1. Introduction
Obstructive sleep apnea (OSA) is a common chronic respiratory disorder in which the upper airway repetitively collapses during sleep. According to the latest epidemiological study, it is estimated that one billion persons have OSA, and that half of these people have moderate or more severe forms [
1]. These individuals with moderate or severe OSA have a greater risk of experiencing a cardiovascular event [
2], and also show a higher risk of all-cause mortality [
3]. Sleep apnea has been also associated with ocular diseases such as glaucoma, floppy eyelid syndrome, nonarteritic anterior ischemic optic neuropathy, papilledema, central serous retinopathy, retinal vein occlusion, keratoconus and ocular surface abnormalities [
4,
5,
6,
7].
OSA is a complex condition that causes several systemic disorders as a consequence of inflammatory and vascular alterations, including some described mechanisms such as changes in intrathoracic pressure and diminished venous drainage, sympathetic activation, intermittent hypoxemia, hypercapnia, oxidative stress and vascular endothelial dysfunction [
8].
Such intermediate mechanisms along with the hypoxemia produced can be controlled through treatment with continuous positive airway pressure (CPAP) [
8]. Effectively, CPAP is the treatment of choice for OSA. The device used for this treatment generates continuous pressure on the upper airway, acting as a pneumatic splint such that it prevents the periodic collapse of the upper airways during sleep.
Treatment with CPAP has been shown to normalize the thickness of the retinal layers and choroid in OSA patients after 3 and 12 months of follow up [
9]. Such effects were greater in the more vascularized retinal layers and choroid [
10].
Adequate anterior surface health including the cornea and tear film is important to protect the eye against infection and damage to deeper structures. Central corneal thickness (CCT) is a clinically relevant measurement in the preoperative exam for refractive surgery[
11] and for adjustment of intraocular pressure measures [
12].
Currently, the most popular method of measuring CCT in the ophthalmology office is ultrasound pachymetry (USP). This instrument is easily available and transportable, and has shown high intra- and inter-examiner and inter-instrument reproducibility when used to assess normal corneas [
13].
Several studies have examined the impacts of hypoxemia on both the thickness of the central cornea and corneal endothelium in individuals with OSA [
14,
15,
16,
17]. These investigations have shown a reduced CCT in those with OSA.[
14,
16,
17] Hence, hypoxemia has been related to reduced CCT[
18]. However, no changes in CCT were detected in subjects with OSA following a mean of 9 months of CPAP therapy [
19].
As OSA is a chronic disorder, it would be interesting to closely monitor these subjects over time, paying special attention to the impacts of CPAP therapy, as this could interfere with observations in patients with OSA. This is the first study with a follow-up period of 12 months to look at the effect of CPAP on CCT in subjects with OSA.
2. Materials and methods
2.1. Study participants
This was a cohort study with 12 months of prospective follow up. Subjects were consecutively enrolled when newly diagnosed with OSA, and when CPAP treatment was indicated according to the results of an overnight polysomnography at the Sleep Unit[
20]. The study protocol adhered to the principles of the Declaration of Helsinki. The study protocol received approval from the hospital Ethics Committee (Act no. 219, Ref: 2240). Written informed consent was obtained from all participants.
Patient data including demographic characteristics, sleep patterns, medical history, medication and daily habits were collected in a standard questionnaire completed before the sleep study. Candidate participants were assessed at the Ophthalmology Dept. within three days of OSA diagnosis. After this baseline ophthalmology visit, participants were started on CPAP. Those who fulfilled the selection criteria were scheduled for a second and a third visit at 3 months and 12 months. One ophthalmologist conducted all assessments. Inclusion criteria were: age older than 18 years, diagnosis of OSA with CPAP treatment indicated [
20], visual acuity > 0.6, sphere range ± 5 diopters, cylinder range ± 3 diopters, and corneal angle > III Shaffer. Exclusion criteria were: OSA diagnosed before the study outset, systemic diseases such as diabetes mellitus, chronic kidney disease, cancer, gout, rheumatoid arthritis, systemic lupus erythematosus or another sleep disorder. Also excluded were subjects with chronic conditions that could affect oxygenation, such as chronic obstructive pulmonary disease, bronchial asthma, interstitial lung disease, central sleep apnea and cardiac disease. To examine the separate effects of OSA and CPAP on CCT, we did not consider for inclusion subjects subjected to eye surgery, or those with a preexisting corneal pathology, experiencing ocular trauma, contact lens wearers, dry eye syndrome, uveitis, optic neuritis, retinal disease, opaque ocular media or non-controlled glaucoma.
2.2. Overnight polysomnography
All subjects underwent an overnight polysomnography as this is the gold standard procedure for sleep studies according to established recommendations[
21]. The procedure started at 12 pm and ended at 7 am, and involved monitoring usual signals, and manual correction of all recordings as recommended [
21].
The polysomnogram was carried out with a Compumedic Sleep VS polysomnograph (Compumedics Sleep Ltd, Abbot sford, Vic., Australia). Three electroencephalogram channels, electro-oculogram, and submental (chin) and anterior tibial electromyograms were monitored. Oronasal flow was studied by a three-way thermistor (triple thermistor air flow thermistor, Compumedics ® Sleep). We also recorded snoring, respiratory effort by thoracic and abdominal belts, electrocardiogram and SpO
2 using a digital pulse oxymeter. Apnea was defined as the absence of oronasal flow lasting at least 10 s and hypopnea as a reduction in air flow of > 50% accompanied by dips in SpO
2 ≥ 3%, with or without accompanying arousals. Following conventional criteria, based on whether or not there was thoracic-abdominal effort, apnea was classified as obstructive, mixed or central. Arousals were established as a sudden change in the EEG rate which could include theta waves, alpha waves and/or rates above 16 Hz excluding sleep spindles, lasting more than 3 s. The apnea/hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour of sleep. The following variables were analyzed after polysomnography: sleep latency in minutes; sleep efficiency; percentage of wakefulness after sleep onset; percentage of REM sleep and NREM stages; arousal index, and periodic limb movement index (PLM). PLM syndrome was diagnosed when the incidence was more than 15/h and the PLMs were not related to respiratory events. Sleep efficiency was defined as the ratio between total sleep time and time in bed, expressed as percentage. As an indication of the stability and quality of sleep, we also calculated the sum of the percentage of slow-wave sleep and REM sleep. The respiratory variables recorded were: SpO
2 in wakefulness; minimum SpO
2 value; number of dips in SpO
2 ≥ 3% per hour of sleep (ODI); the sleep time spent with SpO
2 <90% (T90); percentage of sleep time in apnea and hypopnea, and AHI. ODI quantifies the episodes of intermittent hypoxemia during the SpO
2 recording. T90 is a parameter for overall evaluation of the severity of the disorder in nocturnal SpO
2, since an SpO
2 of < 90% correlates with a PaO
2 of < 60 mm Hg, which defines severe hypoxemia. The primary variable used in the study was the AHI. An AHI of ≥ 10 was considered to be compatible with OSA. The polysomnography was considered of diagnostic validity when at least 180 min of sleep was recorded. OSA severity was classified according to AHI[
20]: as mild (AHI ≥ 5 and < 15 events/h), moderate (AHI ≥ 15 and < 30 events/h) or severe (AHI ≥ 30 events/h).
2.3. Ophthalmologic examination
In each assessment session from 5 to 8 pm the following data were collected: weight and height, auto-refraction, best-corrected visual acuity, slit lamp, CCT, IOP by applanation tonometry (mean of 3 measures), and fundus and gonioscopy exam data. Medical history was noted in the baseline visit.
The first test in each participant was USP with an SP-3000USP (Tomey Inc. Nagoya, Japan). Instrument calibration was verified by the manufacturer at the study outset. Velocity (acoustic index) was set to 1640 m/s. The cornea was anesthetized with 4m/ml topical oxybuprocaine (Alcon Healthcare; Barcelona, Spain). The subject was seated on a chair and asked to fix the gaze on a distant target. Meanwhile, the ultrasound probe was sterilized, aligned perpendicularly to the mid-pupillary axis of the cornea, and gently placed in contact with the cornea. Three consecutive measurements were recorded and the mean of these entered in the analysis.
2.4. CPAP
After the baseline ophthalmologic exam, subjects diagnosed with OSA were started on CPAP therapy. Treatment recommendations in those patients diagnosed with OSA were based on the National Clinical Guidelines [
22].
The patients were evaluated after 12 months of treatment. The level of adherence to CPAP treatment was recorded in each patient by the internal clocks of the devices. In the present study, patients received information about autoCPAP and slept at home with the device (Autoset-10 AirSense TM ; ResMed, Sydney, Australia) overnight. The pressure was set to start automatically, after 10 minutes of adaptation (from 5 cm H2O to a maximum of 17 cm H2O). The recording was considered valid acceptable if all of the following criteria were met: the recording on the autoCPAP device was at least 5 hours and the average leak was less than 0.4 L/sec in the statistics obtained from the autoCPAP. The recording was repeated two more nights if the test was unacceptable. Optimal pressure was determined visually on raw autoCPAP data using pressure analysis that included 90% of periods with a leak less than 0.4 L/s (90th percentile)
The treatment adherence information recorded by the device's software was obtained 3 and 12 months later. Adequate adherence to CPAP was defined as its use for > 3.5 h per night [
20,
23]. An auto-CPAP was used to titrate and set the pressure for each subject. During the treatment period, participants were instructed not to use any topical medication that could affect the eye surface such as artificial tears or systemic drugs.
2.5. Statistical analysis
A descriptive analysis was made of qualitative variables through calculation of counts (n) and proportions (%), and of quantitative variables through arithmetic means and standard deviations. Goodness of fit to a normal distribution was determined by the Shapiro-Wilk test. If the data followed a normal distribution, parametric tests were used and if not, the corresponding non-parametric test was used.
Data were compared by repeated measures ANOVA (parametric) and the Friedman test (non parametric). As post-hoc tests for multiple comparisons, we used Bonferroni correction and the Wilcoxon test (according to the normality of the data distribution).
Possible associations between polysomnographic variables before CPAP treatment and changes in ophthalmologic variables after CPAP treatment in OSA patients were assessed through Spearman’s rank correlation.
All statistical tests were performed using the software package SPSS v.25 (IBM, Armonk, New York). Significance was set at p < 0.05. All comparisons were two-tailed.
3. Results
Out of 30 patients initially enrolled, the final study sample comprised 40 eyes of 20 participants. A flow chart showing the patient selection procedure is provided in
Figure 1.
Baseline patient characteristics are provided in
Table 1. All 20 participants had severe OSA. The mean number of hours of CPAP per night was adequate (5.23 ± 1.77h). Polysomnography data before starting CPAP of CT90, SpO2 (mean and minimum) and ODI indicated hypoxemia. There was a high prevalence of glaucoma (5%).
In
Table 2, we provide the CCT, IOP, and standard automated perimetry data obtained at baseline and in the 3- and 12-month follow-up visits. These data revealed an increase in IOP at 12 months of follow up compared to 3 months.
Correlations between hours of CPAP use or pretreatment polysomnography variables and the changes in CCT produced in response to this treatment can be seen in
Table 3.
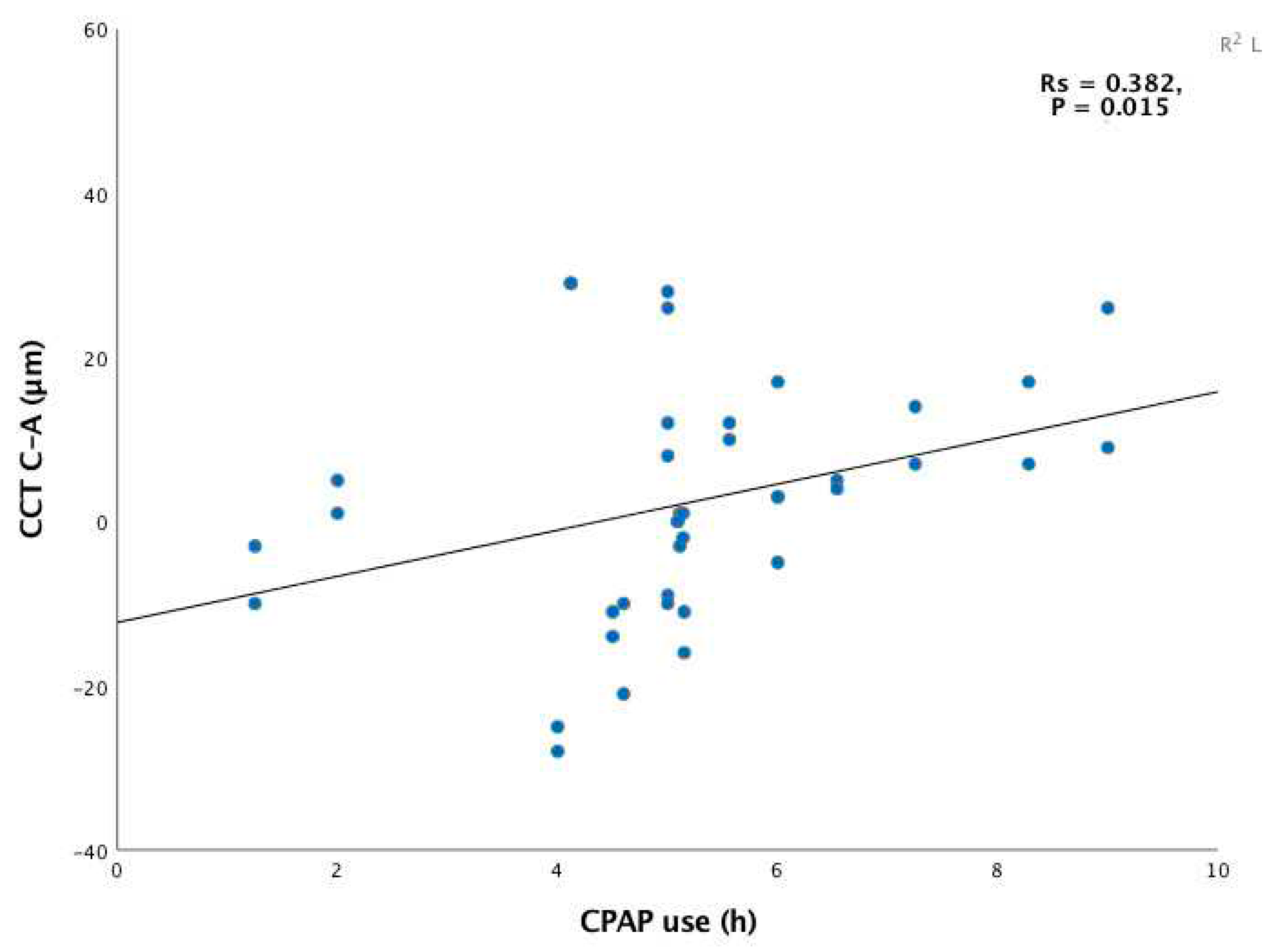
These data revealed significant positive correlation between CPAP duration and CCT changes produced from baseline to 12 months (
Figure 2).
In other words, the longer each patient adhered to treatment, the greater the CCT thickening produced. Further significant positive correlation was observed between the hypoxemic variables ODI and CT90 pre-CPAP and the change produced in CCT after 12 months of treatment. Thus, patients with a greater percentage of desaturation pre-CPAP showed a greater increase in CCT after 12 months of follow up. No significant correlations were observed between CCT and hours of CPAP use or polysomnographic variables pre-CPAP at 3 months.
4. Discussion
This study examines the impacts on CCT of 12 months of CPAP therapy in subjects with severe OSA. Our findings indicate a slight increase in CCT in response to 3 and 12 months of this treatment although without significance. Positive correlation was also observed between the number of hours of CPAP use and CCT change at 12 months of follow up (
Figure 2). This correlation was, however, not significant after only 3 months of treatment. Similarly, significant positive correlation was observed between episodes of intermittent hypoxia (ODI) and severity of hypoxemia (CT90) at 12 months but not at 3 months.
Individuals with OSA feature modified corneal parameters compared to subjects without this sleep disorder [
14,
15,
16,
17] including a thinner CCT [
14,
16,
17]. Sleep apnea has also been associated with hypoxemia [
8]. Hypoxemia induces anaerobic glycolysis and hypoxia-induced stromal acidosis [
24]. This could explain the reduced CCT observed in OSA patients. Further, acute hypoxia can lead to an increased CCT [
25], but this inflammation diminishes over time. Consistently, prolonged exposure to conditions of hypoxia, as occurs in contact lens wearers, has been linked to keratocyte loss and reduced corneal thickness [
18]. Moreover, in situations of hypoxia, nuclear factor-κB [
26] is activated along with cytokines and both play an essential role in hypoxic inflammation. In the tears of individuals with keratoconus or subclinical keratoconus, overexpression has been reported of the proinflammatory cytokines TNF-α and IL-6 [
27]. Keratoconus involves progressive corneal thinning and has been associated with OSA[
28]. These cytokines could in part explain the corneal thinning observed in OSA and the link detected between keratoconus and OSA.
Chalkiadaki et al. [
15] were unable to correlate CCT with variables indicating hypoxemia in subjects with OSA. Bojarun et al. [
14] described a negative relationship between ODI and CCT, and a positive one between SpO2 and CCT. These disparate results could reflect a different severity of OSA, as participants of the study by Chalkiadaki et al.[
15] had severe OSA unlike those of the study by Bojarun et al.[
14]. Besides, Chalkiadaki et al.[
15] specified that all ophthalmological measurements were also made before the onset of CPAP treatment, while this was not, however, mentioned by Bojarun et al.[
14].
It has been recently shown that CPAP therapy improves corneal endothelial parameters such as the coefficient of variation in cell area and the hexagonal cell ratio [
19]. In line with our findings, these last authors observed a slight non significant increase in CCT after starting with CPAP. However, unlike in our study, no correlation was detected between CCT and hours of CPAP device use. It should be considered that we used a USP to measure CCT and Chalkiadaki et al. [
19] used a non contact specular microscope. Both devices show high intraobserver reproducibility and accuracy for measuring CCT although the devices are not interchangeable [
29]. USP has shown the greater degree of intraobserver reproducibility [
29]. It should also be mentioned that all our participants underwent an average of > 5 h/day of CPAP (described as clinically effective) over at least 12 months. Chalkiadaki et al. [
19] reported that 25.9% of subjects received at least 9 months of CPAP yet in the remaining participants the assessment period was between 3 and 18 months. We observed positive correlation between hours of CPAP and CCT after 12 months of this treatment. However, this was not observed after only 3 months of CPAP. It seems that CPAP duration and, thus the correction of the physiopathological mechanisms of OSA
3,8,16, could explain the difference in results with respect to our study.
In our cohort, subjects with greater hypoxemia before CPAP therapy showed a greater CCT increase after one year of CPAP, suggesting correlation between intermittent hypoxemia (ODI) or hypoxemia severity (CT90) pre-CPAP and CCT. These new findings suggest that correcting the overall burden of hypoxemia has a beneficial effect on the cornea. It has been described that subjects with chronic obstructive pulmonary disease show more corneal endothelial damage following cataract surgery [
30]. Our findings point to a need to determine symptoms suggestive of OSA and an indication for CPAP in a setting of intraocular surgery such as cataract surgery. The potential impact of correcting respiratory events and associated hypoxemia could help in the recovery of the cornea. It would have been interesting to undergo a corneal study with a non contact specular microscope to determine whether the changes produced in CCT were accompanied by corneal endothelium changes. This should be a topic of further study.
We observed an increase in IOP after 12 months of CPAP therapy coinciding with a non-significant increase in CCT. It has been shown that CCT significantly affects IOP readings [
31].
Our study has some limitations. The main one was that sample size was small. The reasons for this were difficulties in recruiting subjects diagnosed with OSA with the gold standard test and who also lack the systemic diseases included in the exclusion criteria. Another reason for the small sample size is that it is also difficult to adequately follow these patients and thus confirm suitable adherence to CPAP. Notwithstanding, the 12 months duration of follow up and significant findings obtained are strong points of our study.
In conclusion, this is the first study to examine the effect of 12 months of CPAP on CCT in individuals with OSA. We found that the greater the use of CPAP the greater the improvement produced in CCT, and that subjects with a greater extent of hypoxemia pre-CPAP had a greater capacity for CCT improvement. These observations did not appear early on, but rather they occurred after a full year of CPAP treatment.