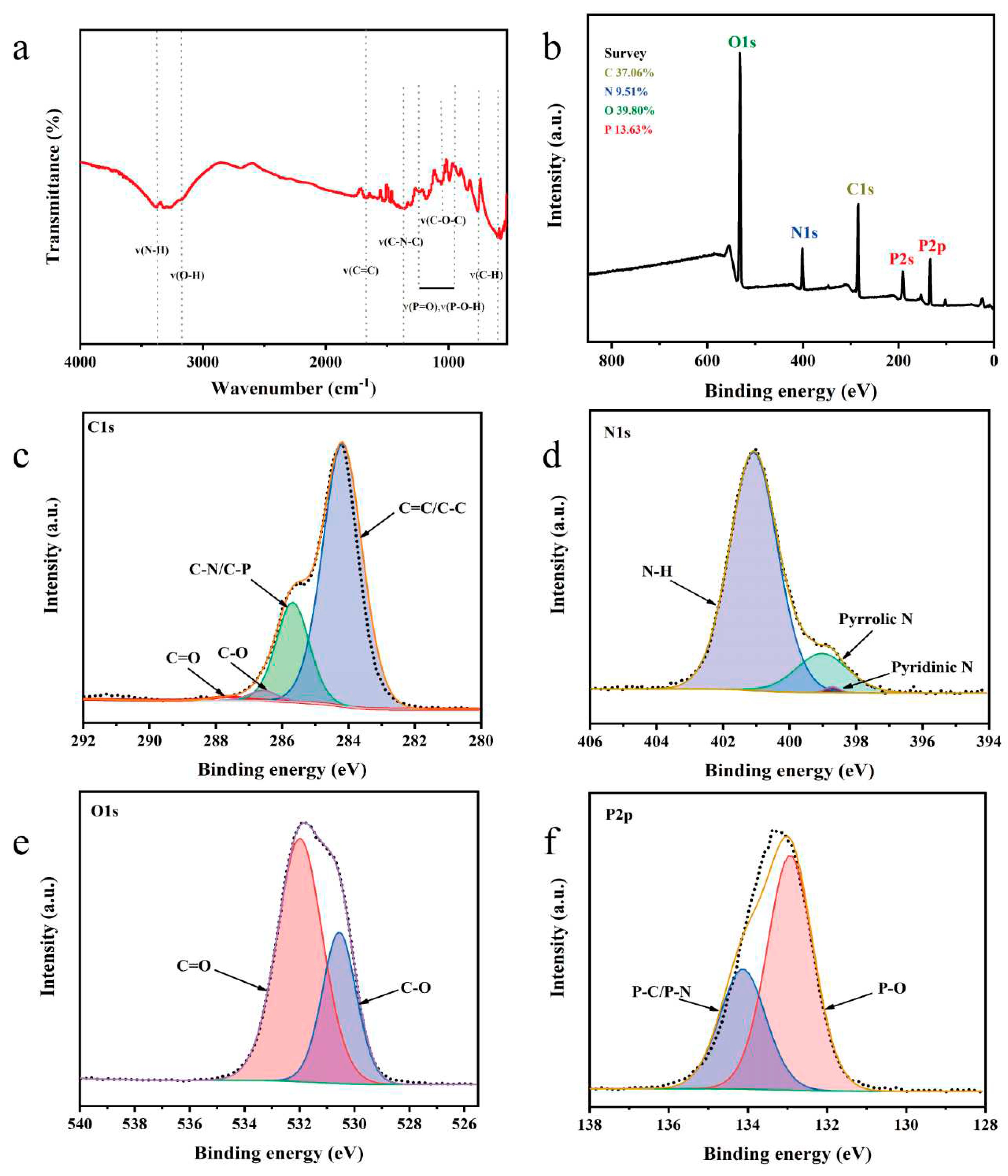
Figure 2a shows the FTIR spectra of the N, P-CDs. The stretching vibrations of the N-H and O-H bonds are observed at 3390 cm
-1 and 3170 cm
-1. The peak at 1670 cm
-1 indicates the presence of stretching vibrations of the C=O or C=N and C=C structures in the carbon core skeleton of N, P-CDs [
15]. 1370 cm
-1 corresponds to the C-N-C stretching vibration in the benzene ring, suggesting the possible presence of imine and piperazine structures in N, P-CDs [
16]. The absorption peaks at 940 cm
-1-1260 cm
-1 correspond to the P=O and P-O-R (R=alkyl) stretching vibrations [
17]. The absorption peaks at 771 cm
-1 and 607 cm
-1 correspond to the bending vibration of C-H, which coincides with the out-of-plane bending vibration of the benzene ring C-H [
18].
The chemical composition of the N, P-CDs was characterized using X-ray photoelectron spectroscopy. As shown in
Figure 2b, the N, P-CDs show four peaks at 286.8 eV, 401.1 eV, 531.81 eV, and 133.39 eV. The atomic percentages of C, N, O, and P were 37.06 %, 9.51 %, 39.80 %, and 16.63 %, respectively. From the high-resolution spectra of C1s (
Figure 2c), it can be seen that there are four chemical bonds in N, P-CDs, i.e., C=C/C-C (284.2 eV), C-N/C-P (285.7 eV), C-O (286.6 eV), and C=O (287.7 eV) [
19,
20]. High-resolution XPS spectra of N1s have three peaks at 399.2 eV, 400.1 eV, and 401.4 eV (
Figure 2d), which are corresponding to pyridine N, pyrrole N, and graphite N, respectively [
21]. The high-resolution spectra of O1s are at 530.4 eV and 531.9 Ev (
Figure 2e), corresponding to the presence of both C-O and C=O forms of O [
22]. The high-resolution XPS energy spectrum of P2p has two peaks at 132.9 eV and 134.1 eV (
Figure 2f), indicating the presence of P=O and P-C/P-N chemical bonds [
13].
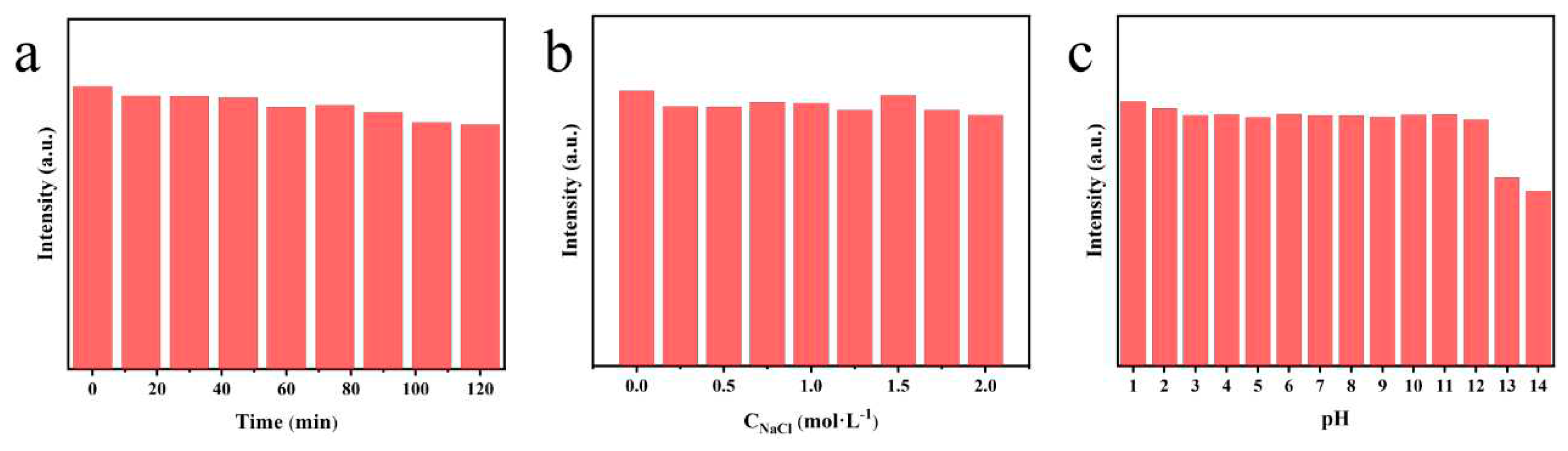
To investigate the environmental stability of N, P-CDs, the effects of ionic strength, UV irradiation, and different pH on the fluorescence intensity of N, P-CDs were investigated separately. As shown in
Figure 3a, after 120 min of UV irradiation, the fluorescence intensity of N, P-CDs remained at 86.54 % of its initial value, indicating that the optimized carbon dots have strong resistance to photobleaching and are suitable for large-scale preparation and storage. The fluorescence intensity of N, P-CDs under different salt concentration solutions (from 0 mol/L to 2 mol/L) was nearly the same, proving that the carbon dots have good salt tolerance, and carbon dots have greater potential for biological applications. When N, P-CDs were dispersed in solutions with different pH values, the fluorescence intensity of the carbon dots remained almost unchanged at a pH of 1-12, showing excellent fluorescence stability. The intensity decreased at a PH of 13 and 14, this is probably due to the presence of more carboxyl groups on the surface of the carbon dots, which led to a chemical reaction on the surface of the carbon dots under strongly alkaline conditions, resulting in a weakening of their fluorescence intensity [
23].
3.2. Effect of N, P-CDs on the growth of water spinach
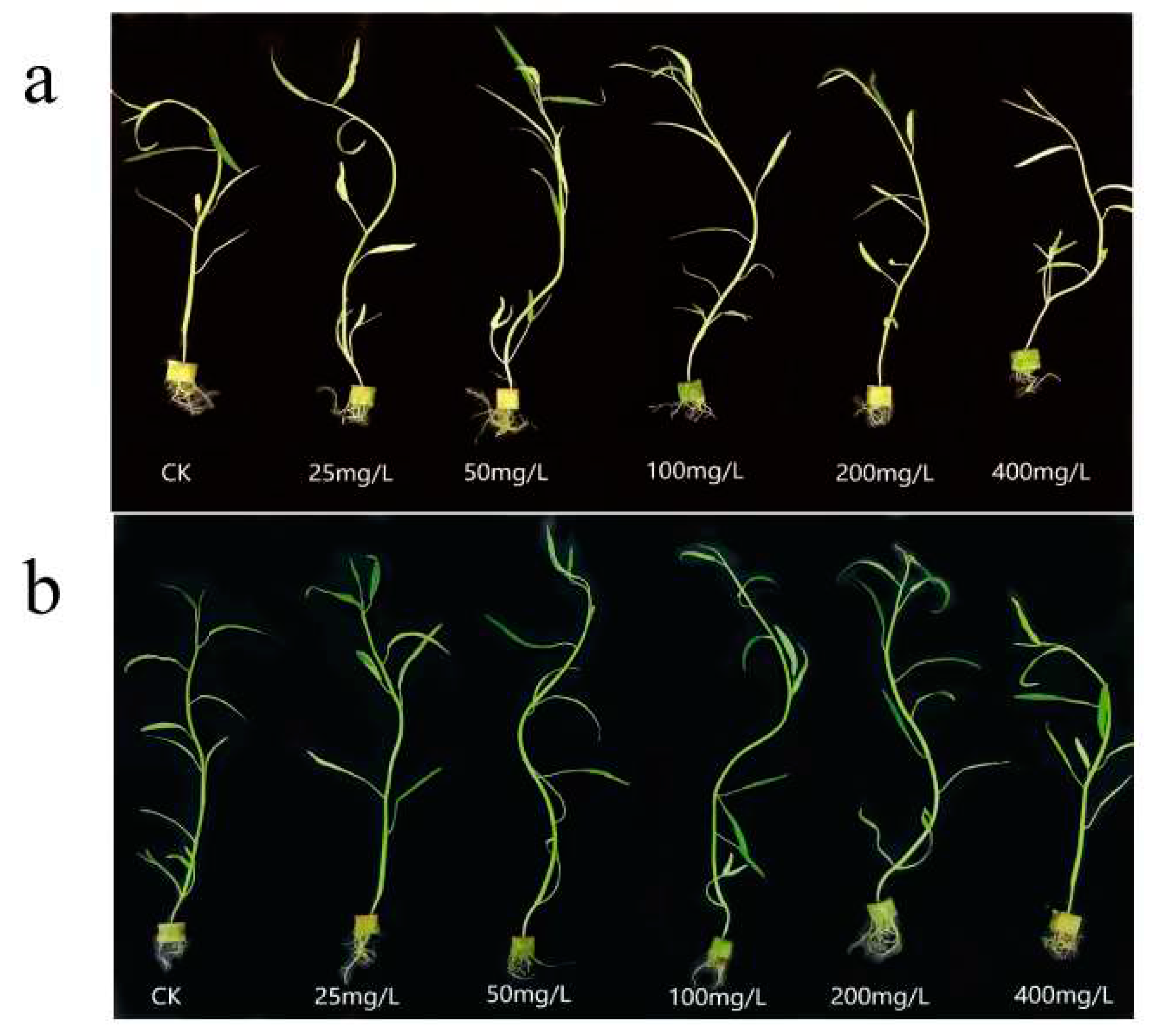
Figure 4 reflects the growth of water spinach at different N, P-CDs concentrations. We can see that at low concentrations of N, P-CDs, both treatments have a growth promoting effect on water spinach, while at a concentration of 200 mg/L, growth inhibition is shown.
As an edible vegetable, the leaves and stems of water spinach are the main part of the food, so it is particularly important to measure the parameters related to the thickness of the leaves and stems. Table S1 shows the values of the morphological indicators of water spinach after the leaf spraying treatment. It can be observed that the mean values of plant height, stem thickness, maximum functional leaf area, and the number of leaves of water spinach increase first and then decrease when further increase the concentration of N, P-CDs is. Water spinach treated with 50 mg/L N, P-CDs solution showed the best performance, with significantly higher mean values of stem thickness and the number of leaves than the blank group (P < 0.05). Table S2 shows the values of each morphological index of water spinach after the root spraying treatment. Similar to the results of the leaf spraying method, all indicators of water spinach improved at low concentrations of N, P-CDs solution, while most of the indicators of water spinach treated at high concentrations were lower than those of the blank group.
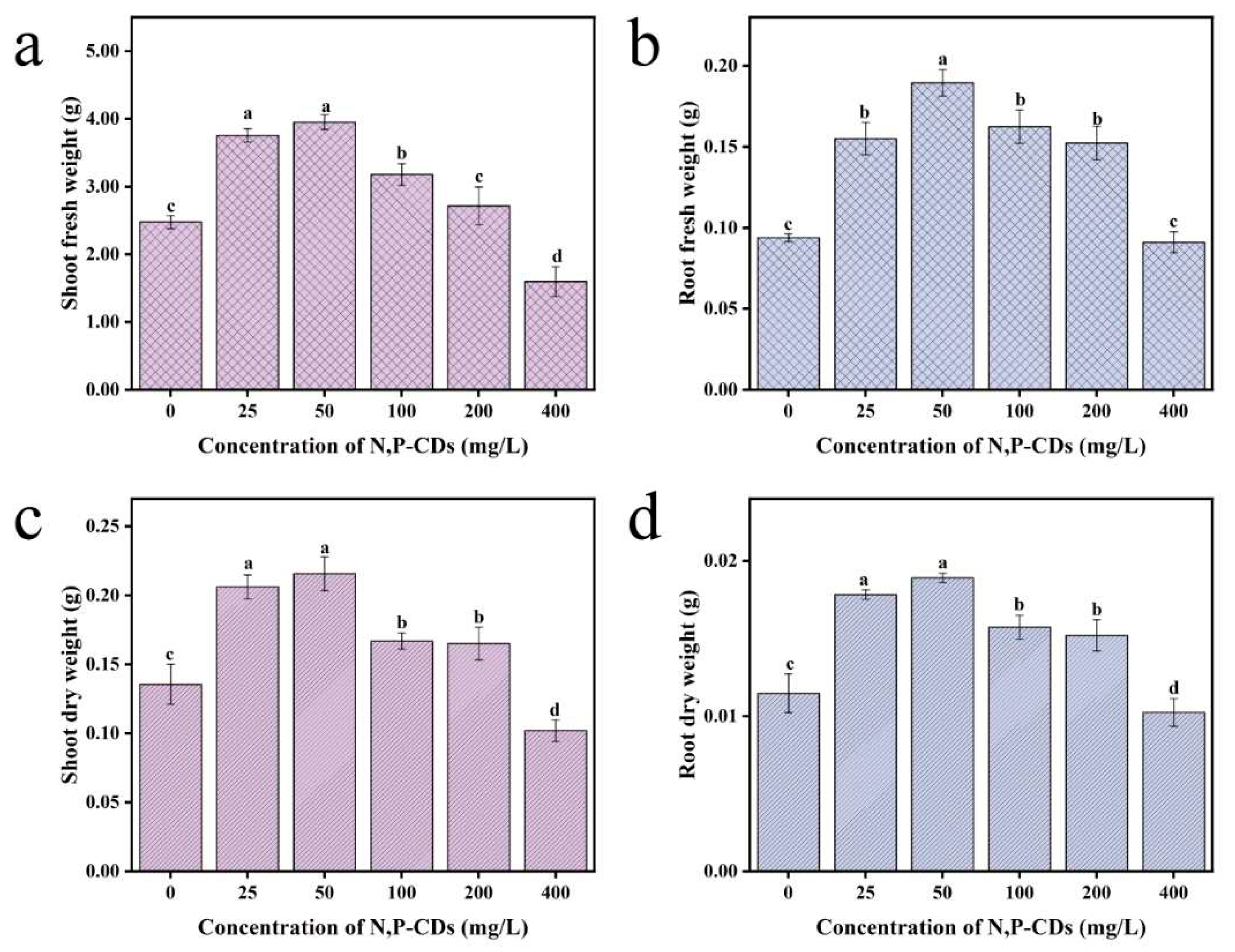
Usually, the amount of plant growth is the main indicator to evaluate the growth status of a plant and the plant's dry weight reflects the accumulation of organic matter in the plant. To investigate whether N, P-CDs can influence the extent of organic matter accumulation in water spinach. The results of the leaf spraying of N, P-CDs are shown in
Figure 5. At 50 mg/L treatment, the fresh weight of the stem and leaf parts of water spinach increased by 59.50 % compared to the blank group. The fresh weight of the root part, dry weight of the stem and leaf part, and dry weight of the root part of water spinach were improved by 101.71 %, 59.03 %, and 64. 78 %, respectively, under the treatment of 50 mg/L (P < 0.05). The results of N, P-CDs applied to the root system are shown in
Figure 6. At 100 mg/L, the fresh weight of stems and leaves of water spinach increased by 90.37 % compared to the blank group, and the difference was particularly significant (P < 0.05). The fresh weight of the root part, dry weight of the stem and leaf part, and dry weight of the root part of water spinach were elevated by 104.91 %, 67.32 %, and 68.88 %, respectively, under the treatment of 100 mg/L compared with the blank group. Taken together, the above experiments showed that the different cultivation methods were able to increase the dry weight of water spinach at low concentrations of N, P-CDs. The increase in fresh weight of the stem and leaf parts of water spinach was much greater with the root spraying method than with the leaf spraying method. The difference in dry weight increase between the two cultivation methods was not significant.
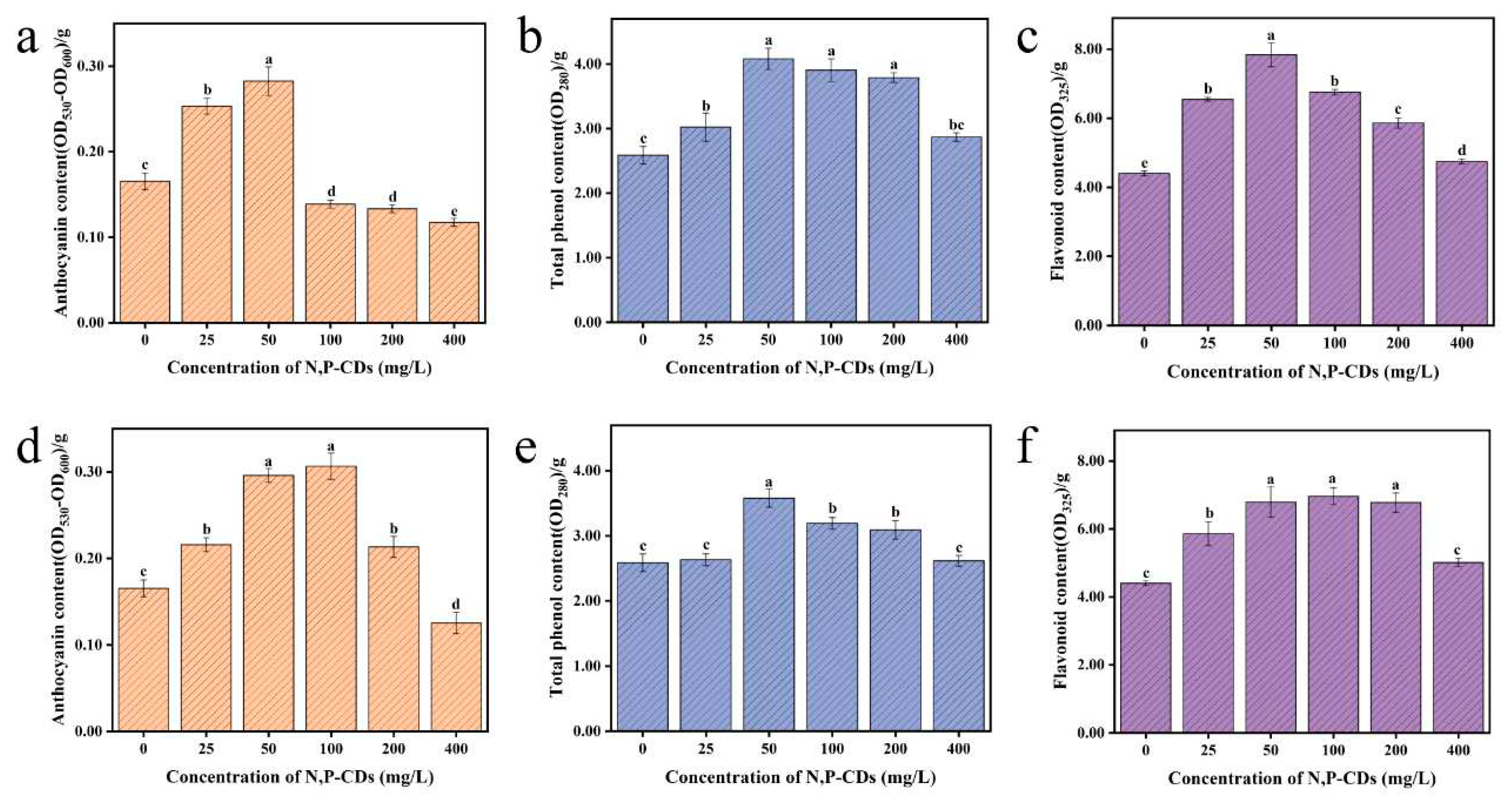
The anthocyanins, flavonoids, and total phenols in plants have free radical scavenging, antioxidant, and detoxifying properties. Therefore, measuring the content of anthocyanins, flavonoids and total phenols in a plant can evaluate the nutritional quality of the plant.
Figure 7a–c show the anthocyanin, total phenol, and flavonoid content of water spinach after leaf spraying. At 50 mg/L treatment, the anthocyanin content increased by 70.97 %, with significant variability between each treatment group (P < 0.05). It was observed that the anthocyanin content gradually decreased with increasing concentration of the carbon dots solution. The maximum value of total phenols was reached at 50 mg/L treatment, with an increase of 57.78 % compared to the blank group, with a significant difference between the blank groups (P < 0.05). The content of flavonoids reached a maximum of 50 mg/L, with an increase of 77.95 % compared to the blank group and a significant difference compared to the other treatment groups (P < 0.05).
Figure 7d–f show the anthocyanin, total phenol, and flavonoid content of water spinach after root spraying. The maximum anthocyanin content was reached under the 100 mg/L treatment with an increase of 85.49 % compared to the blank group, with significant differences from the blank group (P < 0.05). Total phenolic content reached a maximum at 50 mg/L treatment with an increase of 38.40 % compared to the blank group, with significant differences in each treatment group (P < 0.05). Flavonoid content reached a maximum at 100 mg/L treatment, with an increase of 58.08 % compared to the blank group, which was significantly different from the blank group (P < 0.05).
Combined with the above experiments, we found that N, P-CDs were effective in enhancing the anthocyanin, total phenol, and flavonoid content of water spinach. It is known that phenylalanine can be converted into other metabolites such as phenols, anthocyanins, flavonoids, and isoflavones through various enzymatic reactions [
24]. Phenylalanine ammonia-lyase (PAL) as a key enzyme in the phenylalanine metabolic pathway can influence the number of phenolic compounds produced from phenylalanine [
25]. Anthocyanins are a type of flavonoid and have several reactions in common with flavonoids in their synthetic pathway [
26]. The synthesis of flavonoids in plants is regulated by a combination of enzymes. However, the enzymes that mainly affect the reaction rate are chalcone synthase (CHS) and chalcone isomerase (CHI). The two enzymes affect the content of chalcone and naringenin and other substances, respectively, and the synthesis of anthocyanins in plants requires naringenin and other substances as precursors to synthesize [
27]. The flavonoid and anthocyanin contents of water spinach showed approximately the same trend in the experiment, and thus we propose the following mechanism: N, P-CDs can affect the activity of PAL and CHS, and the enhanced activity of PAL and CHS increases the flavonoid and total phenolic contents of water spinach. In addition, we found that the experimental results obtained with water spinach cultivated by leaf spraying and root spraying were not the same, which was attributed to the different ways carbon dots were transported in the plant. When treated with root spraying, the presence of a large number of groups on the surface of the carbon dots causes a positive charge to be attached to their surface, creating an electrostatic attraction with the negative charge on the surface of the plant roots [
28]. This promotes a large uptake of carbon dots at the root surface. The carbon dots that enter the plant's interior pass through the vascular system through the plant's roots, stems, and leaves and eventually reach the leaf veins [
11]. During this transport process some of the carbon dots are broken down by the roots, stems, and leaves of the plant, and the decomposition produces phytohormone analogs which have a growth-promoting effect on the plant[
19]. However, when treated by leaf spraying, the carbon dots can enter the plant's leaf veins directly, avoiding the loss of carbon dots during transport.
3.3. Effect of N, P-CDs on photosynthetic properties of water spinach
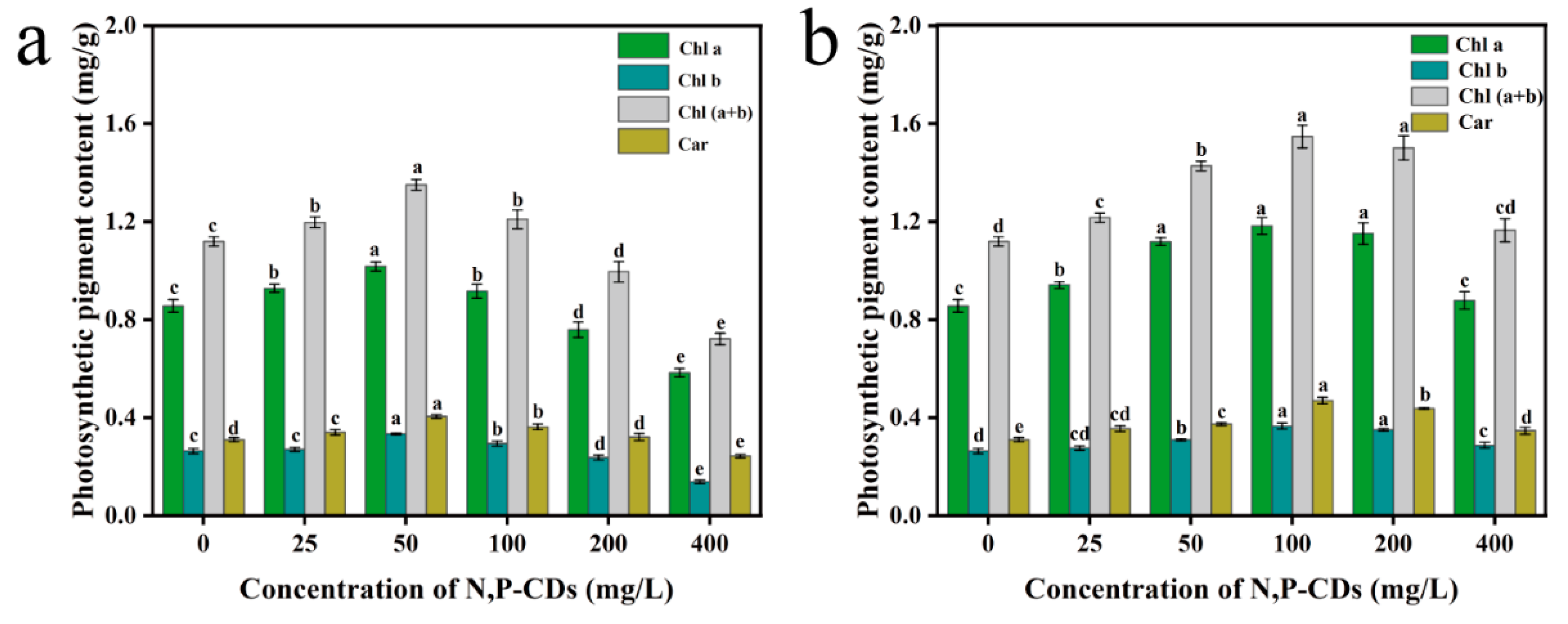
Photosynthetic pigments absorb and transmit light energy during photosynthesis in plants. The number of photosynthetic pigments greatly influences the photosynthetic rate of plants [
29]. We measured the chlorophyll a, chlorophyll b, chlorophyll (a+b), and carotenoid contents in water spinach.
Figure 8a shows the photosynthetic pigment content of the leaves after the application of the modal treatment. Compared to the blank group, chlorophyll a, chlorophyll b, chlorophyll (a+b) and carotenoids content were the most effective under the 50 mg/L treatment, with an increase of 18.84 %, 26.61 %, 20.67 %, and 30.62 %, respectively, with significant differences with the other treatment groups (P < 0.05).
Figure 8b shows the content of each photosynthetic pigment in the root spraying method treatment. Compared to the blank group, chlorophyll a, chlorophyll b, chlorophyll (a+b) and carotenoid content were the most effective in the 100 mg/L treatment, with an increase of 38.17 %, 38.67 %, 38.29 %, and 51.54 %, respectively. We found that N, P-CDs increased the photosynthetic pigment content in water spinach under different spraying methods. The leaf spraying method was generally less effective than the root spraying method in enhancing photosynthetic pigment content.
Plants convert solar energy into chemical energy through photosynthesis and use it to synthesize organic matter for their growth, so the rate of photosynthesis reflects the extent of plant growth. To understand the effect of different culture methods on the photosynthetic parameters of water spinach, we measured the values of net photosynthetic rate Pn, Gs, Ci, and Tr, as shown in Figure S1 and
Figure 2 in supporting information. The experimental results showed that the Pn value of water spinach treated with the leaf spraying method increased by 108.27 % at 50 mg/L compared to the blank group, which was a considerable increase. Compared to the blank group, Gs, Ci, and Tr values increased by 46.61 %, 40.99 %, and 71.52 % at 50 mg/L, respectively, and all four data were different from the blank group (P < 0.05). Compared to the blank group, water spinach treated with the root spraying method increased 131.96 %, 53.93 %, 50.50 %, and 88.66 % in Pn, Gs, Ci, and Tr values at 100 mg/L, respectively. All four data of this group were different from the blank group (P < 0.05).
Photosynthesis is usually divided into two parts: the light reaction and the dark reaction. During the dark reaction, CO
2 is reduced to organic matter through the Calvin cycle, a process that requires the consumption of adenosine triphosphate (ATP). The light reaction produces 2.57 ATP and 2 nicotinamide adenine dinucleotide phosphate ‘NADPH’ through electron transfer, while CO
2 fixation requires 3 ATP and 2 NADPH, indicating an imbalance in the supply of substances for this light and dark reactions [
30]. Combined with the above experiments, it was believed that N, P-CDs increased the content of photosynthetic pigments in water spinach, generating more photons for the chloroplast and thus increasing the light reaction rate. With the increase of the light reaction rate, a large amount of ATP and NADPH were produced to break this imbalance of supply and demand. In addition, the increase in net photosynthetic rate led to a corresponding increase in the stomatal conductance of water spinach. The increase in Ci values in water spinach can be attributed to the entry of carbon dots into the plant, some of which are degraded by horseradish peroxidase ‘HRP’ and H
2O
2 in the plant to produce CO
2[
19]. Studies have shown that plant water channel proteins can affect plant transpiration by regulating internal plant water [
31]. Considering that the carbon dots can activate the expression of genes encoding water channel proteins, we speculate that the increased Tr value of water spinach is related to the expression of genes encoding water channel proteins [
15].