1. Introduction
The transcriptome analysis of humans, plants, fungi, and invertebrate genomes contains a large number of RNA transcripts that have low or null protein-coding potential, these sequences are defined as non-coding RNAs (ncRNAs) (1, 2). The ncRNAs can be separated into two classes, according to the length of the sequences, small and long ncRNAs (2-4). The first one involves the microRNAs (miRNAs), these ~22 nucleotides in length regulate gene expression mainly at the post-transcriptional level. The endogenous small ncRNA comprises mainly: small transfer RNA (tRNA), ribosomal RNA (rRNA), small interfering RNA (siRNA), and microRNA (miRNA) (4). The last one is the evolutionary conserved RNA molecule that presents its precursors usually in clusters within intergenic regions and introns of protein-coding genes and is less found in exons and antisense transcripts. In general, it is widely accepted that siRNA binds to target mRNA, this mainly occurs in the cytoplasm to interfere with protein production with the assistance or not of the RISC complex (4).
On the other hand, long ncRNAs (lncRNAs) are sequences of more than 200 nucleotides that cannot encode proteins. LncRNAs have been classified, according to the genomic localization and orientation, into intergenic lncRNAs (lincRNAs), intronic lncRNAs, natural antisense transcripts, pseudogenes (without producing protein), and retrotransposons (5). Interestingly, lncRNAs can regulate the expression of nearby genes on the same allele (in cis) or regulate genes at other genomic locations across the genome (in trans) (6). In general, lncRNAs can display various biological roles such as proliferation, differentiation, and cell development by different cellular pathways (2). Regarding localization, lncRNAs have been found to localize at both nuclear and cytoplasmic compartments where they bind to DNA, RNA, or proteins to exert their functions. In the nucleus, they interact with transcription factors, chromatin-modifying complex, or ribonucleoproteins, thereby altering the transcription of target genes (7, 8). Likewise, in the cytoplasm, lncRNAs display the regulation of mRNA and proteins (9, 10). Importantly, various preclinical and clinical studies showed that dysregulated expression of lncRNA is associated with the development of several diseases, including cancer. In cancer, lncRNAs can act like tumor suppressor genes (11) or oncogenes (12) depending on the downstream target pathway regulated by them. Furthermore, the expression profile of certain lncRNAs can be used as a biomarker for disease progression, survival, and chemoresistance (13, 14). However, the majority of lncRNAs identified warrant further verification and clinical explorations (2). Further studies revealed that lncRNAs are implicated in the regulation of inflammatory signaling pathways, innate immune response, and T cell differentiation and activation (15-17). In this review, we summarize the current state of knowledge regarding the pivotal role of lncRNAs in regulating immune responses in cancer.
2. Overview of tumor immunity
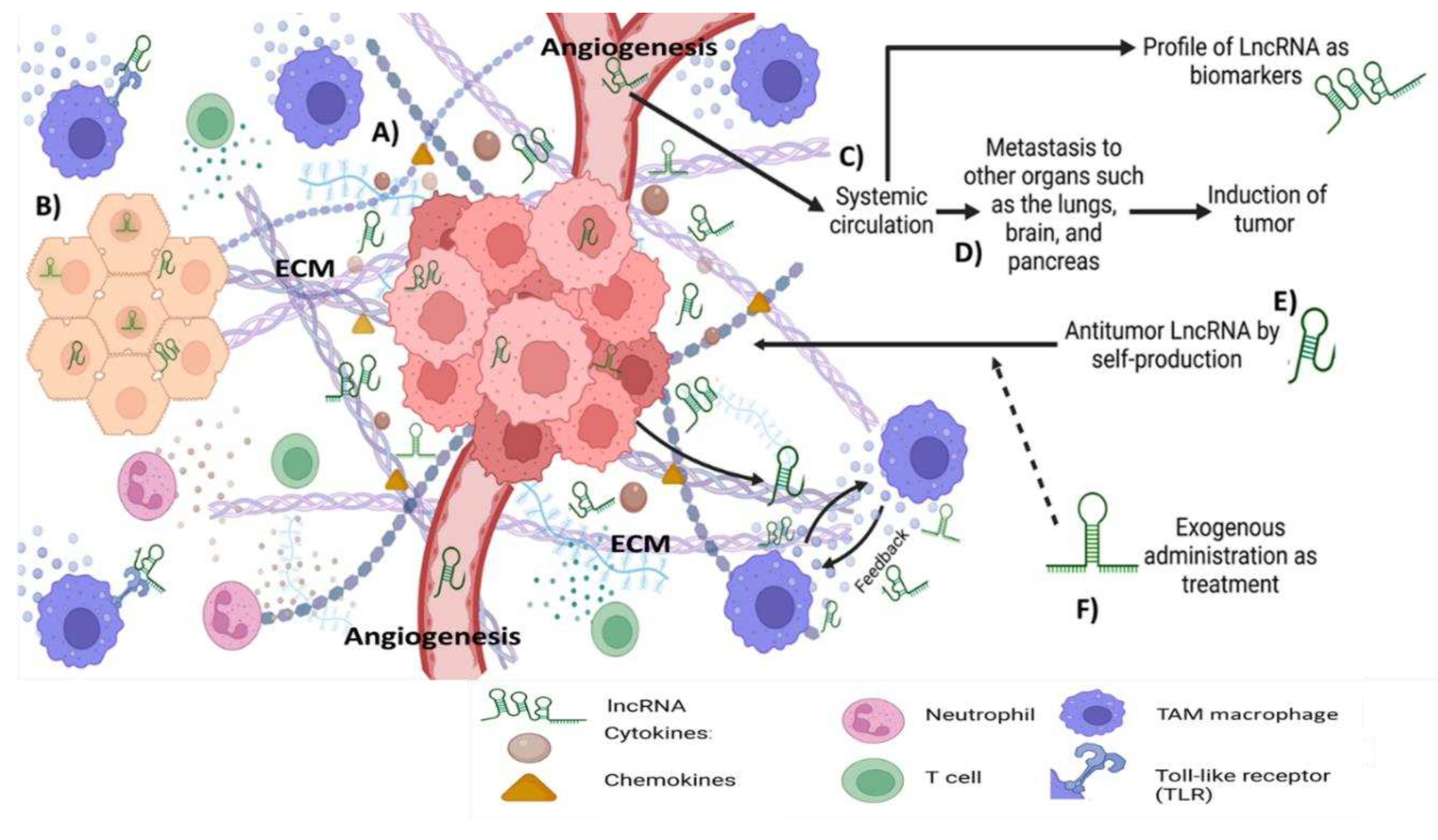
The tumor microenvironment is orchestrated by several intricate factors that involve crosstalk between cancer cells, noncancer vicinity cells, extracellular matrix, immune cells, and inflammatory mediators e.g., chemokines and cytokines, where the regulation is by the stage of the disease and the organ affected (solid or not solid tumor) (18-20) (Figure 1).
The dysregulated control of inflammation contributes importantly to the initiation, promotion, and metastasis of cancer. Clinical experiences have demonstrated that the use of anti-inflammatory drugs displays anticancer activity (21-23). NK cells act directly in the elimination of tumor cells; in the same manner, cytotoxic T lymphocytes can detect tumor antigens expressed on carcinoma cells and target those cells for destruction, and inhibit angiogenesis by secreting IFN-γ (24). Tumor-associated Macrophages (TAM), mainly M2 phenotype, can promote tumor proliferation and metastasis, whereas myeloid-derived suppressor cells (MDSC) and regulatory T (Treg) cells are the main components of the immune suppressive tumor microenvironment (TME) that induce T cells dysfunction thereby increasing tumor progression and metastasis (25, 26). Furthermore, the exogenous regulation of several microenvironmental factors has been emerging as a promising strategy against cancer treatment (27) (Figure 1).
The role of ncRNAs in tumorigenesis has been investigated in a wide array of cancers. In this setting, ncRNAs regulate proliferation, differentiation, apoptosis, necrosis, autophagy, immune response, and inflammation (17, 27-29). In colorectal cancer, it was reported that tumor suppressor miR-195-5p promotes TAM polarization by the suppression of NOTCH2 expression (30). Furthermore, the exosomal miRNAs (miR-934, miR-25-3p, miR-130b-3p, miR-425-5p) can induce the activation of the CXCL13/CXCR5 or CXCL12/CXCR4 axis in colorectal cancer cells, which in turn activate TAM polarization and metastasis to liver (31). On the other hand, lncRNAs such as lnc-EGFR, lncRNA SNHG1, Flicr, and Flatr can orchestrate the correct function and differentiation of Tregs (31). Moreover, lnc-EGFR has also been shown to stimulate Treg differentiation, inhibit cytotoxic T lymphocyte activity, and induce hepatocellular carcinoma (HCC) growth (32). In contrast, NIFK-AS1 lncRNA inhibited the M2-like polarization of macrophages, proliferation, migration, and invasion of endometrial cancer, at least in part, by inhibiting miR-146a (33). The formation of an immunosuppressive microenvironment, has been observed by the activity of lncRNAs over immune check points, like as lncRNA, MALAT1, that can upregulate the expression of PD-L1 through miR-195 and miR-200a-3, respectively (34). Additional information on lncRNA can be found below. The cellular communications through the different components in the TME are undoubtedly complex, however, during the last 10 years the role of immune response in regulating tumor development has made major progress. Nowadays, the concept of switching the negative immune response or tumor profile to an antitumor profile is latent, and possibly it will be a reality shortly (Figure 1). Nevertheless, is imperative a continuing study of the molecular and cellular pathways regulated and their clinical implications. Here, we emphasize the importance to eliminate the malignant cells to abrogate the drug resistance and their elimination without giving rise to an adverse microenvironment, using the owner immune modulation.
3. Role of lncRNAs in cancer
Cancer is the second leading cause of death globally which causes approximately 10 million in 2020 (35). In the US, the lifetime probability of developing cancer is ~44% for men or ~38% for women, respectively (36). It is well known that cancer involves a heterogeneous group of diseases that possess important hallmarks such as: maintained proliferative signaling; evasion of growth suppressors; allowing replicative immortality; activation of metastasis; promoting angiogenesis and resisting cell death. Thus, in general, the unifying feature is the multiplication of abnormal cells that grow beyond their natural boundaries (37). The knowledge of lncRNAs is an area of biological interest that has already received great attention in recent years. Moreover, it has been shown that dysregulated expression of lncRNAs has been detected in a wide array of cancers and they show differential expression patterns in different malignancies. Furthermore, some lncRNAs can act as oncogenes whereas others can act like tumor suppressor genes and this feature renders lncRNAs as potential diagnostic/prognostic biomarkers in cancers (38-40) However, this is not an easy task, because this requires considering the correct identification, stratification, patient personalization, drug delivery, and toxicity of the lncRNA (1, 3, 41).
In the context of tumor-promoting actions of lncRNAs, Elsayed et al. revealed that PRKAR-1B AS2 lncRNA promotes tumor growth and survival of ovarian cancer and knockdown of PRKAR1B-AS2 by specific siRNA reduced tumor growth and sensitized the response to cisplatin in both in vitro and in vivo mouse models of ovarian cancer. Mechanistically, PRKAR1B-AS2 lncRNA was found to promote tumor growth, at least in part, by positively regulating the PI3K/AKT/mTOR pathway (40). Furthermore, it was found that the steroid receptor RNA activator (SRA) serves as a coactivator of the progesterone receptor (PR), estrogen receptor (ER), glucocorticoid receptor (GR), and androgen receptor (AR); interestingly these steroid receptors have been implicated in tumors of reproductive organs in males and females (42). In 2011, almost 200 putative long ncRNAs derived from promoter regions of cell cycle genes were identified during the cell cycle, and their expression profiles showed alteration under certain oncogenic stimuli, stem cell differentiation, or DNA damage (43). The newly discovered long ncRNAs are more and more recognized as active molecules instead of “transcriptional noise” and pieces of evidence are accumulating that some of them have critical roles in carcinogenesis by influencing tumor cell proliferation (37).
Moreover, cancer cells showed replicative immortality; it is known that in normal conditions cell death can be occurred by apoptosis. However, several studies have shown the ability of malignant tumors to attenuate apoptosis and become resistant to different therapeutic modalities. The death cellular process can be induced by various external or internal stimuli. Chemotherapeutic agents such as cisplatinum, and etoposide induce DNA damage, with subsequent induction of apoptosis via the p53-dependent pathway (44). Regarding tumors, they show an increased expression of survival factors or anti-apoptotic regulators like Bcl-2 and Bcl-xL (37, 45, 46). For example, two lncRNAs, with antiapoptotic functions were identified in prostate and squamous carcinoma cells, the PCGEM1 (Prostate-specific transcript 1) and CUDR (cancer upregulated drug resistant). Multiple analyses by northern blot support the exclusive expression of PCGEM in the human prostate, additionally, the overexpression of this oncogenic lncRNA has been related to the risk of prostate cancer (47). Moreover, a functional study of PCGEM1 demonstrates that the overexpression of PCGEM1 In LNCaP cells (Lymph Node Carcinoma of the prostate) results in apoptosis inhibition induced by doxorubicin, briefly, the authors reported the significant delay of P53 and p21waf1/Cip1 induction in LNCaP cells overexpressing PCGEM1, after the treatment by doxorubicin compared with non-overexpressed cells. The inhibition of PARP cleavage by PCGEM1 overexpression was also reported in LNCaP-PCGEM1 cells treated with etoposide and sodium selenite(48). Similarly, CUDR gene overexpression in human squamous carcinoma A431 and A10A cells was more resistant to drug-induced apoptosis by doxorubicin and etoposide. The analysis by western-blot revealed that the stable transfection and overexpression of CURD results in the down-regulation of caspase 3 (49).; it is possible that shortly, these lncRNAs can be used as a therapeutic target for these types of tumors.
Meanwhile, necrosis, which sometimes refers to "uncontrolled" cell death can either eliminate cancer cells or promote their expansion. Necrotic cells usually attract pro-inflammatory cells, which in turn can activate angiogenesis, cancer cell proliferation, and invasiveness (50). Thus, more studies are necessary to fully understand the double-edged nature of this process and how it can be manipulated to achieve a beneficial effect for the patient (51).
4. lncRNAs as tumor biomarkers
One of the features to increase the survival of cancer patients is the development of new biomarkers for the prompt diagnosis of different tumor types. Interestingly, some studies have indicated that aberrant expression of lncRNAs is associated with the development of certain cancers. Thus, lncRNAs could be good candidates as tumor biomarkers because they have high specificity and sensitivity and do not require invasive procedures for their detection (52) (
Figure 1). For example, regarding the aberrant expression of the lncRNAs, namely HULC and Linc00152, the authors report that their expression is significantly higher in hepatocellular carcinoma compared to normal liver tissues (53). Similarly, prostate cancer gene 3 (PCA3, a lncRNA) has been considered a biomarker of prostate cancer (54). Another example is the recent identification of many dysregulated lncRNAs in non-small-cell lung cancer (NSCLCs) where the authors propose that almost 30 lncRNAs could be used for screening of effective and specific biomarkers of NSCLCs (55, 56). Therefore, it is possible in the future that some lncRNAs which have immune modulating properties (e.g. lncRNA-Cox 2, linc 1992 / THRILL, lncRNA-IL7R, HOTAIRM1, and lnc-DC) could be included as routine biomarkers for cancer. Other important criteria to use lncRNAs as biomarkers are the detection methods, possible sources of circulating lncRNAs, the outline of the biological functions, and expression level of the most significant lncRNAs in tissues, cell lines, and body fluids (whole blood, plasma, urine, gastric Juice, and saliva) of different kinds of tumors (52) (
Figure 1). In the immunological context of Cytotoxic T lymphocytes (CTL) by miRNA, it has been reported the feasibility to detect tumoral CTL activity non-invasively using CD8 antibody-conjugated magneto-plasmonic nanoparticles (MPNs) that are suitable for whole-body magnetic resonance (MR), and local optical and photoacoustic (PA) imaging in an ovarian experimental model (57). Similar strategies can be used after lncRNA treatment. Moreover, to enhance the clinical translation potential of these therapeutic modalities, its necessary to address further developing non-invasive imaging methods using MPNs to monitor CTL activity in tumors following therapy.
5. Activating antitumor immunity using long non-coding RNAs
The high recurrence rate presents a major challenge in the clinical management of some cancers, such as high-grade serous ovarian cancer (HGSOC) with a high recurrence due to largely incomplete eradication of tumor cells by standard therapy (58, 59). The stimulation of own immune system to recognize and attack tumor cells is an attractive means to facilitate the complete elimination of tumors (60). It has been observed that infiltrating cytotoxic T-cells (CTLs) can be localized in tumor sites, however, these germinal cells are only present in a small proportion, probably T cell lack either distinctive antigenic peptides or the adhesion or co-stimulatory molecules necessary to elicit a correct primary T-cell response (61). The difficulty to induce an effective anti-tumor immune response largely stems from the highly immunosuppressive microenvironment present in tumors and thus far, no effective immunostimulatory strategies have been developed to effectively enhance CTL activity (61, 62). The use of specific lncRNAs to relieve the immunosuppressive networks in tumors could allow CTLs to infiltrate and kill tumor cells, as we argue in this review. LncRNAs can regulate multiple pathways simultaneously that can prevent pathway redundancy or resistance, a feature which cannot be achieved by many other therapeutic agents, therefore, they may serve as promising agents for enhancing CTL function in several tumors (41).
6. LncRNAs and innate immune response
The first report of the controlling immune gene by lncRNAs in innate immune responses was observed by Guttman et al. in 1999 (63, 64). In this sense, studies of the innate immune responses mediated by Toll-like-receptors (TLRs) showed a promising biological role in cancer regulation (65). TLRs are usually involved in the recognition of specific pathogen-associated molecular patterns (PAMPs) derived from bacteria, viruses, fungi, and protozoa. The activation of the receptors allows a coordinated immune response to clear the infection and eliminate the pathogens (66, 67). Likewise, TLRs play a crucial role in tissue homeostasis by regulating wound healing, non-infectious inflammation, and tissue regeneration (68, 69). The regulation of inflammatory mediators (cytokines, acute proteins, antimicrobial peptides, among others) by lncRNAs via TLRs is not fully studied (70, 71). However, some studies reported that TLR4 and TLR7/8 can be activated by specific agonists (LPS, and R848 synthetic antiviral compound) that increased the expression of lncRNA-Cox2 via MyD88-NFkB pathway (16). The lncRNA-Cox2 showed nuclear and cytosolic localization in murine bone marrow-derived macrophages (BMDMs) (72); the regulation of lncRNA-Cox2 participated both in the activation and repression of immune responses. Moreover, the complex of lncRNA-Cox2 with hnRNP-A/B and hnRNP-A2/B1 can regulate the repression of CCL5 (70, 73). This immune mediator participates in the recruitment of T cells, eosinophils, neutrophils, and basophils to the inflammatory site. Furthermore, CCL5 levels correlated with tumor progression and prognosis in patients with gastric cancer, whereas in breast cancer CCL5 produced by breast cancer cells increase the production of matrix metalloproteinase by T cells and/or monocytes. Interestingly systemic treatment of mice with neutralizing anti-CCL5 antibodies reduced the extent of subcutaneous tumors, liver metastases, and peritoneal carcinosis. In a similar context, knockdown of CCL5 from CT26 (mouse colon tumor cells) inhibits apoptosis of CD8+ and as a consequence reduces the size of the tumor in the mice model (74, 75). For example, the activation of natural killer cells (NK) is mediated by this inflammatory chemokine. In this setting, it is important to comment that NK cells display rapid and potent immunity against tumors and this promising therapy is currently being explored in clinical patients (75). It is feasible that the use of lncRNA-Cox2 could modulate cytokines production and, consequently, prevent or diminish cancer progression by the regulation of immune cells.
On the other hand, in the human monocyte cell line (THP-1), it was reported a positive and negative feedback system produce TNF-α and IL-6 cytokines via TLR2, and this production was found to be regulated by the lncRNA linc1992/THRIL (76). This regulation was analyzed by pull-down assay and RIP assay, where linc1992 and hnRNPL showed a formation of an RNP complex in vivo. Moreover, the ChIP assay revealed that hnRNPL binds to the TNF-α promoter region, while the knockdown of linc1992 showed a reduced binding of hnRNPL to the TNF-α promoter region (76). TNF-α cytokines have potent anti-tumoral properties, as the name implies causing cancer cell death (77). TNF could be an endogenous tumor promoter because TNF stimulates tumor growth, proliferation, invasion, metastasis, and angiogenesis. However, TNF could be a cancer killer, and the antitumor role may involve immune responses e.g., promoting tumor stromal destruction by CTL or tumor-infiltrating macrophages (78). However, TNF-α could stimulate proliferation, survival, migration, and angiogenesis in most cancer cells resulting in tumor promotion. Conclusively, TNF-α plays the role of a double-edged sword that could be either pro- or anti-tumorigenic (77).
In addition, lncRNA microarray analysis showed that the stimulation of THP-1 cells with a TLR4 agonist (LPS) induces the expression of almost 443 lncRNAs by more than twofold and decreased the expression 718 lncRNAs more than twofold (73). In human peripheral blood mononuclear cells, Lnc-IL7R was one of the most up-regulated lncRNA after TLR4 and TLR3 activation by lipopolysaccharide and Pam3CSK4, respectively (79). In addition, a negative expression of E-selectin, VCAM-1, IL-8, and IL-6 was observed following TLR4 stimulation (79). The mechanism by which lncRNA-IL7R regulated E-selectin and VCAM-1 is dependent on the methylation of histone H3 at lysine (H3K27me3) (79). Moreover, increased plasma levels of VCAM-1 and E-selectin are associated with the advanced stage of breast cancer and with the presence of circulating cancer cells (80). Activation of different TLRs in cancer cells results in an inflammatory response that promotes tumorigenesis, however, TLR has been found to induce strong antitumor activity by indirectly activating the tolerant host immune system to destroy cancer cells (65). Therefore, the specific modulation of TLRs by a novel or typical agonist could stimulate the expression of specific lncRNAs that participates in immune checkpoints. Those lncRNAs could be used as a promising new strategy to treat cancer cells. More examples of ncRNA and the regulation of innate immune response can be revised in detail in the recent extensive review published (16).
7. lncRNA and cellular immune response
It is known that immune cells have a direct relationship with the progression or resolution of cancer (6), however, a specific lineage differentiation induced by lncRNA as a therapeutic strategy against cancer, is not been explored yet. LncRNAs are expressed by monocytes, macrophages, dendritic cells, neutrophils, T cells, and B cells. Moreover, it has been reported that these expressions are in direct relation to their different immune cellular stages (6). The stimulus for lncRNA expression includes development, differentiation, activation, and immune responses through different mechanisms, such as dosage compensation, imprinting, enhancer function, and transcriptional regulation (15, 81) (Figure 1).
The differentiation of immune cells (myeloid and lymphoid lineage) occurs in normal conditions, however, under an infectious or inflammatory process, the immune cells can be differentiated (82, 83). Recently, several lncRNAs during the stages of B cells differentiation: pre-B1, pre-B2, immature, naive, and memory, as well the study involves the identification of lncRNA from plasma cells from human bone marrow, naive, memory, and plasma blast cells. This meta-analysis could be a previous step of lncRNA identification associated with malignant lymphomas originating from the distinct stages of normal B-cell, in particular from the germinal centers of B-cells (84).
Furthermore, during the inflammatory process or infectious diseases, hematopoietic differentiation is an important feature that participates in the clearance or progression of infection or the inflammatory process (85). The lncRNA called HOX antisense intergenic RNA myeloid 1 (HOTAIRM1) participates in the differentiation of hematopoietic cells. HOTAIRM1 is the most transcript expressed during induced granulocytic differentiation in NB4 promyelocytic leukemia cell line and neutrophils (86). Besides, neutrophils have a classical role in antimicrobial functions and can also have a significant impact on the tumor microenvironment; through cytokines and chemokines production, which allows inflammatory cell recruitment and activation. Additionally, reactive oxygen species and proteinases released by neutrophils have specific roles in regulating tumor cell proliferation, angiogenesis, and metastasis (87). However, it has been demonstrated that infiltrating neutrophils in bronchoalveolar carcinoma, melanoma, renal carcinoma, and head and neck squamous cell carcinoma (HNSCC) is an indicator of poor prognosis. Future studies need to be performed to induce or not neutrophil differentiation as a cancer strategy, thus the use of lncRNA can be a promissory strategy to do that.
On the other hand, dendritic cells (DCs) are considered "nature’s adjuvants" that can control immune tolerance and active immunity; these cells also participate as natural agents for antigen delivery. In addition, DCs displays immunologic effects on tumors (88). Mouse models of cancer demonstrate that DCs can capture tumor antigens that are released from tumor cells and cross-present these antigens to T cells in tumor-draining lymph nodes. This results in the generation of tumor-specific CTLs that contribute to tumor rejection. Thus, DCs represent important targets for therapeutic interventions against cancer. Moreover, it was recently found that lnc-DC (a lncRNA) was upregulated during human DC development. In specific, lnc-DC bound directly to STAT3 and maintains its phosphorylation (89). Likewise, knockdown of lnc-DC was shown to have functional consequences, such as deficient expression of membrane receptors required for T cell activation that include CD80/86, HLA-DR, and CD40, impairment in antigen, and decreased IL-12 production after stimulation (90). Therefore, further studies are required to better understand the role of lnc-DC in DC differentiation. In the future, a concordance between lncRNA regulation and immune cell differentiation could be used as an alternative strategy that would define cancer progression.
8. Adatative immunity
Usually, adaptative immunity refers to B and T cells, it is known that ncRNA can exert important regulation in lymphocyte biology such as the relation of NKkB, NOTCH, MYC, and TCR/CRR signaling, additionally, lncRNA are related to cell effect functions(16). Examples of lncRNA include lincrR-Ccr2-5AS, GAS5, and NeST, which regulate TH2 cells, TH17, and CD8+ cells, respectively.
The analysis of expression and regulation of intergenic lncRNA during T cell development and differentiation revealed that lincrR-Ccr2-5AS, an associated gene involved in the chemokine-mediated signaling pathway, plays and crucial role in cell differentiation and migration. Briefly, lymphocytes that were knocked down showed down-regulation of Ccr1 to Ccr5 (essential chemokine receptor for migration), moreover, the authors showed that CD45+ cells with low lincrR-Ccr2-5AS expression displayed impaired migration to the lungs in mice model (91).
The correct proliferation of lymphocyte populations is necessary for the correct regulation of the immune response and prevention of leukemic and autoimmune disease, in this context the growth-arrest-specific transcript 5 (GAS5) is crucial for the normal growth arrest in T-cell and nondifferentiated lymphocytes. The overexpression of GAS5 results in an increased rate of apoptosis and reduction of cell-cycle progression promoting an important reduction f lymphocyte populations(92). The use of this lncRNA as a target can be used in the control of leukemia and/or other types of cancer.
The NeST (nettoie Salmonella pas Theiler’s [cleanup Salmonella not Theiler’s] showed in a murine model that binds to WDR5 (histone component H3 lysine 4 methyltransferase complex promoting the alteration of histone 3 methylation at the IFN-γ locus, thus the authors conclude that this lncRNA regulates epigenetic marking of IFN, promoting susceptibility to a viral and bacterial pathogen (93). This could be extrapolated to the human being, with the intrinsical consequences in the dysregulation of IFN-γ activity in cancer and other pathologies.
It is important to mention that the immune and adaptative response works together in an intricate network for the correct function and elimination of pathogens or tumor cells. Thus, slight alterations in the correct function can affect the communication network and alternate the correct function of immunity. It is possible that in the coming years, a new lncRNA related to adaptative response can be incorporated in the field of cancer and other chronic and acute disease.
9. Immune checkpoints and lncRNA
Another mechanism of the cancer cell to evade the elimination by immune cells involve the expression of immune checkpoint molecules. The cell signaling of tumor cells promotes the downregulation of immunoreceptors, that in consequence causes the overstimulation of inhibitory immunoreceptors (94, 95). Thus, the block of the activation of inhibitory immunoreceptors could reactivate the antitumoral functions of immune cells, this has been explored at the experimental level and can be extrapolated into clinical settings shortly. At present, a variety of inhibitory immunoreceptors related to cancer have been identified such as PD-1, (programmed cell death protein 1) CTLA-4 (T lymphocyte-associated antigen), LAG-3 (lymphocyte-activation gene 3), TIM-3 (T cell immunoglobulin domain and mucin domain-3), TIGIT (T-cell immune receptor with immunoglobulin and ITIM domain) and BTLA (94).
The programmed death protein 1 (PD-1) and its ligand 1 (PD-L1) are excellent examples of the importance of negative checkpoints in cancer. PD-1 is expressed on the surface of T cells and myeloid cells, whereas PD-L1 is present on the surface of tumor cells (96). The crosstalk between PDL-1 and PD-1 inhibits the proliferation and production of cytokines and turns off the cytotoxic activity of T cells (96). Currently, there are available antibodies against PD-L1 that showed great success in melanoma, leukemia, and lymphoma, however, their efficacy in solid tumors is limited (97). Recently, it was revealed that not only PD-1 and PD-L1 play a pivotal role in lung adenocarcinoma progression, but also lncRNAs derived from them. The authors found that the PD-L1 lncRNA splice isoform is upregulated by IFNγ, and promotes the proliferation and invasion of lung adenocarcinoma cells via the direct binding to c-Myc enhancing its transcriptional activity. The authors concluded that combined therapy of PD-Li and PD-L1-lnc needs to be considered in lung cancer treatment and it could be applied to other types of cancer as well (98).
Additionally, for the regulation of PD-1/PD-1 pathway, it was reported that Lnc-OC1 enhances PD-L1 expression, allowing cell viability and inhibiting cell apoptosis in endometrial cancer cells (99). Similarly, many lncRNAs showed a positive correlation with PD-L1 in hepatocellular carcinomas (HCC), such as MIR155HG, PCED1B-AS1, and MIAT (100). The latter lncRNA was strongly related to the expression of PD-1, PD-L1, and CTLA4 in HCC, correlating with the immune escape by the regulation of JAK2, SLC6A6, KCND1 genes and resistance to sorafenib (100). During the last 5 years, several research groups have focused their efforts to obtain a prognostic, resistant, and aggressive signature of immune-related lncRNA in different cancers. The computational modeling and the simultaneous analysis of several cellular parameters allow for obtaining valuable information on immune checkpoints that probably can be used in clinical settings for the treatment of particular cancer patients shortly (101-103).
10. Challenges in using lncRNAs for immunotherapy
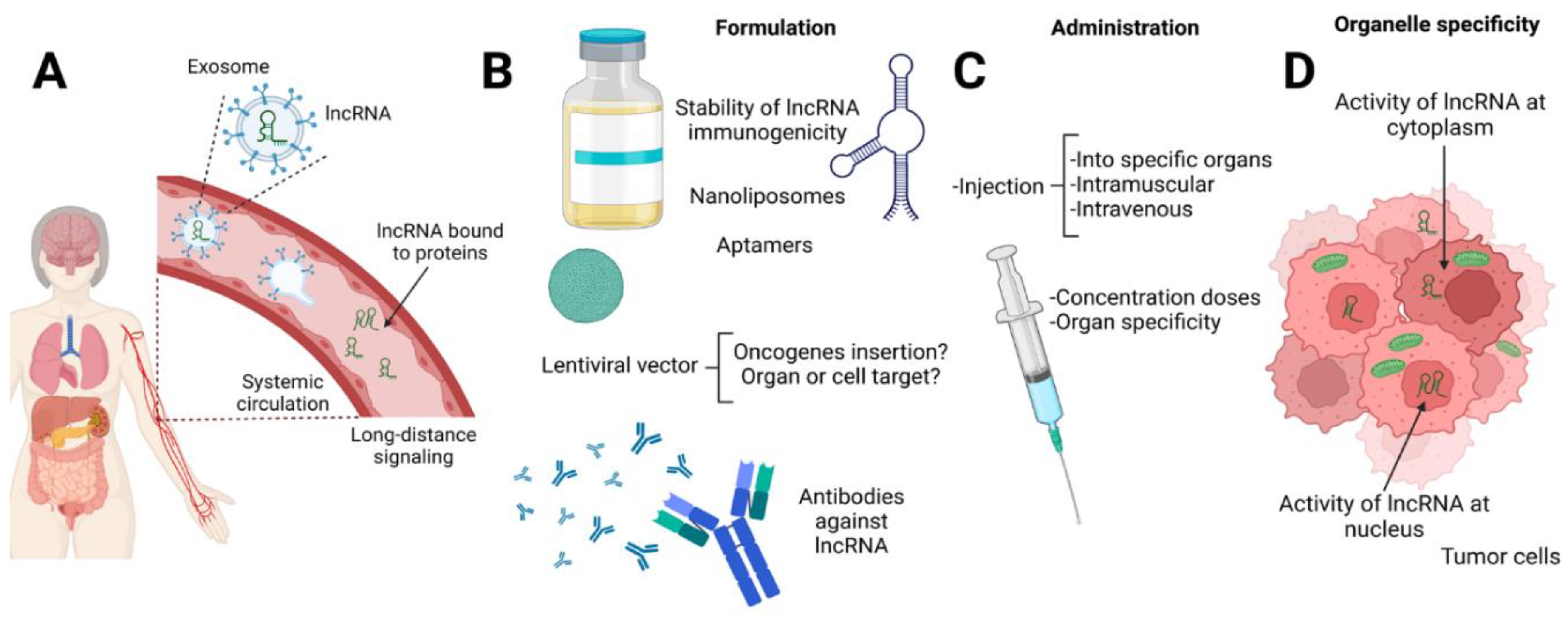
As mentioned before, lncRNAs play complex and extensive roles in gene regulation through epigenetic, transcriptional, and post-transcriptional activity, hence lncRNA can be found in the cytoplasmatic and nuclear levels. Moreover, lncRNAs are transported to distal regions or organs into exosomes for intercellular communication (
Figure 2). In the tumoral microenvironment, exosomal lncRNAs also display a pivotal role in the proliferation, immunosuppression, and chemoresistance of cancer cells (104). Considering the conditions mentioned above, the delivery of ncRNAs in specific cells can be considered one of the most important challenges. Another important challenge is the successful delivery of lncRNAs into tumor sites to enhance the immune response due to their rapid degradation in biological fluids by nucleases and the difficulty in their selective delivery to target cells. Moreover, the subsequent activity in the cytoplasm or nucleus is not an easy task during the design of therapeutic strategies for cancer treatment that involve the use of lncRNA. Several strategies to overcome the challenges of
in-vivo delivery of lncRNA have been explored (105), such as cell-penetrating peptides to induce dominant tolerance assisted by regulatory T cells (Treg) (106), the use of monoclonal antibodies for breast cancer (107) and aptamers to inhibit lung cancer cell invasion, tumor growth and angiogenesis (108). At present, the most common delivery strategies involve the use of viral and non-viral vectors.
Lentiviral vectors are usually used in experimental conditions to achieve targeted lncRNA cellular overexpression and knockdown, but their real use is controversial (109). The third generation of lentiviral vectors has been showing safety in clinical trials to introduce genes in hematopoietic stem cells to correct primary immunodeficiencies, also the use of this strategy was explored to introduce genes into T cells CAR T-cell with clinical success in B malignancies. However, it is necessary more studies in the context of ncRNA and lentiviral vectors due to the theoretical possibility of insertion of oncogenes, however, this has not been demonstrated until now (110).
The delivery of lncRNA using non-viral vectors includes the use of nanoliposomes; which has been used in preclinical studies, showing promising results (40, 111). Some nanoliposomes are composed of either cationic or cationic-anionic compositions. However, the composition that allows the correct delivery in the nucleus is a continuous challenge. Nevertheless, the recent application of ASO-gold-TAT NPs targeting lncRNA MALAT1 in lung cancer noticeably suppressed tumor metastasis in an animal model, prolonging the survival of the animal until 80% (112). Additionally, innovative combinations of liposomes and lipid NPs are coupled with aptamers, antibodies, peptides, and protein ligands, among others to form an active or passive targeted drug delivery system (109). However, the stability, doses of lncRNA, immunogenicity, administration route, real risk, and pathological effects are uncertain, to diminish or eliminate these issues is necessary first a deep knowledge of lncRNA and its meticulous regulation. The approved drug database on the website of the FDA and EMA contains almost 14 types of liposomal products authorized, and two of them include the use of DOPC (1, 2-dioleoyl-sn-glycero-3-phosphocholine) for the delivery of adjuvants for vaccines and morphine and anesthetic delivery(113). It is important to consider the DOPC (nanoliposomes strategy because this has been providing successful in vivo trials of the effective and extensive delivery of miRNAs in the specific tissue in the miRNA system of ovarian cancer (114, 115). It is important to continue with the experimental and clinical trials to discover the most successful strategy for the delivery of lncRNA to resolve chronic and acute diseases, including the development of tumors or cancer by different etiologies.
11. Conclusion and Perspectives
The incessant study of lncRNA has provided new clues about the immune role in the tumor microenvironment; the implications include its use as a biomarker as well as a therapeutic target. At present extensive evidence about the control of immune response by several lncRNA has emerged (innate and adaptative immunity), this is an interesting field that can be explored to fight and eliminate tumors by harnessing the owner's immune modulation. However, before its clinical application, it is imperative to discriminate the role of each lncRNA by the type of cancer. Likewise, the development and design of novel strategies for the correct delivery of the ncRNA in the tumor is an essential challenge that needs to be resolved quickly in parallel studies. The correct combination of lncRNA target and delivery system can be reduced importantly the number of patients dying of cancer or could be used as an efficient tool to improve life expectancy in the coming years.
Author Contributions
Conceptualization, M. M-C., A. H.; writing—original draft preparation, M. M-C., and A. H.; writing—review and editing, A. H.A., P.A., and C.R.-A.; supervision, P.A., and C.R.-A. All authors have read and agreed to the published version of the manuscript.
Funding
Abdelrahman M. Elsayed was supported by the Egyptian Ministry of Higher Education (EMHE).
Acknowledgments
We thank the scientific publication editor, The University of Texas MD Anderson Cancer Center for his critical reading of the manuscript, We thank Itzel Altamirano-Mendoza for her help in the design and preparation of the images.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ncRNAs |
non-coding RNAs |
| lncRNA |
long ncRNAsmiRNAs; microRNAs |
| tRNA |
small transfer RNA |
| rRNA |
ribosomal RNA |
| siRNA |
small interfering RNA |
| miRNA |
microRNA |
| RISC complex |
RNA-induced silencing complex |
| lincRNAs |
intronic lncRNAs |
| TAM |
Tumor-associated Macrophages |
| lncRNA SNHG1 |
long ncRNAs- small nucleolar RNA hot gene1 |
| Flicr |
Foxp3 long intergenic noncoding RNA |
| Flatr |
Foxp3-specific lncRNA anticipatory of Tregs |
| MDSC |
myeloid-derived suppressor cells |
| TME |
tumor microenvironment |
| NOTCH2 |
Neurogenic locus notch homolog protein 2 |
| lnc-EGFR |
long ncRNAs- Epidermal growth factor receptor |
| HCC |
hepatocellular carcinoma |
| PD-L1 |
programmed death protein 1 ligand |
| SRA |
steroid receptor RNA activator |
| PR |
progesterone receptor |
| ER |
estrogen receptor |
| GR |
glucocorticoid receptor |
| AR |
androgen receptor |
| PCGEM1 |
Prostate-specific transcript 1 |
| CUDR |
cancer upregulated drug resistant |
| Treg |
regulatory T cells |
| DOPC |
1, 2-dioleoyl-sn-glycero-3-phosphocholine |
| PCA3 |
prostate cancer gene 3 |
| NSCLCs |
Non-small-cell Lung Cancer |
| MPNs |
magneto-plasmonic nanoparticles |
| MR |
magnetic resonance |
| PA |
photoacoustic |
| HGSOC |
high-grade serous ovarian cancer |
| CTL |
cytotoxic T-cells |
| TLRs |
Toll-like-receptors |
| PAMPs |
pathogen-associated molecular patterns |
| BMDMs |
bone marrow-derived macrophages |
| NK |
natural killer cells |
| THP-1 |
human monocyte cell line |
| VCAM-1 |
Vascular Cell Adhesion Molecule 1 |
| HOTAIRM1 |
HOX antisense intergenic RNA myeloid 1 |
| HNSCC |
head and neck squamous cell carcinoma |
| DCs |
dendritic cells |
| CTLA-4 |
T lymphocyte-associated antigen |
| LAG-3 |
lymphocyte-activation gene 3 |
| TIM-3 |
T cell immunoglobulin domain and mucin domain-3 |
| TIGIT |
T-cell immune receptor with immunoglobulin and ITIM domain |
References
- Huttenhofer, A.; Vogel, J. Experimental approaches to identify non-coding RNAs. Nucleic acids research. 2006, 34, 635–46. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.T.; Colognori, D.; Lee, J.T. Long noncoding RNAs: past, present, and future. Genetics. 2013, 193, 651–69. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nature reviews Cancer. 2015, 15, 321–33. [Google Scholar] [PubMed]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Current genomics. 2010, 11, 537–61. [Google Scholar] [CrossRef] [PubMed]
- Carninci, P.; Kasukawa, T.; Katayama, S.; Gough, J.; Frith, M.C.; Maeda, N.; et al. The transcriptional landscape of the mammalian genome. Science 2005, 309, 1559–63. [Google Scholar]
- Elling, R.; Chan, J.; Fitzgerald, K.A. Emerging role of long noncoding RNAs as regulators of innate immune cell development and inflammatory gene expression. Eur J Immunol. 2016, 46, 504–12. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, K.M.; Abdelmohsen, K.; Yoon, J.H.; Panda, A.C.; Munk, R.; et al. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev. 2016, 30, 1224–39. [Google Scholar] [CrossRef] [PubMed]
- Cabili, M.N.; Dunagin, M.C.; McClanahan, P.D.; Biaesch, A.; Padovan-Merhar, O.; Regev, A.; et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Nishizawa, M.; Ozaki, T.; Kimura, T.; Hashimoto, I.; Yamada, M.; et al. Natural antisense transcript stabilizes inducible nitric oxide synthase messenger RNA in rat hepatocytes. Hepatology. 2008, 47, 686–97. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Z.M.; Chang, Y.N.; Hu, Z.M.; Qi, H.X.; Hong, W. The ways of action of long non-coding RNAs in cytoplasm and nucleus. Gene 2014, 547, 1–9. [Google Scholar] [CrossRef]
- Huang, J.F.; Guo, Y.J.; Zhao, C.X.; Yuan, S.X.; Wang, Y.; Tang, G.N.; et al. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology 2013, 57, 1882–92. [Google Scholar] [CrossRef] [PubMed]
- Guzel, E.; Okyay, T.M.; Yalcinkaya, B.; Karacaoglu, S.; Gocmen, M.; Akcakuyu, M.H. Tumor suppressor and oncogenic role of long non-coding RNAs in cancer. North Clin Istanb. 2020, 7, 81–6. [Google Scholar] [CrossRef]
- Badowski, C.; He, B.; Garmire, L.X. fBlood-derived lncRNAs as biomarkers for cancer diagnosis: the Good, the Bad and the Beauty. NPJ precision oncology. 2022, 6. [Google Scholar] [CrossRef] [PubMed]
- Beylerli, O.; Gareev, I.; Sufianov, A.; Ilyasova, T.; Guang, Y. Long noncoding RNAs as promising biomarkers in cancer. Non-Coding Rna Res. 2022, 7, 66–70. [Google Scholar] [CrossRef]
- Ahmad, I.; Valverde, A.; Ahmad, F.; Naqvi, A.R. Long Noncoding RNA in Myeloid and Lymphoid Cell Differentiation, Polarization and Function. Cells. 2020, 9. [Google Scholar] [CrossRef]
- Bocchetti, M.; Scrima, M.; Melisi, F.; Luce, A.; Sperlongano, R.; Caraglia, M.; et al. LncRNAs and Immunity: Coding the Immune System with Noncoding Oligonucleotides. International journal of molecular sciences. 2021, 22. [Google Scholar] [CrossRef]
- Walther, K.; Schulte, L.N. The role of lncRNAs in innate immunity and inflammation. RNA biology. 2021, 18, 587–603. [Google Scholar] [CrossRef]
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as Organs: Complex Tissues that Interface with the Entire Organism. Developmental cell. 2010, 18, 884–901. [Google Scholar] [CrossRef]
- Wang, J.; Peng, C.; Dai, W.; Chen, X.; Meng, J.; Jiang, T. Exploring Tumor Immune Microenvironment and Its Associations With Molecular Characteristics in Melanoma. Frontiers in oncology. 2022, 12, 821578. [Google Scholar] [CrossRef]
- Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; et al. Anti-Inflammatory Drugs as Anticancer Agents. International journal of molecular sciences. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Sakamoto, H.; Kawamura, R.; Wang, W.; Imai, K.; Shinomura, Y. Nonsteroidal anti-inflammatory drugs and oxidative stress in cancer cells. Histol Histopathol. 2007, 22, 437–42. [Google Scholar]
- Kazberuk, A.; Chalecka, M.; Palka, J.; Surazynski, A. Nonsteroidal Anti-Inflammatory Drugs as PPAR gamma Agonists Can Induce PRODH/POX-Dependent Apoptosis in Breast Cancer Cells: New Alternative Pathway in NSAID-Induced Apoptosis. International journal of molecular sciences. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. British journal of cancer. 2021, 124, 359–67. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; et al. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis - Current Status. Current medicinal chemistry. 2021, 28, 8203–36. [Google Scholar] [PubMed]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013, 138, 105–15. [Google Scholar] [CrossRef]
- Xing, Y.; Ruan, G.; Ni, H.; Qin, H.; Chen, S.; Gu, X.; et al. Tumor Immune Microenvironment and Its Related miRNAs in Tumor Progression. Frontiers in immunology. 2021, 12, 624725. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liu, J.R.; Xie, J.R.; Yuan, X.X.; Wang, B.Y.; Shen, W.J.; et al. Regulation of autophagy by non-coding RNAs in gastric cancer. Frontiers in oncology. 2022, 12. [Google Scholar] [CrossRef]
- Tang, W.W.; Zhu, S.M.; Liang, X.; Liu, C.; Song, L.J. The Crosstalk Between Long Non-Coding RNAs and Various Types of Death in Cancer Cells. Technol Cancer Res T. 2021, 20. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; Sun, M.; Zhang, C.; Wei, C.; Yang, C.; et al. miR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. Journal of hematology & oncology. 2019, 12, 20. [Google Scholar]
- Wu, Z.; Ju, Q. Non-Coding RNAs Implicated in the Tumor Microenvironment of Colorectal Cancer: Roles, Mechanisms and Clinical Study. Frontiers in oncology. 2022, 12, 888276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.Q.; Tang, J.W.; Chen, Y.; Deng, L.; Ji, J.; Xie, Y.; et al. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Zhao, W.; Mao, L.W.; Wang, Y.L.; Xia, L.Q.; Cao, M.; et al. Long non-coding RNA NIFK-AS1 inhibits M2 polarization of macrophages in endometrial cancer through targeting miR-146a. Int J Biochem Cell B. 2018, 104, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.N.; Qi, W.C.; Xia, B.R.; Lou, G.; Jin, W.L. Corrigendum: Long Non-Coding RNAs in the Tumor Immune Microenvironment: Biological Properties and Therapeutic Potential. Frontiers in immunology. 8919. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021, 71, 209–49. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Gutschner, T.; Diederichs, S. The hallmarks of cancer A long non-coding RNA point of view. Rna Biology. 2012, 9, 703–19. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Frontiers in medicine. 2020, 7, 612393. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Amero, P.; Salama, S.A.; Abdelaziz, A.H.; Lopez-Berestein, G.; Rodriguez-Aguayo, C. Back to the Future: Rethinking the Great Potential of lncRNA(S)for Optimizing Chemotherapeutic Response in Ovarian Cancer. Cancers. 2020, 12. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Bayraktar, E.; Amero, P.; Salama, S.A.; Abdelaziz, A.H.; Ismail, R.S.; et al. PRKAR1B-AS2 Long Noncoding RNA Promotes Tumorigenesis, Survival, and Chemoresistance via the PI3K/AKT/mTOR Pathway. Int J Mol Sci. 2021, 22. [Google Scholar] [CrossRef]
- Lavorgna, G.; Vago, R.; Sarmini, M.; Montorsi, F.; Salonia, A.; Bellone, M. Long non-coding RNAs as novel therapeutic targets in cancer. Pharmacol Res. 2016, 110, 131–8. [Google Scholar] [CrossRef]
- McKenna, N.J.; Lanz, R.B.; O'Malley, B.W. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999, 20, 321–44. [Google Scholar] [PubMed]
- Hung, T.; Wang, Y.; Lin, M.F.; Koegel, A.K.; Kotake, Y.; Grant, G.D.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011, 43, 621–9. [Google Scholar] [CrossRef] [PubMed]
- Tsuruo, T.; Naito, M.; Tomida, A.; Fujita, N.; Mashima, T.; Sakamoto, H.; et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003, 94, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014, 2014, 150845. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007, 26, 1324–37. [Google Scholar] [CrossRef]
- Srikantan, V.; Zou, Z.; Petrovics, G.; Xu, L.; Augustus, M.; Davis, L.; et al. PCGEM1, a prostate-specific gene, is overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 2000, 97, 12216–21. [Google Scholar] [CrossRef]
- Fu, X.Q.; Ravindranath, L.; Tran, N.; Petrovics, G.; Srivastava, S. Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell Biol. 2006, 25, 135–41. [Google Scholar] [CrossRef]
- Tsang, W.P.; Wong, T.W.L.; Cheung, A.H.H.; Co, C.N.N.; Kwok, T.T. Induction of drug resistance and transformation in human cancer cells by the noncoding RNA CUDR. Rna. 2007, 13, 890–8. [Google Scholar] [CrossRef]
- Zong, W.X.; Thompson, C.B. Necrotic death as a cell fate. Gene Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kroemer, G. Necroptosis: A Specialized Pathway of Programmed Necrosis. Cell. 2008, 135, 1161–3. [Google Scholar] [CrossRef]
- Shi, T.; Gao, G.; Cao, Y.L. Long Noncoding RNAs as Novel Biomarkers Have a Promising Future in Cancer Diagnostics. Dis Markers. 2016. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Tang, J.; Jiang, R.; Zhang, W.; Ji, J.; et al. HULC and Linc00152 Act as Novel Biomarkers in Predicting Diagnosis of Hepatocellular Carcinoma. Cell Physiol Biochem. 2015, 37, 687–96. [Google Scholar] [CrossRef] [PubMed]
- Leyten, G.H.; Hessels, D.; Jannink, S.A.; Smit, F.P.; de Jong, H.; Cornel, E.B.; et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014, 65, 534–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, X.; Zhu, C.; Guo, L.; Li, Q.; Liu, M.; et al. The prognostic value of long non coding RNAs in non small cell lung cancer: A meta-analysis. Oncotarget. 2016, 7, 81292–304. [Google Scholar] [CrossRef]
- Wei, M.M.; Zhou, G.B. Long Non-coding RNAs and Their Roles in Non-small-cell Lung Cancer. Genomics Proteomics Bioinformatics. 2016, 14, 280–8. [Google Scholar] [CrossRef]
- Sokolov, K.V.; Wu, C.H.; Cook, J.; Zal, T.; Emelianov, S.Y. Biomedical applications of magneto-plasmonic nanoclusters (Conference Presentation). Proc Spie. 2016, 9722. [Google Scholar]
- Rustin, G.; van der Burg, M.; Griffin, C.; Qian, W.; Swart, A.M. Early versus delayed treatment of relapsed ovarian cancer. Lancet. 2011, 377, 380–1. [Google Scholar] [CrossRef]
- Salomon-Perzynski, A.; Salomon-Perzynska, M.; Michalski, B.; Skrzypulec-Plinta, V. High-grade serous ovarian cancer: the clone wars. Arch Gynecol Obstet. 2017, 295, 569–76. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr; Travers, P.M.W. Using the immune response to attack tumors. Immunobiology: The Immune System in Health and Disease. 2001, 5th edition(New York: Garland Science; ).
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013, 14, 1014–22. [Google Scholar] [CrossRef]
- Melero, I.; Rouzaut, A.; Motz, G.T.; Coukos, G. T-Cell and NK-Cell Infiltration into Solid Tumors: A Key Limiting Factor for Efficacious Cancer Immunotherapy. Cancer Discov. 2014, 4, 522–6. [Google Scholar] [CrossRef]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009, 458, 223–7. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Fitzgerald, K.A. Transcription of inflammatory genes: long noncoding RNA and beyond. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2015, 35, 79–88. [Google Scholar]
- Shi, M.; Chen, X.; Ye, K.; Yao, Y.; Li, Y. Application potential of toll-like receptors in cancer immunotherapy: Systematic review. Medicine (Baltimore). 2016, 95, e3951. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Science review: key inflammatory and stress pathways in critical illness - the central role of the Toll-like receptors. Crit Care. 2003, 7, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Dalmaroni, M.J.; Gerswhin, M.E.; Adamopoulos, I.E. The critical role of toll-like receptors--From microbial recognition to autoimmunity: A comprehensive review. Autoimmun Rev. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Donndorf, P.; Abubaker, S.; Vollmar, B.; Rimmbach, C.; Steinhoff, G.; Kaminski, A. Therapeutic progenitor cell application for tissue regeneration: Analyzing the impact of toll-like receptor signaling on c-kit+ cell migration following ischemia-reperfusion injury in vivo. Microvasc Res. 2017, 112, 87–92. [Google Scholar] [CrossRef]
- Seki, E.; Park, E.; Fujimoto, J. Toll-like receptor signaling in liver regeneration, fibrosis and carcinogenesis. Hepatol Res. 2011, 41, 597–610. [Google Scholar] [CrossRef]
- Murphy, M.B.; Medvedev, A.E. Long noncoding RNAs as regulators of Toll-like receptor signaling and innate immunity. J Leukoc Biol. 2016, 99, 839–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X. Long noncoding RNAs in innate immunity. Cell Mol Immunol. 2016, 13, 138–47. [Google Scholar] [CrossRef]
- Menon, M.P.; Hua, K.F. The Long Non-coding RNAs: Paramount Regulators of the NLRP3 Inflammasome. Frontiers in immunology. 2020, 11. [Google Scholar] [CrossRef]
- Imamura, K.; Akimitsu, N. Long Non-Coding RNAs Involved in Immune Responses. Frontiers in immunology. 2014, 5, 573. [Google Scholar] [CrossRef]
- Sugasawa, H.; Ichikura, T.; Kinoshita, M.; Ono, S.; Majima, T.; Tsujimoto, H.; et al. Gastric cancer cells exploit CD4+ cell-derived CCL5 for their growth and prevention of CD8+ cell-involved tumor elimination. International journal of cancer. 2008, 122, 2535–41. [Google Scholar] [CrossRef]
- Aldinucci, D.; Colombatti, A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014, 2014, 292376. [Google Scholar] [CrossRef]
- Li, Z.; Chao, T.C.; Chang, K.Y.; Lin, N.; Patil, V.S.; Shimizu, C.; et al. The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proceedings of the National Academy of Sciences of the United States of America. 2014, 111, 1002–7. [Google Scholar] [CrossRef]
- van Horssen, R.; Ten Hagen, T.L.; Eggermont, A.M. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. The oncologist. 2006, 11, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta pharmacologica Sinica. 2008, 29, 1275–88. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xie, N.; Tan, Z.; Banerjee, S.; Thannickal, V.J.; Abraham, E.; et al. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol. 2014, 44, 2085–95. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.C.; Garcao, F.; Coutinho, E.C.; De Oliveira, C.F.; Regateiro, F.J. Soluble VCAM-1 and E-selectin in breast cancer: relationship with staging and with the detection of circulating cancer cells. Neoplasma. 2006, 53, 538–43. [Google Scholar]
- Geng, H.; Tan, X.D. Functional diversity of long non-coding RNAs in immune regulation. Genes & diseases. 2016, 3, 72–81. [Google Scholar]
- Kondo, M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunological reviews. 2010, 238, 37–46. [Google Scholar] [CrossRef]
- Lai, A.Y.; Kondo, M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. The Journal of experimental medicine. 2006, 203, 1867–73. [Google Scholar] [CrossRef] [PubMed]
- Petri, A.; Dybkaer, K.; Bogsted, M.; Thrue, C.A.; Hagedorn, P.H.; Schmitz, A.; et al. Long Noncoding RNA Expression during Human B-Cell Development. PLoS One. 2015, 10, e0138236. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. Journal of immunology research. 9836. [Google Scholar]
- Zhang, X.; Lian, Z.; Padden, C.; Gerstein, M.B.; Rozowsky, J.; Snyder, M.; et al. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood. 2009, 113, 2526–34. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.D.; Houghton, A.M. Tumor-Associated Neutrophils: New Targets for Cancer Therapy. Cancer research. 2011, 71, 2411–6. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Dhodapkar, K.M.; Palucka, A.K. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ. 2008, 15, 39–50. [Google Scholar] [CrossRef]
- Satpathy, A.T.; Chang, H.Y. Long Noncoding RNA in Hematopoiesis and Immunity. Immunity. 2015, 42, 792–804. [Google Scholar] [CrossRef]
- Laouar, Y.; Welte, T.; Fu, X.Y.; Flavell, R.A. STAT3 is required for FIM-dependent dendritic cell differentiation. Immunity. 2003, 19, 903–12. [Google Scholar] [CrossRef]
- Hu, G.Q.; Tang, Q.S.; Sharma, S.; Yu, F.; Escobar, T.M.; Muljo, S.A.; et al. Expression and regulation of intergenic long noncoding RNAs during T cell development and differentiation. Nature immunology. 2013, 14, 1190–U118. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Hedge, V.L.; Kirkham, L.; Farzaneh, F.; Williams, G.T. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5). J Cell Sci. 2008, 121, 939–46. [Google Scholar] [CrossRef]
- Gomez, J.A.; Wapinski, O.L.; Yang, Y.W.; Bureau, J.F.; Gopinath, S.; Monack, D.M.; et al. The NeST Long ncRNA Controls Microbial Susceptibility and Epigenetic Activation of the Interferon-gamma Locus. Cell. 2013, 152, 743–54. [Google Scholar] [CrossRef]
- Gaikwad, S.; Agrawal, M.Y.; Kaushik, I.; Ramachandran, S.; Srivastava, S.K. Immune checkpoint proteins: Signaling mechanisms and molecular interactions in cancer immunotherapy. Semin Cancer Biol. 2022, 86, 137–50. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–9. [Google Scholar] [CrossRef] [PubMed]
- Gatalica, Z.; Snyder, C.; Maney, T.; Ghazalpour, A.; Holterman, D.A.; Xiao, N.Q.; et al. Programmed Cell Death 1 (PD-1) and Its Ligand (PD-L1) in Common Cancers and Their Correlation with Molecular Cancer Type. Cancer Epidem Biomar. 2014, 23, 2965–70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fu, X.Y.; Li, Q.; Niu, T. Safety and Efficacy of Anti-PD-1 Monoclonal Antibodies in Patients With Relapsed or Refractory Lymphoma: A Meta-Analysis of Prospective Clinic Trails. Front Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Qu S, Jiao Z, Lu G, Yao B, Wang T, Rong W, et al. PD-L1 lncRNA splice isoform promotes lung adenocarcinoma progression via enhancing c-Myc activity. Genome Biol. 2021, 22, 104. [Google Scholar] [CrossRef]
- Jiang, W.; Pan, S.; Chen, X.; Wang, Z.W.; Zhu, X. The role of lncRNAs and circRNAs in the PD-1/PD-L1 pathway in cancer immunotherapy. Mol Cancer. 2021, 20, 116. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Y.; Ou, Q.; Wang, X.; Tang, N. LncRNA MIAT correlates with immune infiltrates and drug reactions in hepatocellular carcinoma. Int Immunopharmacol. 2020, 89, 107071. [Google Scholar] [CrossRef]
- Wang, X.; Cao, K.; Guo, E.; Mao, X.; Guo, L.; Zhang, C.; et al. Identification of Immune-Related LncRNA Pairs for Predicting Prognosis and Immunotherapeutic Response in Head and Neck Squamous Cell Carcinoma. Front Immunol. 2021, 12, 658631. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Fan, X.; Li, W.; Li, X.; Zhu, H.; et al. A novel prognostic signature of immune-related lncRNA pairs in lung adenocarcinoma. Scientific reports. 2021, 11, 16794. [Google Scholar] [CrossRef]
- Xue, Y.; Ning, B.; Liu, H.; Jia, B. Construction of immune-related lncRNA signature to predict aggressiveness, immune landscape, and drug resistance of colon cancer. BMC Gastroenterol. 2022, 22, 127. [Google Scholar] [CrossRef]
- Han, S.Q.; Qi, Y.Q.; Luo, Y.M.; Chen, X.P.; Liang, H.F. Exosomal Long Non-Coding RNA: Interaction Between Cancer Cells and Non-Cancer Cells. Frontiers in Oncology. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Pi, F.M.; Sharma, A.; Rajabi, M.; Haque, F.; Shu, D.; et al. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Advanced drug delivery reviews. 2014, 66, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.Y.; Kronenfeld, J.P.; Gattas-Asfura, K.M.; Bayer, A.L.; Stabler, C.L. Engineering an "infectious" T(reg) biomimetic through chemoselective tethering of TGF-beta1 to PEG brush surfaces. Biomaterials.
- Yao, Y.D.; Sun, T.M.; Huang, S.Y.; Dou, S.; Lin, L.; Chen, J.N.; et al. Targeted delivery of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and metastasis. Sci Transl Med. 2012, 4, 130ra48. [Google Scholar] [CrossRef]
- Lai, W.Y.; Wang, W.Y.; Chang, Y.C.; Chang, C.J.; Yang, P.C.; Peck, K. Synergistic inhibition of lung cancer cell invasion, tumor growth and angiogenesis using aptamer-siRNA chimeras. Biomaterials. 2014, 35, 2905–14. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long non-coding RNAs: From disease code to drug role. Acta Pharm Sin B. 2021, 11, 340–54. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.C.; O'Doherty, U. Clinical use of lentiviral vectors. Leukemia. 2018, 32, 1529–41. [Google Scholar] [CrossRef]
- Talens-Visconti, R.; Diez-Sales, O.; de Julian-Ortiz, J.V.; Nacher, A. Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials. International journal of molecular sciences. 2022, 23. [Google Scholar] [CrossRef]
- Gong, N.Q.; Teng, X.C.; Li, J.H.; Liang, X.J. Antisense Oligonucleotide-Conjugated Nanostructure-Targeting lncRNA MALAT1 Inhibits Cancer Metastasis. Acs Appl Mater Inter. 2019, 11, 37–42. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.L.; Zhang, J.C. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules. 2022, 27. [Google Scholar] [CrossRef]
- Diaz, M.R.; Vivas-Mejia, P.E. Nanoparticles as Drug Delivery Systems in Cancer Medicine: Emphasis on RNAi-Containing Nanoliposomes. Pharmaceuticals (Basel). 2013, 6, 1361–80. [Google Scholar] [CrossRef]
- Vivas-Mejia, P.E.; Rodriguez-Aguayo, C.; Han, H.D.; Shahzad, M.M.; Valiyeva, F.; Shibayama, M.; et al. Silencing survivin splice variant 2B leads to antitumor activity in taxane--resistant ovarian cancer. Clinical Cancer Research. 2011, 17, 3716–26. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).