1. Introduction
The Xenarthra is a superorder of placental mammals endemic to Central and South America, including the orders, Pilosa (anteaters and sloths) and Cingulata (armadillos). The giant anteater (
Myrmecophaga tridactyla) is member of the family, Myrmecophagidae. According to the International Union for Conservation of Nature Red List of Threatened Species, giant anteaters are considered as vulnerable and extinct species in different Latin American countries. Several factors, such as predatory hunting, forest burning, and blunt trauma secondary to wildlife vehicle collisions, contribute to the reduction in the giant anteater population [
1,
2,
3,
4,
5,
6,
7]. The lack of governmental programs for conservation [
8], slow reproductive cycles in nature (long gestation periods, birth of only one cub per year, and extended parental care) [
2], and difficulties with captive-breeding programs are factors that influence species preservation [
9]. The population on giant anteater has been increasingly affected by vehicle collisions. Studies have shown that passage rates are higher in males than in females in giant anteaters living near roads (< 2 km) [
10,
11].
The scarcity of studies involving reproductive understanding and available reproductive protocols and biotechnologies also contribute to challenges in reproduction [
9]. Morphological and histopathological descriptions of the reproductive tracts of this species are highly relevant for determining the typical features or recognizing background lesions in these organs, which may interfere with individual fertility. The lack of knowledge in this area can hinder conservation work if normal and abnormal findings in sexual organs are not adequately addressed. A recent study identified the structural and ultrastructural morphology of the prostate gland and revealed its histological and immunohistochemical features in giant anteaters [
12]. Another study described the morphology and histology of the reproductive tracts [
13].
Different lesions and female reproductive disorders have been described in several domestic and wild species, including lesser anteaters [
14,
15,
16,
17]. However, to the best of our knowledge, studies investigating and analyzing the histochemical features of the background and common lesion alterations in giant female anteaters are rare [
18]. Histopathological characterization of these alterations is important for understanding individual fertility. Thus, this study aimed to increase the information surrounding the ovaries and uterus of giant anteaters through the histological analysis of tissue samples to improve the diagnosis of reproductive alterations.
2. Materials and Methods
2.1. Ethical Approval
This study was approved by three Brazilian committees responsible for conducting wildlife research: System for Genetic Heritage and Associated Traditional Knowledge (#C1018E9), Chico Mendes Institute for Biodiversity Conservation (#7685-1), and Sao Paulo State University Committee on the Use of Animals in Research (177/2020).
2.2. Sample Collection
Six female giant anteaters were included. Their ages were determined based on their body weights according to a previous literature [
13]. Divisions were made to avoid any misinterpretation of histological or histochemical findings related or not with sexual maturity. Representative sections of both the ovaries and uterus were systematically collected and fixed in formalin during necropsy. Samples were collected at São Paulo State University (UNESP, Brazil), University of São Paulo (USP, Brazil), and Universidade Federal de Minas Gerais (UFMG, Brazil) between January 2000 and December 2021.
2.3. Histochemistry
For the histochemical analysis, tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Afterwards, 4-µM thick tissue sections were stained with hematoxylin and eosin for morphological evaluation. Periodic acid-Schiff (PAS) and Masson's trichrome staining were performed to evaluate structural findings.
2.4. Morphological and histological analysis
Ovarian and uterine morphological analyses were performed according to the methods established by Rossi et al. [
17] and Fromme et al. [
13]. The evaluation was performed in a double-blind fashion: one at Sao Paulo State University (N.S.R.) and the other at North Carolina State University (T.T.N.W.). Due to the small number of subjects included, a descriptive analysis using qualitative data was instead performed. The lesions were described to determine the frequency of each lesion.
3. Results
3.1. Gross evaluation
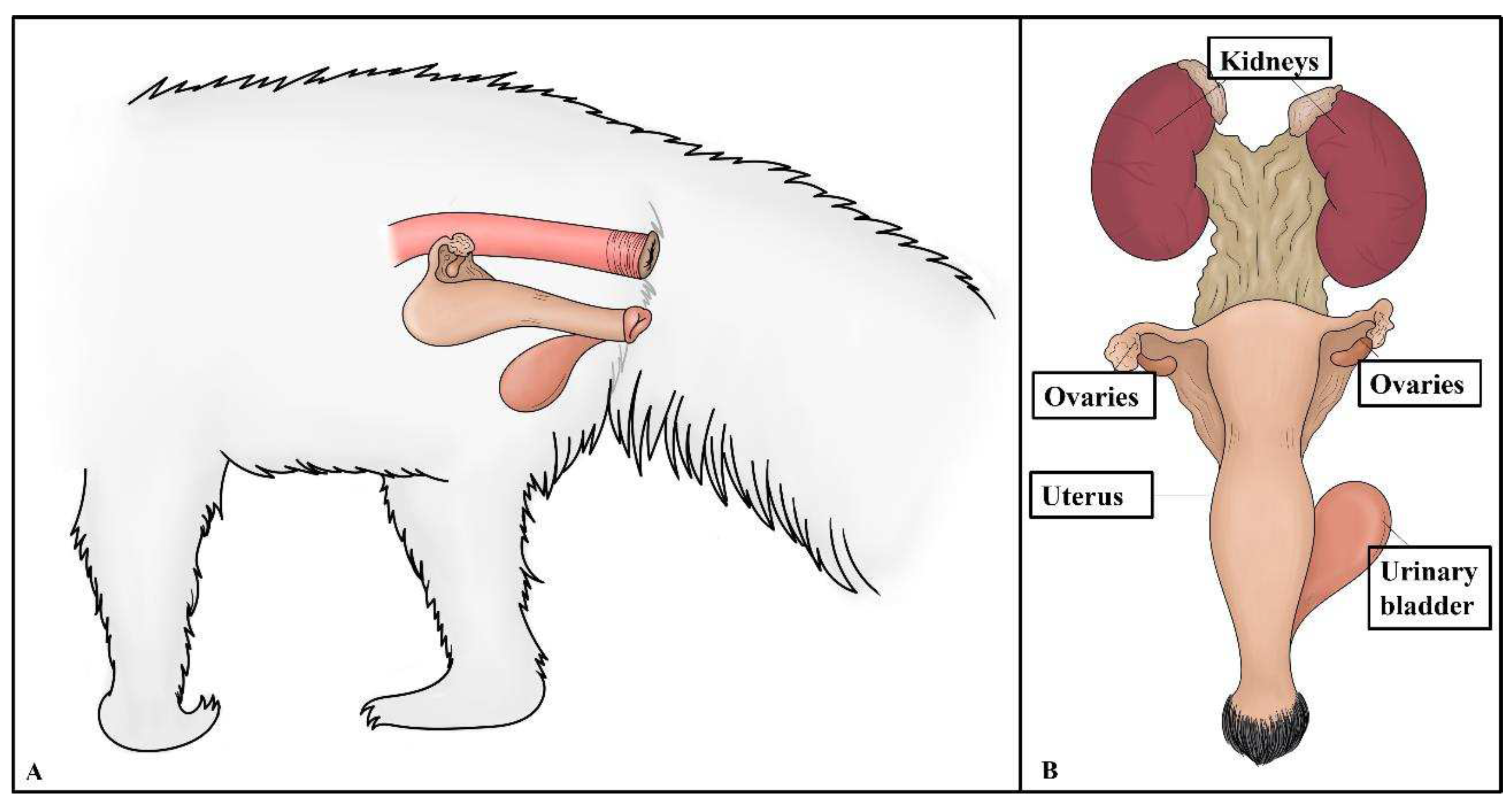
The reproductive tracts of six free-living giant anteater females were collected during the postmortem examination. After the postmortem examination, ovary and uterine samples from five adult (> 2 years) and one young (< 2 years) subjects were grossly and microscopically assessed. The ovaries showed an ovoid morphology and measured approximately 2 cm in length, 0.5 cm in width, and 0.5 in height. The simple uteri were approximately 3 cm in length, 2.5 cm in width, and 2 cm in height. The ovaries and uterus were located dorsal to the urinary bladder and ventral to the rectum (
Figure 1).
3.2. Microscopic evaluation
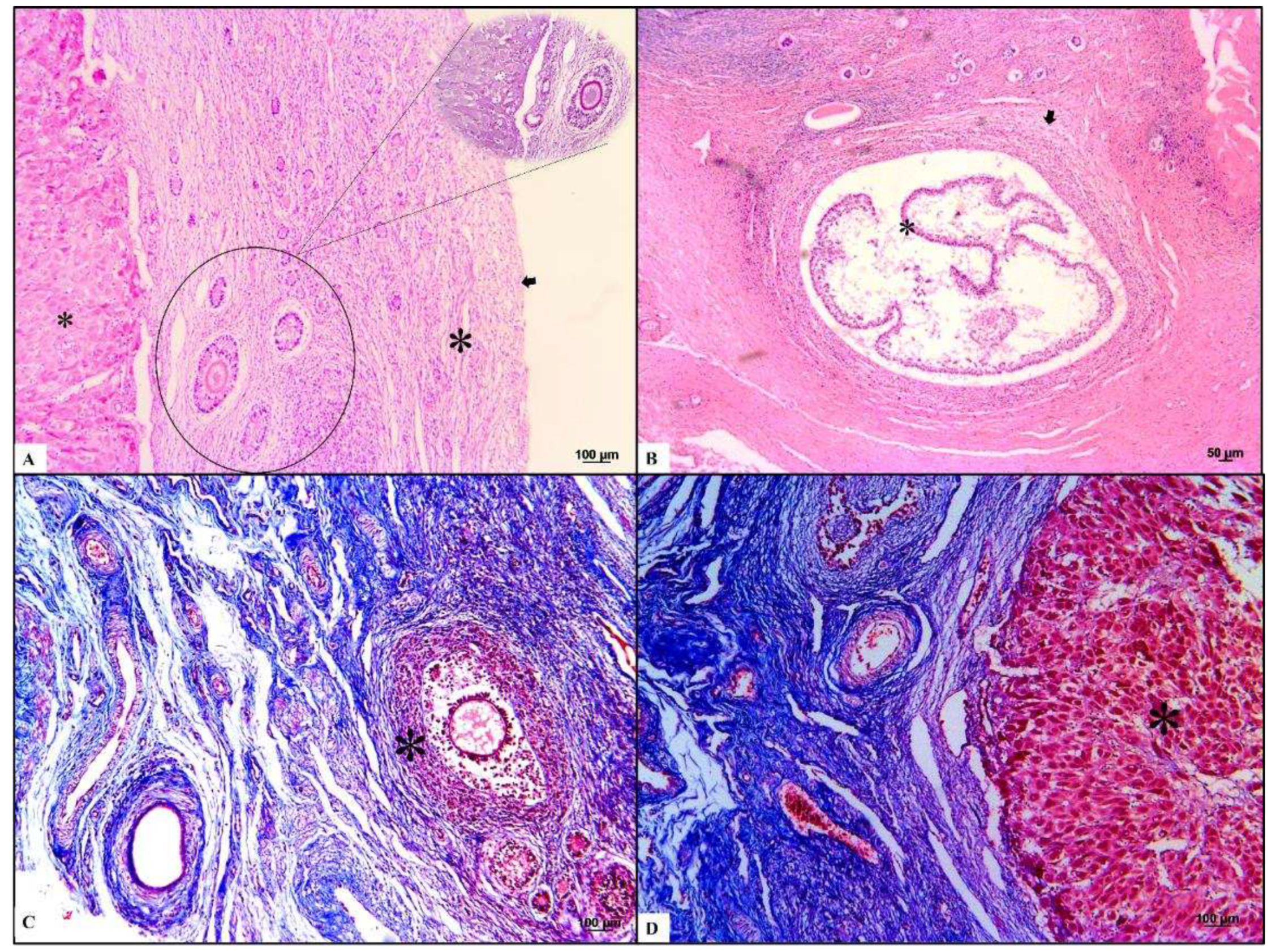
Microscopically, the ovaries demonstrated an architecture consisting of a simple cuboidal epithelium, ovaries, serosa, and dense connective tissue in the tunica albuginea. The ovarian parenchyma comprised two zones. The cortical zone was located under the tunica albuginea and contained ovarian follicles at different stages of development (
Figure 2). They were classified according to the follicle and oocyte size into: primordial (mean diameter [follicle 88.24 µm; oocyte 47.2 µm]; mean follicle number: 58), primary (mean diameter [follicle 125.02 µm; oocyte 38.72 µm]; mean follicle number: 8), secondary (mean diameter [follicle 313.87 µm; oocyte 64.64 µm]; mean follicle number: 4), early antral (mean diameter [follicle 953.68 µm; oocyte 152,27 µm]; mean follicle number: 2), and antral (mean diameter [follicle 1806.89 µm; oocyte 374.42 µm]; mean follicle number: 1). The ovarian cortex surrounds the medullary zone, where the major vessels and nerves enter the ovary centrally and ramify to the periphery proximal to the follicles. We observed that the collagen fibers from the cortical and medullary zones, were colored blue with Masson trichrome staining, and the zona pellucida and teak ovarian cells were positive for PAS staining.
Common lesions, such as typical atretic follicles containing degenerative granulosa cells and oocytes, were observed. Additionally, corpora lutea were visualized as centrally filled with the remains of the blood clot that formed after ovulation, surrounded by granulosa lutein cells with lutein cells on the outside. Some dense, round connective tissue organization, consisting of corpora albicans, was also identified in the cortical zone. The most typical background lesions were thin-walled ovarian cysts filled with pale acidophilic residual degenerative oocytes often rounded by cell debris (
Figure 2).
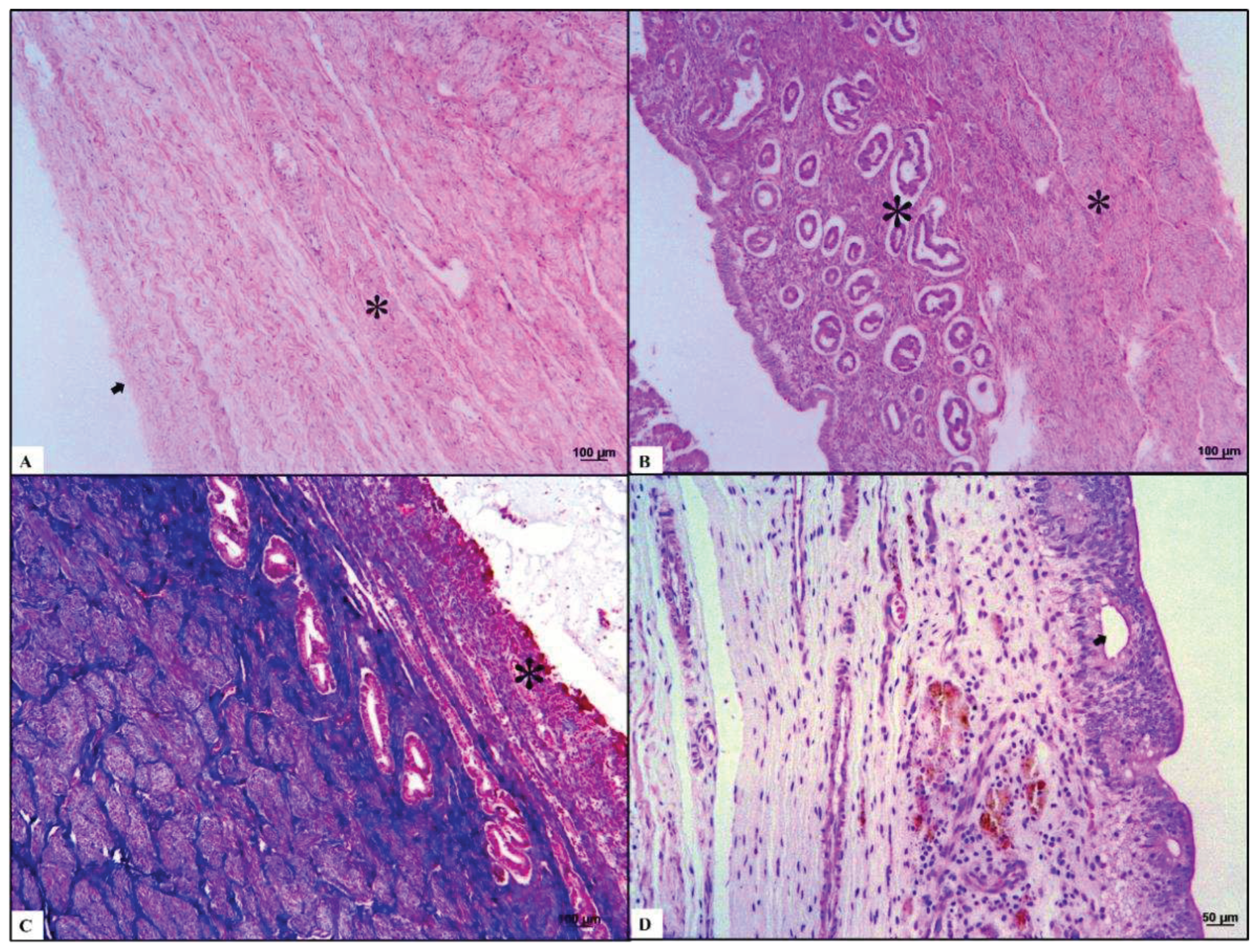
Three different layers were observed in the uterine samples. First, the perimetrium is composed of loose connective tissues located peripherally. Second, the myometrium consists of a thin external longitudinal layer and thick internal circular muscular layer. The simple cuboidal epithelium with the folded endometrium near the uterine lumen presented with simple tubular glands surrounded by dense fibrous connective tissue. We observed that the collagen fibers from the perimetrium and muscle fibers from the myometrium were blue and red, respectively, after Masson trichrome staining.
The most common lesion was mucometra, which is characterized by a discrete uterine lumen dilated with fluid. The most commonly identified background lesions were endometritis and metritis, consisting of a uterine lumen moderately dilated with fluid and discrete leukocytic infiltration in the endometrium and myometrium, respectively. One case showed endometrial cysts comprising thin-walled, round epithelial structures in the endometrium. Luminal secretions and epithelial cyst cells disposed of in the endometrial mucosa were positive in PAS staining (
Figure 3).
4. Discussion
Studies investigating and analyzing the histochemical features of the background and common lesion alterations in female giant anteaters are rare [
18]. Histopathological characterization of reproductive abnormalities is important for understanding individual fertility. Our results contribute to increasing the information surrounding the ovarian and uterine lesions of giant anteaters through histological analyses.
Previous studies have characterized the morphology and histology of the reproductive organs in giant anteaters [
11,
12]. Two studies involving female lesser anteaters (
Tamandua tetradactyla) were performed. Some ovarian backgrounds and common lesions have been reported, including atretic follicles, corpora lutea, and corpora albicans [
5,
17].
We consider our results to be similar to the structural findings observed in female giant anteater in a previous study [
13]. Furthermore, the background and common reproductive lesions visualized were already described in humans, domestic species, and lesser anteaters [
14,
15,
16].
We observed that the giant anteater's ovarian appearance was similar to that of dogs. It is essential to mention that zone distribution occurred for these two species. The follicles of giant anteaters were generally larger than those of dogs. Nevertheless, oocyte size, granulosa cell number, and follicle diameter compared to other developmental follicular stages are homologs of those observed in dogs [
19].
In giant anteaters and dogs, the atretic follicles have an irregular format, and granulosa cells were observed in apoptosis; the follicular cysts present with thick layers of luteinizing granulosa cells, amid loose stroma; the luminal space was filled with eosinophilic material, and the corpora lutea has several vacuolated cells [
19,
20].
This retrospective study could not evaluate uterine tubes because samples were not obtained during the research period. However, the structure of this species is already known. According to Fromme et al. [
13], it is possible to histologically identify the infundibulum, ampulla, and isthmus, which are composed of mucosal, muscular, and serosal layers in giant anteaters. These three portions show variations in the lumen extension and width of the muscular layer, which become smaller and more significant as they become more distant from the ovaries and closer to the uterus, respectively [
13].
We noted that the simple uterus in giant anteaters presented with extensive, well-marked fibrous tissue in the endometrium surrounding the tubular uterine glands. This feature is considered abnormal in domestic species, such as equines, interfering with an individual's fertility [
21,
22]. Additionally, we observed that the myometrial layer was more prominent in this species than in domestic species or lesser anteaters [
17,
23].
In giant anteaters and dogs, the uterine background lesions consisted of inflammatory infiltrates in different uterine layers, resulting in endometritis and metritis; moreover, luminal uterine secretion/edema was present, which may be associated with varying phases of the estrus cycle [
19,
20,
25,
26].
The critical conservation status of giant anteaters also affects their ecological and evolutionary importance in the environment [
27,
28]. Thus, research involving their reproductive tract and fertility is fundamental to improving species conservation and protection and guaranteeing the maintenance of research programs involving these individuals.
5. Conclusions
The results of the present study provide the first histochemical characterization of the background and common reproductive lesions in female giant anteaters. Overall, our results suggest the similarity between the ovarian and uterine pathophysiology of giant anteaters and those of other domestic species and humans. This information may help veterinary pathologists report reproductive alterations in female giant anteaters and allow them to characterize their impact on fertility.
Author Contributions
Conceptualization, F.B.C.M., N.S.R., C.E.F-A and T.T.N.W.; writing—original draft preparation F.B.C.M., N.S.R., C.E.F-A and T.T.N.W.; writing—review and editing, , F.B.C.M, N.S.R., Z.A.L., J.L.C-D, P.E.N.S, K.W., S.R.J.S.S, R.L.S, C.E.F-A and T.T.N.W.; visualization, F.B.C.M., N.S.R., C.E.F-A and T.T.N.W. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: “This research received no external funding” or “This research was funded by Morris Animal Foundation, grant number D21ZO-601” and “The APC was funded by Morris Animal Foundation”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of : System for Genetic Heritage and Associated Traditional Knowledge (#C1018E9), Chico Mendes Institute for Biodiversity Conservation (#7685-1), and Sao Paulo State University Committee on the Use of Animals in Research (177/2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We want to thank Morris Animal Foundation for the support and encouragement for the performance of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miranda, F.; Bertassoni, A.; Abba, A.M. Myrmecophaga tridactyla. The IUCN Red List of Threatened Species, 2014. [CrossRef]
- Medri ÍM, Mourão GM, Harada AY. Edentata: Dieta de Tamanduá-bandeira (Myrmecophaga tridactyla) no Pantanal da Nhecolândia, Brasil (ed. Fonseca, G.A.B. et al.); 2002 pp. 1413-4411.
- Vidolin, G.P.; et al. Planos de Conservação para Espécies de Mamíferos Ameaçados (ed. IAP/Projeto Paraná Biodiversidade); 2009, pp. 0-316.
- Brazil’s new president adds to global threat to science. Nature 2018, 563, 5–6. [CrossRef]
- Arenales, A.; Gardiner, C.H.; Miranda, F.R.; et al. Pathology of Free-Ranging and Captive Brazilian Anteaters. J Comp Pathol 2020, 180, 55–68. [Google Scholar] [CrossRef]
- Pinto, F.A. , Bager, A., Clevenger, A.P.; Grilo, C. Giant anteater (Myrmecophaga tridactyla) conservation in Brazil: Analysing the relative effects of fragmentation and mortality due to roads. Biol Conserv 2018, 228, 148–157. [Google Scholar] [CrossRef]
- de Freitas, C.H.; Justino, C.S.; Setz, E.Z. Road-kills of the giant anteater in south-eastern Brazil: 10 years monitoring spatial and temporal determinants. Wildl Res 2015, 41, 673–680. [Google Scholar] [CrossRef]
- IUCN Red List of Threatened Species. Choice reviews online 49 ISSN 2307-8235, 2012.
- Miranda, F.R.; et al. Avaliação do Risco de Extinção de Myrmecophaga tridactyla Linnaeus, 1758 no Brasil, Processo de avaliação do risco de extinção da fauna brasileira, 2015; Preprint at ICMBio. Available online: http://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/lista-de-especies/7049-mamiferos-myrmecophaga-tridactyla-tamandua-bandeira.html.
- Navas-Suárez, P.E.; Diaz-Delgado, J.; Caiaffa, M.G.; et al. Characterization of Traumatic Injuries Due to Motor Vehicle Collisions in Neotropical Wild Mammals. J Comp Pathol 2022, 197, 1–18. [Google Scholar] [CrossRef]
- Noonan, M.J.; Ascensão, F.; Yogui, D.R.; Desbiez, A.L. Roads as ecological traps for giant anteaters. Anim Conserv 2021, 25, 182e194. [Google Scholar] [CrossRef]
- Moura, F.; Sampaio, L.; Kobayashi, P.; Laufer-Amorim, R.; Ferreira, J.C.; Watanabe, T.T.N.; Fonseca-Alves, C.E. Structural and Ultrastructural Morphological Evaluation of Giant Anteater (Myrmecophaga tridactyla) Prostate Gland. Biology (Basel) 2021, 10, 231. [Google Scholar] [CrossRef]
- Fromme, L.; Yogui, D.R.; Alves, M.H.; Desbiez, A.L.J.; Langeheine, M.; Quagliatto, A.; Siebert, U.; Brehm. R. Morphology of the genital organs of male and female giant anteaters (Myrmecophaga tridactyla). PeerJ 2021, 11, e11945. [Google Scholar] [CrossRef]
- Fromme, L. , Yogui, D.R., Alves, M.H.; et al. Ovarian Filariasis in a Wild Southern Tamandua (Tamanduatetradactyla; Mammalia: Myrmecophagidae). Pathogens 2022, 11, 918. [Google Scholar] [CrossRef]
- Pavone, M.E.; Hirshfeld-Cytron, J.; Tingen, C.; Thomas, C.; Thomas, J.; Lowe, M.P.; Schink, J.C.; Woodruff, T.K. Human ovarian tissue cortex surrounding benign and malignant lesions. Reprod Sci 2014, 21, 582–9. [Google Scholar] [CrossRef]
- McInnes, E.F. Background Lesions in Laboratory Animals: A Color Atlas; Saunders/Elsevier: Edinburgh, 2012; pp. 101–122. [Google Scholar]
- Rossi, L.F.; Luaces, J.P.; Marcos, H.J.; Cetica, P.D.; Gachen, G.; Jimeno, G.P.; Merani, M.S. Female reproductive tract of the lesser anteater (Tamandua tetradactyla, myrmecophagidae, Xenarthra). Anatomy and histology. J Morphol 2011, 272, 1307–13. [Google Scholar] [CrossRef]
- Macêdo, A.A.; Silva, A.P.C.; Pessanha, Â.T.; Soave, S.A.; Paixão, T.A.; Santos, R.L. ENDOMETRITE PURULENTA EM TAMANDUÁ-MIRIM (TAMANDUA TETRADACTYLA) E TAMANDUÁ-BANDEIRA (MYRMECOPHAGA TRIDACTYLA). Arch Vet Sci 2013, 18. [Google Scholar]
- Songsasen, N.; Fickes, A.; Pukazhenthi, B.S.; Wildt, D.E. Follicular morphology, oocyte diameter and localisation of fibroblast growth factors in the domestic dog ovary. Reprod Domest Anim 2009, 44, 65–70. [Google Scholar] [CrossRef]
- Chandra SA, Adler RR. Frequency of different estrous stages in purpose-bred beagles: a retrospective study. Toxicol Pathol 2008, 36, 944–9. [Google Scholar] [CrossRef]
- Rehm, S.; Stanislaus, D.J.; Williams, A.M. Estrous cycle-dependent histology and review of sex steroid receptor expression in dog reproductive tissues and mammary gland and associated hormone levels. Birth Defects Res B Dev Reprod Toxicol 2007, 80, 233–45. [Google Scholar] [CrossRef]
- Blanchard, T.L.; Garcia, M.C.; Kintner, L.D.; Kenney, R.M. Investigation of the representativeness of a single endometrial sample and the use of trichrome staining to aid in the detection of endometrial fibrosis in the mare. Theriogenology 1987, 28, 445–50. [Google Scholar] [CrossRef]
- Snider, T.A.; Sepoy, C.; Holyoak, G.R. Equine endometrial biopsy reviewed: observation, interpretation, and application of histopathologic data. Theriogenology 2011, 75, 1567–81. [Google Scholar] [CrossRef]
- Samuelson, D.A. Textbook of Veterinary Histology; Saunders-Elsevier: St. Louis Mo, 2007. [Google Scholar]
- Schulman, M.L.; Bolton, L.A. Uterine horn aplasia with complications in two mixed-breed bitches. J S Afr Vet 1997, 68, 150–3. [Google Scholar] [CrossRef]
- Darko, M.; Milan, A.; Slobodanka, V.; Svetlana, N.; Vladimir, M. Morphological Characteristics and Expression of Estrogen and Progesterone Receptors in the Canine Endometrium During the Estrus Cycle, Cystic Endometrial Hyperplasia and Pyometra. Acta Vet 2018, 68, 239–50. [Google Scholar]
- Gaudin, T.J.; Croft, D.A. Paleogene Xenarthra and the evolution of South American mammals. J Mammal 2015, 96, 622–634. [Google Scholar] [CrossRef]
- Patzl, M.; Schwarzenberger, F.; Osmann, C.; Bamberg, E.; Bartmann, W. Monitoring ovarian cycle and pregnancy in the giant anteater (Myrmecophaga tridactyla) by faecal progestagen and oestrogen analysis. Anim Reprod Sci 1998, 53, 209–19. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).