1. Introduction
Coronavirus Disease-2019 (COVID-19) is caused by the infection of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), which leads to inflammation primarily in the respiratory system. The recent COVID-19 pandemic has posed a significant global health risk because of its high rate of transmission and risk towards those who are immunocompromised due to underlying medical conditions. According to the World Health Organization’s COVID-19 Dashboard, there have been 462,758,117 confirmed cases and 6,056,725 deaths as of March 17, 2022. Furthermore, the WHO reports that of these deaths, 1,719,002 were from individuals 65+ years of age, which constitutes roughly 28.4% of all reported COVID-19 deaths.
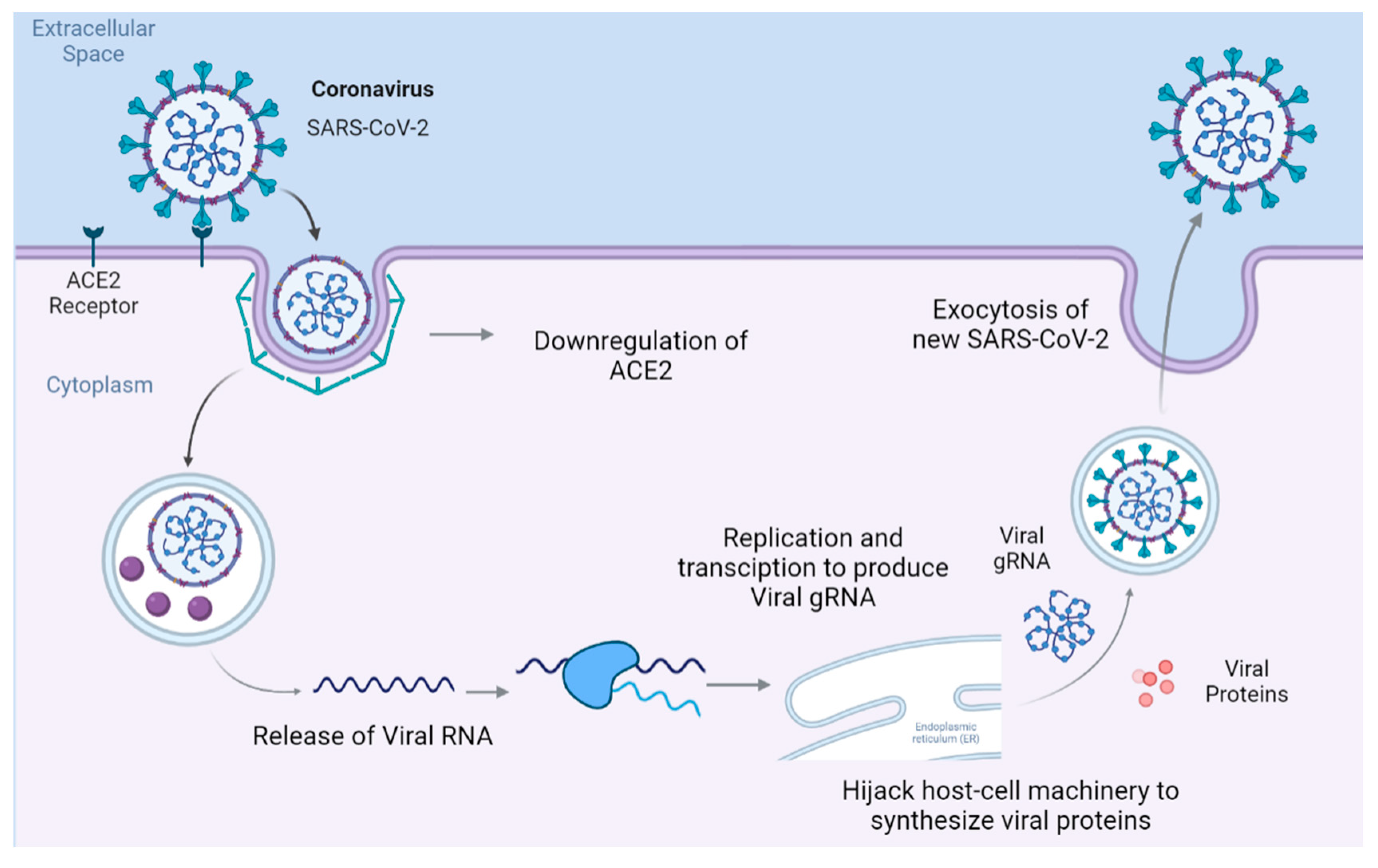
SARS-CoV-2 enters the host cell by binding to the transmembrane receptor angiotensin-converting enzyme 2 (ACE2), where the virus-protein complex is internalized and ACE2 is subsequently downregulated [
1]. As shown in
Figure 1, inside the cell, SARS-CoV-2 releases its genomic RNA (gRNA), which hijacks the host’s replication and transcription complexes to produce more copies of SARS-CoV-2.
The binding of SARS-CoV-2 to ACE2 leads to the downregulation of the latter, leading to systemic inflammations also known as a “cytokine storm” [
1]. The systemic inflammation then induces neuroinflammation, seen through mice models, via a nuclear factor kappa B (NFκB)-induced elevation of cytokines and the subsequent inhibition of Interferon gamma (IFNG)-induced antiviral response within the brain [
2]. As a result, the central nervous system is damaged due to neurotoxicity leading to blood-brain barrier (BBB) disruption, astrogliosis, oxidative stress, and neuronal damage and/or apoptosis [
1]. Lee et al., 2022 analyzed the post-mortem brain samples of the COVID-19 patients. They found multifocal vascular damage accompanied by widespread endothelial cell activation. Predominant expression of macrophages and CD8
+ T cells, CD4+ T cells, and CD20+ B cells were also found in COVID-19 patient’s brain. They also reported the presence of microglial nodules in the hindbrain suggesting neuronal loss and neurophagia [
3]. These results suggest that COVID-infection leads to a loss in vascular integrity and leaky BBB, which in turn results in the entry of systemic pathogens and inflammatory cytokines into the brain and triggers neuroinflammation in the various brain regions.
Parkinson’s disease (PD) is a neurodegenerative disorder that leads to bradykinesia, tremors, and muscular rigidity. It is characterized by the loss of dopaminergic neurons in the substantia nigra and the presence of Lewy bodies in neurons [
4]. Lewy bodies are aggregates formed from α-Synuclein protein fibers, produced by the gene synuclein alpha (SNCA). SNCA mutation may lead to improper translation resulting in the misfolding of the protein’s three-dimensional shape. SNCA modulation can thus be an indicator of PD progression as increased SNCA expression leads to a greater chance of Lewy body formation. A previous study from Semerdzhiev, Slav A et al., has shown that the Nucleocapsid Protein (N-protein) in COVID-19 affects α-Synuclein. SARS-CoV-2 N-protein possesses an affinity for α-Synuclein (αS), which leads to the aggregation of amyloid fibers [
5]. The aggregation of amyloid fibers, also known as Lewy Bodies form within neurons [
4]. The N-protein is a major structural protein that facilitates viral RNA production, suppresses intracellular immune responses, and packages viral RNA into developing virions. N-protein consists of an N-terminal RNA binding domain (RBD) and a C-terminal dimerization domain (CTD) [
5]. Electrostatic interactions play a part in N-protein and αS binding: N-protein has a calculated net positive charge of +24e while αS has a calculated net negative charge of -9e. [
5]. Through multiple Thioflavin T (THT) assays and in-vitro microinjection of SH-SY5Y cells with N-protein, literature has concluded that neuro-COVID-19 leads to the aggregation of amyloid fibers and subsequent formation of Lewy bodies [
5]. We hypothesize that SARS-CoV-2 viral exposure triggers SNCA activation and neuroinflammation in the brains of COVID-19 patients which in turn results in neurodegeneration and progression of PD pathology.
Mechanisms underlying COVID-19 augmentation of PD are still unknown. Patients develop PD that is a common neurodegenerative disease at late stages of their life, usually after 60. However, about 5-10 % of PD patients show early onset, below 50.
COVID-19 has various inflammatory response symptoms. Systemic inflammation can lead to neuroinflammation and neurodegeneration. It is crucial to understand whether COVID-19 patients will develop PD symptoms in future. Some reports also suggests that SARS-CoV-2 infection and its long-term complications results in premature ageing [
6,
7]. Hence, understanding the commonly shared molecules including genes, proteins and their associated signaling pathways that can potentially contribute to the development of PD in SARS-CoV-2 infections is critical. Over the last few decades, bioinformatics network meta-analysis has received greater attention among scientists to identify the key molecules and signaling pathways involved in various pathological conditions. In this study, we employed Ingenuity Pathway Analysis (IPA), which is a bioinformatics tool that employ machine learning and network algorithms to identify relationships among the molecules, signaling pathways, upstream regulators, diseases and functions and their predicted activation state associated with a set of molecules in our dataset constructed from Qiagen Knowledge Base (QKB). QKB is a repository of over 7 million findings from scientific literatures. QKB also has hand drawn signaling pathways that have been created by scientists based on the findings in QKB. IPA’s algorithm employs right tailed fisher exact test to examine and predict the involvement of a signaling pathway in the uploaded set of molecules. Our meta-analyses have identified the commonly shared molecules associated with COVID-19 and PD, signaling pathways, and upstream regulators associated with commonly shared molecules. IPA tools also revealed how the activation/inhibition of a gene or protein in the biological system influences the related molecules downstream. Our meta-analyses suggested that COVID-19 elevates SNCA expression with possible aggregation of α-synuclein and formation of Lewy bodies through the NISP that would subsequently enhance neurodegeneration leading to augmentation of PD onset and progression.
2. Results
2.1. Upstream Regulators and Signaling Pathways Involved in COVID-19 Influencing PD
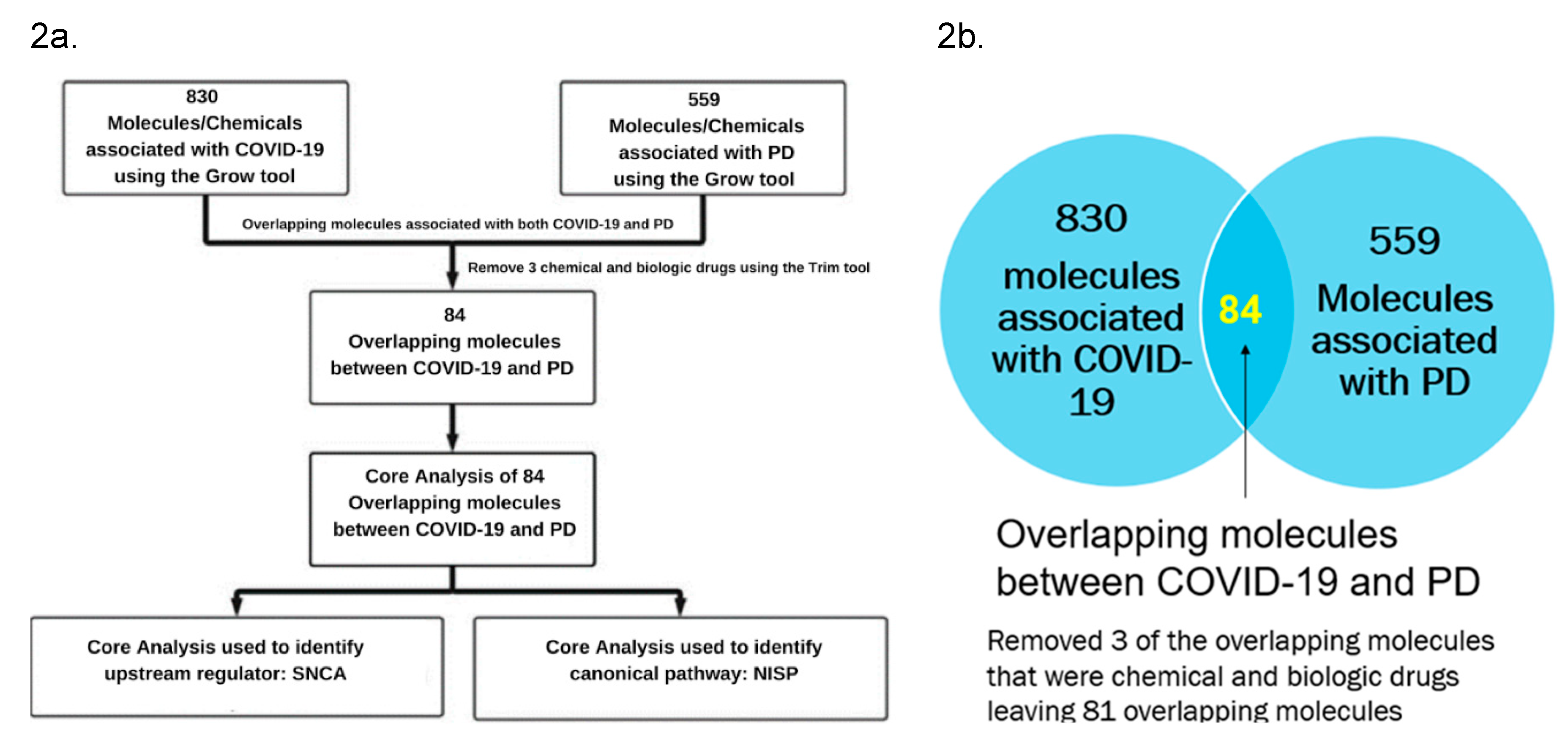
Figure 2a lists various IPA’s tools that were used to identify the signaling pathways and upstream regulators. IPA’s “Grow” tool was used to expand and collect the molecules and chemicals that were associated with COVID-19 and PD. respectively. There were 830 molecules associated with COVID-19 and 559 molecules associated with PD. As shown in
Figure 2b, using IPA’s “Compare” tool, 84 overlapping molecules between the molecules associated with COVID-19 and those with PD were identified. Three of these 81 overlapping molecules were chemical and biologic drugs which are not naturally found in the human body, so they were removed, leaving 81 overlapping molecules for subsequent analyses. IPA’s “Core Analysis” tool was used to identify the upstream regulators and signaling pathways involved in these 81 overlapping molecules. Our core analysis revealed the NISP to be one of the top signaling pathways involved as shown by
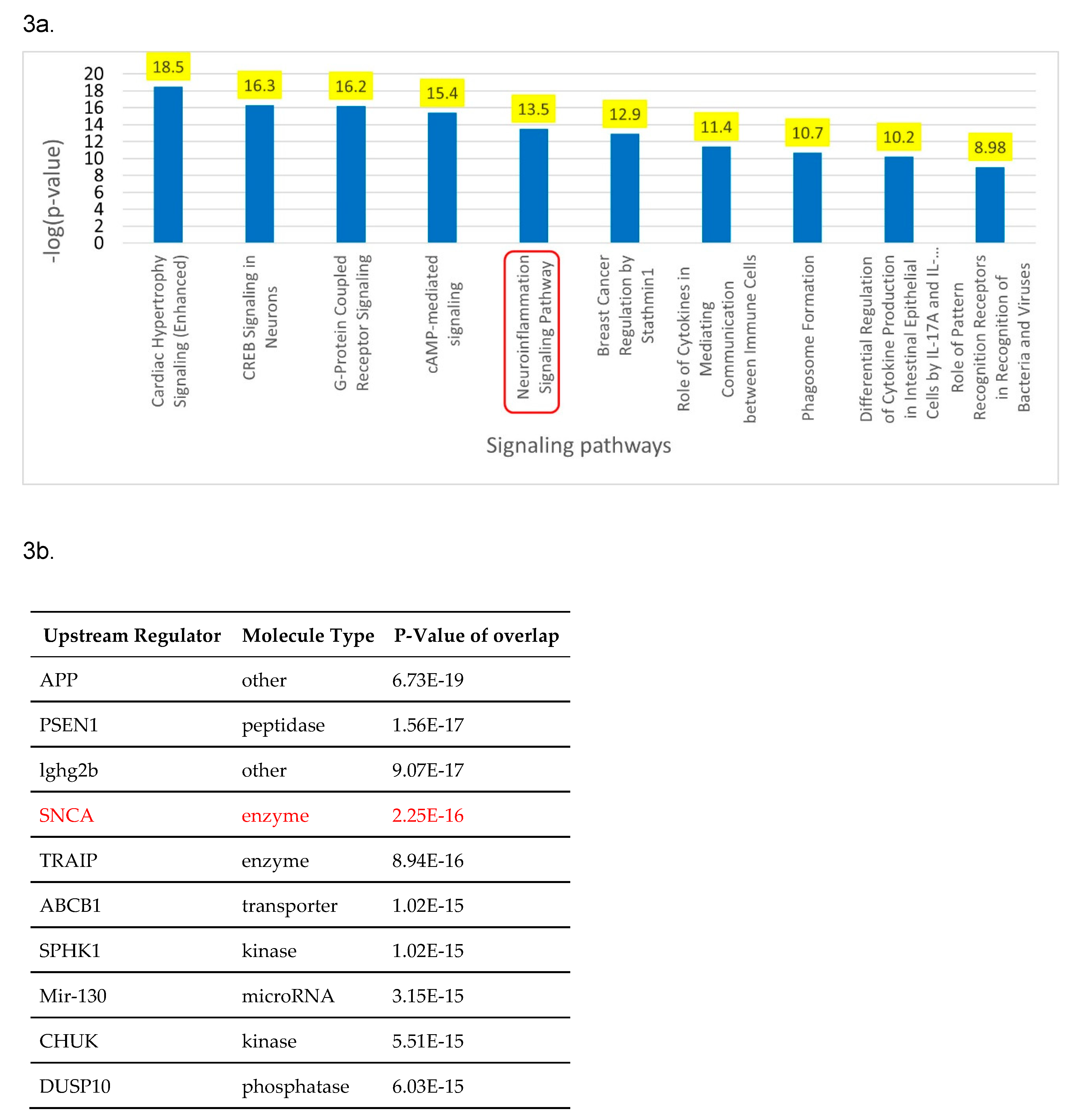
Figure 3a. SNCA was identified as the top upstream regulator associated with COVID-19 and PD by
Figure 3b.
2.2. Upstream Regulators and Signaling pathways Involved in COVID-19 Influencing on SNCA
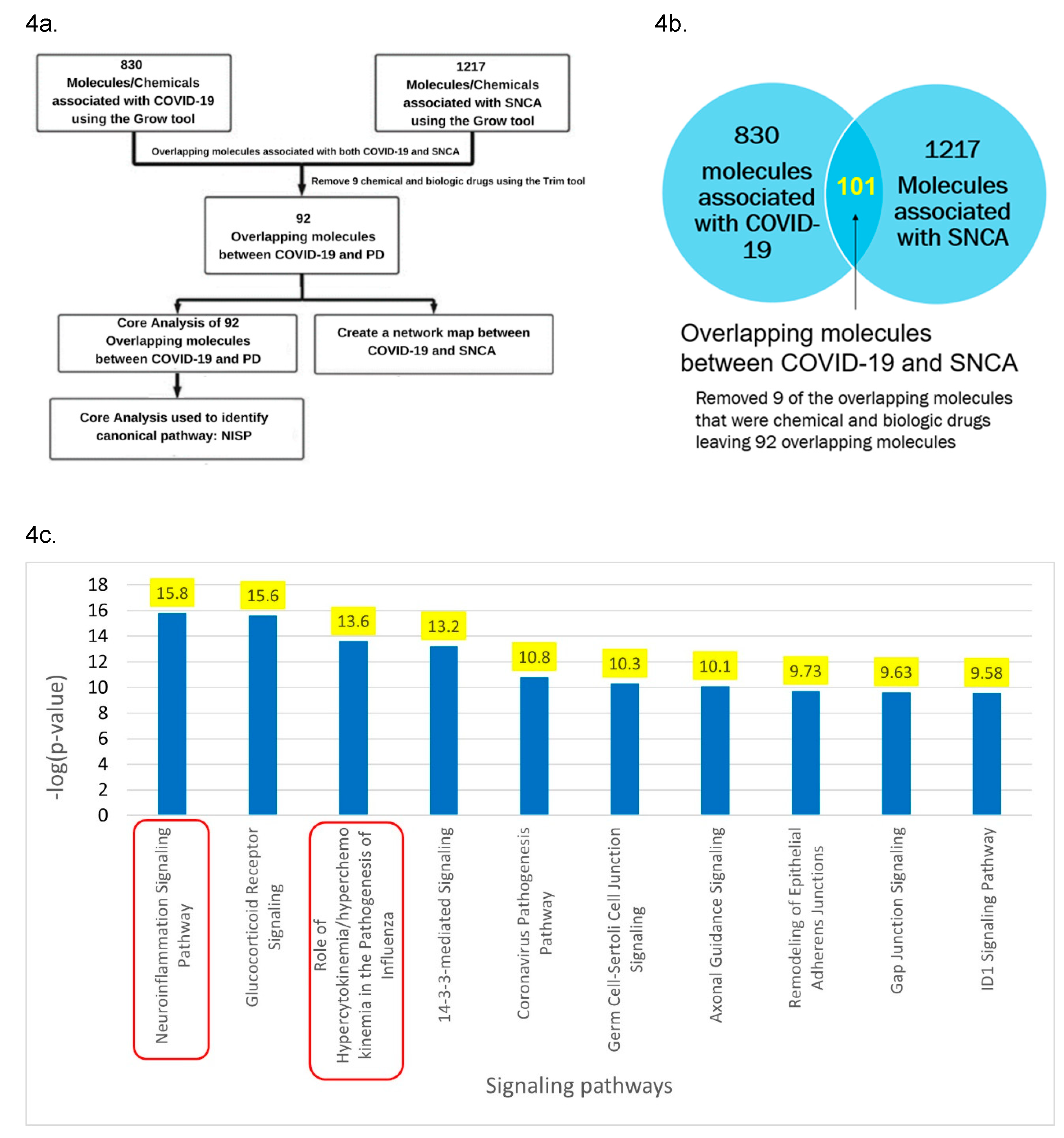
Core analysis of the overlapping molecules associated with COVID-19 and PD revealed SNCA as one of the top upstream regulators as shown in
Figure 3b. As shown in
Figure 4a, IPA’s “Grow” tool was used to expand the molecules and chemicals that were associated with COVID-19 and SNCA. There were 830 molecules and chemicals that were grown from COVID-19 and 1217 molecules that were grown from SNCA. Using IPA’s “Compare” tool, 101 overlapping molecules between COVID-19 and SNCA were identified. Nine of the 101 overlapping molecules were chemical and biologic drugs which are not naturally found in the human body, so they were removed, leaving 92 overlapping molecules as shown in
Figure 4b. IPA’s “Core Analysis” tool was used to identify the signaling pathways involved in the 92 overlapping molecules. The NISP was identified to be the top among the signaling pathways associated with COVID-19 and SNCA. Other top signaling pathways include glucocorticoid receptor signaling and role of hypercytokinemia/hyperchemokinemia in the pathogenesis of influenza as shown in
Figure 4c.
2.3. Pathway Connectivity Map to Examine COVID-19 Modulation of SNCA
Using the 92 overlapping molecules associated with COVID-19 and SNCA, a connectivity map was constructed between COVID-19, overlapping molecules, and SNCA. Literature and IPA’s Core Analysis of molecules associated with COVID-19 identified IFNG as the top upstream regulator with IFNG being downregulated upon SARS-CoV-2 infection. IFNG was manually added to the connectivity map. ACE2, an important receptor responsible for SARS-CoV-2 internalization into the cell and subsequent signal transduction cascades, was added to the connectivity map. Relationships were drawn from COVID-19 to IFNG and IFNG to ACE2 and then to the overlapping molecules using IPA’s “Connect” and “Pathway Explorer” tool. Similarly, the relationships from overlapping molecules to SNCA and PD were also drawn. IPA’s molecule activity predictor (MAP) tool was employed to simulate the inhibition of IFNG to mimic COVID-19, which downregulated ACE2 expression. The molecules that did not show any simulated effects were removed from further analysis. The concurrent downregulation of IFNG and ACE2 activated the expression of 20 molecules and inhibited the expression of 14 molecules eventually leading to an increase in SNCA expression and PD pathogenesis as shown in
Figure 5a. Core Analysis was conducted on the 34 molecules involved in the overall activation of SNCA and PD from the downregulation of IFNG. Signaling pathways were identified using core analysis. As shown in
Figure 3a and
Figure 4c, the NISP was identified to be a significantly enriched signaling pathway among the top signaling pathways identified in the core analysis of COVID-19 influence on PD and COVID-19 influence on SNCA respectively. “Cytokine storms” were also identified to be the topmost upregulated signaling pathway.
2.4. Quantitative Analysis of Upstream Regulators in COVID-19 Modulation of SNCA
To observe the overall impact of COVID-19 on SNCA, the 34 molecules involved in the overall activation of SNCA and PD were analyzed using the Downstream Effect analysis. As shown in
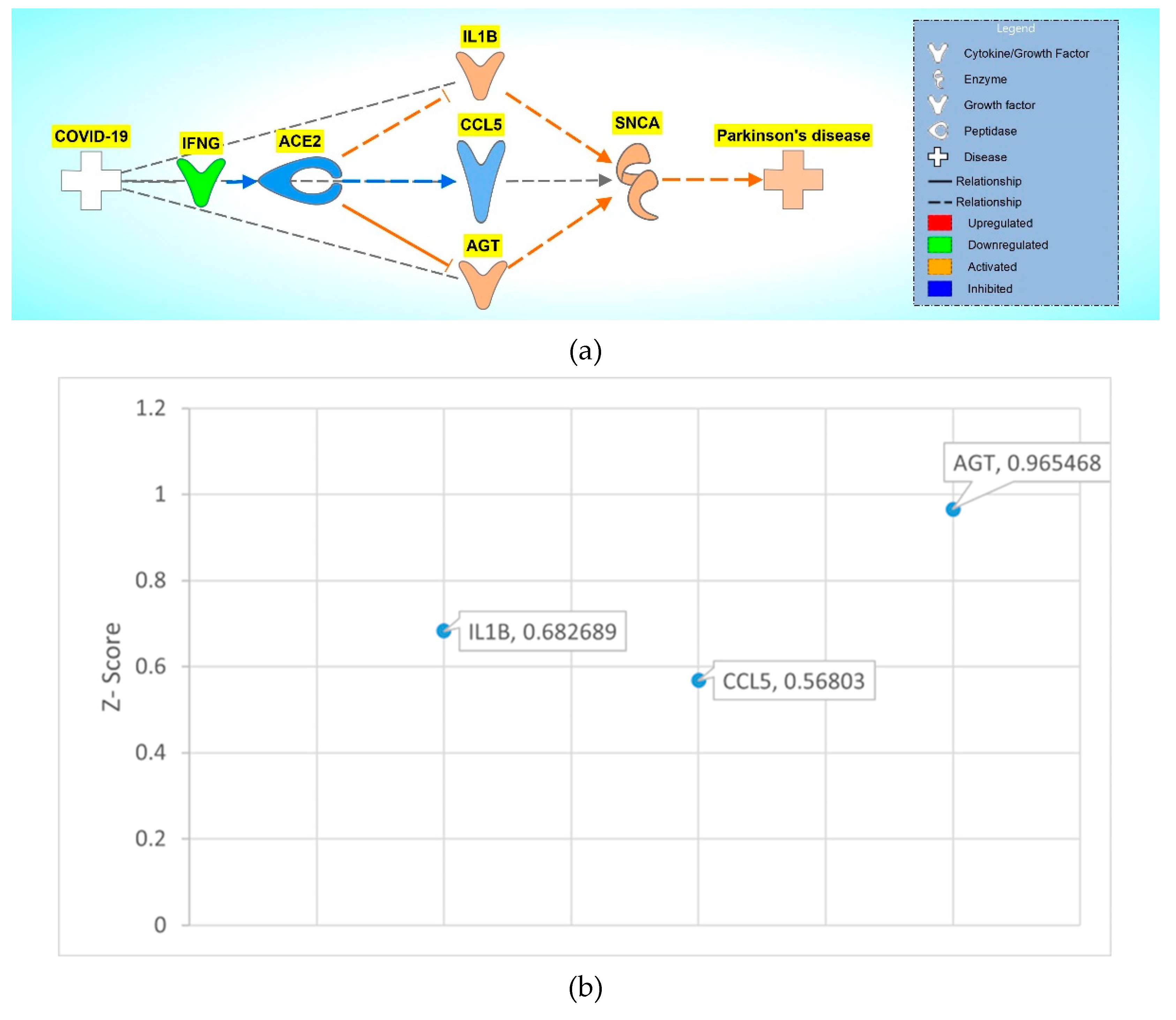
Figure 6a, interleukin-1 beta (IL1B), c-c motif chemokine ligand 5 (CCL5), and angiotensin (AGT) were used as intermediary molecules to identify the strength of influence of ACE2 on SNCA. The overall z-score was found to be -0.43229.
To quantify the influence of ACE2 on SNCA and expand the network of associated molecules, each of the intermediary molecules were grown using IPA’s “Grow” tool. These molecules were trimmed using IPA’s “Trim” tool to keep only the associated molecules that had a downstream effect from ACE2 and an upstream effect on SNCA. The pathway maps were constructed from IL1B’s, CCL5’s, and AGT’s associated molecules using IPA’s “Pathway Explorer” tool.
To begin with, IL1B was grown using IPA’s “Grow” tool to identify all the associated molecules and then connected to ACE2 via IPA’s “Connect” tool. AGT, extracellular signal-regulated kinase 1/2 (ERK1/2), matrix metallopeptidase 2 (MMP2), CCL5, and matrix metallopeptidase 9 (MMP9) showed downstream effect from ACE2 and upstream effect on SNCA. A network was constructed from IL1B and its associated molecules to ACE2 and SNCA. Simulated inhibition of ACE2 showed potential increase in the expression of SNCA with a z-score of -0.683 as shown in
Figure S1a. The same workflow was applied to CCL5, and a network was constructed between ACE2, associated molecules of CCL5, and SNCA as shown in
Figure S1b. The simulated inhibition of ACE2 activated the associated molecules of CCL5 (IL1B, ERK1/2, MMP2, and MMP9) leading to an increase in SNCA expression. The z-score for ACE2 downregulation mediated increased expression of SNCA via CCL5’s associated molecules was found to be -0.946. Similarly, ACE2 inhibition in the network constructed between ACE2, associated molecules between AGT, and SNCA increased the expression of SNCA. Simulated inhibition of ACE2 increased the expression of ERK1/2, MMP9, IL1B, and MMP2 and decreased the expression of CCL5 leading to an overall activation of SNCA as shown in
Figure S1C. The z-score for AGT mediated increase in SNCA expression was found to be -0.683. The overall z-score for the involvement of IL1B, CCL5 and AGT in ACE2 downregulation mediated increased SNCA expression was found to be -2.312, corresponding to a p-value 0.0706 of a two tailed distribution at 95% confidence interval. Downstream Effect analysis provided a z-score for each of the following intermediary molecules involved in ACE2’s influence on SNCA as shown in
Figure 6b. AGT was the intermediary molecule with the greatest influence on ACE2’s effect on SNCA with a z-score of 0.97 followed by CCL5 with a z-score of 0.57 and IL1B with 0.68.
2.5. Pathway Connectivity Map to Examine COVID-19 Modulation of the NISP
Previous results concluded that COVID-19 modulates SNCA expression. Next, we studied the influence of the overlapping molecules with the NISP and how the NISP is involved COVID-19 modulation of PD through a pathway connectivity map as shown in
Figure 7. The NISP was added to the pathway constructed in
Section 2.3. Relationships from the NISP to other nodes in the pathway were implemented using IPA’s “Connect” and “Pathway Explorer” tools. Molecules that did not have relationships with the NISP were removed.
2.6. Quantitative Analysis of Upstream Regulators in COVID-19 Modulation of the NISP
To observe the overall impact of COVID-19 on NISP, the 12 molecules involved in the overall activation of the NISP were analyzed using the Downstream Effect analysis. As shown in
Figure 8a, 6 molecules, c-c motif chemokine ligand 2 (CCL2), CCL5, prostaglandin-endoperoxide synthase 2 (PTGS2), IL1B, tumor necrosis factor (TNF), and interleukin 6 (IL6), were used as intermediary molecules in Downstream Effect analysis.
Similarly, to the Downstream Effect analysis as shown in
Section 2.4, each of the intermediary molecules had a network of associated molecules that were grown and trimmed down to have a downstream effect on ACE2 and an upstream effect on the NISP. The pathway maps were constructed from each of the intermediary molecules from
Figure 8a. IPA’s “Grow” tool and “Connect” tool was used to connect ACE2 and the NISP to each of the grown molecules of the intermediary molecules. The following constructed pathways of the intermediary molecules CCL2, CCL5, PTGS2, IL1B, TNF, and IL6 are shown in
Figure S2a, S2b, S2c, S2d, S2e, and S2f respectively. The z-score was computed for each of the intermediary molecules involved in ACE2 downregulation mediated increased expression of NISP via the Downstream Effect analysis as shown in
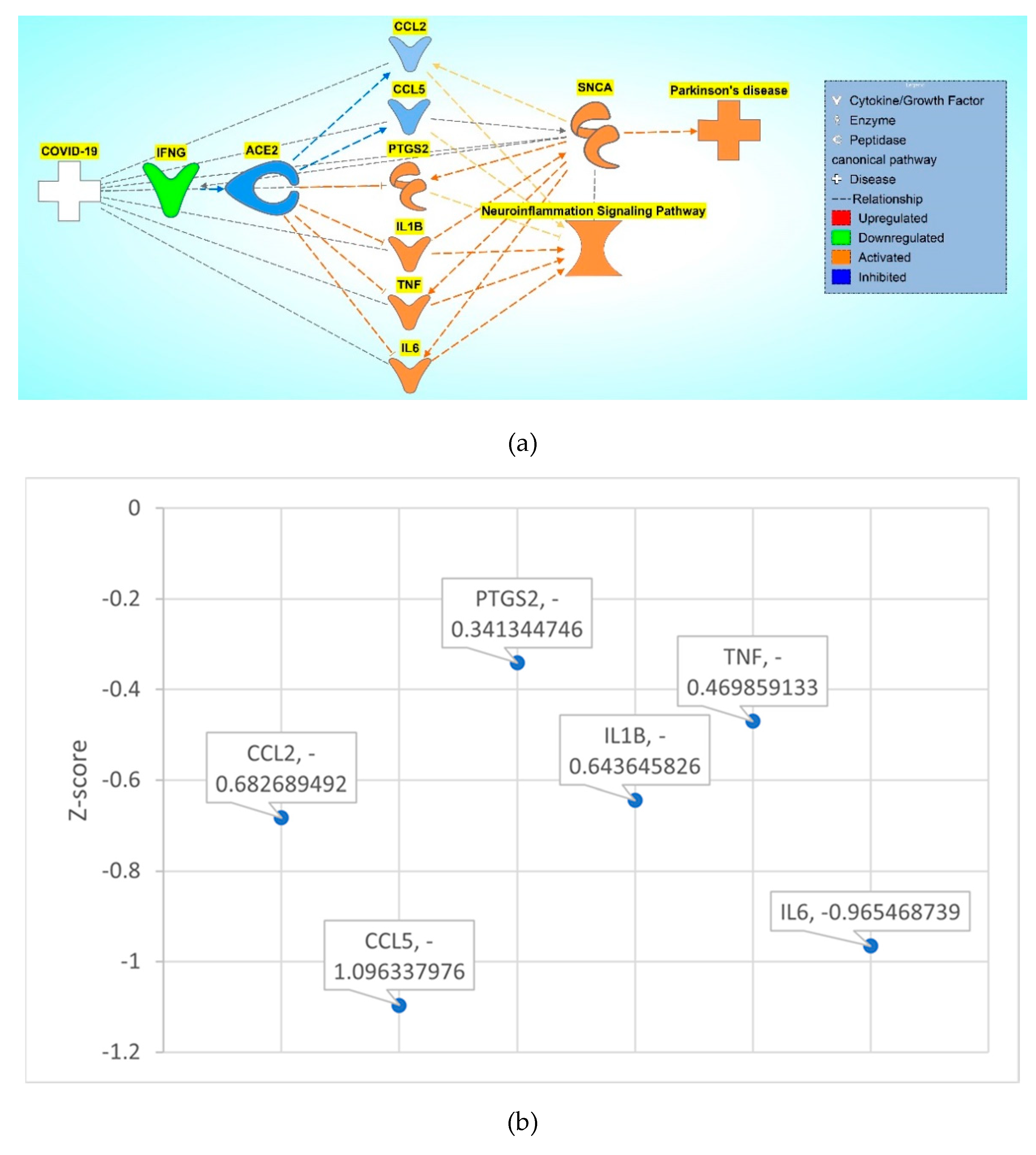
Figure 8b. CCL5 had the greatest influence in ACE2’s effect on the NISP with a z-score of -1.10 and PTGS2 had the least influence with a z-score of -0.34. The other intermediary molecules had the following z-score of -0.47, -0.64, -0.68, and -0.97 for TNF, IL1B, CCL2, and IL6 respectively.
3. Discussion
The present network meta-analysis study suggests a strong association of COVID-19 in the progression of PD. Our results suggest that COVID-19 mediated downregulation of ACE2 receptor increases SNCA expression. Molecules associated with both COVID-19 and PD were analyzed through core analysis which showed NISP as one of the top signaling pathways involved within both diseases with a p-value of 3.1 x 10^-14. This suggests NISP is highly involvement in COVID-19 and PD. Literature shows that COVID-19 causes cytokine storms, which leads to neuroinflammation and subsequently neurodegenerative diseases. To further examine the impact of COVID-19 on PD through the NISP, SNCA was chosen as a molecule of interest. Proteins synthesized from SNCA aggregate to form Lewy Bodies, which are deposited in the neurons of various brain regions resulting in PD. The core analysis of the overlapping molecules associated with both COVID-19 and PD revealed SNCA as a top upstream regulator (p-value of 2.25 x 10^-16), suggesting the involvement of SNCA in the pathology of COVID-19 and PD. The main aim of the present study was to determine how COVID-19 affects SNCA and how ACE2 expression modulates PD pathology.
COVID-19 patients exhibit significant increase in the plasma cytokine levels, including interleukins (IL1B, IL1RA, IL7, IL8, IL9, IL10), fibroblast growth factor, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, IFNG, IP10, monocyte chemoattractant protein 1, macrophage inflammatory proteins (MIP1A and MIP1B), platelet-derived growth factor, TNF, and vascular endothelial growth factor (VEGF), compared to a healthy control group [
8]. Hamster models infected with SARS-CoV-2 and patients who died from COVID-19 showed impaired BBB permeability, microglial activation, and increased brain expression of IL-1β and IL6 within the hippocampus and the inferior olivary nucleus of the medulla when compared to the control groups. These alterations result in neuroinflammation, loss of hippocampal neurogenesis, and neurocognitive symptoms [
9]. Our results were consistent with the previous findings. Core analysis conducted on the associated molecules in both COVID-19 and PD identified the topmost significant signaling pathway as the NISP with a p-value of 1.72 x 10^-16. This means that the NISP plays a crucial role in both COVID-19 and PD. The third most significant signaling pathway is cytokine storms with a p-value of 2.51 x 10^-14. Cytokine storms are the body’s immune response causing the release of inflammatory proteins called cytokines. Infection by SARS-CoV-2 causes the release of cytokine storms. This further supports the theory the COVID-19 causes cytokine storms which then causes neuroinflammation.
To identify the impact of the associated molecules identified in both COVID-19 and SNCA, these molecules were put into a pathway map where the IFNG was downregulated to simulate infection by SARS-CoV-2. IFNG is the topmost upstream regulator in the molecules associated with COVID-19. IFNG is a cytokine that is significantly downregulated upon SARS-CoV-2 infection during the cytokine storm. The simulated downregulation of IFNG using the IPA’s MAP tool showed the inhibition of ACE2. The receptor mediated endocytosis of SARS-CoV-2 via ACE2 leads to the downregulation of ACE2. 34 molecules from the set of molecules associated with COVID-19 and SNCA were consequently affected by the inhibition of ACE2. Of those 34 molecules affected, 14 molecules were inhibited, and 20 molecules were activated. These molecules lead to an overall activation of SNCA which subsequently led to the activation of PD.
To quantify ACE2’s impact on the activation of SNCA, Downstream Effect analysis was conducted. Downstream Effect analysis was conducted on the 34 intermediary molecules downstream of ACE2 and upstream of SNCA to quantify the effects that COVID-19 had on PD. Downstream Effect analysis was performed based on the literature findings from the QKB, where ACE2 impacted the intermediary molecule’s expression and where the intermediary molecules impacted the expression of SNCA. 3 molecules (IL1B, CCL5, and AGT) were identified and used for analysis for the Downstream Effect analysis. Downstream Effect analysis would yield a large margin of error given the sample size of 3 when used for the analysis, so associated molecules of each intermediary molecules with downstream affects from ACE2 and upstream effects on SNCA were identified. Downstream Effect analysis was conducted on the associated molecules of the intermediary molecules. The z-score was computed for each of the intermediary molecules showing IL1B with a z-score of 0.682689, CCL5 with a z-score of 0.56803, and AGT with a z-score of 0.965468. The upregulation of pro-inflammatory cytokine IL1B and the downregulation of neuroprotective agent CCL5 from the inhibition of ACE2 plays a role in the neurotoxicity associated with the neuroinflammation response. ACE2 is responsible for converting Angiotensin II into angiotensin [
1,
2,
3,
4,
5,
6,
7]. The inhibition of ACE2 causes the accumulation of Angiotensin II which is encoded by the gene AGT. Angiotensin II receptor type 1 (AGTR1) converts excess Angiotensin II which leads to inflammation, thrombosis, oxidative stress, and fibrosis [
10]. Upon receptor mediated endocytosis of Angiotensin II from AGTR1, the vasoconstriction leads to hypertension contributing to neuronal injury which causes neurodegeneration associated with PD and Alzheimer’s disease [
11]. The overall p-value of these intermediary molecules was less than 0.05 which shows a significant impact of the downregulation of ACE2 on the activation of SNCA. This shows that IFNG downregulation mimicking SARS-CoV-2 infection led to a significant impact on the activation of SNCA leading to PD as shown in
Figure 6a.
After identifying NISP’s involvement in COVID-19’s activation of PD, the associated molecules between COVID-19 and SNCA were trimmed to only the molecules that were involved in the NISP. These molecules were further trimmed to identify those molecules downstream of ACE2 and upstream of the NISP leaving six molecules. IPA’s “Grow” tool was used on these six molecules (CCL2, CCL5, PTGS2, IL1B, TNF, IL6) to identify the associated molecules for the Downstream Effect analysis. These molecules had a significant overall p-value of less than 0.05. Chemokine receptors specifically CCL5 have multiple functions in the CNS including neuroinflammation, insulin signaling, neuromodulation of synaptic activity, and neuroprotection against a variety of neurotoxins [
12,
13]. Avdoshina et al. have shown that morphine administration enhances the release of CCL5 from astrocytes that inhibit HIV gp120BaL-mediated neurotoxicity [
12]. In addition, CCL5 has also shown neuroprotective activity against various neurotoxins including viral proteins gp120 [
14], trans activator of transcription (TAT) [
15], glutamate [
16], and β-amyloid [
17]. The neuroprotective properties of CCL5 is mainly attributed to its potential to increase neurotrophic factors, such as brain-derived neurotrophic factor and epidermal growth factor [
18]. Our results suggests that COVID-19 mediated downregulation of IFNG inhibits CCL5 expression as shown in
Figure 8a. Along with upregulation of inflammatory mediators like IL1B, TNF, and IL6, downregulation of CCL5 can be a major contributor to COVID-19 mediated neuroinflammation. PTGS2 codes for an enzyme called cyclooxygenase 2 (COX-2) which contributes to neuroinflammation. Increased expression of COX-2 in activated microglial cells can increase production of pro-inflammatory cytokines like TNF and IL1B. These pro-inflammatory cytokines contribute to the neuroinflammation response [
19]. The present network meta-analysis study suggests that COVID-19 induced downregulation of ACE2 elevates SNCA expression through the NISP, leading to the formation and aggregation of Lewy bodies and eventually leading to PD progression. The downstream Effect analysis of ACE2’s effect on SNCA expression showed significant overall activation of PD from COVID-19.
The bioinformatics tool, IPA, gathers millions of literature findings curated by thousands of scientists to create connections that time and material efficient compared to other methods of gathering information. COVID-19 is a global pandemic and a large health concern for the past few years that has taken millions of lives and infected many more. To study how this global pandemic affects patients with PD, using IPA is a time efficient tool that allows us to examine lots of literature and simulate predictive infections of SARS-CoV-2 to examine the PD pathways that have been studied before. By using IPA, we can study a new disease’s impact on a well-studied disease and predict the impact of the new disease on a well-diseased in which we have done with COVID-19 and PD. Due to the nature of COVID-19, which is recent newly discovered disease, not much is known about its effect on PD. Thus, the strength in IPA allows us to view the associated molecules from literature on COVID-19 to provide us with more insight on COVID-19’s impact. Furthermore, PD is a disease that develops over time which makes researching the long-term effects of COVID-19 on PD progression difficult to examine. Using IPA’s MAP tool to simulate the infection of SARS-CoV-2 and examining its effect on the pathways involved in the progression of PD, we can predict the effects of COVID-19 on PD without needing to perform long term studies on populations that have been infected with COVID-19 and their development of PD.
IPA’s MAP tool in simulating the infection of SARS-CoV-2 allows us to identify and focus on molecules related to PD that have been impacted by COVID-19. Identifying these molecules and using the upregulation and downregulation tool to simulate the changes of expression in molecules can be used to identify drug targets by simulating changes where SNCA and molecules involved with PD progression as well as the NISP are downregulated. The downregulation of ACE2 shows an increase in the NISP as well as SNCA and subsequently PD. ACE2 is a potential drug target where upregulation of ACE2 would help alleviate the neuroinflammation associated with COVID-19 as well as the activation SNCA and subsequent PD progression.
4. Materials and Methods
4.1. Ingenuity Pathway Analysis Software
The IPA Analysis Match CL license was purchased from QIAGEN for using all features and tools of the IPA software. All data used for the COVID-19 and PD analysis for this study were retrieved from the QIAGEN Knowledge Base (QKB) between January 12th, 2022, to April 24th, 2022. (QIAGEN Inc.,
https://www.qiagenbioinformatics.com/products.).
4.2. Identification of Associated and Overlapping Molecules for Pathway Construction
IPA’s “Grow” tool produces a network of all genes, proteins, complexes, and chemicals affected by the selected molecule. In the present study, COVID-19, PD, and SNCA were all expanded through IPA’s “Grow” tool. These include genes, proteins, chemicals, and complexes affected by the respective nodes. Chemical drugs and toxicants that do not occur in the biological systems were eliminated from further analysis using the “Trim” tool. IPA’s “Path Explorer” tool and IPA’s “Connect” tool was used to join the overlapping molecules associated with COVID-19, PD, and SNCA together to construct a pathway based on relationship findings found in the QKB. “Path Explorer” tool generated molecular connections between molecules or between molecules and diseases and functions of interest based on the functions in QKB. In the present study, we used “Path Explorer” tool to generate molecular connections between IFNG, overlapping molecules, SNCA, PD and SNCA.
4.3. Identification of Upstream Regulator and Signaling pathways via Core Analysis
The molecules identified to overlap in each of the networks were compiled into a dataset that was then uploaded to IPA for separate core analysis. The core analysis revealed the top upstream regulators and top signaling pathways associated with the molecules. The significance of the upstream regulators and signaling pathways was calculated using Benjamini-Hochenberg corrected Fischer’s exact test to generate –log(p-value).
4.4. Quantitative Analysis of COVID-19 on SNCA Expression and the NISP
IPA shows the qualitative relationships when IPA’s MAP tool is used to simulate the activation or inhibition of a molecule. To determine the quantitative impact of these relationships, Downstream Effect analysis was performed as described previously [
20,
21,
22,
23,
24]. The analysis determines the significance of a molecule’s effect on either another molecule or a signaling pathway by quantifying the relationship between the nodes on IPA. The formula computes a z-score for each molecule that are downstream to IFNG for their contribution to SNCA expression. The z-score ranges between −2 to 2, −2 indicated a strong inhibitory relationship and +2 indicated a strong activation. Individual z-scores were then combined to get the “local z-score” which corresponds to a z-statistic of a normal distribution.
5. Conclusions
The present study was designed to understand the mechanisms in which COVID-19 affects PD through the use of IPA. A set of molecules was identified between COVID-19 and PD as well as COVID-19 and SNCA where core analysis identified the top signaling pathways and upstream regulators. This prompted a pathway to be constructed between COVID-19 and SNCA with IFNG, ACE2, and intermediary molecules consisting of cytokines, receptors, and enzymes. From this, we ran core analysis on the pathway and identified “cytokine storm” to be the top signaling pathway followed by the NISP. To further explore the role of the NISP in the COVID’s modulation of PD, the previous pathway was altered to focus on molecules associated with the NISP. When IFNG was downregulated to mimic SARS-CoV-2 infection, ACE2 was downregulated and affected a set of intermediary molecules leading to an overall activation of SNCA and subsequent activation of PD. This confirmed our theory that COVID-19 causes inflammation that leads to neuroinflammation and eventually neurodegenerative diseases like PD. Through the Downstream Effect analysis, relationship between molecules were analyzed to provide insight on the statistical significance of COVID-19’s effects within the constructed pathways leading to PD. Key molecules identified in the pathway help reveal potential therapeutic targets to mitigate the progression of PD induced by COVID-19’s neuroinflammation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, J.Z. and S.L.C.; methodology, J.Z., M.B. and S.L.C.; software, J.Z.; validation, J.Z., M.B., S.B., and S.L.C.; formal analysis, J.Z..; investigation, J.Z., M.B., S.B. and S.L.C.; resources, S.L.C.; data curation, J.Z. ; writing—original draft preparation, J.Z. and M.B.; writing—review and editing, J.Z., M.B., S.B. and S.L.C.; visualization, J.Z; supervision, S.L.C. ; project administration, S.L.C..; funding acquisition, S.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially sponsored by NIH DA046258 to SLC.
References
- Sulie LChang, W.H.; Montero, A.; Bishir, M.; Chidambaram, S.B. Neuro-Covid-19: A Meta-Analysis of COVID-19-Induced Neuroinflammation and Its Implications. Science Documents. 2020, 1, 26–38. [Google Scholar]
- Huang, C.; Irwin, M.G.; Wong, G.T.C.; Chang, R.C.C. Evidence of the impact of systemic inflammation on neuroinflammation from a non-bacterial endotoxin animal model. Journal of Neuroinflammation. 2018, 15. [Google Scholar] [CrossRef]
- Lee, M.H.; Perl, D.P.; Steiner, J.; Pasternack, N.; Li, W.; Maric, D.; et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022, 145, 2555–2568. [Google Scholar] [CrossRef]
- Lotankar, S.; Prabhavalkar, K.S.; Bhatt, L.K. Biomarkers for Parkinson's Disease: Recent Advancement. Neurosci Bull. 2017, 33, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Semerdzhiev, S.A.; Fakhree, M.A.A.; Segers-Nolten, I.; Blum, C.; Claessens, M. Interactions between SARS-CoV-2 N-Protein and alpha-Synuclein Accelerate Amyloid Formation. ACS Chem Neurosci. 2022, 13, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tayeri, K.; Asadollahi, K.; Madani, N.; Haghjooy Javanmard, S. Does COVID-19 Escalate Aging Process? A Possible Concern. Adv Biomed Res. 2022, 11, 106. [Google Scholar]
- Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Bhat, A.; Paneyala, S.; Patteswari, D.; et al. Does COVID-19 contribute to development of neurological disease? Immun Inflamm Dis. 2021, 9, 48–58. [Google Scholar] [CrossRef]
- Tang, D.; Comish, P.; Kang, R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020, 16, e1008536. [Google Scholar] [CrossRef]
- Klein, R.; Soung, A.; Sissoko, C.; Nordvig, A.; Canoll, P.; Mariani, M.; et al. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Res Sq. 2021.
- D'Ardes, D.; Boccatonda, A.; Rossi, I.; Guagnano, M.T.; Santilli, F.; Cipollone, F.; et al. COVID-19 and RAS: Unravelling an Unclear Relationship. Int J Mol Sci. 2020, 21. [Google Scholar] [CrossRef]
- Goldstein, B.; Speth, R.C.; Trivedi, M. Renin–angiotensin system gene expression and neurodegenerative diseases. Journal of the Renin-Angiotensin-Aldosterone System. 2016, 17, 147032031666675. [Google Scholar] [CrossRef] [PubMed]
- Avdoshina, V.; Biggio, F.; Palchik, G.; Campbell, L.A.; Mocchetti, I. Morphine induces the release of CCL5 from astrocytes: Potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010, 58, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Avdoshina, V.; Rozzi, S.; Mocchetti, I. CCL5 and cytokine expression in the rat brain: Differential modulation by chronic morphine and morphine withdrawal. Brain, Behavior, and Immunity 2013, 34, 130–40. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Avdoshina, V.; Day, C.; Lim, S.T.; Mocchetti, I. Pharmacological induction of CCL5 in vivo prevents gp120-mediated neuronal injury. Neuropharmacology 2015, 92, 98–107. [Google Scholar] [CrossRef]
- Rozzi, S.J.; Borelli, G.; Ryan, K.; Steiner, J.P.; Reglodi, D.; Mocchetti, I.; et al. PACAP27 is Protective Against Tat-Induced Neurotoxicity. Journal of Molecular Neuroscience. 2014, 54, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Bruno, V.; Battaglia, G.; Ksiazek, I.; Van Der Putten, H.; Catania, M.V.; Giuffrida, R.; et al. Selective Activation of mGlu4 Metabotropic Glutamate Receptors Is Protective against Excitotoxic Neuronal Death. The Journal of Neuroscience. 2000, 20, 6413–6420. [Google Scholar] [CrossRef]
- Ignatov, A.; Robert, J.; Gregory-Evans, C.; Schaller, H.C. RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP<sub>3</sub> ) formation in cells transfected with G protein-coupled receptor 75. British Journal of Pharmacology. 2006, 149, 490–497. [Google Scholar]
- Tokami, H.; Ago, T.; Sugimori, H.; Kuroda, J.; Awano, H.; Suzuki, K.; et al. RANTES has a potential to play a neuroprotective role in an autocrine/paracrine manner after ischemic stroke. Brain Research 2013, 1517, 122–32. [Google Scholar] [CrossRef]
- López, D.E.; Ballaz, S.J. The Role of Brain Cyclooxygenase-2 (Cox-2) Beyond Neuroinflammation: Neuronal Homeostasis in Memory and Anxiety. Molecular Neurobiology. 2020, 57, 5167–5176. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Masi S, Nair MPN, Vigorito M, Chu T, Chang SL, editors. Alcohol Modulation of Amyloid Precursor Protein in Alzheimer's Disease2020.
- Chang, S.L.; Bishir, M. Commentary on "Alcohol use disorder as a potential risk factor for COVID-19 severity: A narrative review". Alcohol Clin Exp Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Camacho, R.C.; Alabed, S.; Zhou, H.; Chang, S.L. Network Meta-analysis on the Changes of Amyloid Precursor Protein Expression Following SARS-CoV-2 Infection. Journal of Neuroimmune Pharmacology. 2021. [CrossRef] [PubMed]
- Alabed, S.; Zhou, H.; Sariyer, I.K.; Chang, S.L. Meta-Analysis of Methamphetamine Modulation on Amyloid Precursor Protein through HMGB1 in Alzheimer's Disease. Int J Mol Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
The receptor-mediated endocytosis of SARS-CoV-2 leads to the downregulation of ACE2. Once in the cytoplasm, the virus hijacks host cell machinery to synthesize viral proteins and RNA to replicate itself, after which it spreads to other cells.
Figure 1.
The receptor-mediated endocytosis of SARS-CoV-2 leads to the downregulation of ACE2. Once in the cytoplasm, the virus hijacks host cell machinery to synthesize viral proteins and RNA to replicate itself, after which it spreads to other cells.
Figure 2.
a. Workflow diagram to obtain the upstream regulators and Signaling pathways. The molecules associated with COVID-19 and PD were obtained using the IPA “Grow” tool. The overlapping molecules obtained from IPA’s “Compare” tool were analyzed using IPA’s “Core Analysis” tool to identify the upstream regulators and signaling pathways. b. IPA’s “Compare” tool was employed to identify the overlapping molecules associated with COVID-19 and PD. We identified 84 overlapping molecules common between the two set of molecules, and three molecules that were chemical and biological drugs were removed from further analysis.
Figure 2.
a. Workflow diagram to obtain the upstream regulators and Signaling pathways. The molecules associated with COVID-19 and PD were obtained using the IPA “Grow” tool. The overlapping molecules obtained from IPA’s “Compare” tool were analyzed using IPA’s “Core Analysis” tool to identify the upstream regulators and signaling pathways. b. IPA’s “Compare” tool was employed to identify the overlapping molecules associated with COVID-19 and PD. We identified 84 overlapping molecules common between the two set of molecules, and three molecules that were chemical and biological drugs were removed from further analysis.
Figure 3.
a. Core analysis of the overlapping molecules associated with COVID-19 and PD revealed Neuroinflammation signaling pathway (p-value of 3.1 x 10^-14) as one the top enriched signaling pathway. b. Core analysis of the overlapping molecules associated with COVID-19 and PD found SNCA (p-value of 2.25 x 10^-16) as one of the top 5 upstream regulators.
Figure 3.
a. Core analysis of the overlapping molecules associated with COVID-19 and PD revealed Neuroinflammation signaling pathway (p-value of 3.1 x 10^-14) as one the top enriched signaling pathway. b. Core analysis of the overlapping molecules associated with COVID-19 and PD found SNCA (p-value of 2.25 x 10^-16) as one of the top 5 upstream regulators.
Figure 4.
a. Workflow diagram in obtaining the upstream regulators and signaling pathways. The molecules associated with COVID-19 and SNCA were identified using IPA’s “Grow” tool. The overlapping molecules obtained from IPA’s “Compare” tool were analyzed using IPA’s “Core analysis” tool to identify the upstream regulators and signaling pathways. b. IPA’s “Compare” tool was employed to identify the overlapping molecules associated with COVID-19 and SNCA. We identified 92 overlapping molecules between COVID-19 and SNCA, and three molecules that were chemical and biological drugs were removed from further analysis. c. Core analysis of the overlapping molecules associated with COVID-19 and SNCA revealed “Neuroinflammation Signaling Pathway” (p-value of 1.72 x 10^-16) as the top enriched signaling pathway followed by the “Role of hypercytokinemia/hyperchemokinemia in the Pathogenesis of Influenza,” also known as cytokine storms (p-value of 2.51 x 10^-14).
Figure 4.
a. Workflow diagram in obtaining the upstream regulators and signaling pathways. The molecules associated with COVID-19 and SNCA were identified using IPA’s “Grow” tool. The overlapping molecules obtained from IPA’s “Compare” tool were analyzed using IPA’s “Core analysis” tool to identify the upstream regulators and signaling pathways. b. IPA’s “Compare” tool was employed to identify the overlapping molecules associated with COVID-19 and SNCA. We identified 92 overlapping molecules between COVID-19 and SNCA, and three molecules that were chemical and biological drugs were removed from further analysis. c. Core analysis of the overlapping molecules associated with COVID-19 and SNCA revealed “Neuroinflammation Signaling Pathway” (p-value of 1.72 x 10^-16) as the top enriched signaling pathway followed by the “Role of hypercytokinemia/hyperchemokinemia in the Pathogenesis of Influenza,” also known as cytokine storms (p-value of 2.51 x 10^-14).
Figure 5.
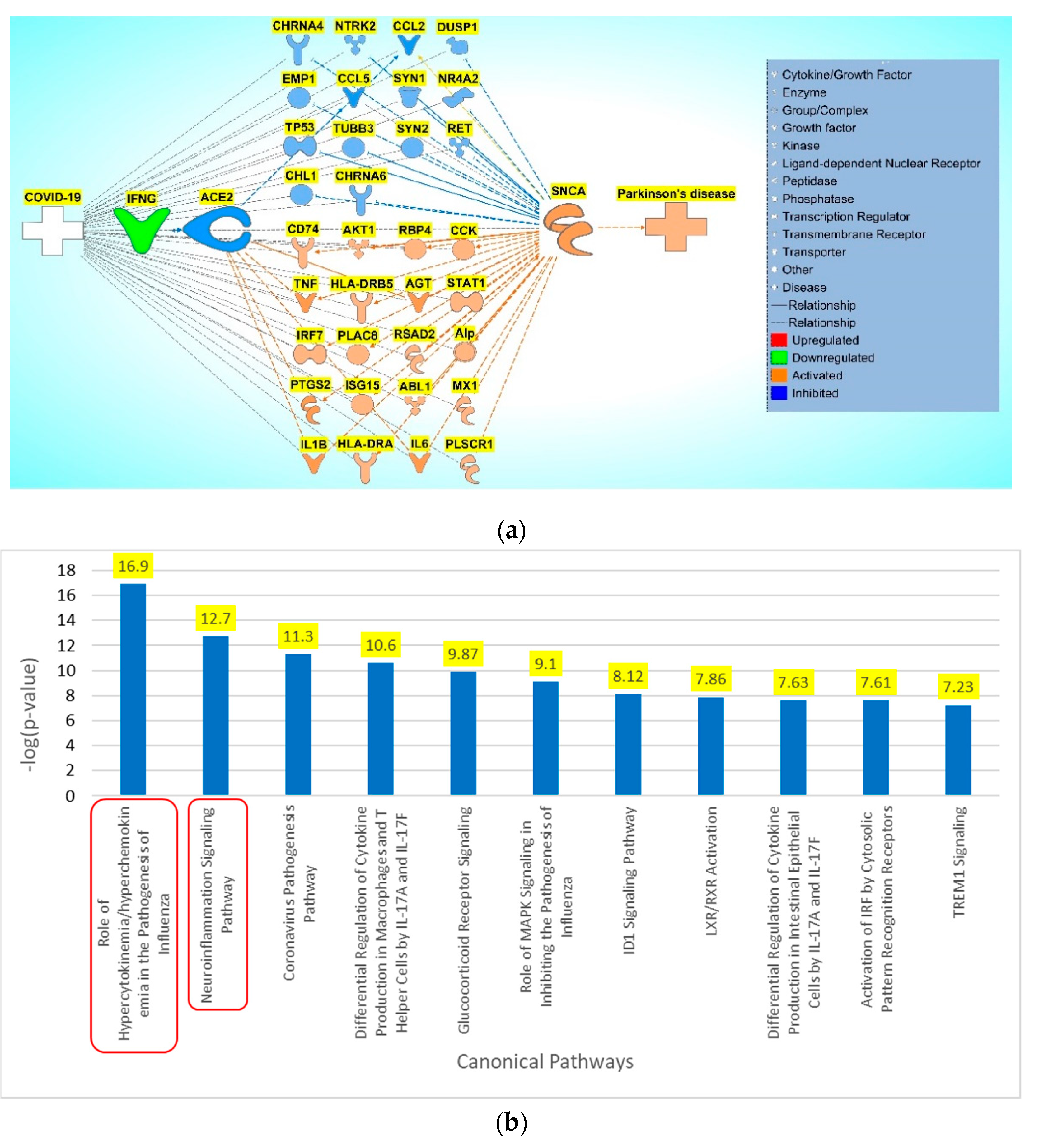
a. COVID-19 mediated downregulation of IFNG leads to the downregulation of ACE2. The downregulation of ACE2 affects the 34 intermediary molecules (14 predicted inhibited molecules (CCL2, CCL5, CHL1, CHRNA4, CHRNA6, DUSP1, EMP1, NR4A2, NTRK2, RET, SYN1, SYN2, TP53 and TUBB3) and 20 predicted activated molecules (ABL1, AGT, AKT1, Alp, CCK, CD74, HLA-DRA, HLA-DRB5, IL6, IL1B, IRF7, ISG15, MX1, PLAC8, PLSCR1, PTGS2, RBP4, RSAD2, STAT1, and TNF)) associated with COVID-19 and SNCA. The downregulation of ACE2 leads to an overall predicted activation of SNCA and subsequent predicted activation of PD pathogenesis. b. Core analysis revealed the top two most significant signaling pathways involved in the modulation of COVID-19 on SNCA are the NISP (p-value of 1.26 x 10−17) and the role of hypercytokinemia/hyperchemokinemia, also known as “cytokine storms” (p-value of 1.99 x 10−13).
Figure 5.
a. COVID-19 mediated downregulation of IFNG leads to the downregulation of ACE2. The downregulation of ACE2 affects the 34 intermediary molecules (14 predicted inhibited molecules (CCL2, CCL5, CHL1, CHRNA4, CHRNA6, DUSP1, EMP1, NR4A2, NTRK2, RET, SYN1, SYN2, TP53 and TUBB3) and 20 predicted activated molecules (ABL1, AGT, AKT1, Alp, CCK, CD74, HLA-DRA, HLA-DRB5, IL6, IL1B, IRF7, ISG15, MX1, PLAC8, PLSCR1, PTGS2, RBP4, RSAD2, STAT1, and TNF)) associated with COVID-19 and SNCA. The downregulation of ACE2 leads to an overall predicted activation of SNCA and subsequent predicted activation of PD pathogenesis. b. Core analysis revealed the top two most significant signaling pathways involved in the modulation of COVID-19 on SNCA are the NISP (p-value of 1.26 x 10−17) and the role of hypercytokinemia/hyperchemokinemia, also known as “cytokine storms” (p-value of 1.99 x 10−13).
Figure 6.
a. COVID-19 –induced inhibition via down regulation of IFNG inhibits ACE2 leading to activation of IL1B and AGT and inhibition of CCL5, causing an overall activation of SNCA. b. Downstream Effect analysis was performed on the intermediary molecules computing an overall z-score of 2.216187 and an overall p-value of 0.026685.
Figure 6.
a. COVID-19 –induced inhibition via down regulation of IFNG inhibits ACE2 leading to activation of IL1B and AGT and inhibition of CCL5, causing an overall activation of SNCA. b. Downstream Effect analysis was performed on the intermediary molecules computing an overall z-score of 2.216187 and an overall p-value of 0.026685.
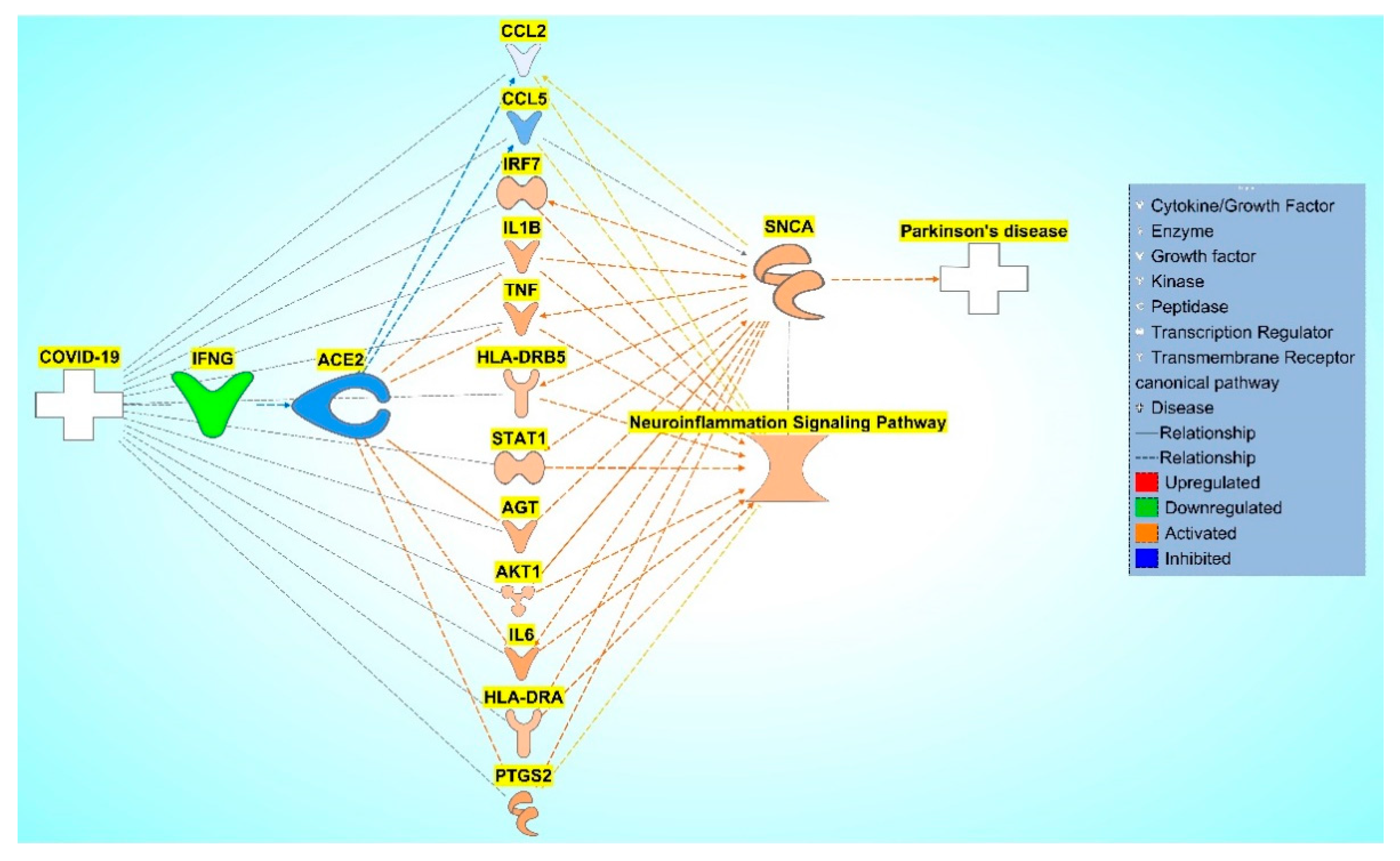
Figure 7.
COVID-19 induced inhibition of ACE2 upregulates IRF7, IL1B, TNF, HLA-DRB5, STAT1, AGT, AKT1, IL6, HLA-DRA, PTGS2, and inhibits CCL2 and CCL5, causing increased activity of the NISP and an overall increase in SNCA expression, eventually leading to PD progression.
Figure 7.
COVID-19 induced inhibition of ACE2 upregulates IRF7, IL1B, TNF, HLA-DRB5, STAT1, AGT, AKT1, IL6, HLA-DRA, PTGS2, and inhibits CCL2 and CCL5, causing increased activity of the NISP and an overall increase in SNCA expression, eventually leading to PD progression.
Figure 8.
a. COVID-19 induced inhibition of ACE2 upregulates IRF7, IL1B, TNF, HLA-DRB5, STAT1, AGT, AKT1, IL6, HLA-DRA, PTGS2, and inhibits CCL2 and CCL5, causing increased activity of the NISP and an overall increase in SNCA expression, eventually leading to PD progression. b. Downstream Effect analysis was performed on the intermediary computing an overall p-value of 0.000027 and an overall z-score of -4.199345911.
Figure 8.
a. COVID-19 induced inhibition of ACE2 upregulates IRF7, IL1B, TNF, HLA-DRB5, STAT1, AGT, AKT1, IL6, HLA-DRA, PTGS2, and inhibits CCL2 and CCL5, causing increased activity of the NISP and an overall increase in SNCA expression, eventually leading to PD progression. b. Downstream Effect analysis was performed on the intermediary computing an overall p-value of 0.000027 and an overall z-score of -4.199345911.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).