Submitted:
20 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Dose-Response Test using X-Ray Irradiation
2.2.1. X-Ray Irradiator
2.2.2. Preparation of Adult Females and Eggs
2.2.3. X-ray Irradiation Treatment
2.2.4. Bioassays after Irradiation Treatment
2.3. Large-Scale Confirmatory Trials using Gamma Radiation
2.3.1. Cobalt-60 Radiation Facility and Treatment
2.3.2. Post-Treatment Rearing and Bioassays
2.4. Statistical Analysis
3. Results
3.1. Dose-Response Testing
3.1.1. Impact on Egg Number Laid by Irradiated Females
3.1.2. Two-Way ANOVA on Dose-Mortality of X-Ray Irradiation
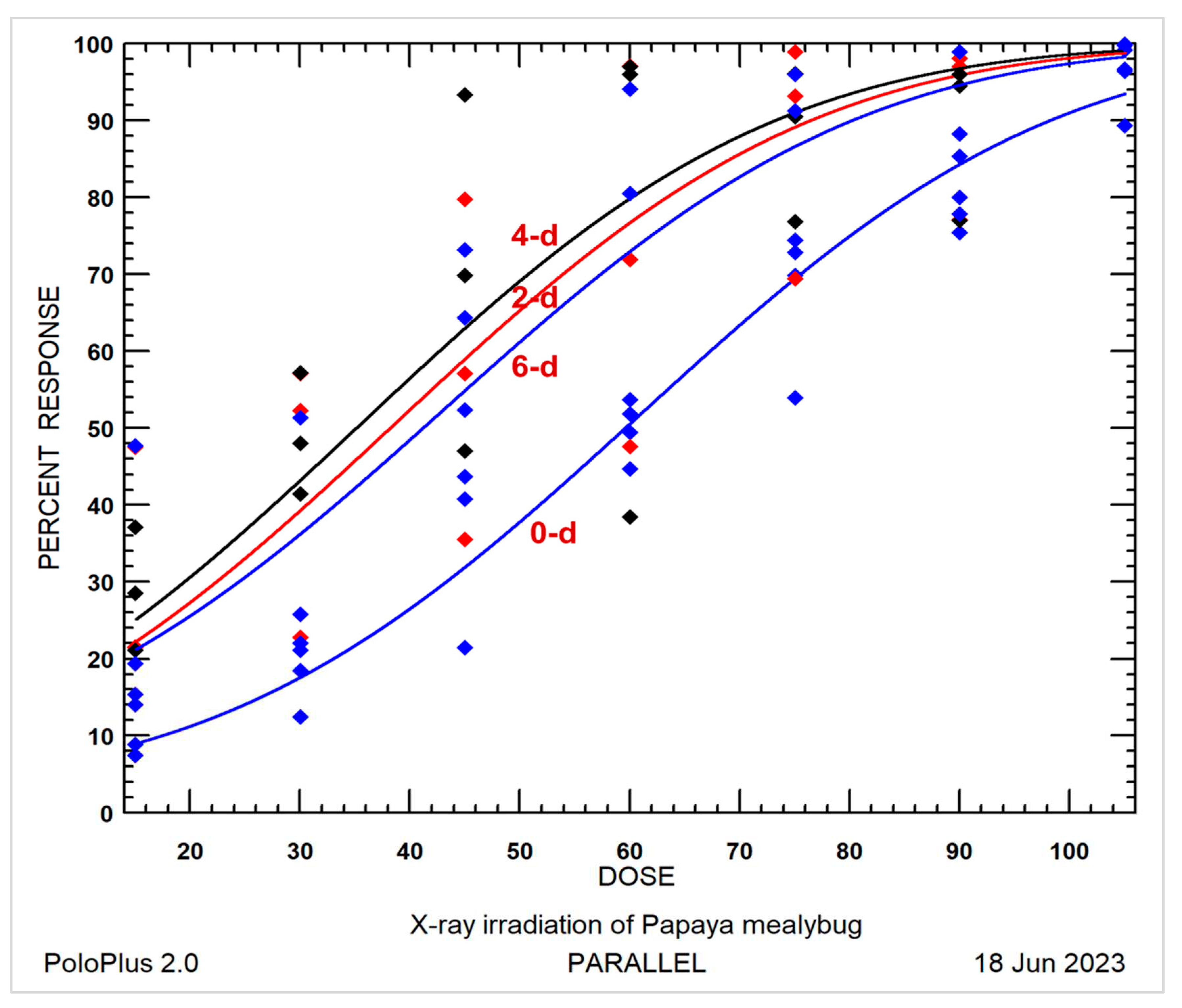
3.1.3. Probit Analysis of Dose-Mortality Data
3.2. Large-Scale Confirmatory Tests
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CABI. Paracoccus marginatus (papaya mealybug) datasheet (updated on 04 Jan 2023). 2023. Available online: https://www.cabi. org/isc/datasheet/39201 (accessed on 28 May 2023).
- Chuai, H.Y.; Shi, M.Z.; Li, J.Y.; Zheng, L.Z.; Fu, J.W. Fitness of the Papaya Mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae), after Transferring from Solanum tuberosum to Carica papaya, Ipomoea batatas, and Alternanthera philoxeroides. Insects 2022, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Finch, E.A.; Beale, T.; Chellappan, M.; Goergen, G.; Gadratagi, B.G.; Khan, M.A.; Rehman, M.A.; Rwomushana, I.; Sarma, A.K.; Wyckhuys, K.A.; et al. The potential global distribution of the papaya mealybug, Paracoccus marginatus, a polyphagous pest. Pest Manag. Sci. 2021, 77, 1361–1370. [Google Scholar] [CrossRef]
- ScaleNet. Paracoccus marginatus. 2023. Available online: http://scalenet.info/catalogue/Paracoccus%20marginatus/ (accessed on 18 April 2023).
- Ahmed, M.Z.; He, R.; Wu, M.T.; Gu, Y.J.; Ren, J.M.; Liang, F.; Li, H.L.; Hu, X.N.; Qiu, Q.; Mannion, C.M.; Ma, J. First report of the papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae), in China and genetic record for its recent invasion in Asia and Africa. Fla. Entomol. 2015, 98, 1157–1162. [Google Scholar] [CrossRef]
- (EFSA Panel on Plant Health) Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; et al. Scientific Opinion on the pest categorisation of Paracoccus marginatus. Efsa J. 2023, 21, 7899–41. [Google Scholar] [CrossRef]
- Kansiime, M.K.; Rwomushana, I.; Mugambi, I.; Makale, F.; Lamontagne-Godwin, J.; Chacha, D.; Kibwage, P.; Oluyali, J.; Day, R. Crop losses and economic impact associated with papaya mealybug (Paracoccus marginatus) infestation in Kenya, Int. J. Pest Manag. 2020, 1–14. [Google Scholar] [CrossRef]
- Miftakhurohmah, M.; Hendrastuti, H.S.; Hamzah, M.K.; Poernomo, W.S.B.; Wahyuno, D. Identification of mealybugs on Piper nigrum as vector of Piper yellow mottle virus (Badnavirus: Caulimoviridae). J. Trop. Plant Pests Dis. 2022, 22, 144–153. [Google Scholar] [CrossRef]
- Song, Z.J; Qing, Y. Ma, F.; Li, Y.; Lu, G.; Sun, H.; Li, Z.; Zhan, G. Predicting the potential geographical distribution of Paracoccus marginatus (Hemiptera:Pseudococcidae). Plant Quar. 2019, 33, 73–78. (In Chinese) [Google Scholar]
- Fang, Y.; Kang, F.; Zhan, G.; Ma, C.; Li, Y.; Wang, L.; Wei, Y.; Gao, X.; Li, Z.; Wang, Y. The effects of a cold disinfestation on Bactrocera dorsalis survival and navel orange quality. Insects 2019, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Zhao, J.; Ma, F.; Liu, B.; Zhong, Y.; Song, Z.; Zhao, Q.; Chen, N.; Ma, C. Radioprotective Effects on Late Third-Instar Bactrocera dorsalis (Diptera: Tephritidae) Larvae in Low-Oxygen Atmospheres. Insects. 2020, 11, 526. [Google Scholar] [CrossRef]
- Follett, P.A.; Neven, L.G. Current trends in quarantine entomology. Annu. Rev. Entomol. 2006, 59, 359–385. [Google Scholar] [CrossRef] [PubMed]
- IPPC (International Plant Protection Convention). 2008. Recommendation on: Replacement or reduction of the use of methyl bromide as a phytosanitary measure (adopted 2008, published 2017). Food and Agricultural Organization, Rome, Italy.
- Hallman, G.J.; Levang-Brilz, N.M.; Zettler, J.L.; Windborne, I.C. Factors affecting ionizing radiation phytosanitary treatments, and implications for research and generic treatments. J. Econ. Entomol. 2010, 103, 1950–1963. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Ma, F.H.; Deng, W.; Li, Z.H.; Song, Z.J.; Ma, C.; Ren, Y.L.; Du, X.; Zhan, G.P. Phytosanitary irradiation treatment of the aerial root mealybug, Pseudococcus baliteus (Hemiptera: Pseudococcidae). J. Econ. Entomol. 2023. [Google Scholar] [CrossRef] [PubMed]
- IPPC (International Plant Protection Convention). 2003. ISPM #18: Guidelines for the use of irradiation as a phytosanitary measure. Food and Agricultural Organization, Rome, Italy.
- IPPC (International Plant Protection Convention). 2023. ISPM #18: Requirements for the use of irradiation as a phytosanitary measure. Food and Agricultural Organization, Rome, Italy.
- Hallman, G.J.; Hénon, Y.M.; Parker, A.G.; Blackburn, C.M. Phytosanitary irradiation: an overview. Fla. Entomol. 2016, 99, 1–13. [Google Scholar]
- IPPC (International Plant Protection Convention). 2015. ISPM #28 PT 19: Irradiation treatment for Dysmicoccus neobrevipes, Planococcus lilacinus and Planococcus minor. Food and Agricultural Organization, Rome, Italy.
- IPPC (International Plant Protection Convention). 2023. ISPM #28 PT 45: Irradiation treatment for Pseudococcus jackbearsleyi. Food and Agricultural Organization, Rome, Italy.
- IPPC (International Plant Protection Convention). 2022. Available online: https://www.ippc.int/en/core-activities/standards-setting/list-topics-ippc-standards/list (accessed on 8 May 2023).
- Dias, V.S.; Hallman, G.J.; Martínez-Barrera, O.Y.; Hurtado, N.V.; Cardoso, A.A.S.; Parker, A.G.; Caravantes, L.A.; Rivera, C.; Araújo, A.S.; Maxwell, F.; et al. Modified atmosphere does not reduce the efficacy of phytosanitary irradiation doses recommended for Tephritid fruit flies. Insects. 2020, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Zarin, M.; Khan, Z.; Seth, R.K. Phytosanitary irradiation treatment against Maconellicoccus hirsutus (Hemiptera: Pseudococcidae). Fla. Entomol. 2016, 99, 102–113. [Google Scholar]
- IPPC (International Plant Protection Convention). 2007. ISPM #28: Phytosanitary treatments for regulated pests. Food and Agricultural Organization, Rome, Italy.
- Song, Z.J. 2020. Potential geographical distribution and phytosanitary irradiation treatment of Paracoccus marginatus. Master Degree Thesis, China Agriculture University, Beijing.
- Ma, C.; Zhan, G.; Zhong, Y.; Liu, B.; Gao, X.; Xu, L.; Wang, Y. Effects of X-ray irradiation on the eggs and females of Dysmicoccus lepelleyi (Hemiptera: Pseudococcidae). J. Econ. Entomol. 2019, 112, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, H.; Liu, B.; Zhao, J.P.; Zhao, Q.Y.; Song, Z.J.; Han, X.; Zhan, G.P. Gamma and X-ray irradiation as a phytosanitary treatment against various stages of Planococcus lilacinus (Hemiptera: Pseudococcidae). J. Asia Pac. Entomol. 2022, 25, 102009. [Google Scholar] [CrossRef]
- Couey, H.M.; Chew, V. Confidence limits and sample size in quarantine research. J. Econ. Entomol. 1986, 79, 887–890. [Google Scholar] [CrossRef]
- ISO/ASTM 51261:2013. Practice for calibration of routine dosimetry systems for radiation processing, 2nd edn. United States of America, International Organization for Standardization and ASTM International.
- Abbott, WS. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- DPS. Data Processing System. <b>2010</b>. User’s guide. Version 13.5. Hangzhou RuiFeng Information Technology Co., Lt. DPS. Data Processing System. 2010. User’s guide. Version 13.5. Hangzhou RuiFeng Information Technology Co., Lt., Hangzhou, China.
- LeOra Software. 2002. User’s guide: PoloPlus probit and logit analysis. Version 2.0. LeOra Software, Berkeley, CA.
- Nicholas, A.H.; Follett, P.A. Postharvest irradiation treatment for quarantine control of western flower thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2018, 111, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.W.; Park, R.M.; Bailer, A.J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 2006, 25, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- NAPO (North American Plant Protection Organization). 2011. RSPM#34: Development of phytosanitary treatment protocols for regulated arthropod pests of fresh fruits or vegetables. Ottawa, ON, Canada.
- Dohino, T.; Masaki, S. Effects of electron beam irradiation on comstock mealybug, Pseudococcus comstocki (Kuwana) (Homoptera: Pseudococcidae). Res. Bull. Plant Prot. Jpn. 1995, 31, 31–36. [Google Scholar]
- Shao, Y.; Ren, L.; Liu, Y.; Wang, Y. , Jiao, Y.; Wang, Q.; Zhan, G. The preliminary results of the impact on the development and reproduction of Jack Beardsley mealybug irradiated with Cobalt-60 gamma rays. Plant Quar. 2013, 27, 51–55. (In Chinese) [Google Scholar]
- Zhan, G.; Shao, Y.; Yu, Q.; Xu, L.; Liu, B.; Wang, Y.; Wang, Q. Phytosanitary irradiation of Jack Beardsley mealybug (Hemiptera: Pseudococcidae) females on rambutan (Sapindales: Sapindaceae) fruits. Fla. Entomol. 2016, 99, 114–120. [Google Scholar]
- Ravuiwasa, K.T.; Lu, K.H.; Shen, T.C.; Hwang, S.Y. Effects of irradiation on Planococcus minor (Hemiptera: Pseudococcidae). J. Econ. Entomol. 2009, 102, 1774–1780. [Google Scholar] [CrossRef]
- Khan, I.; Zahid, M.; Mahmood, F.; Zeb, A. Mortality and growth inhibition of γ-irradiated red scale Aonidiella aurantii (Hemiptera: Diaspdidae) on ‘Kinnow’ citrus (Sapindales: Rutaceae) fruits. Fla. Entomol. 2016, 99, 121–124. [Google Scholar]
- Follett, A. Irradiation as a Phytosanitary treatment for Aspidiotus destructor (Homoptera: Diaspididae). J. Econ. Entomol. 2006, 99, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bitani, S.; Habib, U.R. Mortality and growth inhibition of γ-rradiated Aspidiotus destructor (Hemiptera: Diaspididae), on mango (Sapindales: Anacardiaceae) plantlets. Fla. Entomol. 2016, 99, 125–129. [Google Scholar]
- Van Nieuwenhove, G.A.; Oviedo, A.V.; Perez, J.; Ruiz, M.J.; Dalto, Y.M.; Villagran, M.F.; Cazado, L. E.; Horak, C.I.; Gastaminza, G.A.; Willink, E.; Hallman, G.J. Gamma radiation phytosanitary treatment for Hemiberlesia lataniae (Hemiptera: Diaspididae). Fla. Entomol. 2016, 99, 134–137. [Google Scholar]
- Hofmeyr, H.; Hofmeyr, M.; Slabbert, K. Postharvest phytosanitary irradiation disinfestation of Planococcus citri and P. ficus (Hemiptera: Pseudococcidae). Fla. Entomol. 2016, 99, 166–170. [Google Scholar]
- Zhan, G.; Ren, L.; Shao, Y.; Wang, Q.; Yu, D.; Wang, Y.; Li, T. Gamma irradiation as a phytosanitary treatment of Bactrocera tau (Diptera: Tephritidae) in pumpkin fruits. J. Econ. Entomol. 2015, 108, 88–94. [Google Scholar] [CrossRef]

| Duration | Mean Number(±SE) of Eggs Laid by Irradiated Females a | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 Gy | 15 Gy | 30 Gy | 45 Gy | 60 Gy | 75 Gy | 90 Gy | 105 Gy | |
| 0-2 d | 1305.0±98.1aA | 1339.3±191.9aA | 1250.5±151.8aA | 1163.8±132.0aA | 1457.0±97.4aA | 998.8±74.0aA | 1255.5±107.2aA | 1281.8±237.5aA |
| 3-4 d | 1139.8±186.0aA | 1311.5±322.9aA | 1215.8±340.9aA | 982.0±86.2aA | 1246.5±108.1aA | 869.8±29.0aA | 1145.5±108.7aA | 1228.8±211.5aA |
| 5-6 d | 189.0±11.0aB | 199.8±10.5aB | 186.5±11.5aB | 208.0±9.1aB | 198.8±13.0aB | 124.0±4.7aB | 95.8±8.9aB | 60.5±7.0aB |
| Age | Mortality of age a | Corrected mortality (%) of eggs irradiated at dose b | ||||||
|---|---|---|---|---|---|---|---|---|
| 15 Gy | 30 Gy | 45 Gy | 60 Gy | 75 Gy | 90 Gy | 105 Gy | ||
| 0-d | 50.0±32.0b | 7.9±2.5eA | 16.9±5.0deB | 33.8±9.5cdeB | 47.5±2.6bcdA | 65.4±8.7abcA | 79.9±4.0abA | 98.6±1.3aA |
| 2-d | 68.6±28.7a | 29.6±12.4dA | 43.6±15.3cdAB | 57.1±18.2bcdAB | 72.0±20.4abcA | 87.2±12.9abA | 90.7±9.7abA | 100.0±0.0aA |
| 4-d | 71.9±27.8a | 28.7±6.5cA | 48.8±6.5bcAB | 70.0±19.0abAB | 77.2±27.5abA | 87.9±8.1aA | 90.7±6.5aA | 100.0±0.0aA |
| 6-d | 65.4±30.2a | 23.9±15.1cA | 28.1±14.8bcA | 61.5±9.9abA | 75.0±17.6aA | 86.1±10.5aA | 87.8±9.1aA | 95.1±4.6aA |
| Age (days) | No. eggs | Slope ± SE | Intercept ± SE | Estimating LDs and 95% CI (Gy) a | Hetero-geneity b | ||
|---|---|---|---|---|---|---|---|
| LD90 | LD99 | LD99.9968 | |||||
| 0-d | 53,249 | 0.034 ± 0.000 | -2.090 ± 0.022 | 97.8 (92.0 – 05.4)a | 128.2 (118.7 – 141.0)a | 176.7 (160.7 – 198.9)a | 79.4 |
| 2-d | 2,247 | 0.028 ± 0.001 | -1.030 ± 0.069 | 82.0 (70.5 – 102.5)bc | 119.1 (99.6 – 157.7)b | 178.5 (144.4 – 248.0)a | 14.4 |
| 4-d | 2,095 | 0.026 ± 0.001 | -0.843 ± 0.070 | 80.2 (67.6 – 104.7)c | 119.7 (97.7 - 168.2)b | 182.9 (143.8 – 272.1)a | 15.5 |
| 6-d | 2,195 | 0.028 ± 0.001 | -.130 ± 0.077 | 87.1 (76.0 – 105.0)b | 124.8 (106.4 – 158.3)ab | 185.2 (153.2 – 245.5)a | 11.1 |
| Reference Age (Days) | Pairwise Age (Days) | 95% CI of Lethal Dose Ratio | ||
|---|---|---|---|---|
| LD90 | LD99 | LD99.9968 | ||
| 0-d | 2-d | 1.25×1012 – ∞ | 273.36 – 4.24×1015 | 0.00 – 7.55×109 |
| 4-d | 4.49×1013 – ∞ | 13.80 – 6.31×1013 | 0.00 – 1.18×107 | |
| 6-d | 7.89×106 – 4.78×1014 | 0.00 – 1.73×1010 | 0.00 – 4.59×103 | |
| 2-d | 4-d | 0.00 – 1.38×107 | 0.00 – 1.44×109 | 0.00 – 1.17×1013 |
| 6-d | 0.00 – 1.86 | 0.00 – 5.28×103 | 0.00 – 8.80×109 | |
| 4-d | 6-d | 0.00 – 0.04 | 0.00 – 6.54×104 | 0.00 – 2.79×1015 |
| Irradiation Date | Absorbed Dose (Gy) | DUR | No. Potatoes | No. Females | F1 Generation Eggs | |
|---|---|---|---|---|---|---|
| No. a | Mortality (%) | |||||
| Feb. 13, 2023 | 150.4 – 183.6 | 1.22 | 439 | 9,992 | 2.5×106 | 100 |
| control | 0 | - | 60 | 2,056 | 5.2×105 | 2.84 |
| Apr. 10, 2023 | 146.8 – 185.0 | 1.26 | 817 | 50,376 | 1.3×107 | 100 |
| control | 0 | - | 75 | 4,749 | 1.2×106 | 3.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





